Abstract
In this study, biodiesel was produced from goat fat in the presence of magnesium oxide (MgO) nano-catalyst using transesterification process. The characteristics of the catalyst were studied using field emission scanning electron microscope (FE-SEM), transmission electron microscope (TEM), Brunauer–Emmett–Teller (BET), energy dispersive X-ray/mapping (EDX/Map), Fourier-transform infrared spectroscopy (FTIR), thermal gravimetric analysis (TGA), and dynamic light scattering (DLS). The result showed that the specific surface area and the average pore diameter of the nanocatalyst were 40.44 m2/g and 36.7 nm, respectively, which showed that the catalyst is mesoporous. According to the results of the DLS analysis, the average particle size of the catalyst was determined to be 5.5 nm. Also, the maximum biodiesel yield of 93.12% was obtained at temperature of 70 °C, methanol/oil molar ratio of 12:1, the catalyst content of 1 wt.%, and reaction time of 3 h. In addition, biodiesel was mixed with diesel at different ratios (B25, B50, B75, and B100) to improve fuel properties of the produced biodiesel. The results indicated that the mixtures of B75 and B100 had better density, viscosity, and flash point in comparison to the other mixtures and their properties were within the range of international standards.
Keywords: Biodiesel, Goat fat, Magnesium oxide nanocatalyst, Transesterification process
Introduction
Reduction of fossil fuel resources and environmental problems will lead to serious problems in the near future. For this purpose, a lot of research has been done to find a suitable alternative to fossil fuels (Okitsu et al. 2013). Due to the renewability, free of sulfur and aromatic compounds, non-toxicity, and high flash point, biodiesel has attracted the attention of many researchers and has been introduced as a replacement for diesel (Chongkhong et al. 2009; Enweremadu and Mbarawa 2009; Luu et al. 2014; Ma et al. 2016). There are many ways to produce biodiesel, including pyrolysis, micro-emulsion, and transesterification process, the latter is the most commonly adopted method (Azam et al. 2005).
Biodiesel is mono-alkyl esters of long-chain fatty acids produced from transesterification process of vegetable or animal oil with an alcohol in the presence of a catalyst (Seffati et al. 2019; Vasudevan and Briggs 2008).
There is very little modification in the engine when biodiesel is utilized along with diesel. Having 11% oxygen in the chemical structure of biodiesel, it has more complete combustion (Moser 2009) and produces less pollution like carbon monoxide and unburned hydrocarbons. Other advantages of biodiesel in comparison to diesel are more safety during usage and increasing engine lubrication.
Biodiesel has also some disadvantages such as higher NOx emissions, higher viscosity, and higher pour point (Krishna et al. 2017). Also, biodiesel cannot be stored for a long time because it is degradable and loses its properties.
More than 75% of biodiesel production price is related to raw material. This issue threatens the economic feasibility of the biodiesel industry. Vegetable oils increase the price of biodiesel production and compete directly with human foods (Dorado et al. 2006; Zhang et al. 2003). Therefore, oils from food waste and animal fats are more cost-effective. Chicken fat (Gürü et al. 2010; Marulanda et al. 2010), sheep fat (Ali et al. 2012), and goat fat (Chakraborty and Sahu 2014) can be mentioned as sources of animal fat. Biodiesel produced from animal fat has greater cetane number than that from vegetable sources. In addition, waste fat from animals is a cheap alternative for producing biodiesel (Dorado et al. 2006; Zhang et al. 2003).
Transesterification reaction in the presence of a catalyst requires alcohol to take place. The most common short-chain alcohols used in the transesterification process are methanol, ethanol, propanol, and butanol. In the transesterification process, methanol is used because of its lower price than other alcohols (Ramadhas et al. 2005).
Alkaline, acidic, and enzymatic catalysts can be used as an intermediate in the transesterification reaction. Alkaline and acidic reactants are applied more than the enzymatic reactant as they are cheaper and shorten the reaction time (Zhang et al. 2003). Among the alkaline catalysts, heterogeneous catalysts have attracted the attention of many researchers. They are quickly separated from the reaction mixture without a neutralizing agent therefore the need for washing is eliminated. As well, these catalysts can be recycled and reused and they are less corrosive, therefore the whole process will be safer, cheaper, and environmentally friendly (Agarwal et al. 2012; Chouhan and Sarma 2011; Hara 2009; Lukić et al. 2009; Liu et al. 2010). Among heterogeneous catalysts, nanocatalysts have been recently used to produce biodiesel. The large specific surface area and high catalytic activity are desirable properties of nanocatalysts in biodiesel production (Bankovic-Ilic et al. 2017). Magnesium oxide (MgO) can be widely used as a heterogeneous alkaline catalyst in biodiesel production. The advantages of the MgO catalyst are easily used in the synthesis process, easier to control, its abundant availability and relatively inexpensive (Anggoro et al. 2018). Also, MgO has attracted attention for the transesterification process because it has high basic strength, less environmental effect and low solubility in methanol (Ashok et al. 2018).
There are several factors affecting biodiesel production such as temperature, reaction time, catalyst content and methanol/oil molar ratio (Seffati et al. 2019). Also, biodiesel can be blended with diesel fuel at any ratio and after addition of biodiesel to diesel, the properties of diesel fuel such as flash point, viscosity, density, etc., can be improved (Seffati et al. 2019).
In this study, biodiesel was produced from goat fat in the presence of MgO nanocatalyst and the effect of different parameters such as methanol/oil molar ratio, catalyst content, temperature, and time was investigated on biodiesel production. Then, the produced biodiesel at optimized condition was mixed with diesel in different ratios and their properties (flash point, pour point, cloud point, viscosity, and density) were compared to diesel properties in accordance to international standards.
