Abstract
Breast cancer (BC) is the most common cancer among females with more than 2 million new cases diagnosed worldwide in 2018. Although the prognosis in the majority of cases in the early stages combined with appropriate treatment is positive, there are still about 30% of patients who will develop locoregional diseases and distant metastases. Molecular imaging is very important in the diagnosis, staging, follow-up, and radiotherapy planning. Additionally, it is useful in characterizing lesions, prognosis, and therapy response in BC patients. Nuclear medicine imaging modalities (SPECT and PET) are of indispensable importance in diagnosis (positron emission mammography), staging (sentinel lymph node detection), and follow-up with18F-FDG and tumor characterization. Among many available PET tracers, the most commonly used are 18F-FLT, 18F-FES, 18F-FDHT, 64Cu DOTA trastuzumab (bevacizumab), 68Ga-PSMA, 68Ga-RM2 (gastrin-releasing peptide receptor), 18F-fluorooctreotide (SSTR), and 68Ga-TRAP (RGD)-3αvβ3-integrin. Molecular imaging helps in evaluation of tumor heterogeneity, allowing a shift from one-size-fits-all-approach to era of personalized medicine and precision oncology.
Keywords: Breast cancer, Molecular imaging, Positron emission tomography, Personalized medicine, Precision oncology
Introduction
The most frequently occurring cancer in women is breast cancer (BC) responsible for almost 2 million new cases annually and about half million related deaths worldwide. Breast cancer accounts for 23% among all cancer diagnosed worldwide and represents 14% of all cancer related deaths among women. In females, BC is the leading cause of cancer death in the developed world. One in eight women will develop BC at some stage during their lifetime [1]. Approximately 30% of patients who present with locoregional disease will develop metastases, and despite recent advances in treatment, the vast majority of metastasized BC will be incurable.
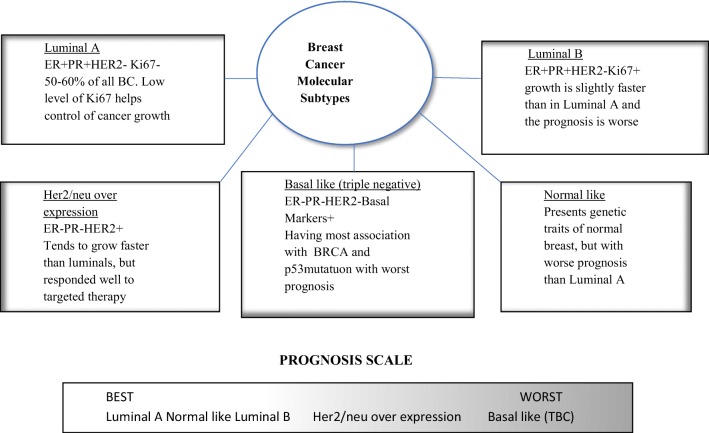
The staging system most often used for BC is the American Joint Committee on Cancer TNM system, based on 7 criteria—extent of tumor (T), spread to nearby lymph nodes (N), spread (metastases) to distant sites (M), grade of the cancer (G), estrogen receptor (ER) status, progesterone receptor status (PR), and HER2/neu (HER2 status). Breast cancer is not just a single disease [2] and it seems to be very heterogenous. It is characterized by different pathological features, disparate responses to treatments, and obvious differences in long-term patient survival. Molecular analyses in addition to routine pathological examination are able to show the whole extent of diversity among BCs. Approximately 70% of BC express ER and majority of ER+ cancers also express PR. Collectively, ER+ cancers are classified as luminal, which are further subclassified based on their HER2 status and proliferation rate as ER−/PR−/HER2+ and ER−/PR−/HER2− (or triple negative breast cancer (TNBC)). The six molecular subtypes of BC currently recognized are as follows: luminal A (ER+), luminal B (ER+, HER2 enriched), HER2 enriched, basal-like, claudin-low, and normal-like BC. In general, both subtypes of ER+, luminal A & B, are associated with excellent long-term survival. HER2 overexpression is associated with a poor prognosis, but it can predict a positive therapeutic response to anti-HER2 drugs. Basal-like and claudin-low are subtypes of TNBC and usually are associated with extremely poor clinical outcome. Normal-like BC is probably not a distinct molecular subtype but could not be otherwise classified because of the normal appearing contaminating epithelium. Molecular classification of BC based on complex patterns of gene expression provides a link between molecular biology and behavior of cancer cells in different subtypes (Fig. 1). Molecular testing has become a routine investigation of BC patient management during the last few years. Although it is clear that routine histopathological evaluation of BC has value and could be used to classify BC based on fundamental phenotypic properties, it could not be fully evaluated without the consideration of the BC genome, proteome, and transcriptome. Available genomic, proteomic, and transcriptomic data suggest that every BC is a distinct entity and the molecular diversity increases with the number of genes analyzed. The same observation could be made for gene mutations, copy number variations, and different pathways of activation. Next-generation sequencing and other molecular technologies may find utility in treatment decisions and choosing appropriate therapeutic strategies for each individual patient.
