Chemosynthetic SUP05 bacteria dominate the microbial communities of deep-sea hydrothermal vents around the world, SUP05 bacteria utilize reduced chemical compounds in vent fluids and commonly form symbioses with invertebrate organisms. This symbiotic relationship could be key to adapting to such unique and extreme environments. Viruses are the most abundant biological entities on the planet and have been identified in hydrothermal vent environments. However, their interactions with the symbiotic microbes of the SUP05 clade, along with their role in the symbiotic system, remain unclear. Here, using metagenomic sequence-based analyses, we determined that bacteriophages may support metabolism in SUP05 bacteria and play a role in the sponge-associated symbiosis system in hydrothermal vent environments.
KEYWORDS: SUP05 bacteria, deep sea, hydrothermal vent, marine phages, sponge
ABSTRACT
In deep-sea hydrothermal vent environments, sulfur-oxidizing bacteria belonging to the clade SUP05 are crucial symbionts of invertebrate animals. Marine viruses, as the most abundant biological entities in the ocean, play essential roles in regulating the sulfur metabolism of the SUP05 bacteria. To date, vent sponge-associated SUP05 and their phages have not been well documented. The current study analyzed microbiomes of Haplosclerida sponges from hydrothermal vents in the Okinawa Trough and recovered the dominant SUP05 genome, designated VS-SUP05. Phylogenetic analysis showed that VS-SUP05 was closely related to endosymbiotic SUP05 strains from mussels living in deep-sea hydrothermal vent fields. Homology and metabolic pathway comparisons against free-living and symbiotic SUP05 strains revealed that the VS-SUP05 genome shared many features with the deep-sea mussel symbionts. Supporting a potentially symbiotic lifestyle, the VS-SUP05 genome contained genes involved in the synthesis of essential amino acids and cofactors that are desired by the host. Analysis of sponge-associated viral sequences revealed putative VS-SUP05 phages, all of which were double-stranded viruses belonging to the families Myoviridae, Siphoviridae, Podoviridae, and Microviridae. Among the phage sequences, one contig contained metabolic genes (iscR, iscS, and iscU) involved in iron-sulfur cluster formation. Interestingly, genome sequence comparison revealed horizontal transfer of the iscS gene among phages, VS-SUP05, and other symbiotic SUP05 strains, indicating an interaction between marine phages and SUP05 symbionts. Overall, our findings confirm the presence of SUP05 bacteria and their phages in sponges from deep-sea vents and imply a beneficial interaction that allows adaptation of the host sponge to the hydrothermal vent environment.
IMPORTANCE Chemosynthetic SUP05 bacteria dominate the microbial communities of deep-sea hydrothermal vents around the world, SUP05 bacteria utilize reduced chemical compounds in vent fluids and commonly form symbioses with invertebrate organisms. This symbiotic relationship could be key to adapting to such unique and extreme environments. Viruses are the most abundant biological entities on the planet and have been identified in hydrothermal vent environments. However, their interactions with the symbiotic microbes of the SUP05 clade, along with their role in the symbiotic system, remain unclear. Here, using metagenomic sequence-based analyses, we determined that bacteriophages may support metabolism in SUP05 bacteria and play a role in the sponge-associated symbiosis system in hydrothermal vent environments.
INTRODUCTION
Gammaproteobacteria belonging to the unculturable clade SUP05 are among the most abundant and ubiquitous chemolithoautotrophs in marine environments (1–4). With an ability to intimately interact with other components of the ecosystem, sulfur-oxidizing SUP05 bacteria form symbioses with invertebrate animals at deep-sea hydrothermal vents (5–7), thereby supporting a diverse and highly dense vent fauna (8). For example, the SUP05 clade bacteria were revealed to be endosymbionts (intracellular symbionts) of mussels and clams in deep-sea vent environments (2, 5–7). Transmission electron microscopy and fluorescence in situ hybridization analyses showed that the bacterial cells were small, dense, and spherical (2, 5–7). Genomes of these small endosymbionts contain the complete set of genes required for sulfur oxidization, allowing the bacteria to utilize surrounding reduced sulfur compounds as an energy source (2, 5, 7). During evolution, the versatile SUP05 symbionts have undergone genome reduction compared with their free-living counterparts, likely as a result of their symbiosis with animals (7).
In addition to their intimate interactions with invertebrates via symbiosis, SUP05 bacteria interact with phages during infection, which may influence aspects of bacterial energy metabolism. Phylogenetic analysis of viral and bacterial genes encoding reverse dissimilatory sulfite reductase subunit A (rdsrA) by Anantharaman et al. revealed potential horizontal gene transfer between SUP05 phages and free-living SUP05 bacteria (4). Further, using single-amplified genome sequencing, Roux et al. showed that free-living SUP05 strains were infected by phages in oxygen-minimum zones and found that the SUP05 phages contained genes for reverse dissimilatory sulfite reductase subunit C (rdsrC) (9). Via the expression of rdsrA (or rdsrC), SUP05 phages may complement the sulfur metabolism of their hosts, thus playing a role in environmental sulfur cycling. However, if and how symbiotic SUP05 bacteria are manipulated by phages remains unclear. Recent studies have provided several clues about the role of SUP05-phage interactions in symbiotic systems. For example, the genome of a SUP05 symbiont of a deep-sea vent mussel contains a prophage region (5). The presence of a viral clpP gene in this region suggests that phages may participate in the intracellular proteolysis of symbiotic SUP05 bacteria. Though the viral clpP gene has not been demonstrated to be functional in the mussel SUP05 symbiont, the role of SUP05 prophages may be essential to a symbiotic system, as prophages have been demonstrated to be a required component in a mutual symbiosis, such as the prophages contained in the endosymbiont Hamiltonella defensa in aphid hosts (10). Only with the presence of the prophages could the aphid endosymbionts be endowed with defensive properties to protect their animal hosts from wasp parasitoids. Furthermore, the loss of prophages was likely to severely influence on the fitness of the aphid hosts, such as by delaying reproduction and reducing body weight (11).
Although the SUP05 symbionts have been studied in deep-sea invertebrates such as mussels and clams, their association with sponges is less clear. Marine sponges host a diverse array of microbial symbionts (12) as well as viruses (13). Analysis of 16S rRNA gene sequences has shown that deep-sea vent sponges harbor thioautotrophic Gammaproteobacteria closely related to the Bathymodiolus mussel SUP05 endosymbionts (14). Recently, sulfur-oxidizing SUP05 strains were revealed to be highly abundant in deep-sea sponges from asphalt seeps, accounting for up to 20% of the total bacterial population (15). Although the SUP05 bacteria have been found in deep-sea sponges at a great abundance, their ecological and functional roles are less known. With the continual discovery of novel deep-sea sponges, including members of the Pachastrellidae, Poecilosclerida, and Haplosclerida (14, 16), there is an opportunity to uncover more chemolithoautotrophic SUP05 symbionts in the sponge environment and to examine the potential impact of sponge-associated viruses during infection. Here, we collected sponges in the order Haplosclerida from two vent sites in the Okinawa Trough and analyzed their associated microbial and viral metagenomes. We obtained the draft genome sequence of the predominant sponge-associated SUP05 species via genome binning and identified putative auxiliary metabolic genes (AMGs) from phages potentially infecting SUP05 bacteria. Based on these data, we propose an interaction between vent sponges, SUP05 bacteria, and SUP05 phages in deep-sea hydrothermal vent environments.
RESULTS
SUP05 bacteria in hydrothermal vent sponges.
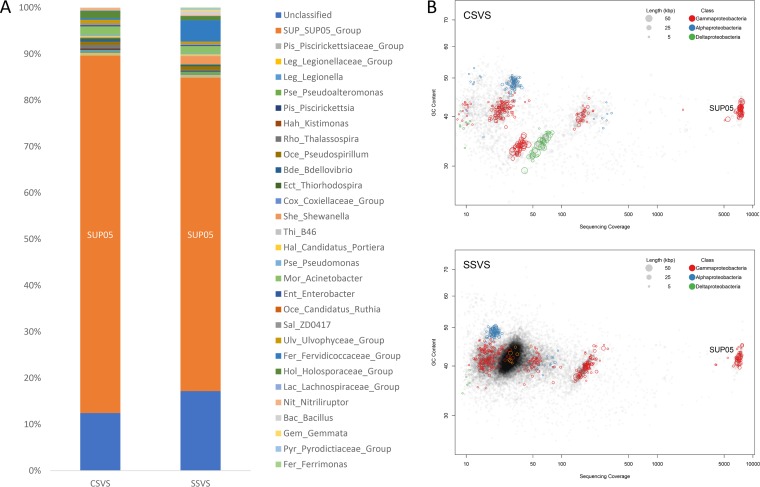
Community structure analysis demonstrated that vent sponge-associated microorganisms at the two sampling sites were diverse, with SUP05, Acinetobacter, Holosporaceae, and Bdellovibrio species identified among the samples. SUP05 clade bacteria were the predominant bacterial group, accounting for 77.1% and 67.7% of all sequences in the microbial communities from sponges at the Crane and Swan sites, respectively (Fig. 1A). The metagenome map of the microbes from sponges at the two sites showed that SUP05 bacteria had a sequence coverage 10 of times greater than that of any other bacterial group (Fig. 1B). The 16S rRNA gene sequences of the predominant SUP05 genomes from the two sites showed 100% nucleotide sequence identity, indicating that they belonged to the same bacterial species (designated VS-SUP05 in this study), which was highly conserved in the hydrothermal vent sponge population of the Okinawa Trough.
FIG 1.