Materials and methods
MgO nanocatalyst
MgO nanocatalyst with a purity of 99%, the particle size of 20 nm, and density of 3.58 g/cm3 was purchased from Vira Carbon Nano Materials (VCN Materials, Iran). Different analyses, such as field emission scanning electron microscope (FE-SEM), transmission electron microscope (TEM), Brunauer–Emmett–Teller (BET), energy dispersive X-ray/mapping (EDX/Map), Fourier-transform infrared spectroscopy (FTIR), thermal gravimetric analysis (TGA), and dynamic light scattering (DLS) were used to determine the properties of the nanocatalyst. FE-SEM (MIRA 3, TE-SCAN, Czech Republic) were used to determine the morphology and the surface properties of the nanocatalyst. TEM analysis (TECNAI G2 F20 S-TWIN, USA) was used to determine the size of the particles. Also, BET analysis (BELSORP MINI II, Microtrace Bel Corp, Japan) was used to determine the specific surface area and pore volume of the nanocatalyst. To investigate the behavior of the nanocatalyst against temperature, TGA analysis (DSC-TGA, Q600, TA, USA) was used. In addition, FTIR analysis (AVATAR, THERMO, USA) was used to determine the functional groups of the nanocatalyst. Moreover, DLS analysis (ZEN 3600, Malvern, England) was used to determine the distribution of the particles size. Furthermore, EDX-Map analysis (MIRA 3, TE-SCAN, Czech Republic) was used to identify the constituent elements of the catalyst.
Extraction of oil from goat fat
Goat fat was obtained from a slaughterhouse at the city of Bushehr (Bushehr province, Iran). Firstly, the goat fat was washed several times with water to separate the blood and waste materials because the existence of these materials may lead to changes in the oil color during the heating process, and decrease the quality of the produced oil. Then, to speed up the process, the fats were cut into small pieces by a knife. To break the tissue of the fats and converting them to oil, they were placed in a large pot and put on the stove with a gentle flame for approximately 3.5 h. The oil was then filtered to remove unmelted particles. Before performing the experiment, the oil should be heated to 100 °C to evaporate the water because the existence of a very low amount of water can cause errors in the experiments.
Biodiesel production method
In this study, the transesterification process was used to produce biodiesel from goat fat in the presence of MgO nanocatalyst. A reflux condenser was used to prevent methanol evaporation and better control of the reaction temperature. Then, 50 g of the oil extracted from the goat fat was transferred into a 250-mL three-neck round-bottom flask. The flask was placed on a heater to raise the oil temperature to the desired value. Then, the mixture of methanol and the catalyst (initially 1 wt.% for checking the effect of methanol/oil molar ratio) was added to the oil and a magnet was applied to blend the mixture. The time of mixing the oil with methanol and catalyst was recorded as the starting time of the experiment. Also, the temperature was controlled by a heater equipped with a magnetic stirrer. The solution temperature was checked every 5 min by a thermometer and kept at the desired temperature (60 °C for checking the effect of methanol/oil molar ratio). After the reaction time (2 h) was completed, the biodiesel was produced.
At the end of the reaction, the produced biodiesel was transferred to the decanter funnel for separation and after 24 h, the solution in the funnel was converted into three phases including biodiesel, glycerol, and catalyst, respectively. The yield of biodiesel was calculated using Eq. (1):
| 1 |
The effect of different factors on biodiesel production
In this study, the effect of different parameters such as methanol/oil molar ratio, reaction temperature, contact time, and catalyst content on the biodiesel production was investigated. To determine the best conditions for biodiesel production, one of the parameters was varied and other factors were kept constant. The transesterification reaction was initially evaluated at 60 °C for 2 h, and the catalyst content of 1 wt.% with a stirring speed of 1500 rpm and different methanol/oil molar ratios (6:1, 9:1, 12:1 and 15:1). After determining the optimal value of methanol/oil molar ratio, the reaction was performed at optimal methanol/oil molar ratio of 12:1, reaction temperature of 60 °C, catalyst content of 1 wt.%, stirring rate of 1500 rpm, and different reaction times (1, 2, 3, and 4 h). Then, the effect of reaction temperature (40, 50, 60, 70, and 80 °C) and catalyst content (0.5, 0.75,1, and 1.25 wt.%) was also evaluated at the optimized conditions.
To make a mixture of biodiesel and diesel fuel, biodiesel was mixed with diesel at different ratios (B00, B25, B50, B75, and B100) and their physical properties like flash point, pour point, cloud point, density, and kinematic viscosity were measured. Biodiesel and diesel mixtures are shown as BXX, where XX represents the biodiesel percentage in the blended fuel. The results were compared with international standards such as ASTMD 6751 and EN 14214. Closed cup flash point tester (China), pour point and cloud point of petroleum products tester (China) and a viscometer (SVM3000) were used for measuring flash point, pour point, cloud point, and viscosity, respectively.
Analyzing oil of goat fat using GC
In the current research, goat fat was used as an oil source. Compositions of fatty acid in the goat fat was obtained by gas chromatography (GC:6890-MSD:5973, Agilent, USA). Fatty acids composition of oil of goat fat using GC is represented in Table 1.
Table 1.
The composition of goat fat fatty acids analyzed by GC device
| Free fatty acid composition | Structure of free fatty acids | Percentage | Molecular weight | Chemical formula of free fatty acids |
|---|---|---|---|---|
| Oleic acid | C18:1 | 47.18 | 282.46 | C18H34O2 |
| Palmitic acid | C16:0 | 30.42 | 256.42 | C16H32O2 |
| Linoleic acid | C18:2 | 14.20 | 280.45 | C18H32O2 |
| Myristic acid | C14:0 | 0.76 | 228.37 | C14H28O2 |
| Linolenic acid | C18:3 | 0.71 | 278.49 | C18H30O2 |
| Stearic acid | C18:0 | 6.60 | 284.48 | C18H36O2 |
| Arachidic acid | C20:0 | 0.05 | 304.47 | C20H40O2 |
| Lauric acid | C12:0 | 0.05 | 200.32 | C12H24O2 |
Results and discussion
Characterization of MgO nanocatalyst
FE-SEM analysis (Fig. 1) was used to determine the morphology, particles size, and internal micro-structure of the catalyst. As shown in Fig. 1, most of the catalyst particles were in the nanometer range and they had high porosity, showing a high specific surface area of the catalyst.
