As previous research suggests a link between microbiota and FE, modulation of the intestinal microbiome may be effective in improving FE in pigs. The FMTp in gestating sows, alone or in combination with postweaning dietary inulin supplementation in offspring, achieved improvements in FE and resulted in a higher relative abundance of intestinal bacteria associated with fiber degradation and a lower relative abundance of potential pathogens. However, there was a detrimental effect on growth, although this may not be wholly attributable to microbiota transplantation, as antibiotic and other interventions were also part of the FMT regimen. Therefore, further work with additional control groups is needed to disentangle the effects of each component of the FMTp in order to develop a regimen with practical applications in pig production. Additional research based on findings from this study may also identify specific dietary supplements for the promotion/maintenance of the microbiota transferred via the maternal FMTp, thereby optimizing pig growth and FE.
KEYWORDS: inoculation, growth, intestinal microbiota, microbial modulation, swine, prebiotic
ABSTRACT
As previous studies have demonstrated a link between the porcine intestinal microbiome and feed efficiency (FE), microbiota manipulation may offer a means of improving FE in pigs. A fecal microbiota transplantation procedure (FMTp), using fecal extracts from highly feed-efficient pigs, was performed in pregnant sows (n = 11), with a control group (n = 11) receiving no FMTp. At weaning, offspring were allocated, within sow treatment, to (i) control (n = 67; no dietary supplement) or (ii) inulin (n = 65; 6-week dietary inulin supplementation) treatments. The sow FMTp, alone or in combination with inulin supplementation in offspring, reduced offspring body weight by 8.1 to 10.6 kg at ∼140 days of age, but there was no effect on feed intake. It resulted in better FE, greater bacterial diversity, and higher relative abundances of potentially beneficial bacterial taxa (Fibrobacter and Prevotella) in offspring. Due to the FMTp and/or inulin supplementation, relative abundances of potential pathogens (Chlamydia and Treponema) in the ileum and cecal concentrations of butyric acid were significantly lower. The maternal FMTp led to a greater number of jejunal goblet cells in offspring. Inulin supplementation alone did not affect growth or FE but upregulated duodenal genes linked to glucose and volatile fatty acid homeostasis and increased the mean platelet volume but reduced ileal propionic acid concentrations, granulocyte counts, and serum urea concentrations. Overall, the FMTp in pregnant sows, with or without dietary inulin supplementation in offspring, beneficially modulated offspring intestinal microbiota (albeit mostly low-relative-abundance taxa) and associated physiological parameters. Although FE was improved, the detrimental effect on growth limits the application of this FMTp-inulin strategy in commercial pig production.
IMPORTANCE As previous research suggests a link between microbiota and FE, modulation of the intestinal microbiome may be effective in improving FE in pigs. The FMTp in gestating sows, alone or in combination with postweaning dietary inulin supplementation in offspring, achieved improvements in FE and resulted in a higher relative abundance of intestinal bacteria associated with fiber degradation and a lower relative abundance of potential pathogens. However, there was a detrimental effect on growth, although this may not be wholly attributable to microbiota transplantation, as antibiotic and other interventions were also part of the FMT regimen. Therefore, further work with additional control groups is needed to disentangle the effects of each component of the FMTp in order to develop a regimen with practical applications in pig production. Additional research based on findings from this study may also identify specific dietary supplements for the promotion/maintenance of the microbiota transferred via the maternal FMTp, thereby optimizing pig growth and FE.
INTRODUCTION
Feed efficiency (FE) is of major importance in pig production, as feed accounts for the majority of the cost associated with producing pigs (1). Previous work from our group and others demonstrated an association between the intestinal microbiota and residual feed intake (RFI) (a metric for FE) in pigs (2–5). It may therefore be possible to improve FE through manipulation of the intestinal microbiota. This could be achieved via fecal microbiota transplantation (FMT) and/or dietary supplementation with feed additives.
