Abstract
Objective
Severe sepsis and septic shock (SS/SS) treatment bundles reduce mortality, and early infectious diseases (ID) consultation also improves patient outcomes. We retrospectively examined whether early ID consultation further improves outcomes in Emergency Department (ED) patients with SS/SS who complete the sepsis bundle.
Method
We included 248 adult ED patients with SS/SS who completed the 3-hour bundle. Patients with ID consultation within 12 hours of ED triage (n = 111; early ID) were compared with patients who received standard care (n = 137) for in-hospital mortality, 30-day readmission, length of hospital stay (LOS), and antibiotic management. A competing risk survival analysis model compared risks of in-hospital mortality and discharge alive between groups.
Results
In-hospital mortality was lower in the early ID group unadjusted (24.3% vs 38.0%, P = .02) and adjusted for covariates (odds ratio, 0.47; 95% confidence interval (CI), 0.25–0.89; P = .02). There was no significant difference in 30-day readmission (22.6% vs 23.5%, P = .89) or median LOS (10.2 vs 12.1 days, P = .15) among patients who survived. A trend toward shorter time to antibiotic de-escalation in the early ID group (log-rank test P = .07) was observed. Early ID consultation was protective of in-hospital mortality (adjusted subdistribution hazard ratio (asHR), 0.60; 95% CI 0.36–1.00, P = .0497) and predictive of discharge alive (asHR 1.58, 95% CI, 1.11–2.23; P-value .01) after adjustment.
Conclusions
Among patients receiving the SS/SS bundle, early ID consultation was associated with a 40% risk reduction for in-hospital mortality. The impact of team-based care and de-escalation on SS/SS outcomes warrants further study.
Keywords: antimicrobial stewardship, bundle, infectious diseases consultation, mortality, sepsis
Early infectious diseases consult within 12 hours of emergency department triage was associated with lower mortality in patients with severe sepsis or septic shock who completed the 3-hour sepsis bundle.
INTRODUCTION
Severe sepsis and septic shock (SS/SS) carry serious risk of morbidity and mortality [1]. Early SS/SS treatment bundles improve clinical outcomes [2, 3] and are recommended by published guidelines [4]. Both the New York State Department of Health (NYSDOH) [5] and the Centers for Medicare and Medicaid Services [6] mandate hospital reporting on sepsis bundle adherence within 3 hours of SS/SS onset in adults and sepsis-related mortality (SEP-1 core measure). The SEP-1 treatment bundle includes measurement of lactate, collection of blood cultures prior to antibiotic administration, administration of broad-spectrum antibiotics, and crystalloid fluid resuscitation for hypotensive patients. Although some studies show a reduction in sepsis-related mortality after implementation of sepsis reporting [7, 8], adverse outcomes, such as antibiotic over-use and hospital-onset Clostridioides difficile infection (HO-CDI), can occur [9, 10].
There are approximately 850,000 Emergency department (ED) visits annually in the U.S. for sepsis [11]. Although inappropriate antibiotic prescribing is common in the ED [12–17], antimicrobial stewardship (ASP) strategies, including electronic health record (EHR) decision support, improve appropriateness of therapy [18–22]. Infectious diseases (ID) consultation in the ED also improves appropriate antibiotic prescribing, promotes beneficial collaborative relationships between ID and ED colleagues, and reduces unnecessary admissions [23]. Similar models of team-based care and shared decision making have been endorsed by the Institute of Medicine, the American College of Physicians, and the Society for Academic Emergency Medicine [24–26].
Participation of ID specialists in patient care teams, especially in the first 2 days of hospitalization, results in better outcomes in infectious conditions, including shorter length of hospital stay (LOS), reduced mortality, reduced readmissions, more appropriate choice of antibiotics and decreased antibiotic use overall [27–30]. Viale et al demonstrated improved bundle compliance, reduced mortality, and improved antimicrobial appropriateness in patients with SS/SS by providing ID consultation within 1 hour of ED arrival 24 hours per day, 7 days per week (24/7) [31]. However, a 24/7 ID consultation model is not feasible in many hospitals, and it is not clear if the mortality benefit in SS/SS extends beyond improvement of adherence to standard sepsis bundles. Combining ID consultation early in the patient's clinical course with ASP decision support could offer clinical benefit in SS/SS that may not be possible in hospitals without continuous ID staffing. The aim of this study was to evaluate the impact of early ID consultation in the first 12 hours after ED triage on clinical outcomes and antimicrobial prescribing among patients presenting with SS/SS who received the 3-hour sepsis treatment bundle.
METHODS
A collaborative Emergency Department-Infectious Diseases (ED-ID) team-based care program was designed and implemented at the Einstein campus at the Montefiore Medical Center (MMC) with the aim of improving sepsis-related outcomes while promoting optimal ASP practices. The program combined ED-specific prescribing tools and decision support with streamlined ID consultation for ED patients with sepsis. The ED-ID program was endorsed by both ID and ED providers, and it improved prescribing practices and adherence to ASP guidelines [23]. Consequently, ID consultation was added to the recommended 3-hour bundle order set for any patient with SS/SS beginning in 2017 with the goal of improving antimicrobial management while reducing sepsis-related mortality.
Study Design
We conducted a retrospective cohort study at the Einstein campus of MMC, a 421-bed nonprofit teaching and academic medical center with 32 intensive-care unit beds, 80,000 annual ED visits, and 30,000 annual admissions. The study was approved by the MMC Institutional Review Board (protocol #2015–5278). Patients were identified using the data submission files for NYSDOH sepsis reporting. Patients who presented to the Einstein campus of MMC between January 2017 and March 2018, who met inclusion criteria for sepsis reporting in the ED, and who successfully received all components of the 3-hour sepsis bundle (blood culture collection prior to antibiotic administration, broad-spectrum antibiotic regimen initiation, lactate measurement, and 30ml/kg crystalloid fluid resuscitation in hypotensive patients) were included [6]. The purpose of including only patients who received all components of the 3-hour bundle was to assess the clinical impact of early ID consultation separately from outcomes related to compliance with the treatment bundle. Patients who died within 6 hours of arrival to the ED, those who were ineligible for the full sepsis bundle due to palliative goals of care or patient or family preference, and patients less than 18 years of age were excluded from the data set. Only the earliest event was included for patients who had more than one episode of sepsis during the data collection time frame.