Fig. 1.

FE-SEM image of MgO nanocatalyst (scale is 500 nm)
TEM was another analysis performed on the catalyst. TEM image of the MgO nanocatalyst is shown in Fig. 2. As can be seen in this figure, the particle size was in the nano-range and they were distributed heterogeneously and asymmetrically. Some areas are darker, indicating a high concentration of particles in that area.
Fig. 2.
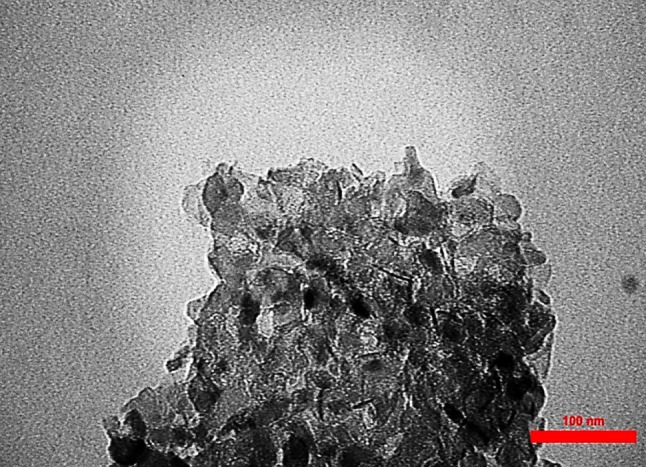
TEM image of MgO nanocatalyst (scale is 100 nm)
BET analysis was performed for measuring the specific surface area of the catalyst and the result is shown in Fig. 3. The basis of this analysis was to measure the adsorbed and desorbed volume of nitrogen gas by catalyst surface at constant temperature (77 K). BET results indicated that total pore volume, the specific surface area, volume of pores, and the average diameter of the pores in the catalyst were 9.29 cm3 (STP)/g, 40.44 m2/g, 0.371 cm3/g, and 36.69 nm, respectively. The average pore diameter of the catalyst indicated that the catalyst was mesoporous, because it was less than 50 nm.
Fig. 3.
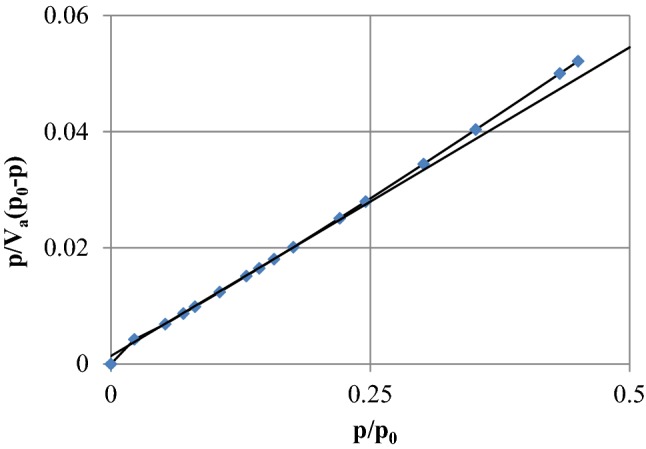
BET analysis of MgO nanocatalyst for determination of specific surface area of the nano-catalyst
TGA analysis was performed to investigate the effect of temperature on nanocatalyst. Figure 4 shows the effect of temperature on MgO nanocatalyst. Before heating, the temperature was set at 0 °C and the weight of the catalyst was considered to be 100%. By increasing the temperature, the catalyst weight was decreased with a very slight slope. This weight loss may be contributed to the moisture content of the catalyst. The weight reduction was continued until 307.45 °C in which the weight of the catalyst decreased by 5.09% and reached 94.91% of its initial value. After this temperature, the slope of the diagram increased dramatically and the sample structure was mostly decomposed between 300 and 400 °C. This temperature could be called thermal decomposition temperature of the MgO nanocatalyst, which caused the deterioration of the nanocatalyst. Decomposition temperature is an important factor which indicates the temperature range that the material can be used without decomposition and higher temperatures can destroy the sample structure. The reduction in the weight of the catalyst continued until 396.13 °C and the weight of the catalyst decreased by 15.42%. Then, the curve of the TGA diagram continues almost horizontally until 954 °C. At temperatures higher than 954 °C the diagram was horizontal, therefore there was no weight loss after this temperature.
Fig. 4.
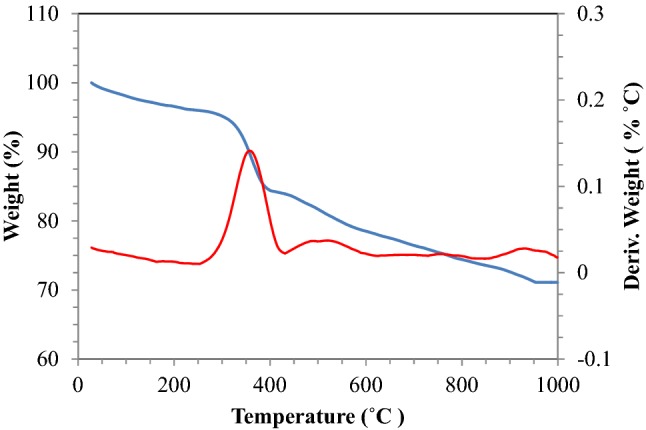
TGA analysis for determining the effect of temperature on the weight loss of MgO nanocatalyst
To determine the functional groups in the nanocatalyst, FTIR analysis was used and the results are shown in Fig. 5. The largest peak indicates a powerful absorbance at a wavenumber of 3695 cm−1 which was related to O–H bond. Also, there is a wide peak in the range of 2953 cm−1 and 3512 cm−1, which can be related to O–H bond (Yacob et al. 2009). Another stable group was at 1420 cm−1 wavenumber which is related to the carbonate group formed by CO2 absorption on Mg. The stretching peak at 1064 cm−1 is related to –CO bond. The peak between 2850 and 2962 cm−1 indicates the stretching bond of –CH and the peak between 430 and 660 cm−1 is also related to metal–oxygen (Mg–O) vibration (Jung et al. 2003; Mguni 2012).