Fig. 1.
Breast cancer molecular subtypes with prognosis scale
Diagnosis and management of breast cancer are undergoing a shift from one-size-fits-all-approach to an era of personalized medicine. Precise diagnosis with genomic profiles and molecular imaging definitely improved tumor characterization. Currently available diagnostic modalities with newer surgical techniques and radiation therapies support a new multidisciplinary approach in ways to prolong survival. Surgical intervention remains the primary BC treatment whereas breast conservation surgery (BCS) is developing during the last 40 years, beginning with the pioneered work of Veronesi [3]. Precise tumor localization is a mainstay of BCS. Wire or radioactive seeds are with no significant difference in operative times, but the latter has a lower re-excision rate [4]. One of the major clinical advances in breast surgery was the introduction of sentinel lymph node biopsy (SLNB) instead of conventional axillary node dissection. SLNB is one of the most common nuclear medicine procedures in an oncology setting for patients with BC, melanoma, prostate cancer, cervical cancer, and head and neck cancer and very rarely in other malignancies. Continuation of breast cancer management has resulted in precision medicine where BC patients’ treatment could be personalized. This is done by integrating IHC markers and genome analysis combined with anatomic imaging modalities and unique information from functional molecular imaging studies. All of this will help in better treatment planning and response assessment of BC patients [4].
Positron Emission Mammography
18F-FDG (fluorodeoxyglucose) PET and FDG PET/CT are minimally invasive methods that provide whole body imaging of metabolically active lesions, commonly used in various malignancies as opportunistic screening. Breast cancer detection rate of the FDG PET cancer screening program was reported to be 0.18–0.23%. This is lower than mammography (MMG) and physical examination screening program with a detection rate of 0.31%. Relatively low FDG use in BC is also a limitation for PET and PET/CT, with limited detectability of small cancers and noninvasive and lobular carcinomas. But FDG has been increasingly used in positron emission mammography (PEM) providing with single FDG dose PET/CT whole body and PEM scan respectively. PEM is high-resolution breast imaging technology which uses an organ-specific instrument by compressing the breasts with two parallel photon detectors similar to MMG, with low spatial resolution of 2.4 mm. The first report of breast cancer (BC) screening by positron emission tomography (PEM) was published by Yamamoto [5]. In their study, 265 women were enrolled for BC screening, and the final detection rate of BC was 2.3%. All of these patients underwent an ultrasound before the PEM study and were followed for at least 1 year afterwards to assess specificity (overall sensitivity was 100% and specificity was 84.5%). He discussed other imaging modalities, such as MMG, MRI, and ultrasound (US), respectively. MMG does not achieve sufficient accuracy among women younger than 50 years because of the density of their breasts. The diagnostic accuracy of MRI is higher than that of MMG and US, but there are some challenges associated with MRI. These challenges are low specificity, claustrophobia, and difficulties in reporting images in patients with large body size, implanted metal devices, and breasts under estrogen modulation. The merits of PEM are not only its high sensitivity, but also the ability to provide better spatial resolution and an attractive alternative imaging modality in women with dense breast tissue, difficult to interpret on MMG screening, and in women who are pre- or perimenopausal who may have various difficulties undergoing an MRI. PEM is usually combined with an ultrasound (US) that is able to detect lesions in dense breasts, but tends to produce a higher rate of false-positive results than MMG, so these US findings can be adjusted with PEM. However, the most important limitation of PEM is significant radiation exposure (3.5 mSv), as well as high cost that is not covered. By now, the only screening modality proven to reduce breast cancer–specific mortality is MMG as there are insufficient criteria to support other imaging modalities as screening [4].