Sepsis Decision Support
Decision support for empiric antimicrobial prescribing in SS/SS patients was incorporated into an order set in the EHR; paper and online versions also were available on the hospital's intranet website. The tools included guidance based on suspected source of infection (if known), health care exposure history, clinical risk factors, renal function, and allergy history. Use of the SS/SS order set allowed providers to obtain the first dose of restricted antimicrobials without calling for ASP approval to prevent delays in antibiotic administration. Decision support tools were available to providers regardless of their decision to formally consult ID; however, ID consultation request was included as a suggested component of the 3-hour bundle in the order set.
ID Consultation
Early ID consultation was voluntary, at the discretion of the responsible ED physician. Infectious diseases attending physicians provided formal clinical consultation and recommendations for antibiotic selection, dosing, duration, and additional testing 7 days per week from 8am through 5pm. Consults requested outside of these hours were seen the following morning. An ASP member or ID fellow and ID attending physician were available 24/7 for approvals and other questions regarding antibiotic therapy as per hospital protocol.
For the intervention cohort (early ID), patients were included if they received ID consultation within 12 hours from ED triage. The comparison group (standard care) consisted of patients who were not seen by an ID specialist within 12 hours of ED triage. Patients were included in the standard care group regardless of whether they had an ID consult later in the hospital course or no ID consult was requested during admission. This design was a practical approach to evaluate if expedited ID consultation within a 12-hour window, which is likely feasible for most hospitals and ID services, offered a significant clinical benefit in sepsis care.
Data Collection
Clinical Looking Glass (Emerging Health Information Technology, Yonkers, NY), a computerized health care surveillance software at MMC linked to the EHR, and chart review were utilized to validate the submission file data and obtain data that were not available in the NYSDOH data set. Data collected included patient demographics, history and comorbidities, microbiologic data, antibiotic treatment, clinical data, and outcomes.
Clinical Outcomes
Primary outcomes were all-cause in-hospital mortality, LOS, hospital readmission within 30 days of index discharge, time to death, and time to hospital discharge alive. Secondary outcomes were receipt of appropriate antibiotic therapy, time to appropriate therapy, receipt of effective antibiotic therapy, time to effective antibiotic therapy, microbiologic tailoring of therapy, time to microbiologic tailoring of therapy, total antibiotic days, antibiotic de-escalation, time to de-escalation, acceptance of ID recommendations regarding duration and de-escalation of antibiotics, definitive infectious diagnosis, time to definitive infectious diagnosis, and HO-CDI (per National Healthcare Safety Network definition [32]). An antimicrobial regimen was deemed appropriate if in accordance with hospital ASP guidelines based on suspected or confirmed source of infection, history of a multidrug-resistant organism (MDRO), hospitalization in the preceding 90 days, allergy history, and renal function, as well as activity against any pathogens that were identified. An antimicrobial regimen was deemed effective based on in vitro activity against any pathogens that were isolated, or in the absence of microbiologic diagnosis, if sufficient to treat the typical pathogens at the confirmed source of infection, even if the regimen had a greater spectrum of antimicrobial activity than recommended by ASP guidelines. De-escalation was defined as a change in antibiotic therapy from a broad-spectrum regimen to a more narrow-spectrum regimen by changing the antibiotic agent, discontinuing one agent in a combination regimen, or both.
Statistical Analysis
Baseline demographics, key clinical variables, and outcomes were compared between the early ID consultation and standard care groups using a two-sample t test for normally-distributed continuous data, Wilcoxon rank sum test for nonnormally distributed continuous data, χ 2 test or Fisher exact test for categorical data, and log-rank tests for time-to-event data. A χ 2 test was used to compare rates of in-hospital mortality, as well as readmission within 30 days of index hospital discharge, and multivariable logistic regression was used to adjust for covariates. Fine and Gray models for competing risks were used to analyze time to mortality and time to discharge alive [29, 33]. The final multivariable logistic regression and Fine and Gray models included all variables with a P value of <.20 in the univariable test (age, race/ethnicity, prior hospitalization, prior use of intravenous antibiotics, history of MDRO, lactate > 4, and intra-abdominal source of infection), and we adjusted for either overall Charlson score (model 2) or hemiplegia or paraplegia (model 3) for comorbidities. Initial hypotension, vasopressor requirement within 72 hours, and need for mechanical ventilation within 72 hours were included in the multivariable model for their clinical significance. As a sensitivity analysis, mortality-related outcomes also were compared among patients who either died in the hospital or who were discharged to hospice for sepsis or complications of hospitalization. An additional sensitivity analysis was performed analyzing mortality, time to discharge alive, and time to death for all patients who had an ID consult within the first 7 days of admission versus those who did not. All analyses were conducted in SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
A total of 248 patients met inclusion criteria: 111 received an ID consult within 12 hours of ED triage (early ID) and 137 received standard care. Baseline demographics and clinical features (Table 1) were similar in the 2 groups except that patients in the early ID group were more likely to have a history of hemiplegia or paraplegia (7.2% vs 1.5%, P value = .046), recent hospitalization (57.7% vs 38.0%, P = .002), recent intravenous antibiotic use (40.9% vs 23.5%, P = .004), and history of MDRO (27.3% vs 6.6%, P < .001). Of those patients in the standard care group without an ID consult within 12 hours of ED triage, 75 (54.7%) had an ID consult later in their clinical course, and the remaining 62 (45.3%) had no ID consult during the index admission. Index admission refers to the initial admission for sepsis and is meant to distinguish from any subsequent readmissions where an ID consult could also have occurred. Median time to ID consult was 2 hours (interquartile range [IQR], 1–8) in the early ID group, and 27 hours (IQR, 19–68) for those in the standard care group who had an ID consult. Utilization of antibiotic decision support within the sepsis order set (28.8% vs 21.9%, P = .21), positive blood culture results (38.7 vs 35.0%, P = .55) and positive nonblood microbiology test results (49.5% vs 47.4%, P = .74) did not differ between the early ID and standard care groups.
Table 1.