Fig. 5.
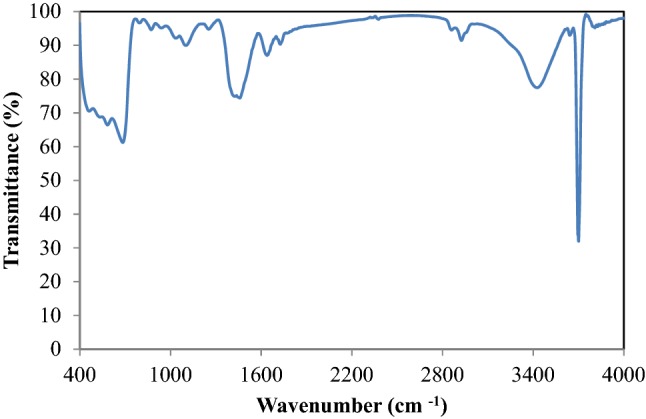
FTIR analysis for determination of functional groups in the MgO nanocatalyst
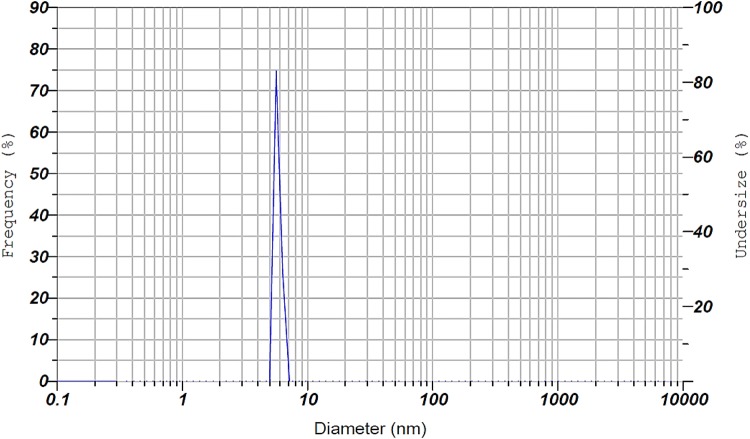
Another analysis performed on the MgO nanocatalyst was DLS analysis. This analysis is a useful method to determine the particle size distribution. Figure 6 shows the particle size distribution of the MgO nanocatalyst. According to DLS analysis, the average particle size was 5.5 nm and most of the catalyst particles were 5.5 nm. This analysis showed that the particle size of the nanocatalyst was very small.
Fig. 6.
Particle size distribution of MgO nanocatalyst by DLS analysis
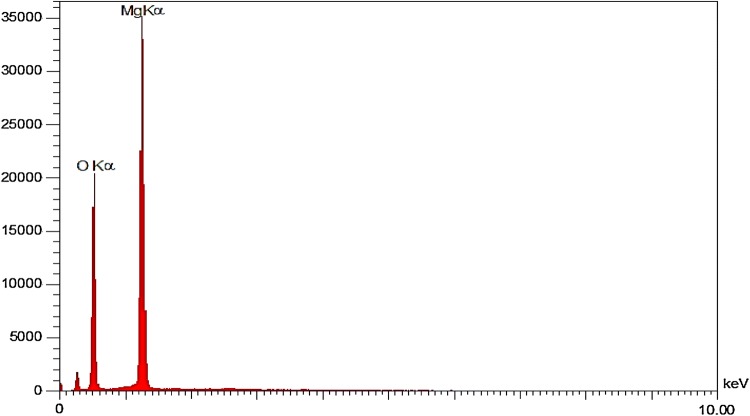
EDS or EDX-Map was another analysis used in this study. EDS analysis can determine the percentage of elements in the catalyst. Table 2 represents the percentage of elements in the MgO nanocatalyst. According to EDX analysis, the elements of this nanocatalyst were oxygen and magnesium and no impurities were observed in the sample. Figure 7 shows the EDX analysis diagram and the elements in the nanocatalyst. X-ray spectroscopy of the powder also represented the peaks for Mg and O elements and no other peak was observed, proving the high purity of the nanocatalyst.
Table 2.
Elemental analysis of MgO nanocatalyst
| Elements | Weight percent | Atomic percent |
|---|---|---|
| O | 35.70 | 45.76 |
| Mg | 64.30 | 54.24 |
| 100 | 100 |
Fig. 7.