Pet Tracers in Oncology
For PET molecular imaging of BC, several tracers targeting certain tumor characteristics have been developed (Table 1)
Table 1.
Mechanisms/targets of the reviewed SPECT and PET radiopharmaceuticals with references
| PET tracer | Target | References |
|---|---|---|
| 18F-FDG | Glucose consumption | [6] |
| 18F-FLT | DNA synthesis | [7] |
| 64Cu DOTA trastuzumab (99mTc-HYNIC-H6F) | HER receptor | [8–11] |
| 18F-FES | ES receptor | [12–14] |
| 18F-FDHT | Androgen receptor | [15] |
| 68Ga-PSMA | PSMA | [16, 17] |
| 68Ga-RM2 | Gastrin-releasing peptide receptor | [18] |
| 111 In pentreotide (18F fluorooctreotide) | SSTR | [19] |
| 68Ga-TRAP (RGD)3 | αvβ3-Integrin | [20] |
18F-FDG
18F-FDG PET/CT has not been approved for opportunistic screening and staging apart from IIIA stage (NCCN guidelines 2.2016 for invasive breast cancer). However, in patients with triple negative breast cancer (TNBC), it might be of great significance. Ulaner reported 232 newly diagnosed patients with I to III stage TNBC (the vast majority with high grade ductal histology) who underwent 18F-FDG PET/CT [6]. Thirteen patients were upstaged by the identification of unsuspected nodal metastases, while 30 had distant metastases. In 26/30, metastases were approved by histology and in the rest, follow-up was used for verification of metastases. So, initial staging could not be the only criteria for 18F-FDG PET/CT use, as a substantial percentage (15%) was upstaged to stage IV, a fact that should be taken in account for preparing future guidelines, where at least IIB TNBC patients should undergo 18F-FDG PET/CT before starting the treatment. With an initial stage IIB, treatment should be surgical with neoadjuvant and adjuvant chemotherapy, while with IV, it will be palliative chemotherapy, as surgery has not been shown to improve survival in advanced BC. So 18F-FDG PET/CT might avoid unnecessary surgeries and toxicities from curative-intent neoadjuvant and adjuvant chemotherapy.
18F-FLT
The assessment of early response to chemotherapy is the cornerstone of the BC patient’s further treatment. Treatment response has been assessed by RECIST criteria, which relies on measuring changes of the longest diameter of the target lesion. Tumor response assessment is inappropriate due to the length of time until the appearance of physical changes. Also, molecular therapy does not always result in shrinking the target lesions and measurement will not be an appropriate method of assessment. Assessment of cell proliferation by Ki67 immunostaining could provide an alternative strategy to assess chemosensitivity and chemoresistance in BC and will show changes in breast tumor proliferation status, as early as 24 h after and certainly 1–4 weeks after chemotherapy. Molecular imaging could replace anatomical and histological approach, taking in account that 18F-FDG uptake could be diminished 1 week after the treatment starts, and also could exhibit increased uptake due to the enhanced inflammatory response.
Proliferation markers such as thymidine analogue 3′-deoxy-3′-18Ffluorothymidine (18F-FLT) are not susceptible to inflammatory changes and can be used to measure early treatment response. Objective changes in 18F-FLT-PET could be seen at 1 week after FEC (standard protocol with 5-fluorouracil, epirubicin, and cyclophosphamide) treatment. Given that 18F-FLT uptake is highly correlated with proliferation KI-67 index breast cancer, the present findings indicate that 18F-FLT-PET can be used to measure changes in proliferation as early as 1 week post treatment and also serial scans with 18F FLT-PET which could be done with high reproducibility [7].