Baseline Demographic and Clinical Characteristics in Early Infectious Diseases Consult and Standard Care Groups
| Total (N = 248) |
Early ID Consult (N = 111) |
Standard Care (N = 137) |
P valuea | |
|---|---|---|---|---|
| Age at index date, mean (SD) | 71.4 (14.7) | 69.9 (15.3) | 72.6 (14.1) | .15 |
| Gender, n (%) | .94 | |||
| Female | 128 (51.6) | 57 (51.4) | 71 (51.8) | |
| Male | 120 (48.4) | 54 (48.6) | 66 (48.2) | |
| Race/ethnicity, n (%) | .08 | |||
| Hispanic | 85 (34.3) | 45 (40.5) | 40 (29.2) | |
| Non-Hispanic black | 70 (28.2) | 34 (30.6) | 36 (26.3) | |
| Non-Hispanic white | 63 (25.4) | 22 (19.8) | 41 (29.9) | |
| Other/unknown | 30 (12.1) | 10 (9.0) | 20 (14.6) | |
| Charlson comorbidity score, mean (SD) | 4.6 (3.1) | 4.8 (3.1) | 4.4 (3.1) | .22 |
| Individual comorbidity, n (%) | ||||
| Myocardial infarction | 33 (13.3) | 18 (16.2) | 15 (10.9) | .22 |
| Congestive heart failure | 91 (36.7) | 45 (40.5) | 46 (33.6) | .26 |
| Peripheral vascular disease | 36 (14.5) | 17 (15.3) | 19 (13.9) | .75 |
| Cerebrovascular disease | 42 (16.9) | 20 (18.0) | 22 (16.1) | .68 |
| Dementia | 82 (33.1) | 32 (28.8) | 50 (36.5) | .20 |
| Chronic pulmonary disease | 102 (41.1) | 45 (40.5) | 57 (41.6) | .87 |
| Rheumatic disease | 7 (2.8) | 4 (3.6) | 3 (2.2) | .70 |
| Peptic ulcer disease | 10 (4.0) | 3 (2.7) | 7 (5.1) | .52 |
| Mild liver disease | 25 (10.1) | 14 (12.6) | 11 (8.0) | .23 |
| Diabetes without chronic complications | 39 (15.7) | 20 (18.0) | 19 (13.9) | .37 |
| Diabetes with chronic complications | 82 (33.1) | 39 (35.1) | 43 (31.4) | .53 |
| Hemiplegia or paraplegia | 10 (4.0) | 8 (7.2) | 2 (1.5) | .046 |
| Renal disease | 108 (43.5) | 50 (45.0) | 58 (42.3) | .67 |
| Any malignancy | 43 (17.3) | 18 (16.2) | 25 (18.2) | .67 |
| Moderate or severe liver disease | 6 (2.4) | 3 (2.7) | 3 (2.2) | 1.00 |
| Metastatic solid tumor | 26 (10.5) | 12 (10.8) | 14 (10.2) | .88 |
| AIDS/HIV | 4 (1.6) | 3 (2.7) | 1 (0.7) | .33 |
| Prior hospital admission within 90 days of the index visit, n (%) | 116 (46.8) | 64 (57.7) | 52 (38.0) | .002 |
| Received IV antibiotics in the 90 days prior to index visit, n (%) (n = 2 missing) |
77 (31.3) | 45 (40.9) | 32 (23.5) | .004 |
| History of MDRO in the 6 months prior to index visit, n (%) (n = 2 missing) |
39 (15.9) | 30 (27.3) | 9 (6.6) | <.0001 |
| Hypotension, n (%) | 73 (29.4) | 30 (27.0) | 43 (31.4) | .45 |
| Lactate ≥4, n (%) | 75 (30.2) | 28 (25.2) | 47 (34.3) | .12 |
| ICU admission within 72 hours, n (%) | 79 (31.9) | 34 (30.6) | 45 (32.8) | .71 |
| Vasopressors within 72 hours, n (%) | 100 (40.3) | 44 (39.6) | 56 (40.9) | .84 |
| Mechanical ventilation within 72 hours, n (%) | 38 (15.3) | 17 (15.3) | 21 (15.3) | 1.00 |
| Creatinine, median (IQR) | 1.5 (1.0, 2.5) | 1.5 (1.0, 2.9) | 1.5 (1.0, 2.4) | .89 |
| Bilirubin, median (IQR) (n = 23 missing) |
0.6 (0.3, 1.0) | 0.7 (0.3, 1.1) | 0.5 (0.3, 0.9) | .26 |
| Platelet count, median (IQR) (n = 1 missing) |
241.0 (174.0, 341.0) | 240.0 (177.0, 342.0) | 242.5 (173.0, 332.0) | .96 |
| Presumed source of infection at the time of ID consultation, n (%) | ||||
| Pneumonia (CAP/HCAP) | 145 (58.5) | 60 (54.1) | 85 (62.0) | .20 |
| Meningitis/encephalitis | 3 (1.2) | 1 (0.9) | 2 (1.5) | 1.00 |
| Bloodstream infection | 5 (2.0) | 2 (1.8) | 3 (2.2) | 1.00 |
| Skin/soft tissue infection | 22 (8.9) | 12 (10.8) | 10 (7.3) | .33 |
| Urinary tract infection | 56 (22.6) | 27 (24.3) | 29 (21.2) | .55 |
| Intra-abdominal | 32 (12.9) | 18 (16.2) | 14 (10.2) | .16 |
| Unknown | 21 (8.5) | 9 (8.1) | 12 (8.8) | .85 |
| Sepsis order set utilized, n (%) | 62 (25.0) | 32 (28.8) | 30 (21.9) | .21 |
| Positive blood culture, n (%) | 91 (36.7) | 43 (38.7) | 48 (35.0) | .55 |
| Any positive nonblood culture, n (%) | 120 (48.4) | 55 (49.5) | 65 (47.4) | .74 |
Bold values indicate those that are statistically significant.
Abbreviations: CAP, community-acquired pneumonia; HCAP, healthcare-associated pneumonia; ICU, intensive care unit; ID, infectious diseases; IQR, interquartile range; IV, intravenous; MDRO, multidrug-resistant organism; SD, standard deviation.
a t test, Wilcoxon rank sum test, χ 2 test, or Fisher exact test.
Primary Outcomes
In the unadjusted analysis, rate of in-hospital mortality was statistically significantly lower in the early ID group compared with the standard care group (24.3% vs 38.0%, P = .02) (Table 2). In the adjusted analysis, this difference remained statistically significant (adjusted odds ratio [AOR], 0.47; 95% CI, 0.25–0.89; P = .02) (Table 3). Vasopressors within 72 hours (AOR, 2.76; 95% CI, 1.42–5.34; P = .003) and age (AOR, 1.03; 95% CI, 1.01–1.05; P = .01) also were significantly associated with mortality. Among patients who were discharged alive, there was no statistically significant difference in primary outcomes of median hospital length of stay (10.2 vs 12.1 days, P = .15), rate of 30-day readmission (22.6% vs 23.5%, P = .89), or median days of antibiotic therapy (7.0 vs 9.0, P = .72) (Table 4).
Table 2.