Dominant SUP05 bacteria within the sponge-associated microbial community. (A) Relative abundances of operational taxonomic units corresponding to sponge-associated microorganisms at the genus level. Composition percentage is shown on the vertical axis. SUP05 bacteria were highly abundant in the microbial communities of both the Crane site vent sponge (CSVS) and the Swan site vent sponge (SSVS). (B) Genome binning of dominant SUP05 bacteria. SUP05 bacteria were sequenced with superhigh coverage (∼7,000×).
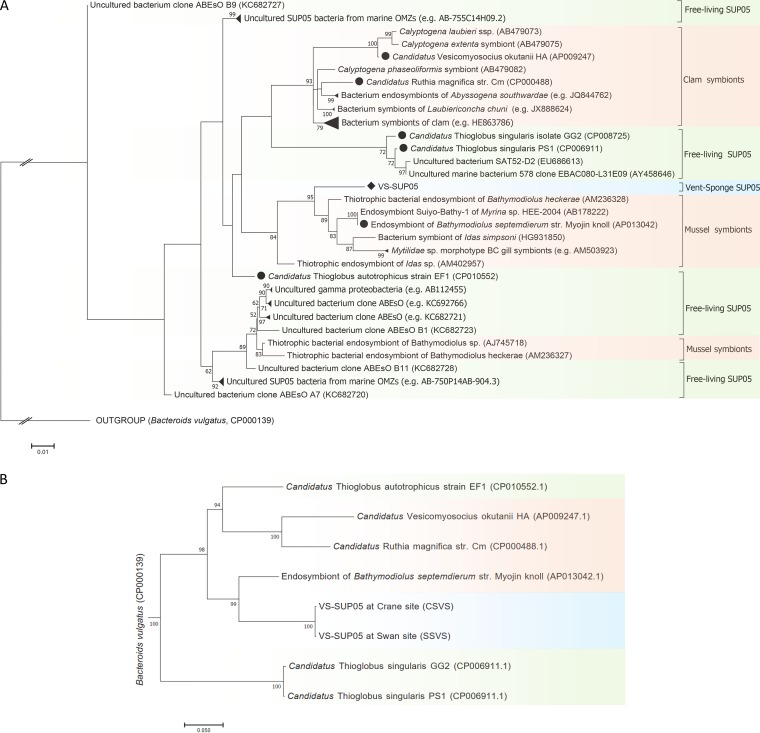
The VS-SUP05 16S rRNA gene sequence was then used to build a phylogenetic tree (Fig. 2A). The closest relatives (96.5% to 98.2% nucleotide sequence identity) of VS-SUP05 were gill endosymbionts of Bathymodiolus mussels from deep-sea hydrothermal vents (5, 17). In addition, symbionts from mussels belonging to the genera Idas and Mytilidae were also closely related to VS-SUP05 and were grouped into the same clade of sulfur-oxidizing symbiotic SUP05 bacteria. In contrast, free-living SUP05 species such as “Candidatus Thioglobus” were clearly separated from the symbiont group containing VS-SUP05 (Fig. 2A). Phylogenomic analysis, based on single-copy genes, also showed that VS-SUP05 and a mussel (Bathymodiolus septemdierum strain Myojin knoll) endosymbiont were intimately related (Fig. 2B), which is consistent with the phylogenetic analysis based on 16S rRNA gene sequences. Collectively, these results suggested that VS-SUP05 has a close evolutionary relationship with endosymbionts of deep-sea animals.
FIG 2.
Maximum-likelihood-based phylogenetic analysis of VS-SUP05. (A) Phylogenetic tree based on the 16S rRNA gene sequences of VS-SUP05 and selected symbiotic and free-living SUP05 bacteria. (B) Phylogenomic tree based on the concatenated sequences of conserved marker genes from VS-SUP05 and selected symbiotic and free-living SUP05 bacteria. SUP05 symbionts are indicated in orange, and free-living SUP05 bacteria are indicated in green. Black circles represent bacteria with available genome sequences, while black rhombuses represent VS-SUP05 (marked in blue). Bootstrap values of <50 are hidden at the nodes.
Although VS-SUP05 has the closest relatives from the other hosts in hydrothermal vents based on the analysis of full-length 16S rRNA genes, it may have a wide distribution in global sponges regardless of shallow-water or deep-sea environments. According to the comparison of the V4 region of 16S rRNA genes, relatives of VS-SUP05 can be found in a diverse array of sponges, such as Haliclona tubifera, Polymastia spp., and Phakellia ventilabrum (see File S2 in the supplemental material). Among these relatives, symbionts from the Clathrinida sponges and Crella incrustans in Breaker Bay, the Tethyida sponges and Plakina trilopha in the Skomer Islands, and the Haplosclerida sponges in Mediterranean Sea are the most identical (99% identity) to VS-SUP05 in our study.
Genomic features of VS-SUP05.
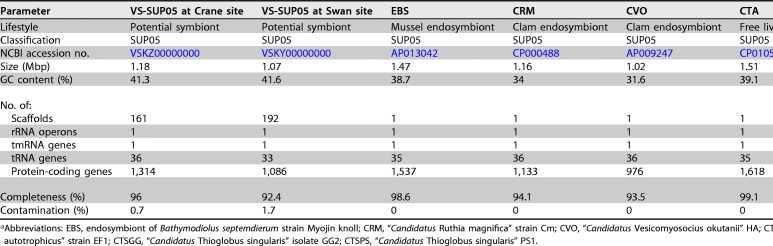
Two draft genome sequences of VS-SUP05 were obtained separately from sponges at the two sampling sites by genome binning. The genome features of VS-SUP05 are shown in Table 1. CheckM analysis showed that the two VS-SUP05 genome bins had high recovery quality (Crane site data, 96% complete and 0.7% contaminated; Swan site data, 92.4% complete and 1.7% contaminated). The VS-SUP05 genome from the Crane site was 1.18 Mb in size with a GC content of 41.3%, including 1,314 coding genes, 36 tRNA genes, 1 transfer-messenger RNA (tmRNA) gene, and 1 rRNA operon. The VS-SUP05 genome from the Swan site was 1.07 Mb in size with a GC content of 41.6%, including 1,086 coding genes, 33 tRNA genes, 1 tmRNA gene, and 1 rRNA operon. The average nucleotide identity between the VS-SUP05 at the Swan and Crane sites was 99.2%. In addition, most of the protein sequences in the two VS-SUP05 genomes have very high similarity (>97%) (see details in File S3 in the supplemental material). Therefore, we selected the higher-quality genome from the Crane site to represent VS-SUP05 in further genomic analyses and comparisons.
TABLE 1.
Genomic features of the VS-SUP05, three mollusk endosymbionts, and three free-living SUP05 bacteriaa
Abbreviations: EBS, endosymbiont of Bathymodiolus septemdierum strain Myojin knoll; CRM, “Candidatus Ruthia magnifica” strain Cm; CVO, “Candidatus Vesicomyosocius okutanii” HA; CTA, “Candidatus Thioglobus autotrophicus” strain EF1; CTSGG, “Candidatus Thioglobus singularis” isolate GG2; CTSPS, “Candidatus Thioglobus singularis” PS1.
The structure of the VS-SUP05 genome was compared with those of sequenced genomes of close relatives in the SUP05 clade, including three endosymbionts and three free-living bacteria (Table 1). The size of the VS-SUP05 genome (1.18 Mb) was similar to those of the other SUP05 genomes (1 to 1.8 Mb) but was closest to those of the symbiotic SUP05 bacteria (e.g., the clam endosymbiont with a genome size of 1.16 Mb). VS-SUP05 had the highest GC content (41.3%) among the examined genomes, while the GC contents of the symbionts and free-living SUP05 ranged from 31.6% to 38.7% and from 36.3% to 39.1%, respectively. More homolog regions (Fig. 3A) were shared between VS-SUP05 and the symbionts EBS, CVO, and CRM and free-living autotrophic bacterium CTA than between VS-SUP05 and the two remaining free-living bacteria (CTSPS and CTSGG), which are more distantly related. It has been proposed that free-living bacterium CTA, which shared more homologous features with symbionts, including VS-SUP05, was originally a symbiont and has reverted back to a free-living lifestyle (18–20). In addition, homolog annotation analysis showed that there were more orthologs between the endosymbionts and VS-SUP05 than between VS-SUP05 and the free-living bacteria (Fig. 3B and C). The mussel endosymbiont (EBS), which was the closest relative of VS-SUP05 in the phylogeny, shared the largest number of homologs with the VS-SUP05 genome. In total, 15 unique genes were identified in the VS-SUP05 genome that were not present in the other six SUP05 genomes. Functionally, these unique genes were associated with transcription, cell wall/membrane/envelope biogenesis, amino acid transport and metabolism, defense mechanisms, energy production and conversion, and inorganic ion transport and metabolism (see File S4 in the supplemental material for detailed information on ortholog distribution and function in each genome). Overall, homology comparisons against other SUP05 bacteria suggested that the VS-SUP05 genome shared more homologous regions with the symbionts than with the free-living SUP05 bacteria.
FIG 3.
Distribution of homolog synteny clusters and annotated clusters of orthologous groups. (A) Synteny of homologs in the genomes of VS-SUP05 and six closely related SUP05 bacteria. (B and C) Distribution of homologs among the genomes of VS-SUP05 and six closely related SUP05 bacteria. EBS, endosymbiont of Bathymodiolus septemdierum strain Myojin knoll; CRM, “Candidatus Ruthia magnifica” strain Cm; CVO, “Candidatus Vesicomyosocius okutanii” HA; CTA, “Candidatus Thioglobus autotrophicus” strain EF1; CTSGG, “Candidatus Thioglobus singularis” isolate GG2; CTSPS, “Candidatus Thioglobus singularis” PS1.
Metabolic pathways inferred from the VS-SUP05 genome.