To date, the use of FMT in pigs has mainly been limited to the establishment of the human gut microbiota in pigs in order to develop a model for humans (6, 7). However, the pig gut microbiota has also been transferred to rodents (8) and, to a lesser extent, other pigs (9–11). One of the latter studies is from our group and used an inoculum derived from fecal extracts collected from highly feed-efficient pigs, with a view to improving FE (11). The results showed that FMT in pregnant sows and/or their offspring impacted the lifetime growth of offspring, as pigs were ∼4 to 8 kg lighter at slaughter (11). Intestinal microbiota composition and predicted functionality, along with physiological parameters, were also impacted, and overall, the results indicated that FMT may not be a suitable approach to optimize FE in pigs. However, although potentially beneficial FMT-associated modulation of the sow intestinal microbiota occurred, with some evidence of microbiome transfer from FMT-treated sows to their offspring, other bacterial taxa either were not transferred to or did not colonize within the offspring microbiome. Therefore, it is possible that dietary prebiotic supplementation in offspring might provide a substrate for transplanted microbiota, thereby encouraging their proliferation and potentially improving FE.
A prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (12). Inulin is a dietary fiber derived mainly from chicory, which is not digestible by the host (13). It has proven effective as a prebiotic in humans, but in pigs, results have been more contradictory (14). Nonetheless, a number of studies have found beneficial effects of inulin supplementation of pig diets, in terms of both improving growth performance and modulating the intestinal microbiota (15–17). In particular, supplementing weaner diets with inulin may be a useful way to counteract the susceptibility to infection and reduced growth rate associated with the stress of weaning, and a number of studies have demonstrated beneficial modulation of the intestinal microbiota and improved growth, gut health, and FE (16, 18, 19). For example, Grela et al. found that dietary inulin addition improved weight gain, reduced feed intake, and improved FE in pigs between 10 and 84 days of age (15). Inulin is fermented in the lower part of the digestive tract by enzymes produced by certain types of bacteria, resulting in increased production of volatile fatty acids (VFA), mainly butyrate (20). The addition of inulin to the diet of pigs (at various stages throughout their productive life) has been shown to increase bacterial populations considered beneficial in the small and/or large intestine (mainly the latter) while reducing potentially pathogenic bacteria (14, 21). However, a recent meta-analysis showed that although strong negative relationships were found between dietary inulin and colonic enterobacteria throughout all production phases, the same was true for fecal lactobacilli, which are generally considered beneficial in the gut (14).
The hypothesis here was that by promoting the proliferation and persistence of a microbial profile for improved FE early in life, lifetime FE in pigs would improve. The objectives were to determine if FMT, using fecal extracts from highly feed-efficient pigs, in pregnant sows and/or dietary inulin supplementation in offspring postweaning would improve offspring FE and to determine if inulin supplementation would support the survival/growth of any potentially beneficial bacteria transferred to offspring as a result of maternal FMT.
(This research was conducted by U. M. McCormack in fulfillment of the requirements for a Ph.D. from the Waterford Institute of Technology [WIT], Waterford, Ireland, in 2017 [22].)
RESULTS
This study comprised a total of 4 treatments: control sow and control offspring (CON/CON), control sows and inulin-supplemented offspring (CON/INU), fecal microbiota transplant procedure (FMTp)-treated sows and control offspring (FMTp/CON), and FMTp-treated sows and inulin-supplemented offspring (FMTp/INU).
Due to the large number of significant sow-offspring treatment interactions observed, we focused on the effect of sow or offspring treatment, and we have indicated if an interaction was also observed, only if relevant. All significant interactions are summarized in Table S1 in the supplemental material. Although there were several significant differences in the inulin-supplemented offspring at weaning, and these are shown in the relevant tables and figures, they are not outlined here, as inulin was supplemented in the diet only from weaning. In addition, bacterial taxa and predicted functional pathways present at relative abundances of <0.001% are not discussed.
Impact of FMT in sows and/or inulin inclusion in offspring diets on offspring growth.