Outcomes in Early Infectious Diseases Consult and Standard Care Groups
| Total (N = 248) |
Early ID Consult (N = 111) |
Standard Care (N = 137) |
P valuea | P value for time-to-eventb | |
|---|---|---|---|---|---|
| In-hospital mortality, n (%) | 79 (31.9) | 27 (24.3) | 52 (38.0) | .02 | |
| In-hospital mortality or discharge to hospice related to sepsis, n (%) | 84 (33.9) | 28 (25.2) | 56 (40.9) | .01 | |
| Appropriate antibiotics, n (%) | 202 (81.5) | 91 (82.0) | 111 (81.0) | .85 | .84 |
| Effective antibiotics, n (%) | 246 (99.2) | 110 (99.1) | 136 (99.3) | 1.00 | .15 |
| Confirmed infection diagnosis, n (%) | 202 (81.5) | 91 (82.0) | 111 (81.0) | .85 | .62 |
| Antibiotic de-escalation, n (%) | 152 (61.3) | 72 (64.9) | 80 (58.4) | .30 | .07 |
| Days of antibiotic therapy, median (IQR) | 8.0 (4.5, 12.0) | 7.0 (5.0, 12.0) | 8.0 (4.0, 12.0) | .72 | |
| Hospital-onset Clostridioides difficile infection, n (%) (n = 1 missing) |
9 (3.6) | 5 (4.5) | 4 (2.9) | .74 |
Bold values indicate those that are statistically significant.
Abbreviations: ID, infectious diseases; IQR, interquartile range.
a χ 2 test, Fisher exact test, or Wilcoxon rank sum test.
b Log-rank test.
Table 3.
Logistic Regression Models of Outcome: In-hospital Mortality (N = 248)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | AOR (95% CI) | P value | AOR (95% CI) | P value | |
| Early ID consult (reference = standard care) | 0.53 (0.30–0.91) | .02 | 0.47 (0.25–0.89) | .02 | 0.48 (0.25–0.91) | .02 |
| Age at index date | 1.03 (1.01–1.05) | .01 | 1.03 (1.01–1.05) | .01 | ||
| Race/ethnicity (reference = Non-Hispanic white) | ||||||
| Hispanic | 1.45 (0.68–3.10) | .33 | 1.43 (0.67–3.03) | .36 | ||
| Non-Hispanic black | 0.88 (0.39–2.02) | .77 | 0.93 (0.40–2.12) | .86 | ||
| Other/unknown | 0.83 (0.28–2.44) | .73 | 0.81 (0.27–2.38) | .70 | ||
| Charlson comorbidity score | 1.04 (0.94–1.15) | .42 | ||||
| Hemiplegia or paraplegia (reference = no) | 0.55 (0.10–3.04) | .50 | ||||
| Recent hospital admission (reference = no) | 1.16 (0.52–2.56) | .72 | 1.28 (0.60–2.73) | .52 | ||
| Recent IV antibiotics (reference = no) | 1.05 (0.46–2.37) | .91 | 1.09 (0.48–2.48) | .83 | ||
| History of MDRO (reference = no) | 1.24 (0.54–2.88) | .61 | 1.27 (0.55–2.95) | .58 | ||
| Hypotension (reference = no) | 0.77 (0.39–1.54) | .47 | 0.81 (0.41–1.61) | .55 | ||
| Lactate ≥4 (reference = no) | 0.94 (0.49–1.79) | .85 | 0.93 (0.49–1.77) | .83 | ||
| Vasopressors (reference = no) | 2.76 (1.42–5.34) | .003 | 2.80 (1.44–5.43) | .002 | ||
| Mechanical ventilation (reference = no) | 1.79 (0.82–3.94) | .15 | 1.76 (0.80–3.88) | .16 | ||
| Intra-abdominal source of infection (reference = no) | 0.64 (0.25–1.63) | .35 | 0.62 (0.25–1.56) | .31 |
Bold values indicate those that are statistically significant. Model 1: AUC = 0.58; Model 2: AUC = 0.72; Model 3: AUC = 0.71.
Abbreviations: AOR, adjusted odds ration; AUC, area under the curve; CI, confidence interval; ID, infectious diseases; IV, intravenous; MDRO, multidrug-resistant organism; OR, odds ratio.
Table 4.
Length of Hospital Stay, Days of Antibiotic Therapy, and 30-Day Readmission Rate Among Patients Who Survived the Index Hospitalization
| Total (N = 169) |
Early ID Consult (N = 84) |
Standard Care (N = 85) |
P value a | |
|---|---|---|---|---|
| Length of hospital stay (days), median (IQR) | 11.1 (7.3, 16.1) | 10.2 (6.8, 15.7) | 12.1 (8.3, 18.3) | .15 |
| Days of antibiotic therapy, median (IQR) | 8.0 (5.0, 12.0) | 7.0 (5.0, 11.5) | 9.0 (5.0, 12.0) | .72 |
| 30-day readmission, n (%) | 39 (23.1) | 19 (22.6) | 20 (23.5) | .89 |
Abbreviations: ID, infectious diseases; IQR, interquartile range.
aWilcoxon rank sum test, χ 2 test, or Fisher exact test.
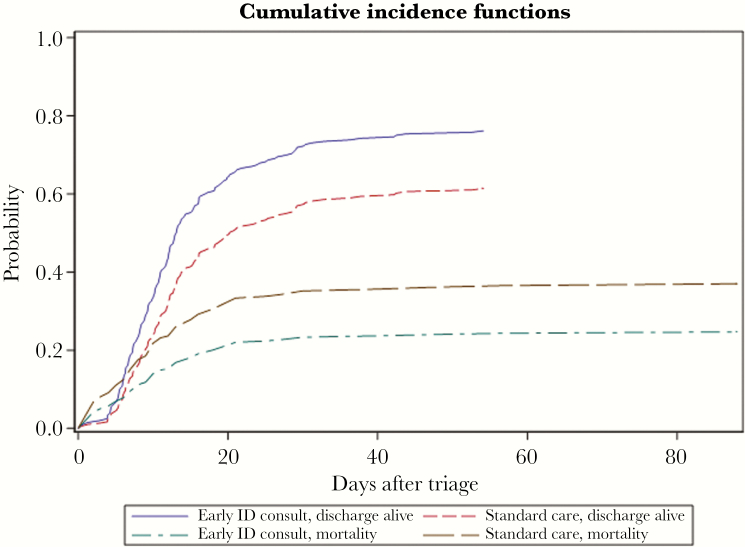
In the unadjusted time-to-event analysis using a Fine and Gray model for competing risks, early ID consult was significantly associated with a lower risk of mortality (subdistribution hazard ratio [sHR], 0.61; 95% CI, 0.39–0.98; P = .04) and positively associated with likelihood of hospital discharge alive (sHR, 1.51; 95% CI, 1.11–2.03; P = .008) (Table 5 and Figure 1). In the adjusted Fine and Gray model, early ID consult remained protective of mortality (adjusted sHR [asHR], 0.60; 95% CI, 0.36–1.00; P = .0497), and predictive of hospital discharge alive (asHR, 1.58; 95% CI, 1.11–2.23; P = .01) (Table 5). Vasopressors were predictive of mortality (asHR, 2.26; 95% CI, 1.35–3.76; P = .002) and negatively associated with likelihood of discharge alive (asHR, 0.48; 95% CI, 0.33–0.70; P = .0001). Age also was predictive of mortality (asHR, 1.02; 95% CI, 1.00–1.04; P = .02) and negatively associated with likelihood of discharge alive (asHR, 0.99; 95% CI, 0.97–1.00; P = .008).