A comparison of Kyoto Encyclopedia of Genes and Genomes (KEGG)-annotated functional groups between VS-SUP05 and the six closely related SUP05 bacteria is presented in Fig. 4 (see the detailed gene count for each pathway in File S5 in the supplemental material). Based on correlation analysis of the gene count in each genome, free-living bacteria (CTSGG and CTSPS) were classified into one group (here named the free-living group), while VS-SUP05, CTA, and the three endosymbionts (EBS, CRM, and CVO) were clustered into another group. The free-living group had a higher abundance of nonredundant genes involved in microbial metabolic pathways such as cell motility, xenobiotic biodegradation and metabolism, membrane transport, and lipid metabolism, suggesting that the group including endosymbionts might be subject to genome reduction. Detailed annotation information for each pathway is shown in File S5.
FIG 4.
Comparison of KEGG-annotated protein-coding sequences from the genomes of VS-SUP05 and six reference bacteria. EBS, endosymbiont of Bathymodiolus septemdierum strain Myojin knoll; CRM, “Candidatus Ruthia magnifica” strain Cm; CVO, “Candidatus Vesicomyosocius okutanii” HA; CTA, “Candidatus Thioglobus autotrophicus” strain EF1; CTSGG, “Candidatus Thioglobus singularis” isolate GG2; CTSPS, “Candidatus Thioglobus singularis” PS1).
Metabolic comparison showed that the number of genes (n = 80) involved in membrane transport in the VS-SUP05 genome was most similar to that (n = 85) in mussel endosymbiont EBS but was significantly fewer than the >190 membrane transport genes observed in the free-living bacteria (CTSGG and CTSPS). Genes involved in metabolic pathways such as xenobiotic biodegradation and metabolism, amino acid metabolism, and lipid metabolism were also reduced in the mussel symbionts, clam symbionts, and VS-SUP05, with an abundance only half that seen in the free-living bacteria. Among the pathways involved in cellular processes, there were over 3-fold more genes related to cell motility in the free-living group (18 genes in each genome) than in the VS-SUP05 genome (5 genes) and the other symbiotic bacteria (1 to 5 genes in each genome). Interestingly, a series of sulfur oxidization-related genes, sat, aprAB, dsrAB, soxA, soxB, soxX, soxY, and soxZ, was identified in the VS-SUP05 genome, indicating that these sponge-associated SUP05 bacteria are affiliated with the sulfur-oxidizing bacteria. This sulfur-oxidizing capacity was shared among the SUP05 endosymbionts (EBS, CRM, and CVO). However, genes for alpha-ketoglutarate dehydrogenases involved in the interconversion of succinyl coenzyme A (succinyl-CoA) and 2-oxoglutarate were not detected, indicating that VS-SUP05 may be an obligate chemolithoautotroph in the SUP05 clade (1, 21).
The secondary metabolite gene clusters detected by antiSMASH (22) showed that VS-SUP05 has the capacity to produce bacteriocins, a group of antibiotic compounds that inhibit the growth of closely related bacteria (23). BLAST analysis of the secondary metabolite genes revealed that overall, genes from endosymbiont EBS showed the greatest similarity to those from VS-SUP05, with nucleotide sequence identities ranging from 53% to 87% (see detailed BLAST results in File S6 in the supplemental material). The shared biosynthetic and regulatory genes in the VS-SUP05 and EBS genomes are illustrated in Fig. S4 and in the text in the supplemental material. Among the biosynthetic genes, four genes with the high sequence identity to the EBS coded for an aldehyde dehydrogenase (SMCOG1017), a pyruvate oxidase/decarboxylase (SMCOG1055), a rhodanese domain-containing protein (SMCOG1163), and a domain of unknown function (DUF692). In addition, a regulatory gene encoding the RNA (SMCOG1032) was detected. Genes for antibiotic compounds were also searched against the genomes of closely related bacteria CRM, CVO, CTA, CTSPS, and CTSGG; however, the exclusive detection of the bacteriocin genes in VS-SUP05 and EBS suggested that bacteriocin production was probably specific to these two closely related animal-associated SUP05 bacteria.
Sponge-associated viruses and putative VS-SUP05 phages.
In the Swan site metagenome, 126 contigs were identified as putative viral sequences by VirSorter. These sequences were then confirmed as not derived from prophages by PHASTER and genome annotation. A total of 964 protein-encoding sequences were predicted among the viral contigs (see File S7 in the supplemental material). Taxonomic classification showed that at least nine viral families were represented, including Podoviridae, Siphoviridae, and Myoviridae. Single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA) viruses were both identified in the viral community. Many of the sponge-associated viral sequences belonged to dsDNA viruses, including members of the order Caudovirales or the families Mimiviridae, Phycodnaviridae, and Poxviridae. Compared with the ssDNA viruses (average depth, ∼30), some of the dsDNA viruses had a very high sequencing depth (average depth, ∼300). Comparisons against viral reference genomes showed that viruses from the sponges showed similarity to phages infecting bacteria belonging to the genera Bdellovibrio, Shewanella, Pseudomonas, and Pseudoalteromonas, which were also identified in the sponge metagenome. Fifteen of the 126 contigs were identified as putative VS-SUP05 phage genomes (marked in blue in File S7) because they showed the greatest identity to dsDNA Caudovirales and ssDNA Microviridae that have been reported to infect sulfur-oxidizing SUP05 bacteria (9). The potential VS-SUP05 phages belonged to four families: Myoviridae, Siphoviridae, Podoviridae, and Microviridae. Some of the sequenced genomes were circular, suggesting that they were potentially complete genomes with intact genetic information. For example, the Podoviridae genome corresponding to Contig_183683 was circular, with a length of 31.8 kb. A total of 35 genes were identified from the genome, two of which corresponded to hallmark viral tail and terminase large subunit proteins. Comparisons against reference viral genomes showed that the two hallmark genes showed the greatest identity to corresponding sequences from SUP05 phages recovered from oxygen-minimum zones (9). Bacterial genes were also identified in the viral genomes, including a ydaS/ydaT homolog coding for the antitoxin subunit of a bacterial toxin-antitoxin (TA) system.
In the Crane site metagenome, a total of 33 viral contigs were identified, carrying 237 predicted protein-coding sequences (File S7). These sequences were then confirmed as not derived from prophages by PHASTER and genome annotation. Taxonomic classification showed that at least five viral families were represented in the sponge-associated viral community, including Microviridae, Corticoviridae, Podoviridae, Siphoviridae, and Myoviridae. Sequence comparison against viral reference genomes revealed that the sponge-associated phages likely infected Bdellovibrio, Bacillus, Pseudomonas, and Pseudoalteromonas species, which were also harbored in the host sponge. Two of the 33 contigs were identified as putative VS-SUP05 phage genomes (marked in blue in File S7) and were affiliated with Podoviridae and Microviridae viruses. The genome of the putative Microviridae VS-SUP05 phage (Contig_25599) was circular, with a length of 4.6 kb. The genome contained six genes, among which three were hallmark coat protein-, capsid protein-, and portal protein-encoding viral genes. Comparison against reference viral genomes showed that the viral genes had the greatest identity to SUP05 phages found in samples from oxygen-minimum zones (9).
Comparison of the viral genomes from the Crane and Swan sites revealed that some short contigs from one site completely mapped to long contigs from the other site. BLASTn analysis of the overlapping contigs showed a high degree of sequence similarity (see File S8 in the supplemental material). For example, Contig_105717 from the Swan site metagenome (3,721 bp) completely overlapped with Contig_962 (11,307 bp) from the Crane site genome, with a nucleotide sequence identity of 99.2% in the overlapping region. Among the viral sequences with high similarity, Contig_25599 and Contig_42464, corresponding to putative VS-SUP05 phages from the Crane site, were almost identical to contigs 43469 and 2354, respectively, from the Swan site. Contig_43469, with a length of 2,822 bp, completely overlapped with Contig_25599 (4,615 bp) (99.7% nucleotide sequence identity), while Contig_42464, with a length of 5,343 bp, almost completely overlapped (5,285 bp overlapping) with Contig_2354 (6,635 bp) (98.7% nucleotide sequence identity). Interestingly, we found that the putative sponge-associated SUP05 phages (Microviridae) were also present in various other animals based on NCBI-nr database (https://www.ncbi.nlm.nih.gov) comparison of the capsid protein sequences from the Microviridae phages (Contig_25599 and Contig_43469) with those of other animal-associated phages. These host animals included both invertebrates, such as abalone, sea squirts (Ciona robusta), and honey bees (Apis mellifera), and vertebrates, such as minnows and red snapper. All of the hits had an average nucleotide identity of >50%. Among them, the highest identity was observed for a phage capsid protein detected in abalone (GenBank accession no. AXF52680.1), with an identity of 57%.
Viral Fe-S cluster assembly genes related to VS-SUP05.
AMGs in phages play a key role in viral replication and complementing host metabolism. Various AMGs have been identified in cultured phages or viromes, including the widespread ribonucleotide reductase gene for nucleotide metabolism (24) and metabolic genes essential for energy metabolism and iron-sulfur (Fe-S) cluster assembly (25). AMGs related to sulfur-, nitrogen-, phosphate-, and carbohydrate-based energy metabolism, nucleotide metabolism, and Fe-S cluster assembly were identified in the sponge-associated viral communities from the Swan and Crane sites (see details in File S7 and Fig. S5 in the supplemental material). The genes necessary for Fe-S cluster assembly were related to those found in VS-SUP05 and symbiotic bacteria from other marine invertebrates and were therefore selected for further analysis. Fe-S clusters are key cofactors of dissimilatory sulfite reductases, which are crucial enzymes in sulfur oxidation (26). A complete set of Fe-S cluster assembly genes (encoding IscS, IscU, IscA, Hsc66, Hsc20, and ferredoxin) was identified in the VS-SUP05 genome, suggesting that dissimilatory sulfite reductases and biosynthetic cofactor Fe-S clusters can function together in sulfur metabolism.