EDX analysis for determination of MgO nanocatalyst elements
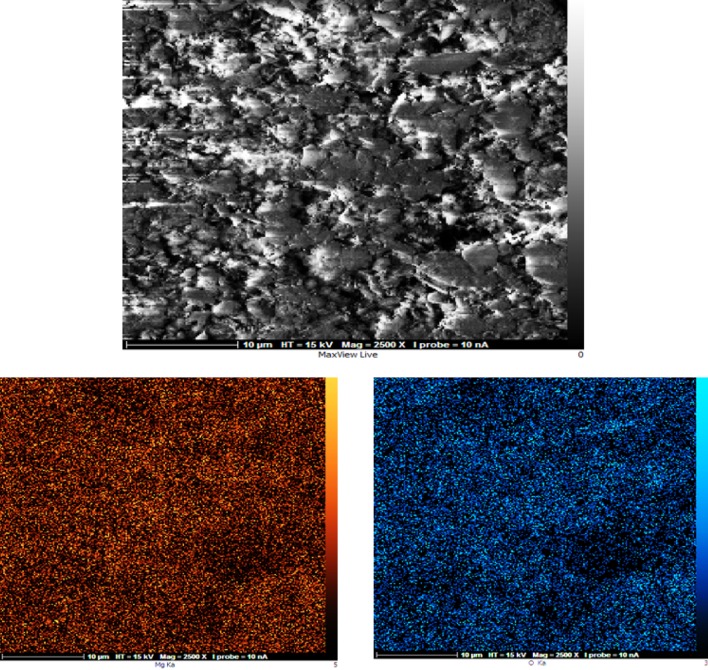
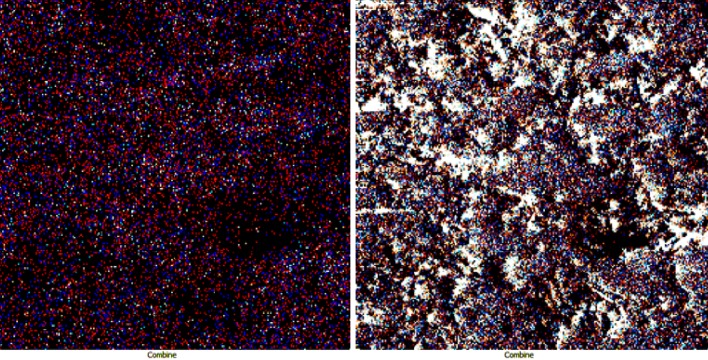
Map analysis allowed to find out the frequency distribution of elements in an image. Figure 8 shows the image of the map analysis for MgO nanocatalyst. In this figure, the orange and blue colors are related to the elements of magnesium and oxygen, respectively. Also, the distribution of both magnesium and oxygen elements on the nano-catalyst surface is shown in Fig. 9. The right image is related to the distribution of the elements in the background of the SEM image which shows how the elements are distributed on the catalyst surface.
Fig. 8.
Map analysis to determine the distribution of elements on the surface of MgO nanocatalyst
Fig. 9.
Frequency distribution of oxygen and magnesium elements in MgO nanocatalyst
Effect of various parameters on the production of biodiesel
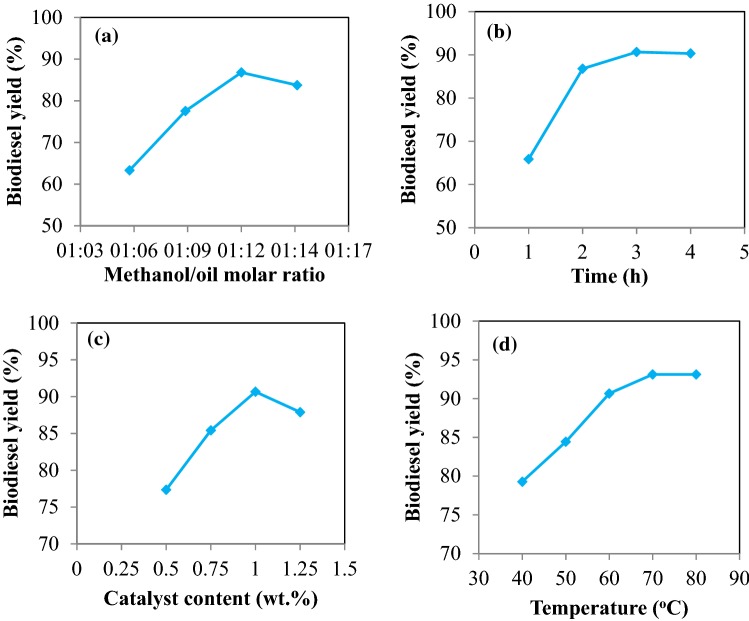
The methanol-to-oil molar ratio is the one of most important parameters on biodiesel yield. In this study, the effect of different methanol/oil molar ratios (6:1, 9:1, 12:1 and 15:1) was investigated on the biodiesel yield. Other parameters including catalyst content (1 wt.%), temperature (60 °C), reaction time (2 h), and agitation rate (1500 rpm) were kept constant. Figure 10a illustrates the effect of methanol/oil molar ratio on the biodiesel yield. According to the results, by increasing the methanol/oil molar ratio, the biodiesel yield was increased and the biodiesel yield was reduced at ratios more than 12:1. The reason for this event was that glycerin would be thoroughly dissolved in excess methanol when the amount of methanol is too high. This leads to the reduction of methanol amount and inhibits the reaction of methanol with the catalyst (Obadiah et al. 2012; Viriya-Empikul et al. 2010). Therefore, the methanol-to-oil molar ratio of 12:1 was considered as the optimum value.
Fig. 10.
The effect of different parameters on biodiesel production from goat fat in the presence of MgO nanocatalyst including methanol/oil molar ratio (a), contact time (b), percentage of catalyst (c), and reaction temperature (d)
The other important parameter affecting biodiesel yield is the reaction time. In this study, to determine the effect of reaction time, the experiments were done at different reaction times (1, 2, 3, and 4 h). To determine the optimum time, other parameters including catalyst weight percent (1%), temperature (60 °C), methanol/oil molar ratio (12:1), and agitation rate (1500 rpm) were kept constant. Based on Fig. 10b, the lowest amount of biodiesel yield was achieved 65.85% at 1 h reaction time. As the reaction time was increased, the yield of biodiesel production was increased and the highest yield of biodiesel production (90.66%) was achieved at 3 h. After 3 h, the yield of biodiesel decreased slightly to 90.31% which can be related to saponification of fatty acids (Li et al. 2011) or due to a reduction in catalyst activity or running out of reactants so the best time to produce biodiesel from goat fat in the presence of MgO nanocatalyst was 3 h.