Table 5.
Fine and Gray (Competing Risks) Models of Outcomes Time to Discharge Alive and Time to Mortality (N = 248)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Outcome Event = Discharge Alive (Competing Event = Mortality) |
sHR (95% CI) | P value | asHR (95% CI) | P value | asHR (95% CI) | P value |
| Early ID consult (reference = standard care) | 1.51 (1.11–2.03) | .008 | 1.58 (1.11–2.23) | .01 | 1.57 (1.11–2.24) | .01 |
| Age at index date | 0.99 (0.97–1.00) | .008 | 0.99 (0.97–1.00) | .01 | ||
| Race/ethnicity (reference = Non-Hispanic white) | ||||||
| Hispanic | 0.86 (0.56–1.32) | .48 | 0.88 (0.57–1.36) | .57 | ||
| Non-Hispanic black | 0.85 (0.54–1.35) | .49 | 0.85 (0.54–1.36) | .50 | ||
| Other/unknown | 1.03 (0.60–1.74) | .92 | 1.09 (0.65–1.82) | .75 | ||
| Charlson comorbidity score | 0.96 (0.90–1.02) | .21 | ||||
| Hemiplegia or paraplegia (reference = no) | 1.20 (0.61–2.35) | .60 | ||||
| Recent hospital admission (reference = no) | 0.97 (0.64–1.47) | 0.90 | 0.88 (0.60–1.29) | .51 | ||
| Recent IV antibiotics (reference = no) | 0.99 (0.66–1.49) | 0.96 | 0.97 (0.64–1.47) | .89 | ||
| History of MDRO (reference = no) | 0.95 (0.61–1.46) | 0.81 | 0.92 (0.60–1.42) | .70 | ||
| Hypotension (reference = no) | 1.24 (0.84–1.81) | 0.28 | 1.17 (0.80–1.72) | .41 | ||
| Lactate ≥4 (reference = no) | 1.02 (0.72–1.43) | 0.92 | 1.01 (0.72–1.42) | .95 | ||
| Vasopressors (reference = no) | 0.48 (0.33–0.70) | 0.0001 | 0.50 (0.35–0.72) | .0002 | ||
| Mechanical ventilation (reference = no) | 0.70 (0.44–1.12) | 0.13 | 0.71 (0.44–1.14) | .15 | ||
| Intra-abdominal source of infection (reference = no) | 1.27 (0.78–2.06) | 0.34 | 1.30 (0.80–2.13) | .29 | ||
| Outcome Event = Mortality (Competing Event = Discharge Alive) |
||||||
| Early ID consult (reference = standard care) | 0.61 (0.39–0.98) | 0.04 | 0.60 (0.36–1.00) | 0.0497 | 0.61 (0.37–1.02) | .06 |
| Age at index date | 1.02 (1.00–1.04) | 0.02 | 1.02 (1.00–1.04) | .02 | ||
| Race/ethnicity (reference = Non-Hispanic white) | ||||||
| Hispanic | 1.33 (0.75–2.37) | 0.33 | 1.32 (0.73–2.35) | .36 | ||
| Non-Hispanic black | 0.88 (0.44–1.74) | 0.71 | 0.92 (0.46–1.82) | .81 | ||
| Other/unknown | 0.90 (0.37–2.17) | 0.81 | 0.88 (0.36–2.14) | .78 | ||
| Charlson comorbidity score | 1.02 (0.95–1.10) | 0.54 | ||||
| Hemiplegia or paraplegia (reference = no) | 0.57 (0.15–2.14) | .41 | ||||
| Recent hospital admission (reference = no) | 1.10 (0.61–2.00) | 0.75 | 1.15 (0.64–2.08) | .64 | ||
| Recent IV antibiotics (reference = no) | 1.16 (0.61–2.20) | 0.65 | 1.20 (0.63–2.29) | .57 | ||
| History of MDRO (reference = no) | 1.12 (0.60–2.08) | 0.73 | 1.12 (0.60–2.10) | .71 | ||
| Hypotension (reference = no) | 0.79 (0.45–1.37) | 0.39 | 0.81 (0.47–1.41) | .46 | ||
| Lactate ≥4 (reference = no) | 1.06 (0.65–1.72) | 0.83 | 1.04 (0.64–1.71) | .87 | ||
| Vasopressors (reference = no) | 2.26 (1.35–3.76) | 0.002 | 2.28 (1.38–3.79) | .001 | ||
| Mechanical ventilation (reference = no) | 1.68 (0.94–3.01) | 0.08 | 1.67 (0.94–2.98) | .08 | ||
| Intra-abdominal source of infection (reference = no) | 0.63 (0.29–1.39) | 0.26 | 0.62 (0.28–1.38) | .24 |
Bold values indicate those that are statistically significant.
Abbreviations: asHR, adjusted subdistribution hazard ratio; CI, confidence interval; ID, infectious diseases; IV, intravenous; MDRO, multidrug-resistant organism; subdistribution hazard ratio, sHR.
Figure 1.
Unadjusted Cumulative Incidence Curves for In-hospital Mortality and Discharge Alive.
Early infectious diseases consult was statistically significantly associated with a lower risk of in-hospital death (subdistribution hazard ratio [sHR], 0.61; 95% confidence interval [CI], 0.39–0.98; P = .04) and a higher likelihood of hospital discharge alive (sHR, 1.51; 95% CI, 1.11–2.03; P = .008). Abbreviation: ID, infectious diseases.