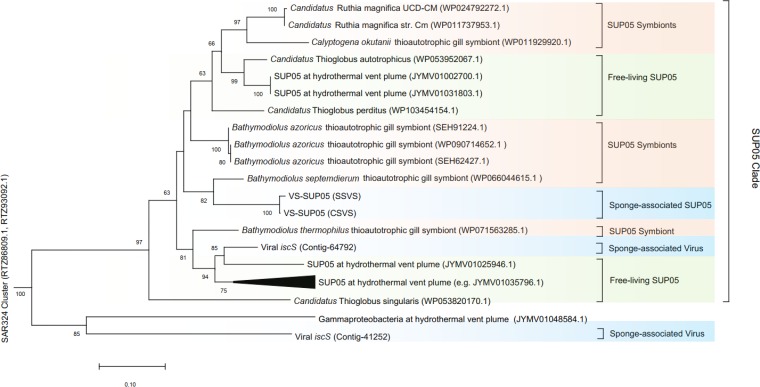
iscU, encoding an Fe-S cluster assembly protein, was confirmed to be an AMG (25). In the sponge-associated virome from the Swan site, three contigs (Contig_64792, Contig_41252, and Contig_147375) contained iscU as well as other components (iscR, iscA, and iscS) required for the assembly of Fe-S clusters. All of the viral iscU genes showed identity to iscU genes from bacterial symbionts in the NCBI-nr database. For example, iscU from Contig_64792 showed 96% protein sequence identity to a deep-sea mussel symbiont (accession no. WP_090714649.1), iscU from Contig_41252 showed 81% identity to a snail symbiont (accession no. WP_043107631.1), and iscU from Contig_147375 had 83% protein sequence identity to a snail symbiont (accession no. WP_043107631.1). We also found that all the proteins related to Fe-S cluster assembly from viral Contig_64792 showed high sequence identity to their corresponding proteins in SUP05 bacteria (including both free-living and symbiotic bacteria). Phylogenetic analysis (Fig. 5) of iscS from Contig_64792 showed that the sponge-associated phage gene was clustered with genes from a clade of SUP05 bacteria and was closely related to a free-living SUP05 group bacterium from hydrothermal vents which was shown to be infected with deep-sea phages (4). The tight clustering of viral and host iscS sequences suggests that the viral iscS genes originated from SUP05 bacteria, which is analogous to previous observations regarding core photosynthesis genes and rdsr sequences in phages (4, 27). iscS from the putative VS-SUP05 phage showed the greatest identity to a SUP05 symbiont (protein identity, 88%; accession no. WP_071563285.1) and showed 85% identity to iscU from the VS-SUP05 genomes from both the Swan and Crane sites. Furthermore, the map of the genes required for Fe-S cluster assembly showed that the VS-SUP05 viral contig shared high nucleotide sequence similarity and genome structure with both symbiotic and free-living SUP05 bacteria (Fig. 6). The high level of sequence identity suggests that the putative VS-SUP05 phage can potentially infect symbiotic SUP05 harbored in marine invertebrates.
FIG 5.
Maximum-likelihood-based phylogenetic tree generated from the iscS genes from vent sponge-associated microbes. Different groups of microbes are distinguished by color: blue, sponge-associated SUP05 and viruses; orange, SUP05 symbionts; green, free-living SUP05 bacteria. Bootstrap values of <50 are hidden at the nodes.
FIG 6.
Map of sequences containing Fe-S cluster assembly-associated genes. EBS, endosymbiont of Bathymodiolus septemdierum strain Myojin knoll; CRM, “Candidatus Ruthia magnifica” strain Cm; CVO, “Candidatus Vesicomyosocius okutanii” HA; CTA, “Candidatus Thioglobus autotrophicus” strain EF1; CTS, “Candidatus Thioglobus singularis” PS1.
DISCUSSION
SUP05 bacteria dominate vent sponge microbial communities.
Our study shows that sulfur-oxidizing SUP05 bacteria dominate the microbial communities of deep-sea hydrothermal vent sponges from the Okinawa Trough. A predominance of SUP05 bacteria has been previously reported in hydrothermal plumes, such as at the Suiyo Seamount (28), and bacteria from this clade form chemosynthetic symbioses with mussels and clams at deep-sea hydrothermal vents (2, 5, 6, 29, 30). The sponge-associated SUP05 genome (VS-SUP05) recovered in the current study displayed homolog synteny, genomic structure and metabolic pathway similarity, and a close phylogenetic relationship with SUP05 symbionts from mussels and clams. Additionally, genome annotation suggested that the VS-SUP05 symbiont contained a series of genes related to sulfur oxidization, which was consistent with findings for the mussel and clam symbionts. These sulfur-oxidizing genes benefit the SUP05 symbionts by conferring the capacity to utilize reduced sulfur compounds as an energy source.
Reduced chemical compounds are abundant in the deep-sea hydrothermal vent and cold seep environments and can be utilized by microbial symbionts. Research shows that nutritionally symbiotic methane-oxidizing bacteria account for a significant proportion (50% to 70%) of the sponge-associated microbiota in cold seep environments (15). The dominant symbionts use methane from seeps as an energy source to fuel chemosynthetic primary production for host nutrition, thereby maintaining the symbiotic system. The dominant sulfur-oxidizing SUP05 bacterium in our study may serve as an important nutrient supply for the host sponge as part of a nutritional symbiosis in deep-sea hydrothermal vent environments, where vent plumes and fluids are rich in hydrogen sulfide. The VS-SUP05 genomes from both the Crane and Swan sites harbored genes for sulfur metabolism and carbon fixation. This suggests that the bacteria are capable of oxidizing sulfur and using sulfide and thiosulfate as an electron donor and an energy source, respectively. This process is beneficial for the sponge, as it removes toxic sulfide molecules. Ribulose-bisphosphate carboxylase genes (rbcL and rbcS) were also found in the VS-SUP05 genomes and are likely to be involved in the fixation of carbon dioxide. Additionally, the VS-SUP05 genome from the Crane site harbored genes coding for a periplasmic nitrate reductase (napAB) and a nitrite reductase (nirBD), which give rise to the nitrate respiration that has been observed in symbionts from clams and tubeworms at hydrothermal vents (2, 31, 32). With sulfur oxidation and nitrate respiration available for energy metabolism, products such as sulfate, ammonia, and liberated electrons can be generated for use in other pathways. For example, any generated electrons can be transferred to the Calvin cycle for carbon fixation, producing carbohydrates that can be used either by the bacteria themselves or by the host sponges.
Cellular components produced by symbionts are also important to the host. Amino acids synthesized by Buchnera play a role in the metabolic dependence of the host aphids (33). Likewise, cofactors synthesized by the symbiont Wigglesworthia are required by the host tsetse flies (34). As shown by KEGG orthology analysis, many genes coding for amino acids and cofactors were detected in the VS-SUP05 genome. The VS-SUP05 genome from the Crane site contained almost a complete set of genes coding for essential amino acids such as valine, isoleucine, and leucine but was lacking many genes required for the synthesis of nonessential amino acids such as alanine and asparagine (see Fig. S6 in the supplemental material). Although one or two genes were missing from pathways for the biosynthesis of some amino acids such as lysine and methionine, alternative products in VS-SUP05 or intermediates from the host might complement the functions of these genes. For example, the missing genes for the biosynthesis of proline and tyrosine could be found in the other bacterial genomes in the sponge-associated microbiota. Cofactors are another set of cellular components required by a host, and genes required for the production of thiamine, riboflavin, pyridoxine, biotin, lipoic acid, folate, and heme were detected in the VS-SUP05 genome (Fig. S6). ATP binding cassette (ABC) transporters related to the transport of heme, lipoprotein, lipopolysaccharide, and general l-amino acids were also detected. With these transporters, synthesized compounds, including cofactors and amino acids, can be exported and therefore utilized by the sponges as a source of nutrients.
Compared with their free-living relatives, symbiotic SUP05 bacterial genomes exhibit obvious gene loss, which corresponds to their symbiotic lifestyle. In the intracellular environment or sponge body environment, environmental factors such as salinity and nutrient concentration are usually stable, meaning that symbiotic SUP05 bacteria do not need to move directionally in response to environmental changes. This may result in the loss of functional genes required for cell motility, such as those involved in chemotaxis and flagellar motility. Genes necessary for adaptation to environmental stress conditions were also less abundant in symbionts, probably because symbiotic bacteria reside in the relatively stable and favorable environments within the body of their hosts. We also observed the loss of functional genes associated with xenobiotic biodegradation and metabolism in our samples. Interestingly, similar functional gene losses were found in clams and mussels in deep-sea vent environments. However, Slaby et al. (35) revealed that sponge-associated bacteria from Aplysina aerophoba in the Mediterranean Sea (5-m depth) have more genes related to bacterial defense, such as toxin-antitoxin systems, than the reference bacteria including free-living bacteria. This indicates contrasting chemical environments in symbiotic systems of different sponge species and/or different habitats (such as shallow sea versus deep sea).
Stable association between SUP05 bacteria and their phages in vent sponges.