The effect of the maternal FMTp and postweaning dietary inulin supplementation on offspring lifetime growth is shown in Table 1 and Table S1. At 100 days of age, FMTp/CON pigs (51.2 kg) had a lower body weight than CON/CON (59.0 kg) and CON/INU (58.6 kg) pigs, and offspring from FMTp sows (52.5 kg) were lighter than their control counterparts (58.8 kg) (P < 0.05). At ∼140 days of age (slaughter), FMTp/CON and FMTp/INU offspring were 10.6 and 7.1 kg lighter (P < 0.05), respectively, than control and inulin-supplemented offspring from CON sows (P < 0.05). Due to the sow FMTp, offspring were also lighter at slaughter (P < 0.05). Consequently, the cold-carcass weights of offspring from FMTp sows were 8.9 and 5.1 kg lower (P < 0.05) than those of offspring from CON sows when offspring treatments were the control and inulin, respectively (P < 0.05). FMTp/INU pigs had a greater muscle depth than CON/INU offspring (P < 0.05). No treatment differences were observed for average daily feed intake (ADFI), average daily gain (ADG), and feed conversion efficiency (FCE) or other carcass traits measured in offspring.
TABLE 1.
Effects of fecal microbiota transplantation in sows and/or dietary supplementation of inulin in offspring for 42 days postweaning on pig growth performance and carcass traitsa
| Parameterb | Sow effectc |
Offspring effectd |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean value for group |
SEM | P value | Mean value for group |
SEM | P value | |||
| Control | FMTp | Control | Inulin | |||||
| Wt (kg) | ||||||||
| Birth | 1.50 | 1.30 | 0.893 | 0.85 | 1.39 | 1.41 | 0.898 | 0.99 |
| Weaning | 9.1 | 7.5 | 0.89 | 0.18 | 8.3 | 8.3 | 0.90 | 0.97 |
| Day 100 | 90.5 | 82.7 | 0.89 | <0.001 | 86.3 | 87.0 | 0.90 | 0.41 |
| Day 140 | 104.4 | 95.5 | 0.89 | <0.001 | 99.6 | 100.3 | 0.90 | 0.59 |
| ADFI (g/day) | 1,999 | 1,930 | 29.8 | 0.13 | 1,963 | 1,965 | 27.4 | 0.96 |
| ADG (g/day) | 819 | 814 | 11.1 | 0.63 | 816 | 817 | 10.2 | 0.93 |
| FCE (g/g) | 2.38 | 2.34 | 0.055 | 0.63 | 2.35 | 2.37 | 0.051 | 0.77 |
| RFI (g/day), days 35–140 | 19.5 | −17.4 | 10.96 | 0.05 | −0.07 | 2.19 | 11.25 | 0.88 |
| Carcass traits | ||||||||
| Wt (kg) | 80.5 | 73.5 | 1.15 | 0.01 | 76.6 | 77.5 | 1.15 | 0.54 |
| Kill-out yield (%) | 76.6 | 77.3 | 0.45 | 0.25 | 76.9 | 77.0 | 0.44 | 0.83 |
| Fat depth (mm) | 13.6 | 13.8 | 0.29 | 0.36 | 13.9 | 13.5 | 0.28 | 0.41 |
| Muscle depth (mm) | 52.8 | 53.2 | 0.51 | 0.05 | 53.0 | 53.0 | 0.49 | 0.92 |
| Lean meat yield (%) | 56.6 | 56.5 | 0.25 | 0.93 | 56.4 | 56.7 | 0.25 | 0.47 |
ADFI, average daily feed intake (between weaning and day ∼140 of age); ADG, average daily gain (between weaning and day ∼140 of age); FCE, feed conversion efficiency (between weaning and day ∼140 of age); RFI, residual feed intake.
Least-square means and pooled standard errors of the means are presented. Parameters in boldface type depict a significant sow-offspring interaction (details are given in Table S1 in the supplemental material). Days correspond to days of age.
n = 11 for the control group, and n = 11 for the FMT procedure (FMTp) group.
n = 62 for the control group, and n = 59 for the group that received inulin for the first 6 weeks postweaning.
Offspring from FMTp sows (FMTp/CON and FMTp/INU) had a lower RFI value (better FE) than offspring from CON sows (P < 0.05). This was reflected at the sow treatment level, where pigs from FMTp sows had a lower RFI than those from CON sows (P < 0.05). Inulin supplementation alone, however, did not influence offspring RFI (P > 0.05).
Influence of FMT in sows and/or inulin inclusion in offspring diets on offspring intestinal microbial diversity.