Secondary Outcomes
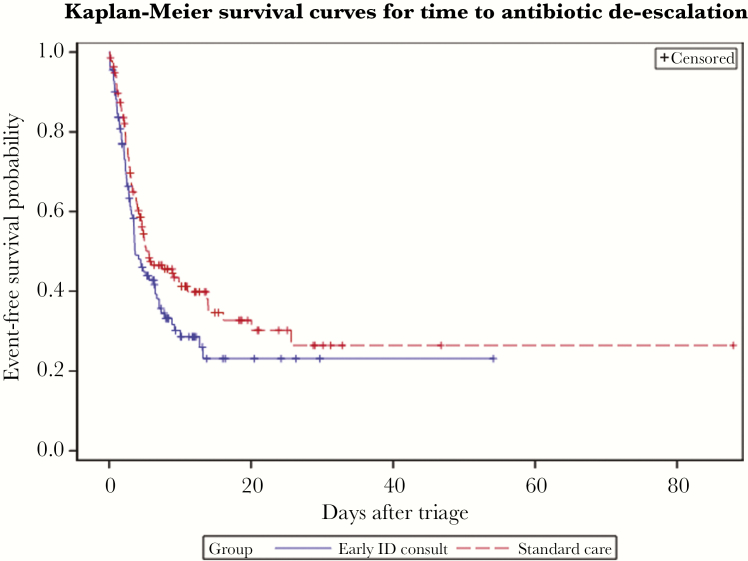
Clinical management-related outcomes are summarized in Table 2. Early ID consult did not differ from standard care for appropriate antibiotic prescribing (82.0% vs 81.0%, P = .85), effective antibiotic prescribing (99.1% vs 99.3%, P = 1.00), de-escalation at any point during therapy (64.9% vs 58.4%, P = .30), median days of antibiotic therapy (7.0 vs 8.0, P = .72), confirmed infection diagnosis (82.0% vs 81.0%, P = .85), or HO-CDI (4.5% vs 2.9%, P = .74). In time-to-event analyses, there was no significant difference between early ID and standard care groups for time to appropriate antibiotics (log-rank test P = .84), time to effective antibiotics (log-rank test P = .15), or time to confirmed infection diagnosis (log-rank test P = .62). There was a trend toward shorter time to de-escalation in the early ID group (log-rank test P = .07) (Figure 2).
Figure 2.
Kaplan-Meier Plot for Time to Antibiotic De-escalation.
Early infectious diseases consult was with a shorter time to de-escalation, but this trend did not reach statistical significance (N = 248, censored for death and discharge; log-rank test P = .07). Abbreviation: ID, infectious diseases.
We performed several subgroup analyses for clinical management-related outcomes, summarized in Table 6a–c. Among the 157 patients who had a positive blood or nonblood culture, there was no significant difference between patients who had early ID consult versus standard care for overall microbiologic tailoring of antibiotics at any point in therapy (80.3% vs 77.9%, P = .72), nor in time to microbiologic tailoring of antibiotics (log-rank test P = .60) (Table 6a). There were no differences in acceptance of ID recommendations between early ID and standard care groups for antibiotic de-escalation (90.0% vs 98.1%, P = .14) or duration of therapy (87.9% vs 92.9%, P = .30) when these recommendations were made, but there was a trend towards fewer antibiotic days in the early ID group (7 vs 10, P = .09) (Table 6b–c).
Table 6.
Clinical Management Outcome Subgroup Analyses in Early ID Consult and Standard Care Groups
| Subgroup | Secondary Outcome | Total Subgroup | Early ID Consult | Standard Care | P valuea | P value for time-to-eventb |
|---|---|---|---|---|---|---|
| a. Positive Blood or Nonblood Culture | N = 157 | N = 71 | N = 86 | |||
| Microbiologic tailoring of therapy, n (%) | 124 (79.0) | 57 (80.3) | 67 (77.9) | .72 | .60 | |
| b. De-escalation Recommendation Given | N = 122 | N = 70 | N = 52 | |||
| Antibiotic de-escalation, n (%) | 117 (95.9) | 66 (94.3) | 51 (98.1) | .39 | .39 | |
| De-escalation recommendations followed, n (%) | 114 (93.4) | 63 (90.0) | 51 (98.1) | .14 | ||
| c. Antibiotic Duration Recommendation Given | N = 161 | N = 91 | N = 70 | |||
| Days of antibiotic therapy, median (interquartile range) | 8.0 (6.0, 13.0) | 7.0 (5.0, 12.0) | 10.0 (7.0, 13.0) | .09 | ||
| Recommendations for duration of antibiotic therapy followed, n (%) | 145 (90.1) | 80 (87.9) | 65 (92.9) | .30 |
a χ 2 test, Fisher exact test, or Wilcoxon rank sum test.
b Log-rank test.
Sensitivity Analyses
When examining the outcome of mortality or discharge to hospice related to sepsis or complications, results were similar to those for the primary mortality outcome (25.2% vs 40.9%, P = .01) (Table 2), both in the unadjusted model (odds ratio [OR], 0.49; 95% CI, 0.28–0.84; P = .01) and when adjusted for the same covariates as included in the primary mortality analysis (AOR, 0.44; 95% CI, 0.24–0.83; P-value = .01) (Supplementary Table 1). Mortality did not differ between patients who had an ID consult in the first 7 days compared with those who did not in the unadjusted (OR, 0.65; 95% CI, 0.36–1.17; P = .15), or adjusted (AOR, 0.68; 95% CI, 0.35–1.32; P = .25) analyses (Supplementary Table 2). Time to discharge alive likewise did not differ between these groups in the unadjusted (sHR, 1.27; 95% CI, 0.88–1.82; P = .19) and adjusted (asHR, 1.22; 95% CI, 0.83–1.78; P = .31) analyses, nor was there a difference in time to death in the unadjusted (sHR, 0.70; 95% CI, 0.44–1.12; P = .14) or adjusted (asHR, 0.79; 95% CI, 0.46–1.34; P = .38) analyses (Supplementary Table 3).
DISCUSSION
In patients who completed the 3-hour bundle for SS/SS in the ED, ID consultation within 12 hours of ED triage was associated with lower rate and risk of in-hospital mortality and greater likelihood of hospital discharge alive. This result is consistent with data from other clinical studies where involvement of an ID consultant has shown improved clinical outcomes for various syndromes [28–31]. There was no difference in hospital length of stay or rate of 30-day readmission associated with early ID consultation in our study.