Marine invertebrates frequently form symbioses with microorganisms, which have proven to be taxonomically widespread (36). Sponges are estimated to have played host to symbionts, mainly bacteria, for hundreds of millions of years (15). These bacterial associates are consistently present in geographically separated conspecific sponges, including Aplysina aerophoba (37), Rhopaloeides odorabile (37), Theonella swinhoei (37), Myxilla incrustans (38), and Haliclona rufescens (38). Our finding that microbial communities are rather stably sponge species specific has been shown before (37, 38), demonstrating stable associations between certain sponges and bacterial symbionts. Lee et al. (38) compared the bacterial communities in conspecific sponges from different shallow-water regions and found high levels of similarity among the bacterial associates from M. incrustans and H. rufescens sponges. Specifically, the 16S rRNA gene sequences of a Bacillus sp. from the conspecific M. incrustans sponges showed >99% identity. In the current study, the 16S rRNA genes of the dominant SUP05 bacteria from Haplosclerida sponges from two different geographic regions showed 100% nucleotide sequence identity. Further, the 16S rRNA gene sequences from these dominant SUP05 bacteria consistently made up ∼70% of reads of 16S rRNA genes from the bacterial community, indicating that these bacteria are highly conserved among Haplosclerida sponge populations in deep-sea hydrothermal vents. Additionally, based on searching against on the EMP global data sets, we found that the sponge symbionts, including SUP05, in our study are widely distributed across sponges in shallow and deep environments. This suggests that sponge-associated bacteria are likely to be highly conserved and may be resistant to environmental perturbations during evolution, regardless of whether the sponges occur in shallow seawater or in the deep-sea environment.
Sulfur-oxidizing SUP05 bacteria not only have a stable association with deep-sea Haplosclerida sponges but also form intimate symbiotic relationships with other marine animals such as bathymodioline mussels and vesicomyid clams (Fig. 2A). Peterson et al. (19) showed that bathymodioline-associated sulfur-oxidizing symbionts were distributed among a wide range of other invertebrate hosts, including sponges and polychaetes, which supports our observation that SUP05 was present among multiple marine invertebrate animals. However, although these chemosynthetic SUP05 symbionts are flexibly associated with different animals, they are highly conserved. 16S rRNA gene sequence analysis (sequences retrieved from bacteria listed in Fig. 2A) revealed nucleotide sequence identity ranging from 98.4% to 99.8% among the congeneric-vesicomyid-associated SUP05 bacteria and from 98.4% to 100% among congeneric-bathymodioline-associated SUP05 bacteria. In addition, the conservation of symbiotic SUP05 bacteria is likely to be high in a single-animal genus. This observation suggests that congeneric invertebrate animals acquire SUP05 bacteria by vertical transmission directly from their parents. As a result of vertical transmission, symbiotic SUP05 populations are subject to smaller variations during the evolution of a single-animal genus than are free-living SUP05 bacteria (16S rRNA gene similarity ranging from 96.5% to 100%) in seawater habitats.
Sponges, as common symbiont hosts, harbor a diverse range of viruses (13, 39). Using transmission electron microscopy, Pascelli et al. (13) showed that 50 morphotypes were present in the viral communities of reef sponge species. However, metagenomic sequence analyses suggested that these morphologically diverse viruses are highly conserved within conspecific sponges (39). Our finding that similar bacteriophages occurred in sponges from two different locations provides further evidence that the viral community is stable in sponges belonging to the same species, even when geographically separated. Additionally, we found that conserved sponge-associated SUP05 phages (e.g., Microviridae) were also present in different animals, such as abalone and sea squirts. Stable symbiotic environments give rise to animal-related phage conservation. Although the interactions between SUP05 bacteria and their phages have not been studied in a wide range of animal hosts, the high conservation of SUP05 bacteria and associated phages in sponges hints at an intimate association that may influence their host.
Potential SUP05-phage interactions in hydrothermal vent sponges.
Viruses potentially have close interactions with microbial symbionts harbored by animal hosts and may therefore affect the symbiosis system (40). Animal-related viruses are thought to be required for mutualistic symbiosis. For example, the presence of prophages within bacterial symbionts conferred protection against wasp parasitoids in aphids (10). Therefore, viruses found within the sponges are potentially essential for the symbiotic bacteria and intimately interact with the symbionts. One viral contig (Contig_64792) from the Swan site metagenome contained iscU, which is classified as an auxiliary metabolic gene for Fe-S cluster assembly (25). In addition to iscU, iscS and iscR were also found in the same contig. Phylogenetic analysis based on iscS gene sequences suggested that the viral iscS gene was derived from the SUP05 clade, which also contained symbiotic SUP05 bacteria as well as VS-SUP05 (Fig. 5). The tight phylogenetic clustering and high homolog similarity (>74%) of iscS implies that both the free-living and symbiotic SUP05 bacteria were potentially infected by the putative SUP05 phages and that the bacterial iscS genes were transferred to the phages, which influenced both ecology and evolution.
The potential SUP05-phage interaction in vent sponges may play a key role to help sponges to adapt to the vent environment, which is rich in sulfur compounds. Based on phylogenetic and similarity analyses of iscS, free-living SUP05 bacteria from a deep-sea hydrothermal plume (4) are closely related to VS-SUP05 (>84% similarity) and the putative VS-SUP05 phage-containing Contig_64792 (>87% similarity). Anantharaman et al. (4) reported that horizontal transfer of rdsrA occurred between free-living SUP05 bacteria and marine phages. Since AMG rdsrA encodes a reverse dissimilatory sulfite reductase that oxidizes elemental sulfur, SUP05 phages are likely to be involved in sulfur oxidation as part of the host metabolism, enhancing the efficiency of sulfur cycling. The coexpression of iscS, iscR, and iscU by SUP05 phages might supplement the biosynthesis of the Fe-S cluster and therefore supplement the sulfur metabolism in VS-SUP05 during infection. Through this metabolic compensation, viruses play an essential role in regulating host metabolism in the hydrothermal vent environment (41).
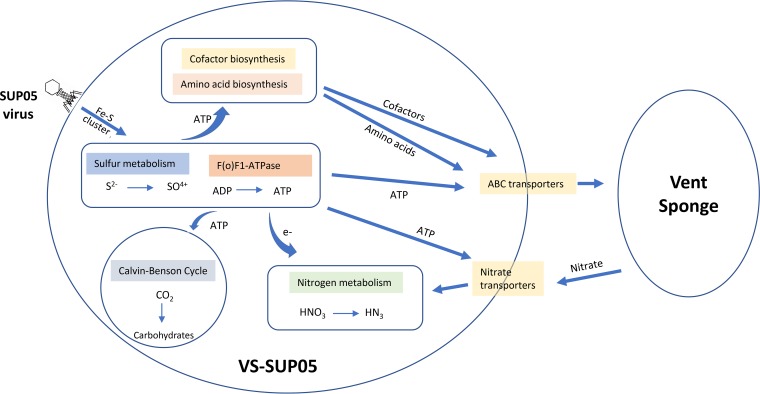
Given that VS-SUP05 and the putative SUP05 phages live in symbiosis with host vent sponges, the sponge-SUP05-phage interaction may be necessary for adaptation to the deep-sea hydrothermal vent environment. Thus, we propose that VS-SUP05 and the SUP05 phages take advantage of the surrounding reduced sulfur compounds and provide energy for the symbiotic system. In this sulfur oxidation process, the expression of viral iscS, iscU, and iscR genes supplements the assembly of Fe-S clusters in VS-SUP05, thereby increasing the efficiency of energy generation from sulfur oxidation. VS-SUP05 uses the generated energy to synthesize essential amino acids and cofactors, which are transported outside the cell via ABC transporters or released during lysis, supplying nutrition for the host sponge. The energy from sulfur oxidation can also power the Calvin cycle for carbon dioxide fixation and support the nutrient needs of the sponge. In addition, nutrients such as nitrate produced by the sponge are taken up by VS-SUP05 and utilized during nitrate respiration (Fig. 7).
FIG 7.
Proposed sponge-bacterium-virus interaction in hydrothermal vents.
In addition to having interactions with phages in sulfur metabolism, the VS-SUP05 bacteria are likely to be influenced by the sponge-associated phages in aspects of bacterial defense. Sponge symbionts have a significant enrichment of genes related to bacterial defense that has been revealed by statistical analyses (35). In our study, the deep-sea sponge symbionts contain five COGs (COG5450, COG2337, ENOG410YAX4, ENOG410Y2NY, and ENOG4111NPA) involved in the toxin-antitoxin (TA) systems. However, all the genes for TA systems are absent in the dominant VS-SUP05 genome, and the ydaS gene, encoding antitoxins that regulate bacterial growth and death (42), for the TA system was found in a putative SUP05 phage genome. The viral ydaS may complement the bacterial TA systems of the VS-SUP05 and other sponge symbionts and contribute to the survival of the symbionts.
In conclusion, this study reveals that sulfur-oxidizing SUP05 bacteria (VS-SUP05) and their phages are present in deep-sea hydrothermal vent sponges. Metagenomic analyses indicate that VS-SUP05 potentially forms a symbiotic partnership with the host sponges and is much more likely to be infected with sponge-associated phages than with free-living SUP05 bacterial phages. Viral AMGs associated with Fe-S cluster assembly suggest that horizontal gene transfer has occurred between SUP05 bacteria (including symbiotic SUP05 and free-living SUP05 strains) and sponge-associated phages. The expression of genes encoding Fe-S cluster assembly proteins by the putative SUP05 phages may supplement sulfur oxidation in VS-SUP05 symbionts of hydrothermal vent sponges. The close relationship among sponge-associated phages, SUP05 bacteria, and host sponges indicates that the interactions among phages, bacteria, and animals might provide an opportunity for marine animals to adapt to extreme deep-sea hydrothermal vent environments.
MATERIALS AND METHODS
Sample collection, DNA extraction, and sequencing.