The catalyst concentration is another important factor in the production of biodiesel. In this research, the catalyst concentrations of 0.5, 0.75,1, and 1.25 wt.% was investigated on biodiesel production while other parameters such as methanol/oil molar ratio of 12:1, temperature of 60 °C, reaction time of 3 h, and the stirring rate of 1500 rpm were kept constant. According to Fig. 10c, biodiesel yield was the lowest (77.35%) at the catalyst content of 0.5 wt.%. Then, biodiesel yield increased by increasing the catalyst percentage until 1 wt.%, which had the highest production yield (90.66%). As can be observed, biodiesel yield was decreased at the catalyst content of 1.25 wt.%. This reduction can be attributed to soap production (Keihani et al. 2018) or conversion of the solution to a viscose solution (Li et al. 2011). At first, the biodiesel yield increased due to the high active surface area of the catalyst in the reaction. When the catalyst percentage was higher than the desired value, the particles of the catalyst stuck together and formed a bulk mass that caused a reduction in the active surface area of the catalyst and led to an increase in the viscosity of the mixture. Therefore, more power and energy were required for the mixing process which was a factor in reducing the biodiesel production (Liu and Wang 2013).
The yield of biodiesel production in the transesterification process strongly depends on temperature (Rodríguez-Guerrero and Rosa 2013; Srivastava and Prasad 2000). Since biodiesel production is an endothermic reaction, temperature is an important factor. To investigate the effect of temperature, reactions were performed in different temperatures (40, 50, 60, 70, and 80 °C) under the operating condition of 12:1 methanol/oil molar ratio, the reaction time of 3 h, and mixing rate of 1500 rpm. According to Fig. 10, the biodiesel yield was low at low reaction temperatures and the lowest biodiesel yield was 79.28% which obtained at 40 °C. The yield of biodiesel increased by increasing the reaction temperature and the highest biodiesel yield (93.12%) was obtained at 70 °C. At temperature above 70 °C, the biodiesel yield was not changed. Therefore, the optimum temperature for producing biodiesel from goat fat in the presence of MgO nanocatalyst was considered to be 70 °C.
Comparing results with previous studies
The biodiesel produced from goat fat in the presence of MgO nanocatalyst was compared with other catalysts reported in the literature and the results are given in Table 3. As seen in the table, the biodiesel yield in this research is comparable to previous studies.
Table 3.
Comparing the results with previous studies
| Catalyst | Temperature (oC) | Reaction time (h) | Catalyst content (wt.%) | Methanol/oil molar ratio | Biodiesel yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Na2SiO3 | 220 | 0.5 | 0.5 | 35:1 | 95.6 | Yin et al. (2010) |
| NaOH | 55–60 | 1.5 | 0.46 | 35% volume of oil | 91 | Math (2016) |
| H2SO4 (IRARa (with silica gel) | 69.97 | 2.5 | 59.93 | 31.88:1 | 96.7 | Chakraborty and Sahu (2014) |
| H2SO4 (IRAR, (without silica gel) | 69.97 | 2.5 | 59.93 | 31.88:1 | 91.087 | Chakraborty and Sahu (2014) |
| H2SO4 (conventional, with silica gel) | 69.97 | 2.5 | 59.93 | 31.88:1 | 88.28 | Chakraborty and Sahu (2014) |
| H2SO4 (conventional, without silica gel) | 69.97 | 2.5 | 59.93 | 31.88:1 | 82.96 | Chakraborty and Sahu (2014) |
| Nano-MgO | 70 | 3 | 1 | 12:1 | 93.12 | Present study |
aInfrared radiation-assisted reactor
Physical properties of biodiesel
After production of biodiesel under the optimized condition, it was mixed with diesel fuel in different proportions (B25, B50, and B75). Table 4 represents the physical properties of biodiesel and its mixtures with diesel at different ratios. Density, kinematic viscosity, flash point, pour point, and cloud point were measured for different mixtures of biodiesel/diesel and the results were compared with international standards.
Table 4.
Physical properties of biodiesel and its mixtures with diesel at different proportions
| Properties | Unit | EN14214 | ASTM D6751 | B00 | B25 | B50 | B75 | B100 |
|---|---|---|---|---|---|---|---|---|
| Density at 15 °C | kg/m3 | 860–900 | – | 832 | 841 | 858 | 877 | 883 |
| Cinematic viscosity at 40 °C | mm2/s | – | 1.9–6 | 3 | 3.3 | 3.7 | 4.3 | 4.8 |
| Flash point | °C | At least 120 | At least 130 | 82 | 90 | 102 | 131 | 162 |
| Pour point | °C | – | – | − 7 | 1 | 3 | 6 | 8 |
| Cloudy point | °C | – | – | 2 | 6 | 8 | 10 | 11 |
Density plays an important role in the amount of fuel injected from the fuel injection system and fuel atomization in the combustion chamber. If the density is within the standard range, the fuel injection system will face the problem and injection of fuel to the combustion chamber will be very slow (Baroutian et al. 2008; Tate et al. 2006). Based on the results, the density of B00, B25, and B50 were lower than the standard ranges and the density increased with increasing the ratio of biodiesel in the mixture. Also, the density of B75 and B100 mixtures was within the standard range. Increasing the density causes the amount of fuel transmitted by the fuel injection system to be very slow (Altun et al. 2010).
Kinematic viscosity displays the ability of a substance to flow and is effective on the quality of fuel atomization. Diesel with high kinematic viscosity will lead to the formation of large droplets in injection (Alptekin and Canakci 2008; Canakci et al. 2009). If the fuel has a viscosity lower than the standard range, it will lose its softening property which leads to early wear out and seeping of the fuel system. On the other hand, fuel viscosity higher than standard range causes the fuel injection system needs more energy to inject the fuel (Rao 2011). The results indicated that the viscosity of B100 was higher than that of B00, therefore it can be concluded that biodiesel had better lubrication property than diesel but the fuel injection system required more energy to spray the fuel.
The higher the flash point, the safer the fuel for storage and transportation (Esmaeili and Foroutan 2018). When the flash point of biodiesel is larger than diesel fuel, using biodiesel fuel is considerably safer than diesel fuel. The results indicated that the flash point of biodiesel was significantly higher than diesel fuel. The reason for this was the existence of aliphatic hydrocarbons with low carbon number without double bond in the diesel.