HER2 Tracers
Human epidermal growth factor receptor 2 (HER2) is associated with a highly aggressive infiltrating type of BC prone to metastatic spreading. Overexpression of HER2 accounts for 30% invasive BC associated with aggressive behavior and poor clinical outcomes. However, the development of monoclonal antibodies against the extracellular domain of HER2 significantly alters the natural history of HER2+ BC. HER2 expression is usually evaluated in post-surgery specimens by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). Molecular imaging techniques substitute for invasive biopsy procedures and allow assessment of secondary lesions not easily accessible to biopsy. Also, PET and SPECT imaging allow HER2 in vivo detection during tumor progression and treatment.
Tamura reported PET imaging with 64Cu-DOTA trastuzumab in six HER2-positive BC patients. Biodistribution studies showed high activity in blood but low in normal tissues, and radiation exposure with 64Cu-DOTA trastuzumab was equivalent to that of 18F-FDG PET/CT [8]. As this radiopharmaceutical has good acceptable dosimetry and pharmacologic safety results, it might be used for the diagnosis of primary and secondary BC lesions and could predict the biologic effect of anti-HER2 antibodies. That would be helpful in choosing treatment between anti-HER2 antibodies and HER2 tyrosine kinase inhibitors. Also, 64Cu-DOTA trastuzumab could be used in other HER2-positive malignancies, such as HER2-positive advanced gastric cancer.
Use of 99mTc for labeling means safety and easy availability for routine use. The development of biotechnology and the discovery of nanoparticles, which are able to host different functionalities and to be loaded in different therapeutic molecules, make theranostic concept possible [9].
Radiolabeled trastuzumab and pertuzumab are promising tracers, because of their high accumulation in HER2-positive tumor tissue. However, they would be of limited effectiveness, as significant activity happens 3–5 days post administration, too late for necessary treatment modification.
Because of that, many HER-targeted proteins and small-molecule alternatives such as ScFv-Fc, F(ab9)2, minibody, diabody, and affibody molecules were developed and radiolabeled [10]. Small targeting molecules reveal different biodistributions and clearance pathways, providing better quality images. The main characteristics of these smaller peptides are lower immunogenicity and shorter circulatory half-lives. In addition, their chemical modifications are simpler, which is more appropriate for routine clinical practice.
Li et al. labeled 99mTc-HYNIC-H6F peptide that specifically accumulates in HER2-positive tumors and might be promising for the diagnosis of HER2-positive cancers [11]. Apart from technetium availability, the main advantage of 99mTc-HYNIC-H6F is targeting different regions of the HER2 receptor trastuzumab. This provides a great opportunity to monitor the therapeutic effects of trastuzumab, without competition with the same binding area of the HER2 receptor.
Estrogen Receptor Tracers
Patients with distant metastases that are estrogen-positive are rarely cured, but usually live longer than the others, due to the expectedly better prognosis for luminal 1 and 2 subgroups. Clinical management of ER-positive tumors has not been standardized as there are different endocrine treatment regimens, cytotoxic chemotherapy, and other targeted agents. Many patients tolerate endocrine therapy well with few toxicities, but also many of them do not benefit from the endocrine treatment, developing refractoriness. The best way to assess responsiveness to endocrine therapy is measurement of tumor ER expression, with immunohistochemistry as a gold standard. The aim of Kurland’s study was to assess imaging classifiers 18F-fluoroestradiol (18F-FES) uptake as a substitute for IHC and to predict the response to endocrine treatment, as there is obvious agreement between 18F-FES uptake and in vitro assay of ER [12]. In the same group of patients, 18F-FDG PET/CT was used to distinguish between metabolically aggressive and indolent disease. After both imaging procedures, patients were sorted into 3 groups (indolent disease, aggressive but strongly ER avid, and aggressive and weakly ER avid) which are highly relevant for clinicians’ and patients’ decisions of therapeutic options. The combination of 18F-FDGPET and 18F-FES PET will be useful in discriminating between patients with disease that is indolent or amenable to endocrine therapy and patients whose disease is more aggressive and is unlikely to respond to endocrine therapy. Combination of both images may help guide the timing of endocrine therapy and selection of other targeted and/or cytotoxic chemotherapy. Unlike tissue sampling, these images may evaluate the entire burden of metastatic disease.