Potential hydrothermal activity was detected in the Okinawa Trough based on acoustic water column anomalies, most likely resulting from CO2 bubbles discharging from venting sites. An EM122 multibeam echosounder system (Kongsberg Maritime) onboard the research vessel (R/V) Yokosuka (cruises YK14-16 and KR15-16) was used to detect anomalies, as outlined by Nakamura et al. (43). Each of the detected locations was then explored using the remotely operated vehicle (ROV) KAIKO (with vehicle Mk-IV) operated from R/V Kairei during cruise KR15-16 (45). Two diffuse venting sites were discovered in the southern Okinawa Trough: the first on Tarama Knoll (25°5.49ʹN, 124°32.81ʹE; depth, 1,730 to 1,770 m) and the second on Tarama Hill (25°4.54ʹN, 124°31.01ʹE; depth, 1,950 to 1,990 m) (16, 45). The site on Tarama Knoll was named the Swan site and was >100 m by 100 m in size. The Swan site was extensively colonized by Haplosclerida sponges (Shirley Pomponi and Maria Cristina Diaz, personal communication), Alaysia tubeworms, and the mussel species Bathymodiolus aduloides. Some hot spots exhibited shimmering water and hosted common Okinawa Trough vent fauna such as squat lobsters (Shinkaia crosnieri), limpets (Lepetodrilus nux), snails (Provanna subglabra and Provanna clathrata), and barnacles (Neoverruca intermedia) (45). The site on Tarama Hill was named the Crane site and was much smaller (∼10 m by 10 m). The Crane site was dominated by Haplosclerida sponges (Cristina Diaz, personal communication), tubeworms belonging to the genera Alaysia and Lamellibrachia, and the mussel species B. aduloides (for details see reference 16).
Samples of the dominant sponge, belonging to the order Haplosclerida (NCBI GenBank accession numbers MN153804 for the 18S rRNA gene and MN153806 for the 28S rRNA gene) (see Fig. S1 and S2 in the supplemental material), characterized by its digit-like form, were collected from both sites (for the Swan site, ROV KAIKO dive 670, 30 October 2015, 25°5.4861ʹN, 124°32.8089ʹE, 1,736-m depth; for the Crane site, ROV KAIKO dive 669, 29 October 2015, 25°4.5388ʹN, 124°31.0103ʹE, 1,973-m depth). Upon recovery on board the research vessel, the vent sponge samples were immediately stored at −80°C. For microbial community analysis, sponge samples were rinsed three times with Tris-EDTA (TE) buffer and cut into small pieces (∼0.1 cm3) before being homogenized using a plastic pestle and a 1.5-ml centrifuge tube. Samples were then filtered through mesh (pore size, 100 μm) to remove the sponge skeleton, with the filtrates then further filtered through a 10-μm filter. The resulting filtrates were centrifuged at 10,000 × g at room temperature, and the cell pellets were washed with TE buffer. Total DNA was then extracted using a FastDNA kit (MP Biomedicals) as per the manufacturer’s instructions, and the DNA quantity and quality were checked using a NanoDrop spectrophotometer (Thermo Fisher Scientific) and agarose gel electrophoresis. Two libraries, with insert sizes of approximately 300 bp and 500 bp, were constructed with the NEBNext Ultra DNA Library Prep kit and then sequenced on an Illumina XTen platform, generating 2 × 150-bp paired-end reads.
Microbial community analysis, metagenome assembly, genome binning, and draft genome reassembly.
Illumina raw reads were trimmed using Trimmomatic (version 0.36, ILLUMINACLIP: TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:40) (46) to remove low-quality and adaptor-contaminated reads. For microbial community analysis, trimmed reads were input into Parallel-META3 (47) for taxonomic profiling with default parameters. Because only single-ended shotgun reads are currently supported in Parallel-META3, only forward reads from the paired-end trimmed read data were taxonomically profiled. To investigate the global distribution of the sponge symbionts obtained in our study, the reference data of QIIME maps and BIOMs of two studies (study 10793 [48] of the Sponge Microbiome Project and study 10346 [49] of the Global Sponge Microbiome) were downloaded from the Earth Microbiome Project data sets (http://www.earthmicrobiome.org/) (50). The reads of 16S rRNA genes were retrieved using Parallel-META3 and were mapped to the operational taxonomic unit (OUT) representative sequences (V4 regions, 16S rRNA genes) contained in the reference data using BLASTN (E value, <1e−30; identity, >97%; alignment length ≥ representative sequence length). Prior to metagenome assembly, the trimmed reads were reduced to a depth of 100× using a normalization process conducted in BBMap (https://sourceforge.net/projects/bbmap/). Normalized read data were assembled using MEGAHIT (51), with the customized parameter of –k-list (from 21 to 141 with a 10-mer step size). Contigs resulting from the MEGAHIT analysis were further filtered to remove sequences that were <500 bp in size. A binning method developed by Albertsen et al. (52) was then used to cluster contigs based on GC content, sequencing depth, tetranucleotide frequency, and essential gene taxonomy (see Fig. S3 in the supplemental material). Reads from each of the genome bins were then extracted using Perl scripts for reassembly. Because of a high abundance of reads in the bins (∼7000× at depth), BBMap was used to reduce the coverage to 100× using the command bbnorm.sh as a default. The normalized reads were reassembled using SPAdes version 3.11.1 (53) with a list of sequential k-mer sizes from 21 to 121 (k-mer step size of 10) and the parameter –careful. Finally, the level of contamination and the completeness of the reassembled draft genomes (scaffold length, >1 kb) were assessed by CheckM (54).
Phylogenetic analysis of the dominant SUP05 bacteria.
Two draft genome bins from two samples were phylogenetically profiled based on 16S rRNA gene sequences. The 16S rRNA genes were predicted by meta-RNA (55), using SINA 1.2.11 (56) searching against the databases of Greengenes (57), SILVA (58), and RDP (59). Because the 16S rRNA genes of the two recovered genomes were identical (100% nucleotide sequence identity) and both were affiliated with the SUP05 clade, we used VS-SUP05 (vent sponge-associated SUP05) to collectively refer to the dominant SUP05 bacteria from the two vent sponges. The VS-SUP05 16S rRNA gene sequences were then used for homology analyses against the NCBI-nt (http://blast.ncbi.nlm.nih.gov/) and IMG (https://img.jgi.doe.gov/) databases, respectively. Hits with high sequence identity (>92%) were aligned using Mafft (–adjustdirectionaccurately –auto) (60) and used to construct a maximum-likelihood phylogenetic tree using RAxML with the algorithm of rapid Bootstrap analysis, the GTRCAT model of nucleotide substitution, and 1,000 bootstrap replications (parameters, -f a -m GTRCAT -N 1,000) (61). The genome sequences of VS-SUP05 and six relatives selected from the 16S rRNA gene-based phylogenetic tree were then searched against 120 conserved marker proteins (62) from single-copy genes using GTDB-Tk v0.3.1 (62) with the default parameter classify_wf. The identified homologs of the marker protein sequences concatenated by GTDB-Tk were then imported into RAxML to construct a genome-wide evolutionary tree with the algorithm of rapid Bootstrap analysis, the PROTGAMMAWAG model of amino acid substitution, and 1,000 bootstrap replications (-f a -m PROTGAMMAWAG -N 1,000).
Functional genome annotation.
The homolog annotation of the whole metagenome was carried out based on the gene prediction by the PRODIGAL (63) and the following functional assignment using the NCBI-nr database (https://www.ncbi.nlm.nih.gov). The VS-SUP05 draft genome was functionally annotated using PROKKA (64). PRODIGAL (63) and ARAGORN (65) in PROKKA were used to identify coding genes and tRNA genes, respectively. MEGAN6 (ultimate edition) (66) was then used to reconstruct metabolic pathways based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (67). Ortholog annotation of the VS-SUP05 genome along with six reference genomes was carried out based on the eggNOG database (68). Secondary metabolic gene clusters were detected using the online tool antiSMASH (http://antismash.secondarymetabolites.org/#!/start). To compare the two draft genomes, the average nucleotide identity analysis was performed by Anvi’o5 (8), and their total predicted proteins were mapped to each other using BLASTp to see the similarity of each protein sequence.
Genome-wide comparison with closely related SUP05 bacteria.
Six closely related SUP05 bacteria, five with complete genome sequences available (2, 5, 7, 69, 70) and one with a draft genome sequence (https://www.ncbi.nlm.nih.gov/nuccore/NZ_CP008725.1), were selected based on the 16S rRNA phylogenetic tree. The genomes were functionally annotated against the KEGG database using MEGAN6 (ultimate edition) under the same conditions used for analysis of VS-SUP05. A heat map of the gene counts of each KEGG pathway was then plotted using Rscripts (https://github.com/rasbt/R_snippets/tree/master/heatmaps) to visualize differences in genome function between VS-SUP05 and the six related bacteria. Putative homologous regions of the seven genomes were predicted and visualized using MCScanX with default parameters (71). In addition, homology searching against the eggNOG database was conducted to annotate homologs in the genomes of VS-SUP05 and the six related bacteria. Venn diagrams of the distribution of annotated homologs among the seven genomes were plotted using Venny 2.1 (http://bioinfogp.cnb.csic.es/tools/venny/), while detailed information on the intersections was calculated using VENN DIAGRAMS (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Detection and annotation of viral contigs.