The pour point is the lowest temperature at which the fuel can flow. According to the results, pour point increased by increasing the amount of biodiesel in the mixture. Pour point of the biodiesel was 8 °C while diesel pour point wa − 7 °C. As the pour point of biodiesel is much higher than diesel, the performance of the biodiesel will be worse than diesel in the cold weather.
Cloud point determines the temperature limits in the use of fuel. Based on the results, the cloud point increased by increasing the ratio of biodiesel in the mixture. The lowest cloud point was for B00 (2 °C) and the highest cloud point was for B100 (11 °C). Therefore, biodiesel cannot be used in cold weather. Waxy crystals are formed in the fuel at low temperatures which can clog filters and lines of the fuel injection system. There is no limit for the cloud point according to ASTM D6751 and EN14214 standards and it should be determined separately for each weather condition.
Conclusion
In this research, biodiesel was produced from goat fat in the presence of MgO nanocatalyst using transesterification process. Surface properties of MgO nano-catalyst was investigated using FESEM, TEM, BET, TGA, DLS, FTIR, and EDX-Map analysis. Also, the effect of different parameters on biodiesel production including methanol/oil molar ratio (6:1, 9:1, 12:1, 15:1), reaction time (1, 2, 3, and 4 h), the catalyst addition (0.5, 0.75, 1, and 1.25 wt.%) and temperature (40, 50, 60, 70, and 80 °C) were studied. The results showed that a biodiesel yield of 93.12% was obtained at optimum conditions such as methanol/oil molar ratio of 12:1, the reaction time of 3 h, catalyst content of 1 wt.%, and temperature of 70 °C).
To improve the properties of diesel fuel, the produced biodiesel was blended with diesel in different ratios and their properties were investigated in accordance with ASTM D6751 and EN14214 standards. The results showed that by increasing the biodiesel ratio in the fuel mixture, some of the properties such as pour point and cloud point increase, which limit the use of biodiesel in cold weather. Also, the density of fuel increased when the biodiesel ratio in mixture increased and the density of the B25 and B50 mixtures were slightly lower than standard range, but the mixtures of B75 and B100 were in the range of standard. In addition, the viscosity of the biodiesel and all mixtures were within the standard range. Moreover, the flash point of biodiesel/diesel mixture increased compared to pure diesel making a safer condition for storage and transportation of the fuel. Furthermore, the flash points of B75 and B100 were higher than the standard range.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agarwal M, Chauhan G, Chaurasia SP, Singh K. Study of catalytic behavior of KOH as homogeneous and heterogeneous catalyst for biodiesel production. J Taiwan Inst Chem Eng. 2012;43:89–94. [Google Scholar]
- Ali AS, Ahmad F, Farhan M, Ahmad M. Biodiesel production from residual animal fat using various catalysts. Pak J Sci. 2012;64:282. [Google Scholar]
- Alptekin E, Canakci M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew Energy. 2008;33:2623–2630. [Google Scholar]
- Altun Ş, Yaşar F, Öner C. The fuel properties of methyl esters produced from canola oil-animal tallow blends by base-catalyzed transesterification. Uluslararası Mühendislik Araştırma ve Geliştirme Dergisi. 2010;2:2–5. [Google Scholar]
- Anggoro DD, Buchori L, Sasongko SB, AAFF MV. Synthesis of Ca/MgO catalyst using sol gel method for monoglycerides production. AIP Conf Proc. 2018;1977:030012. [Google Scholar]
- Ashok A, Kennedy LJ, Vijaya JJ, Aruldoss U. Optimization of biodiesel production from waste cooking oil by magnesium oxide nanocatalyst synthesized using coprecipitation method. Clean Technol Environ. 2018;20:1219–1231. [Google Scholar]
- Azam MM, Waris A, Nahar NM. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy. 2005;29:293–302. [Google Scholar]
- Banković-Ilić IB, Miladinović MR, Stamenković OS, Veljković VB. Application of nano CaO-based catalysts in biodiesel synthesis. Renew Sust Energy Rev. 2017;72:746–760. [Google Scholar]
- Baroutian S, Kheireddine Aroua M, Abdul Raman AA, Sulaiman NMN. Estimation of vegetable oil-based ethyl esters biodiesel densities using artificial neural networks. J Appl Sci. 2008;8:3005–3011. [Google Scholar]
- Canakci M, Ozsezen AN, Arcaklioglu E, Erdil A. Prediction of performance and exhaust emissions of a diesel engine fueled with biodiesel produced from waste frying palm oil. Expert Syst Appl. 2009;6:9268–9280. [Google Scholar]
- Chakraborty R, Sahu H. Intensification of biodiesel production from waste goat tallow using infrared radiation: process evaluation through response surface methodology and artificial neural network. Appl Energy. 2014;114:827–836. [Google Scholar]
- Chongkhong S, Tongurai C, Chetpattananondh P. Continuous esterification for biodiesel production from palm fatty acid distillate using economical process. Renew Energy. 2009;34:1059–1063. [Google Scholar]
- Chouhan AS, Sarma AK. Modern heterogeneous catalysts for biodiesel production: a comprehensive review. Renew Sust Energy Rev. 2011;15:4378–4399. [Google Scholar]
- Dorado MP, Cruz F, Palomar JM, Lopez FJ. An approach to the economics of two vegetable oil-based biofuels in Spain. Renew Energy. 2006;31:1231–1237. [Google Scholar]
- Enweremadu CC, Mbarawa MM. Technical aspects of production and analysis of biodiesel from used cooking oil—a review. Renew Sust Energy Rev. 2009;13:2205–2224. [Google Scholar]