Estrogen receptor α (ERα) is by far an important prognostic and predictive biomarker in BC. Assessment of ERα expression in tissue specimens is an important determining factor for appropriate endocrine treatment of ERα signaling blockage. Nevertheless, it is not a good indicator for successful results, as disease recurrence and metastatic disease could develop in spite of regular therapy. Therefore, functional imaging is of great importance in selecting the patients who will benefit from endocrine therapy versus the patients who will do better with adjuvant chemotherapy [13]. In another study, 26 postmenopausal women with ER+ BC underwent 18F-FES PET scan and afterwards followed for a median of 74 months [14]. Level of standardized uptake value (SUV) max of 7.3 was used as a threshold, to distinguish responders from nonresponders on endocrine therapy. Recurrence occurred in none of 8 treated with neoadjuvant chemotherapy (NC) and in 2 of 5 with neoadjuvant endocrine therapy (NET), with SUV max less than 7.3. So, postmenopausal women who are ER+ but 18F-FES PET–negative may benefit from NC, rather than NET.
Androgen Receptor Tracers
Androgen receptors (AR) are not routinely determined in patients with BC although they are present in 70–80% of them. Keeping in mind that only ER+ patients benefit from anti-estrogen endocrine therapy, as ER are functionally and structurally comparable with AR, response to AR-targeting drugs may rely on AR expression as well. Molecular imaging offers the possibility to noninvasively determine the presence of relevant drug targets in all sites of metastatic spread throughout the body [15]. AR expression in prostate cancer could be assessed by 18F-fluorodihydrotestosteron (FDHT) PET. Molecular imaging could be a promising tool in patient selection for clinical trials with AR antagonist treatment protocols. AR-targeted therapy has not been approved as a standard treatment regimen, but there are clinical trials with preliminary acceptable results, showing stable post treatment disease in one third of metastatic BC patients. Many studies of AR-targeted therapy and a combination of AR and ER targeted therapies are going on, allowing better perspective for patients with AR and ER-positive metastatic BC. Having in mind that hormone receptor conversion occurrence is common, during the disease development while both receptor-positive and receptor-negative lesions are present, pretreatment ER and AR assessment is of great importance. 18F-FDHT and 18F-FES PET imaging could be used instead of metastasis biopsy when the lesions are not easily accessible. Also, molecular imaging with 18F-FDHT and 18F-FES PET is very important for the assessment of treatment effectiveness, avoiding the suboptimal treatment when the receptors are changing in the process of metastatic disease development.
Prostate-Specific Membrane Antigen Tracers
Prostate-specific membrane antigen (PSMA) has been proven as an important agent in the diagnosis and treatment of metastatic prostate cancer. 68Ga-labeled PSMA inhibitors show more accuracy compared with 18F choline for recurrent and for primary PC when combined with. Apart from prostate carcinoma, PSMA has been overexpressed in many tumor-associated neo vasculatures in a variety of solid tumors including BC. In May 2015 in EANMMI, image of the month was the first case report of the patient with metastatic breast cancer positive of 18F-FDG as well as 68Ga-PSMA PET/CT scan [16]. The image showed high PSMA uptake in liver and skeletal metastases. Regarding the possibility of PSMA directed therapy, Sathekge continued his study later with a group of 19 patients with breast cancer who underwent PET/CT scanning with 68Ga-labeled PSMA inhibitor (PSMA HBED CC). From 81 detected lesions, 84% of them were PSMA-positive including primary tumors, local recurrences, lymph nodes, and distant metastases. In 7 patients, the 18F-FDG scan was also done and revealed 35 lesions, among them 6 PSMA-negative with weak relation between tumor metabolism assessed by FDG and tumor angiogenesis assessed by PSMA [17]. Higher FDG uptake was seen in primary tumors, compared with distant metastases, and it was in keeping with upregulation of PSMA in tumor-associated vascular endothelial cells in solid tumors. PSMA participates in matrix degradation, facilitating integrin signaling and p21 activating kinase 1 (PAK1) activation, leading to increased tumor invasion and regulating neovasculature probably involving VEGF. 68Ga-PSMA scanning should be helpful in prediction and monitoring of anti-angiogenic treatment in patients suffering from BC and also allows development of theranostic principles, promoting the use of 177Lu-PSMA in BC patients.