Previous studies have demonstrated an association between time to effective antibiotics and mortality in patients with sepsis and hypotension [34]. In their evaluation of the impact of an ID sepsis team in the ED, Viale et al demonstrated lower mortality in the postintervention cohort that was attributed to improved bundle compliance, an increase in appropriateness of antibiotics, and higher rates of blood culture acquisition and pathogen identification [31]. However, in our study the difference in mortality observed in the early ID group cannot be explained by a difference in rates of bundle compliance or blood culture acquisition, because all patients completed the 3-hour bundle, including blood culture acquisition, as a criterion of inclusion. The observed difference in mortality also cannot be attributed to antibiotic appropriateness, antibiotic effectiveness, or pathogen identification in our study as these outcomes were not significantly different between groups. Appropriate and effective antibiotic selection was likely similar between groups as a result of the hospital system's robust ASP with built-in decision support for SS/SS empiric regimens in the sepsis order set. Even if ID consultation was not requested or completed within 12 hours, decision support tools or guidance by phone from ASP or ID fellows were alternative mechanisms to provide a timely pathway to optimal empiric regimens. Other studies have similarly shown high rates of appropriate antimicrobial prescribing using comprehensive treatment guidance and empiric protocols for patients with SS/SS in the ED setting [35, 36].
We observed a statistical trend toward shorter time to antibiotic de-escalation in the early ID group compared with standard care group and shorter duration of antibiotic therapy in patients with early ID consult compared to later ID consult in the subsample where an antibiotic duration recommendation was made, but neither reached statistical significance (possibly due to small sample size). Antibiotic reassessment, which is tailored to microbiological data, is protective against mortality in patients with bacteremia in the ED [37] and appropriate antibiotic de-escalation has been associated with improved mortality in SS/SS [38]. The mechanism by which de-escalation reduces mortality has not been proven, but reduction in toxicity and adverse drug events has been suggested [38].
Behavioral and social factors, such as tolerance of risk and uncertainty, fear of adverse outcome related to underprescribing, and social team dynamics lead to differences in antibiotic-related decision making, such as when to de-escalate [39]. Patients often are managed by different provider care teams in the ED and inpatient areas, but the early ID model offers continuity through the consulting ID physician and perhaps a greater degree of certainty regarding the improvement in a patient's condition over time. In this way, early ID consultation may function similarly to procalcitonin for increasing confidence in de-escalation and reducing mortality in SS/SS [40]. Additionally, the co-management of an ID physician early in hospitalization could provide a model for appropriate prescribing behavior and social support for changing antibiotics initially prescribed by another provider. We postulate that these behavioral and social factors contribute to the care team's decision to de-escalate earlier with early ID consultation. In order to optimize early ID intervention in SS/SS, further studies are needed to measure the effects of shared decision making and team-based care on prescribing behaviors in clinical sepsis care and to assess which patients most benefit from early ID intervention. The American College of Physicians and the Society for Academic Emergency Medicine have advocated for development of a team-based care research agenda to optimize effectiveness of dynamic clinical care teams with the goal of improving safety and clinical outcomes [25, 26].
Limitations of our study are the retrospective nature of the study, relatively limited statistical power in subgroup analyses for antibiotic management, inability to quantify the impact of decision support tools or recommendations offered by ASP and ID consultants on the phone prior to or in the absence of formal ID consultation, inability to distinguish antibiotic-related toxicity from organ failure related to SS/SS to quantify antibiotic-related adverse events, and inability to measure behavioral and social determinants of antibiotic prescribing. Other general drawbacks are the limitations of the SEP-1 definition of SS/SS based on systemic inflammatory response syndrome criteria and composite nature of the bundle [41], and the lack of consensus regarding what constitutes “appropriate” broad-spectrum antibiotics to receive credit for completion of the 3-hour sepsis bundle [42]. The single center nature of this study allowed for the use of tailored antimicrobial regimens based upon the local epidemiology and antibiogram to optimize therapy for our patient population. Validation at other sites should be pursued.
There are several important strengths of our study. By design, all patients included in the study received the benefit of rapid comprehensive sepsis therapy and treating providers had access to multiple ASP resources. Despite bundle compliance in all patients and no difference in timely antibiotics, appropriate antibiotics, effective antibiotics, or identification of pathogens between groups, those in the early ID group showed a survival benefit. The early ID model for SS/SS patients in the ED at our hospital has been sustainable, represents a realistic team-based model for sepsis care, and provides evidence that this type of model is associated with positive patient outcomes. This model can be easily adapted for other hospitals, especially in settings where a dedicated 24/7 ID-led sepsis consult service is not feasible.
In conclusion, we find that the addition of early ID consultation to sepsis bundles in the ED is associated with lower mortality in patients with SS/SS. Our study adds to the mounting evidence that ID consultation as part of a team-based care approach is associated with improved patient outcomes, and addition of ID consultation to sepsis bundles should be considered. Further study is needed to determine if this benefit is due to differences in antibiotic de-escalation, to assess whether other elements of ID management and shared decision making also contribute to outcomes, and to measure the impact of behavioral and social factors in antibiotic management in sepsis. Regardless, this study highlights the important role of ID specialists in the appropriate and timely clinical management of patients with SS/SS.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgements
The authors thank Nadine Katz, MD; Rafael Ruiz, PhD; Belinda Ostrowsky, MD, MPH; Peter Shamamian, MD; Kami Kim, MD; and the providers and staff of the Montefiore Medical Center, Einstein campus Emergency Department.