- Esmaeili H, Foroutan R. Optimization of biodiesel production from goat tallow using alkaline catalysts and combining them with diesel. Chem Chem Technol. 2018;12:120–126. [Google Scholar]
- Gürü M, Koca A, Can Ö, Çınar C, Şahin F. Biodiesel production from waste chicken fat based sources and evaluation with Mg based additive in a diesel engine. Renew Energy. 2010;35:637–643. [Google Scholar]
- Hara M. Environmentally benign production of biodiesel using heterogeneous catalysts. ChemSusChem Chem Sust Energy Mater. 2009;2:129–135. doi: 10.1002/cssc.200800222. [DOI] [PubMed] [Google Scholar]
- Jung HS, Lee JK, Kim JY, Hong KS. Crystallization behaviors of nanosized MgO particles from magnesium alkoxides. J Coll Interface Sci. 2003;259:127–132. doi: 10.1016/s0021-9797(03)00034-1. [DOI] [PubMed] [Google Scholar]
- Keihani M, Esmaeili H, Rouhi P. Biodiesel production from chicken fat using nano-calcium oxide catalyst and improving the fuel properties via blending with diesel. Phys Chem Res. 2018;6:521–529. [Google Scholar]
- Krishna K, Kumar BSP, Reddy KVK, Kumar SC, Kumar KR. Effects of alumina nano metal oxide blended palm stearin methyl ester bio-diesel on direct injection diesel engine performance and emissions. IOP Conf Ser Mater Sci Eng. 2017;225:012212. [Google Scholar]
- Li Y, Qiu F, Yang D, Li X, Sun P. Preparation, characterization and application of heterogeneous solid base catalyst for biodiesel production from soybean oil. Biomass Bioenergy. 2011;35:2787–2795. [Google Scholar]
- Liu K, Wang R. Biodiesel production by transesterification of duck oil with methanol in the presence of alkali catalyst. Pet Coal. 2013;55(1):68–72. [Google Scholar]
- Liu C, Lv P, Yuan Z, Yan F, Luo W. The nanometer magnetic solid base catalyst for production of biodiesel. Renew Energy. 2010;35:1531–1536. [Google Scholar]
- Lukić I, Krstić J, Jovanović D, Skala D. Alumina/silica supported K2CO3 as a catalyst for biodiesel synthesis from sunflower oil. Bioresour Technol. 2009;100:4690–4696. doi: 10.1016/j.biortech.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Luu PD, Takenaka N, Van Luu B, Pham LN, Imamura K, Maeda Y. Co-solvent method produce biodiesel form waste cooking oil with small pilot plant. Energy Procedia. 2014;61:2822–2832. [Google Scholar]
- Ma Y, Wang Q, Gao Z, Sun X, Wang N, Niu R, Ma H. Transesterification of waste cooking oil using FeCl3-modified resin catalyst and the research of catalytic mechanism. Renew Energy. 2016;86:643–650. [Google Scholar]
- Marulanda VF, Anitescu G, Tavlarides LL. Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J Supercrit Fluid. 2010;54:53–60. [Google Scholar]
- Math MC. Application of response surface methodology for optimization of biodiesel production by transesterification of animal fat with methanol. Int J Renew Energy Res. 2016;6:74–79. [Google Scholar]
- Mguni LL (2012) Biodiesel production over supported nano-magnesium oxide particles. Doctoral dissertation, University of Johannesburg
- Moser BR. Biodiesel production, properties, and feedstocks. Vitro Cell Dev Biol Plant. 2009;45:229–266. [Google Scholar]
- Obadiah A, Swaroopa GA, Kumar SV, Jeganathan KR, Ramasubbu A. Biodiesel production from palm oil using calcined waste animal bone as catalyst. Bioresour Technol. 2012;116:512–516. doi: 10.1016/j.biortech.2012.03.112. [DOI] [PubMed] [Google Scholar]
- Okitsu K, Sadanaga Y, Takenaka N, Maeda Y, Bandow H. A new co-solvent method for the green production of biodiesel fuel—optimization and practical application. Fuel. 2013;103:742–748. [Google Scholar]
- Ramadhas AS, Jayaraj S, Muraleedharan C. Biodiesel production from high FFA rubber seed oil. Fuel. 2005;84:335–340. [Google Scholar]
- Rao PV. Experimental investigations on the influence of properties of jatropha biodiesel on performance, combustion, and emission characteristics of a DI-CI engine. World Acad Sci Eng Technol. 2011;75:855–868. [Google Scholar]
- Rodríguez-Guerrero JK, Rosa PT. Production of biodiesel from castor oil using sub and supercritical ethanol: effect of sodium hydroxide on the ethyl ester production. J Supercrit Fluid. 2013;83:124–132. [Google Scholar]
- Seffati K, Honarvar B, Esmaeili H, Esfandiari N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel. 2019;235:1238–1244. [Google Scholar]
- Srivastava A, Prasad R. Triglycerides-based diesel fuels. Renew Sust Energy Rev. 2000;4:111–133. [Google Scholar]
- Tate RE, Watts KC, Allen CAW, Wilkie KI. The densities of three biodiesel fuels at temperatures up to 300 °C. Fuel. 2006;85:1004–1009. [Google Scholar]
- Vasudevan PT, Briggs M. Biodiesel production—current state of the art and challenges. J Ind Microbiol Biotechnol. 2008;35:421. doi: 10.1007/s10295-008-0312-2. [DOI] [PubMed] [Google Scholar]
- Viriya-Empikul N, Krasae P, Puttasawat B, Yoosuk B, Chollacoop N, Faungnawakij K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour Technol. 2010;101:3765–3767. doi: 10.1016/j.biortech.2009.12.079. [DOI] [PubMed] [Google Scholar]
- Yacob AR, Mustajab MKAA, Samadi NS. Calcination temperature of nano MgO effect on base transesterification of palm oil. World Acad Sci Eng Technol. 2009;56:408–412. [Google Scholar]
- Yin JZ, Ma Z, Hu DP, Xiu ZL, Wang TH. Biodiesel production from subcritical methanol transesterification of soybean oil with sodium silicate. Energy Fuel. 2010;24:3179–3182. [Google Scholar]
- Zhang Y, Dube MA, McLean DDL, Kates M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol. 2003;89:1–16. doi: 10.1016/s0960-8524(03)00040-3. [DOI] [PubMed] [Google Scholar]