Gastrin-Releasing Peptide Receptor Tracers
Gastrin-releasing peptide receptor belongs to the bombesin receptor family with physiologic ligand gastrin-releasing peptide. The main physiological function of GRF is gastrin-releasing and regulation of enteric function, but also appears to play a role in carcinogenesis and tumor proliferation. Gastrin-releasing peptide receptor (GRPR) shows overexpression in breast cancer, prostate cancer, and peritumoral vessels around ovarian cancer. Labeled GRPR agonists have been tested preclinically and clinically; however, antagonist revealed better image contrast and higher uptake, without any gastrointestinal adverse effects. RM2 is one of the antagonists with promising results in prostate cancer. Stoykow published results of 15 newly presented BC patients who underwent RM2 scanning and revealed potential clinical upstaging of 47% of the examined patients after 68Ga-RM2-PET/CT due to the detection of suspicious lymph nodes [18]. Low radiotracer uptake in fat and muscular tissue allows visualization of 5 mm lymph nodes. Imaging with 68Ga-RM2-PET/CT upstaged almost 40% of scanned patients, showing especially high detection rates in the internal mammary lymph nodes region. With the exception of diagnosis and staging in patients with BC, labeling of GPRP antagonists with therapeutic radioisotopes (for example, 177Lu) could allow for the development of new successful theranostic concept.
Somatostatin Receptor Tracers
Neuroendocrine differentiation (NED) is a rare event, but overexpression of somatostatin receptors (SSTR) in breast carcinoma has been reported in many studies. Many authors do not consider evidence of SSTR to be sufficient to define NED, so assessment of synaptophysin and chromogranin should be added.
Saveli reported a case of ductal infiltrating carcinoma with NED, positive for estrogen and negative for progesterone and cERB receptors (immunohistochemistry positive on synaptophysin and chromogranin) in liver metastasis that appeared 4 years after the initial diagnosis and treatment [19]. Chemotherapy with docetaxel and epirubicin resulted in partial remission. The overexpression of SSTR in the metastases (liver) was detected by 111In pentreotide scintigraphy CA15.3, CEA, CgA, and NSE were increased. 90Y-DOTATOC was done and evident shrinkage of the liver metastases was visualized on CT 1 month after the treatment. So, PRRT could be effective in other neoplasms other than neuroendocrine tumors, as a second- or third-line therapy, providing potentially objective tumor shrinkage even in aggressive disease.