VirSorter (72) with default parameters was used to identify viral contigs from the metagenomic assembly (contigs of >500 bp) by searching against the RefSeqABVir and Viromes databases (72). Detected phage contigs in categories 1 (“pretty sure”) and 2 (“quite sure”) were selected and annotated based on the standard operating procedure of Metavir 2 (73), and then the detected phage contigs were analyzed by PHASTER (74) (contigs of >15 kb) or functional annotation (contigs of <15 kb) to check prophage features (genes for integrases and attachment sites). To identify taxonomic affiliations, viral contigs were first imported into Prodigal for open reading frame (ORF) prediction, with the resulting ORFs then aligned against the viral NCBI-RefSeq database (http://blast.ncbi.nlm.nih.gov/) using BLASTp (E value of <0.001) (75). The BLASTp output was then imported into MEGAN6 using the LCA algorithm for taxonomic analysis of viral contigs. For functional annotation, viral contigs were subjected to BLASTp analysis against the NCBI viral RefSeq database and to HMMScan analysis against the PFAM database, with a threshold of E value of 0.001 and bit score of 30. AMGs were identified by BLASTp analysis of predicted viral genes against the NCBI-nr database. Genes related to sulfur metabolism or nitrogen metabolism, as well as homologs of ribonucleotide reductase genes, were selected as putative AMGs. BLASTp analysis (cutoff, E value of <1e−5) of predicted viral genes against the viral NCBI-RefSeq database and the reference genomes of marine viruses infecting SUP05 bacteria (4, 9) was also conducted to identify potential VS-SUP05 phages. Genes whose highest hit was from a reference SUP05 phage were identified as putative VS-SUP05 phage genes. For the phylogenetic analysis based on viral iscS sequences, IscS-like amino acid sequences from sponge-associated SUP05 bacteria and phages were aligned and compared with top hit sequences from NCBI. Mafft with default parameters was used to align IscS amino acid sequences. The alignments were refined using Gblocks with default parameters (76). Maximum-likelihood analysis was then conducted to infer phylogenetic relationships among the bacteria using RaxML with the algorithm of rapid bootstrap analysis, the PROTGAMMAJTT model of amino acid substitution, and 1,000 bootstrap replications, (-f a -m PROTGAMMAJTT -N 1,000).
Data availability.
The NCBI genome accession numbers for the sponge metagenome and the reassembled draft genome at the Crane site are VSKW00000000 and VSKZ00000000, respectively. The NCBI genome accession numbers for the sponge metagenome and the reassembled draft genome at the Swan site are VSKX00000000 and VSKY00000000, respectively.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Qingdao National Laboratory for Marine Science and Technology (QNLM2016ORP0303), the China Ocean Mineral Resources R & D Association (DY135-E2-1-04), and the National Natural Science Foundation of China (41576146). This study and cruise KR15-16 were also supported by Council for Science, Technology, and Innovation (CSTI) as the Cross Ministerial Strategic Innovation Promotion Program (SIP), Next-Generation Technology for Ocean Resource Exploration.
We thank Peiyuan Qian (Hong Kong University of Science and Technology) for his valuable and constructive suggestions. We thank the captains and crews of R/VEs Kairei and Yokosuka, as well as the operation team of the ROV KAIKO, for their untiring support during the relevant cruises (YK14-16 and KR15-16). We gratefully acknowledge Kyoko Okino (AORI, University of Tokyo) for leading the YK14-16 cruise. We thank Kentaro Nakamura (University of Tokyo) for analyzing the MBES data which led to the discovery of the new hydrothermal sites. Shirley Pomponi (Florida Atlantic University) and Maria Cristina Diaz (Florida Atlantic University) are thanked for their helpful comments on sponge taxonomy.
We declare no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00992-19.
REFERENCES
- 1.Walsh DA, Zaikova E, Howes CG, Song YC, Wright JJ, Tringe SG, Tortell PD, Hallam SJ. 2009. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326:578–582. doi: 10.1126/science.1175309. [DOI] [PubMed] [Google Scholar]
- 2.Newton ILG, Woyke T, Auchtung TA, Dilly GF, Dutton RJ, Fisher MC, Fontanez KM, Lau E, Stewart FJ, Richardson PM, Barry KW, Saunders E, Detter JC, Wu D, Eisen JA, Cavanaugh CM. 2007. The Calyptogena magnifica chemoautotrophic symbiont genome. Science 315:998–1000. doi: 10.1126/science.1138438. [DOI] [PubMed] [Google Scholar]
- 3.Anantharaman K, Breier JA, Sheik CS, Dick GJ. 2013. Evidence for hydrogen oxidation and metabolic plasticity in widespread deep-sea sulfur-oxidizing bacteria. Proc Natl Acad Sci U S A 110:330–335. doi: 10.1073/pnas.1215340110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anantharaman K, Duhaime MB, Breier JA, Wendt KA, Toner BM, Dick GJ. 2014. Sulfur oxidation genes in diverse deep-sea viruses. Science 344:757–760. doi: 10.1126/science.1252229. [DOI] [PubMed] [Google Scholar]
- 5.Ikuta T, Takaki Y, Nagai Y, Shimamura S, Tsuda M, Kawagucci S, Aoki Y, Inoue K, Teruya M, Satou K, Teruya K, Shimoji M, Tamotsu H, Hirano T, Maruyama T, Yoshida T. 2016. Heterogeneous composition of key metabolic gene clusters in a vent mussel symbiont population. ISME J 10:990–1001. doi: 10.1038/ismej.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiwara Y, Takai K, Uematsu K, Tsuchida S, Hunt JC, Hashimoto J. 2000. Phylogenetic characterization of endosymbionts in three hydrothermal vent mussels: influence on host distributions. Mar Ecol Prog Ser 208:147–155. doi: 10.3354/meps208147. [DOI] [Google Scholar]
- 7.Kuwahara H, Yoshida T, Takaki Y, Shimamura S, Nishi S, Harada M, Matsuyama K, Takishita K, Kawato M, Uematsu K, Fujiwara Y, Sato T, Kato C, Kitagawa M, Kato I, Maruyama T. 2007. Reduced genome of the thioautotrophic intracellular symbiont in a deep-sea clam, Calyptogena okutanii. Curr Biol 17:881–886. doi: 10.1016/j.cub.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Lutz RA, Kennish MJ. 1993. Ecology of deep‐sea hydrothermal vent communities: a review. Rev Geophys 31:211–242. doi: 10.1029/93RG01280. [DOI] [Google Scholar]
- 9.Roux S, Hawley AK, Torres Beltran M, Scofield M, Schwientek P, Stepanauskas R, Woyke T, Hallam SJ, Sullivan MB. 2014. Ecology and evolution of viruses infecting uncultivated SUP05 bacteria as revealed by single-cell- and meta-genomics. Elife 3:e03125. doi: 10.7554/eLife.03125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver KM, Degnan PH, Hunter MS, Moran NA. 2009. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325:992–994. doi: 10.1126/science.1174463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weldon SR, Strand MR, Oliver KM. 2013. Phage loss and the breakdown of a defensive symbiosis in aphids. Proc Biol Sci 280:20122103. doi: 10.1098/rspb.2012.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor MW, Radax R, Steger D, Wagner M. 2007. Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascelli C, Laffy PW, Kupresanin M, Ravasi T, Webster NS. 2018. Morphological characterization of virus-like particles in coral reef sponges. PeerJ 6:e5625. doi: 10.7717/peerj.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishijima M, Lindsay DJ, Hata J, Nakamura A, Kasai H, Ise Y, Fisher CR, Fujiwara Y, Kawato M, Maruyama T. 2010. Association of thioautotrophic bacteria with deep-sea sponges. Mar Biotechnol (NY) 12:253–260. doi: 10.1007/s10126-009-9253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin-Blum M, Antony CP, Sayavedra L, Martinez-Perez C, Birgel D, Peckmann J, Wu YC, Cardenas P, MacDonald I, Marcon Y, Sahling H, Hentschel U, Dubilier N. 2019. Fueled by methane: deep-sea sponges from asphalt seeps gain their nutrition from methane-oxidizing symbionts. ISME J 13:1209. doi: 10.1038/s41396-019-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Watanabe HK, Miyazaki J, Kawagucci S. 2017. Unanticipated discovery of two rare gastropod molluscs from recently located hydrothermally influenced areas in the Okinawa Trough. PeerJ 5:e4121. doi: 10.7717/peerj.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duperron S, Sibuet M, MacGregor BJ, Kuypers MM, Fisher CR, Dubilier N. 2007. Diversity, relative abundance and metabolic potential of bacterial endosymbionts in three Bathymodiolus mussel species from cold seeps in the Gulf of Mexico. Environ Microbiol 9:1423–1438. doi: 10.1111/j.1462-2920.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 18.Klose J, Polz MF, Wagner M, Schimak MP, Gollner S, Bright M. 2015. Endosymbionts escape dead hydrothermal vent tubeworms to enrich the free-living population. Proc Natl Acad Sci U S A 112:11300–11305. doi: 10.1073/pnas.1501160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen JM, Wentrup C, Verna C, Knittel K, Dubilier N. 2012. Origins and evolutionary flexibility of chemosynthetic symbionts from deep-sea animals. Biol Bull 223:123–137. doi: 10.1086/BBLv223n1p123. [DOI] [PubMed] [Google Scholar]