Financial support. None reported.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10. [DOI] [PubMed] [Google Scholar]
- 2. Rivers E, Nguyen B, Havstad S, et al. ; Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–77. [DOI] [PubMed] [Google Scholar]
- 3. Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care 2005; 9:R764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dellinger RP, Carlet JM, Masur H, et al. ; Surviving Sepsis Campaign Management Guidelines Committee Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004; 32:858–73. [DOI] [PubMed] [Google Scholar]
- 5. New York State Department of Health. Section 405.4. In: New York Codes, Rules and Regulations, Title 10. Eagan, MN: West Publishing; 2013. [Google Scholar]
- 6. The Joint Commission. Specifications manual for national hospital inpatient quality measures v5.3.https://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx. Accessed 3 April 2019. Published 2017. [Google Scholar]
- 7. Seymour CW, Gesten F, Prescott HC, et al. . Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahn JM, Davis BS, Yabes JG, et al. . Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA 2019; 322:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. IDSA Sepsis Task Force. Infectious Diseases Society of America (IDSA) POSITION STATEMENT: why IDSA did not endorse the surviving sepsis campaign guidelines. Clin Infect Dis 2018; 66:1631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiensch R, Poeran J, Saunders-Hao P, et al. . Impact of an electronic sepsis initiative on antibiotic use and health care facility-onset Clostridium difficile infection rates. Am J Infect Control 2017; 45:1091–100. [DOI] [PubMed] [Google Scholar]
- 11. Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med 2017; 45:1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park SY, Oh WS, Kim YS, et al. . Health care-associated acute pyelonephritis is associated with inappropriate empiric antibiotic therapy in the ED. Am J Emerg Med 2016; 34:1415–20. [DOI] [PubMed] [Google Scholar]
- 13. Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother 2014; 58:1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanderweil SG, Tsai CL, Pelletier AJ, et al. . Inappropriate use of antibiotics for acute asthma in United States emergency departments. Acad Emerg Med 2008; 15:736–43. [DOI] [PubMed] [Google Scholar]
- 15. Mohareb AM, Dugas AF, Hsieh YH. Changing epidemiology and management of infectious diseases in US EDs. Am J Emerg Med 2016; 34:1059. –65. [DOI] [PubMed] [Google Scholar]
- 16. Thorpe JM, Smith SR, Trygstad TK. Trends in emergency department antibiotic prescribing for acute respiratory tract infections. Ann Pharmacother 2004; 38:928–35. [DOI] [PubMed] [Google Scholar]
- 17. Timbrook TT, Caffrey AR, Ovalle A, et al. . Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther 2017; 6:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trinh TD, Klinker KP. Antimicrobial stewardship in the emergency department. Infect Dis Ther 2015; 4:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis LC, Covey RB, Weston JS, Hu BB, Laine GA. Pharmacist-driven antimicrobial optimization in the emergency department. Am J Health Syst Pharm 2016; 73:S49–56. [DOI] [PubMed] [Google Scholar]
- 20. Jorgensen SCJ, Yeung SL, Zurayk M, et al. . Leveraging antimicrobial stewardship in the emergency department to improve the quality of urinary tract infection management and outcomes. Open Forum Infect Dis 2018; 5:ofy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung P, Scandlyn J, Dayan PS, Mistry RD. Working at the intersection of context, culture, and technology: provider perspectives on antimicrobial stewardship in the emergency department using electronic health record clinical decision support. Am J Infect Control 2017; 45:1198–202. [DOI] [PubMed] [Google Scholar]
- 22. Dinh A, Duran C, Davido B, et al. . Impact of an antimicrobial stewardship programme to optimize antimicrobial use for outpatients at an emergency department. J Hosp Infect 2017; 97:288–93. [DOI] [PubMed] [Google Scholar]
- 23. Madaline T, Feldmesser M, Chung P, et al. . Building bridges: improving antibiotic prescribing in the emergency department. Open Forum Infect Dis 2014; 1(Suppl 1):S99. Abstract 228. [Google Scholar]
- 24. Mitchell P, Wynia M, Golden R, et al. . Core principles & values of effective team-based health care. Washington, DC: Institute of Medicine; 2012. www.iom.edu/tbc. [Google Scholar]
- 25. Doherty RB, Crowley RA; Health and Public Policy Committee of the American College of Physicians Principles supporting dynamic clinical care teams: an American College of Physicians position paper. Ann Intern Med 2013; 159:620–6. [DOI] [PubMed] [Google Scholar]
- 26. Grudzen CR, Anderson JR, Carpenter CR, Hess EP. The 2016 academic emergency medicine consensus conference, shared decision making in the emergency department: development of a policy-relevant patient-centered research agenda May 10, 2016, New Orleans, LA. Acad Emerg Med 2016; 23(12):1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eron LJ, Passos S. Early discharge of infected patients through appropriate antibiotic use. Arch Intern Med 2001; 161:61–5. [DOI] [PubMed] [Google Scholar]
- 28. Fluckiger U, Zimmerli W, Sax H, Frei R, Widmer AF. Clinical impact of an infectious disease service on the management of bloodstream infection. Eur J Clin Microbiol Infect Dis 2000; 19:493–500. [DOI] [PubMed] [Google Scholar]
- 29. Bai AD, Showler A, Burry L, et al. . Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60:1451–61. [DOI] [PubMed] [Google Scholar]
- 30. Schmitt S, McQuillen DP, Nahass R, et al. . Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58:22–8. [DOI] [PubMed] [Google Scholar]
- 31. Viale P, Tedeschi S, Scudeller L, et al. . Infectious diseases team for the early management of severe sepsis and septic shock in the emergency department. Clin Infect Dis 2017; 65:1253–9. [DOI] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention. Multidrug-Resistant Organism & Clostridium difficile Infection (MDRO/CDI) Module. https://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf. Published January 2019. Accessed September 17, 2019. [Google Scholar]
- 33. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 34. Kumar A, Roberts D, Wood KE, et al. . Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 35. Kano KI, Shime N, Nishiyama K. Implementation of an empirical antimicrobial protocol in a critical care setting: a single-center retrospective observational cohort study in bacteremic patients. J Infect Chemother 2018; 24:965–8. [DOI] [PubMed] [Google Scholar]
- 36. Francis M, Rich T, Williamson T, Peterson D. Effect of an emergency department sepsis protocol on time to antibiotics in severe sepsis. CJEM 2010; 12:303–10. [DOI] [PubMed] [Google Scholar]
- 37. Aillet C, Jammes D, Fribourg A, et al. . Bacteraemia in emergency departments: effective antibiotic reassessment is associated with a better outcome. Eur J Clin Microbiol Infect Dis 2018; 37:325–31. [DOI] [PubMed] [Google Scholar]
- 38. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. . De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40:32–40. [DOI] [PubMed] [Google Scholar]
- 39. Warreman EB, Lambregts MMC, Wouters RHP, et al. . Determinants of in-hospital antibiotic prescription behaviour: a systematic review and formation of a comprehensive framework. Clin Microbiol Infect 2019; 25:538–45. [DOI] [PubMed] [Google Scholar]
- 40. de Jong E, van Oers JA, Beishuizen A, et al. . Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16:819–27. [DOI] [PubMed] [Google Scholar]
- 41. Rhee C, Filbin MR, Massaro AF, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program Compliance with the national SEP-1 quality measure and association with sepsis outcomes: a multicenter retrospective cohort study. Crit Care Med 2018; 46:1585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Septimus EJ, Coopersmith CM, Whittle J, et al. . Sepsis national hospital inpatient quality measure (SEP-1): multistakeholder work group recommendations for appropriate antibiotics for the treatment of sepsis. Clin Infect Dis 2017; 65:1565–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.