Integrin Tracers
There has recently been a huge development of new positron diagnostic tracers. Radiolabeled arginyl glycyl aspartic acid (RGD) is a very promising agent that enables specific targeting of αvβ3-integrin an endothelial and tumor cell receptor with a probably significant role in angiogenesis. Integrins are family of transmembrane proteins interacting with extracellular matrix, mediating cell adhesion and extracellular to intracellular signaling pathways. This seems to be very important in tumor progression and metastases. αvβ3-Integrin has been overexpressed by angiogenic endothelium and tumor cells and is involved in various angiogenic signaling cascades, including vascular endothelial growth factor (VEGF) pathway. Inhibition of VEGF significantly suppresses tumor αvβ3-integrin expression with reduction of microvascular density, so that is why it was proposed as a marker of angiogenic activity. Inhibition of VEGF pathway using anti-VEGF antibody (bevacizumab) has been established as anti-angiogenic treatment in non–small cell lung, colorectal and BC. Kazmierczak used 68Ga-TRAP (RGD)3 PET/CT for monitoring in vivo αvβ3-integrin expression as a biomarker of anti-angiogenic therapy effects in breast cancer xenografts in mice treated with VEGF antibody (bevacizumab) over the course of 1 week [20]. When they reached tumor size of 0.5 cm, animals were assigned in imaging or immunohistochemistry group. In the imaging cohort, animals were randomly assigned to therapy or control group. After the baseline scan, animals were treated with bevacizumab 5 mg/kg or with volume equivalent placebo solution and 7th day repeated scan was done with 68Ga-TRAP (RGD)3. RGD uptake in treated animals has been reduced following VEGF inhibition, whereas it remains the same in the untreated group. RGD-based hybrid imaging is an important diagnostic tool used in addition to other morphology-based and functional tumor response assessment modalities in BC patients who underwent anti-angiogenic treatment. Specific targeting of αvβ3-integrin with RGD imaging could be helpful in the selection of patients who are more likely to benefit from targeted anti-angiogenic treatment protocol, allowing for proper use and leading to personalized cancer treatment approaches.
Conclusion
Molecular imaging is very important in the diagnosis, staging, follow-up, and radiotherapy planning, as well as in characterizing lesions, predicting prognosis and therapy response in BC patients. This is particularly useful when quantitative indexes of tumor metabolism or function are used, such as tumor functional volume (TFV), apparent diffusion coefficient (ADC), or standardized uptake value (SUV).
It is well known that human cancers frequently express intratumor phenotypic heterogeneity with profound implications on BC development and therapeutic response. Measuring heterogeneity could be extremely useful in selecting the most promising therapeutic approach. In vivo imaging techniques should be the cornerstone of future precision oncology as 18F-FDG PET/CT is able to define patients’ prognosis and predict responses to NAC. Hybrid imaging with PET/MRI is very promising and could be used as a single imaging examination for theranostic purposes in BC patients.
Molecular imaging is becoming an indispensable tool that is not only limited to cancer research and clinical trials, but also more commonly used in routine clinical practice. Among many other imaging modalities available, PET imaging is a very sensitive and one of the most important imaging methods. The development of new tracers has achieved visualization improvements in tumor assessment and has become the corner stone for specific diagnosis, precise staging, and treatment response in cancer patients. Innovations in imaging modalities (PET with optical imaging) will probably be further evaluated for clinical use.
Molecular imaging with PET is a powerful tool for novel tracer discovery and development, with the potential to be used for target and tracer validation. Additionally, it will be very helpful in clinical practice, which would provide a shift from a one-size-fits-all-approach to an era of personalized medicine and precision oncology.
Ethical Approval
This work does not contain any studies with human participants or animals performed by any of the authors.
Compliance with Ethical Standards
Conflict of interest