- 20.Sachs JL, Skophammer RG, Regus JU. 2011. Evolutionary transitions in bacterial symbiosis. Proc Natl Acad Sci U S A 108(Suppl 2):10800–10807. doi: 10.1073/pnas.1100304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigro OD, Jungbluth SP, Lin HT, Hsieh CC, Miranda JA, Schvarcz CR, Rappe MS, Steward GF. 2017. Viruses in the oceanic basement. mBio 8:e02129-16. doi: 10.1128/mBio.02129-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, Lee SY, Fischbach MA, Muller R, Wohlleben W, Breitling R, Takano E, Medema MH. 2015. antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res 43:W237–W243. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotter PD, Ross RP, Hill C. 2013. Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 24.Dwivedi B, Xue B, Lundin D, Edwards RA, Breitbart M. 2013. A bioinformatic analysis of ribonucleotide reductase genes in phage genomes and metagenomes. BMC Evol Biol 13:33. doi: 10.1186/1471-2148-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurwitz BL, Brum JR, Sullivan MB. 2015. Depth-stratified functional and taxonomic niche specialization in the ‘core’ and ‘flexible’ Pacific Ocean virome. ISME J 9:472–484. doi: 10.1038/ismej.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira TF, Franklin E, Afonso JP, Khan AR, Oldham NJ, Pereira IAC, Archer M. 2011. Structural insights into dissimilatory sulfite reductases: structure of desulforubidin from Desulfomicrobium norvegicum. Front Microbiobiol 2. doi: 10.3389/fmicb.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breitbart M. 2012. Marine viruses: truth or dare. Annu Rev Mar Sci 4:425–448. doi: 10.1146/annurev-marine-120709-142805. [DOI] [PubMed] [Google Scholar]
- 28.Sunamura M, Higashi Y, Miyako C, Ishibashi J, Maruyama A. 2004. Two bacteria phylotypes are predominant in the Suiyo seamount hydrothermal plume. Appl Environ Microbiol 70:1190–1198. doi: 10.1128/aem.70.2.1190-1198.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duperron S, Nadalig T, Caprais J-C, Sibuet M, Fiala-Médioni A, Amann R, Dubilier N. 2005. Dual symbiosis in a Bathymodiolus sp. mussel from a methane seep on the Gabon continental margin (southeast Atlantic): 16S rRNA phylogeny and distribution of the symbionts in gills. Appl Environ Microbiol 71:1694–1700. doi: 10.1128/AEM.71.4.1694-1700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesniewski RA, Jain S, Anantharaman K, Schloss PD, Dick GJ. 2012. The metatranscriptome of a deep-sea hydrothermal plume is dominated by water column methanotrophs and lithotrophs. ISME J 6:2257. doi: 10.1038/ismej.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hentschel U, Felbeck H. 1993. Nitrate respiration in the hydrothermal vent tubeworm Riftia-Pachyptila. Nature 366:338–340. doi: 10.1038/366338a0. [DOI] [Google Scholar]
- 32.Hentschel U, Hand S, Felbeck H. 1996. The contribution of nitrate respiration to the energy budget of the symbiont-containing clam Lucinoma aequizonata: a calorimetric study. J Exp Biol 199:427–433. [DOI] [PubMed] [Google Scholar]
- 33.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- 34.Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. 2002. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet 32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 35.Slaby BM, Hackl T, Horn H, Bayer K, Hentschel U. 2017. Metagenomic binning of a marine sponge microbiome reveals unity in defense but metabolic specialization. ISME J 11:2465–2478. doi: 10.1038/ismej.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saffo MB. 1992. Invertebrates in endosymbiotic associations. Am Zool 32:557–565. doi: 10.1093/icb/32.4.557. [DOI] [Google Scholar]
- 37.Hentschel U, Hopke J, Horn M, Friedrich AB, Wagner M, Hacker J, Moore BS. 2002. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl Environ Microbiol 68:4431–4440. doi: 10.1128/aem.68.9.4431-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee OO, Wong YH, Qian PY. 2009. Inter- and intraspecific variations of bacterial communities associated with marine sponges from San Juan Island, Washington. Appl Environ Microbiol 75:3513–3521. doi: 10.1128/AEM.00002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laffy PW, Wood-Charlson EM, Turaev D, Jutz S, Pascelli C, Botte ES, Bell SC, Peirce TE, Weynberg KD, van Oppen MJH, Rattei T, Webster NS. 2018. Reef invertebrate viromics: diversity, host specificity and functional capacity. Environ Microbiol 20:2125–2141. doi: 10.1111/1462-2920.14110. [DOI] [PubMed] [Google Scholar]
- 40.Roossinck MJ, Bazan ER. 2017. Symbiosis: viruses as intimate partners. Annu Rev Virol 4:123–139. doi: 10.1146/annurev-virology-110615-042323. [DOI] [PubMed] [Google Scholar]
- 41.He T, Li H, Zhang X. 2017. Deep-sea hydrothermal vent viruses compensate for microbial metabolism in virus-host interactions. mBio 8:e00893-17. doi: 10.1128/mBio.00893-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi Y, Inouye M. 2011. Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nat Rev Microbiol 9:779–790. doi: 10.1038/nrmicro2651. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura K, Kawagucci S, Kitada K, Kumagai H, Takai K, Okino K. 2015. Water column imaging with multibeam echo-sounding in the mid-Okinawa Trough: implications for distribution of deep-sea hydrothermal vent sites and the cause of acoustic water column anomaly. Geochem J 49:579–596. doi: 10.2343/geochemj.2.0387. [DOI] [Google Scholar]
- 44.Reference deleted.
- 45.Makabe AT, Chen C, Torimoto J, Matsui Y, Shibuya T, Miyazaki J, Kitada K, Kawagucci S. 2016. Discovery of new hydrothermal vent fields in the mid- and southern-Okinawa Trough. Goldschmidt Conf Abstr 2016:1945. [Google Scholar]
- 46.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jing G, Sun Z, Wang H, Gong Y, Huang S, Ning K, Xu J, Su X. 2017. Parallel-META 3: comprehensive taxonomical and functional analysis platform for efficient comparison of microbial communities. Sci Rep 7:40371. doi: 10.1038/srep40371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moitinho-Silva L, Nielsen S, Amir A, Gonzalez A, Ackermann GL, Cerrano C, Astudillo-Garcia C, Easson C, Sipkema D, Liu F, Steinert G, Kotoulas G, McCormack GP, Feng G, Bell JJ, Vicente J, Bjork JR, Montoya JM, Olson JB, Reveillaud J, Steindler L, Pineda MC, Marra MV, Ilan M, Taylor MW, Polymenakou P, Erwin PM, Schupp PJ, Simister RL, Knight R, Thacker RW, Costa R, Hill RT, Lopez-Legentil S, Dailianis T, Ravasi T, Hentschel U, Li Z, Webster NS, Thomas T. 2017. The sponge microbiome project. Gigascience 6:1–7. doi: 10.1093/gigascience/gix077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas T, Moitinho-Silva L, Lurgi M, Bjork JR, Easson C, Astudillo-Garcia C, Olson JB, Erwin PM, Lopez-Legentil S, Luter H, Chaves-Fonnegra A, Costa R, Schupp PJ, Steindler L, Erpenbeck D, Gilbert J, Knight R, Ackermann G, Victor Lopez J, Taylor MW, Thacker RW, Montoya JM, Hentschel U, Webster NS. 2016. Diversity, structure and convergent evolution of the global sponge microbiome. Nat Commun 7:11870. doi: 10.1038/ncomms11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vazquez-Baeza Y, Gonzalez A, Morton JT, Mirarab S, Zech Xu Z, Jiang L, Haroon MF, Kanbar J, Zhu Q, Jin Song S, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R, Earth Microbiome Project Consortium . 2017. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li D, Liu CM, Luo R, Sadakane K, Lam TW. 2015. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 52.Albertsen M, Hugenholtz P, Skarshewski A, Nielsen KL, Tyson GW, Nielsen PH. 2013. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol 31:533–538. doi: 10.1038/nbt.2579. [DOI] [PubMed] [Google Scholar]
- 53.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y, Gilna P, Li W. 2009. Identification of ribosomal RNA genes in metagenomic fragments. Bioinformatics 25:1338–1340. doi: 10.1093/bioinformatics/btp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pruesse E, Peplies J, Glockner FO. 2012. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glockner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM. 2009. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil PA, Hugenholtz P. 2018. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 63.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 65.Laslett D, Canback B. 2004. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res 32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huson DH, Mitra S, Ruscheweyh HJ, Weber N, Schuster SC. 2011. Integrative analysis of environmental sequences using MEGAN4. Genome Res 21:1552–1560. doi: 10.1101/gr.120618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanehisa M, Goto S. 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powell S, Szklarczyk D, Trachana K, Roth A, Kuhn M, Muller J, Arnold R, Rattei T, Letunic I, Doerks T, Jensen LJ, von Mering C, Bork P. 2012. eggNOG v3.0: orthologous groups covering 1133 organisms at 41 different taxonomic ranges. Nucleic Acids Res 40:D284–9. doi: 10.1093/nar/gkr1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marshall KT, Morris RM. 2015. Genome sequence of “Candidatus Thioglobus singularis” strain PS1, a mixotroph from the SUP05 clade of marine gammaproteobacteria. Genome Announc 3:e01155-15. doi: 10.1128/genomeA.01155-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah V, Morris RM. 2015. Genome sequence of “Candidatus Thioglobus autotrophica” strain EF1, a chemoautotroph from the SUP05 clade of marine gammaproteobacteria. Genome Announc 3:e01156-15. doi: 10.1128/genomeA.01156-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. 2012. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res 40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roux S, Enault F, Hurwitz BL, Sullivan MB. 2015. VirSorter: mining viral signal from microbial genomic data. PeerJ 3:e985. doi: 10.7717/peerj.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roux S, Tournayre J, Mahul A, Debroas D, Enault F. 2014. Metavir 2: new tools for viral metagenome comparison and assembled virome analysis. BMC Bioinformatics 15:76. doi: 10.1186/1471-2105-15-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y, Wishart DS. 2016. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res 44:W16–W21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 76.Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NCBI genome accession numbers for the sponge metagenome and the reassembled draft genome at the Crane site are VSKW00000000 and VSKZ00000000, respectively. The NCBI genome accession numbers for the sponge metagenome and the reassembled draft genome at the Swan site are VSKX00000000 and VSKY00000000, respectively.