Daniela Miladinova declares no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rivenbank AG, O’Connor SM, Coleman WB. Molecular and cellular heterogeneity in breast cancer challenges for personalized medicine. Am J Pathol. 2013;183:1113–1124. doi: 10.1016/j.ajpath.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305:6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 4.McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. J Nucl Med. 2016;57:9S–16S. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Tasaki Y, Kuwada Y, Ozawa Y, Inoue T. A preliminary report of breast cancer screening by positron emission mammography. Ann Nucl Med. 2016;30:130–137. doi: 10.1007/s12149-015-1040-0. [DOI] [PubMed] [Google Scholar]
- 6.Ulaner GA, Castillo R, Goldman DA, Wills J, Riedl CC, Pinker-Domenig K, et al. 18F-FDG-PET/CT for systemic staging of newly diagnosed triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2016;43:1937–1944. doi: 10.1007/s00259-016-3402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenny L, Coombes RC, Vigushin DM, Al-Nahfas A, Shousha S, Aboagye O. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34:1339–1347. doi: 10.1007/s00259-007-0379-4. [DOI] [PubMed] [Google Scholar]
- 8.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, et al. 64Cu-DOTA-Trastuzumab PET imaging in patients with HER2-positive breast Cancer. J Nucl Med. 2013;54:1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 9.Rainone P, Riva B, Belloli S, Sudati F, Ripamonti M, Verderio P, et al. Development of 99mTc-radiolabeled nanosilica for targeted detection of HER2-positive breast cancer. Int J Nanomedicine. 2017;12:3447–3461. doi: 10.2147/IJN.S129720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen J, Sandberg D, Sandstrom M, Wennborg A, Feldwicsh J, Tolmachev V, et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111 in-ABY-025 affibody molecule. J Nucl Med. 2014;55:730–735. doi: 10.2967/jnumed.113.131243. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Wu Y, Wang Z, Jia B, Hu C, Dong C, et al. SPECT/CT imaging of the novel HER2-targeted peptide probe 99mTc-HYNIC-H6F in breast cancer mouse models. J Nucl Med. 2017;58:821–826. doi: 10.2967/jnumed.116.183863. [DOI] [PubMed] [Google Scholar]
- 12.Kurland BF, Peterson JM, Lee JH, Schubert EK, Currin ER, Link JM, et al. Estrogen receptor binding (18F-FES PET) and glycolytic activity (18F-FDG PET) predict progression-free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res Online. 2016;23:407–415. doi: 10.1158/1078-0432.CCR-16-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler AM, Linden HM. Functional estrogen receptor imaging before neo adjuvant therapy for primary breast cancer. J Nucl Med. 2017;58:560–562. doi: 10.2967/jnumed.116.183533. [DOI] [PubMed] [Google Scholar]
- 14.Chae SY, Kim S-B, Ahn SH, Kim HO, Yoon DH, Ahn DH, et al. A randomized feasibility study of 18Ffluoroestradiol PET to predict pathological response to neoadjuvant systemic therapy in estrogen receptor–rich postmenopausal breast cancer. J Nucl Med. 2016;58:563–568. doi: 10.2967/jnumed.116.178368. [DOI] [PubMed] [Google Scholar]
- 15.Venema CA, Mammatas LH, Schroder CP, van Kruchten M, Appolonio G, Glaudemans AWJM, et al. Androgen and estrogen receptor imaging in metastatic breast cancer patients as a surrogate for tissue biopsies. J Nucl Med. 2017;58:1906–1912. doi: 10.2967/jnumed.117.193649. [DOI] [PubMed] [Google Scholar]
- 16.Sathekge M, Modiselle M, Vorster M, Mokgoro N, Nyakale N, Mokaleng B, et al. T. 68Ga PSMA imaging of metastaic breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:1482–1483. doi: 10.1007/s00259-015-3066-x. [DOI] [PubMed] [Google Scholar]
- 17.Sathekge M, Lengana T, Modiselle M, Vorster M, Zeevaart I, Maes A, et al. 68Ga-PSMA-HBED-CC PET imaging in breast carcinoma patients. Eur J Nucl Med Mol Imaging. 2017;44:689–694. doi: 10.1007/s00259-016-3563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoykow C, Erbes T, Maecke HR, Bulla S, Bartholoma M, Mayer S, et al. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist 68Ga-RM2 and PET. Theranostics. 2016;6:1641–1650. doi: 10.7150/thno.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savelli G, Zaniboni A, Bertagna F, Bosio G, Nisa L, Rodella C, et al. Peptide receptor radionuclide therapy (PRRT) in a patient affected by metastatic breast cancer with neuroendocrine differentiation. Breast Care. 2012;7:408–410. doi: 10.1159/000343612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazmierczak PM, Todica A, Gildehaus FJ, Eppeneder HH, Brendel M, Eschbach RS, et al. 68Ga-TRAP-(RGD)3 hybrid imaging for the in vivo monitoring of αvb3-integrin expression as biomarker of anti-angiogenic therapy effects in experimental breast cancer. PLoS One. 2016;11(12):1–19. doi: 10.1371/journal.pone.0168248. [DOI] [PMC free article] [PubMed] [Google Scholar]