In general, the offspring from FMTp sows had a larger number of operational taxonomic units (OTUs) in the feces in the early postweaning period, whereas inulin-supplemented offspring had a smaller number than their control counterparts (Table S2). This was reflected to some extent in microbial diversity measures (Fig. 1). At 130 days of age, all treatment groups had higher Shannon diversity index values (species abundance and evenness, accounting for rare species) than CON/CON pigs (P < 0.05) (Fig. 1A), and irrespective of offspring treatment, offspring from FMTp sows had a higher Shannon diversity index (4.2) than offspring from CON sows (3.8) (P < 0.05) (data not shown). However, a lower Simpson diversity index (species richness and evenness, which takes into account the number as well as the relative abundance of each species present) was observed in the ileum of inulin-supplemented offspring (0.66) than in control offspring (0.71) (P < 0.05) (Fig. 1B and data not shown).
FIG 1.
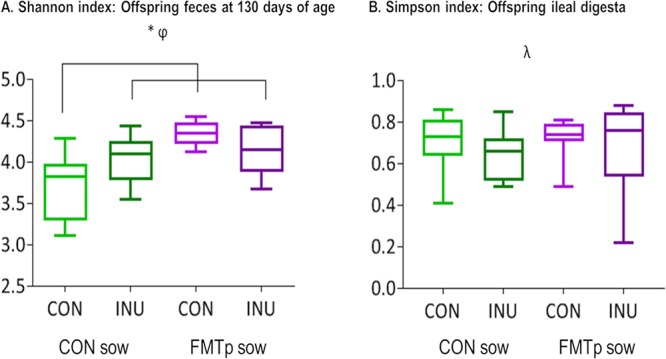
Variations in the Shannon diversity indices of the offspring microbiota in feces at 130 days of age (A) and in the Simpson diversity indices of offspring ileal microbiota as a result of fecal microbiota transplantation (FMT) in sows and/or dietary supplementation with inulin in offspring for 42 days postweaning (B). Data are from 32 pigs (at the sow treatment level, n = 16 for the control [CON] group and n = 16 for the FMT procedure [FMTp] group; at the offspring treatment level, n = 16 for the CON group and n = 16 for the inulin [INU] treatment group). * indicates significant differences at the sow × offspring treatment level (P ≤ 0.05), φ indicates a sow treatment effect (P ≤ 0.05), and λ indicates an offspring treatment effect (P ≤ 0.05).
Microbial β-diversity was also measured in all fecal and intestinal samples and is depicted from OTUs using principal-component analysis (PCA) plots using a Euclidean distance metric, which is calculated from regularized log-transformed counts and plotted using ggplot2 (Fig. S1). Throughout the lifetime of the pigs, there was an influence of sow treatment on offspring microbial diversity, with offspring from FMTp sows clustering away from offspring born to CON sows, in the feces at weaning (R2, 0.45; P < 0.05) and at 130 days of age (just prior to slaughter) (R2, 0.32) (P < 0.05). On day 65 (R2, 0.55) and day 130 (R2, 0.15), dietary supplementation with inulin led to a clustering effect in the feces (P < 0.05). Although there were no significant differences in the ileum, CON/CON and FMTp/CON offspring clustered separately for the cecum (R2, 0.51; P < 0.05), and CON/INU and FMTp/CON offspring clustered away from CON/CON offspring for the colon (R2, 0.53; P < 0.05).
Effect of the FMTp in sows and/or inulin supplementation in offspring on offspring intestinal microbial composition.
The microbial composition, at the phylum and genus levels, was investigated in offspring feces throughout their lifetime and in the intestinal digesta collected at slaughter. The numbers of OTUs present at each sampling time point/in each digesta type were as follows: 542 at weaning, 347 on day 50 of age, 75 on day 65, 531 on day 100, and 585 on day 130, 66 in the ileum, 361 in the cecum, and 456 in the colon. The composition at the phylum level for feces and digesta samples is shown in Fig. S2. The number of phyla detected varied over time: 12 were detected in the feces at weaning, 8 were detected at day 50 of age, 6 were detected at day 65 of age, 15 were detected at day 100 of age, and 14 were detected at day 130 of age, with 7 detected in the ileum and 7 detected in both the cecum and colon, respectively. However, many were detected at a very low relative abundance.
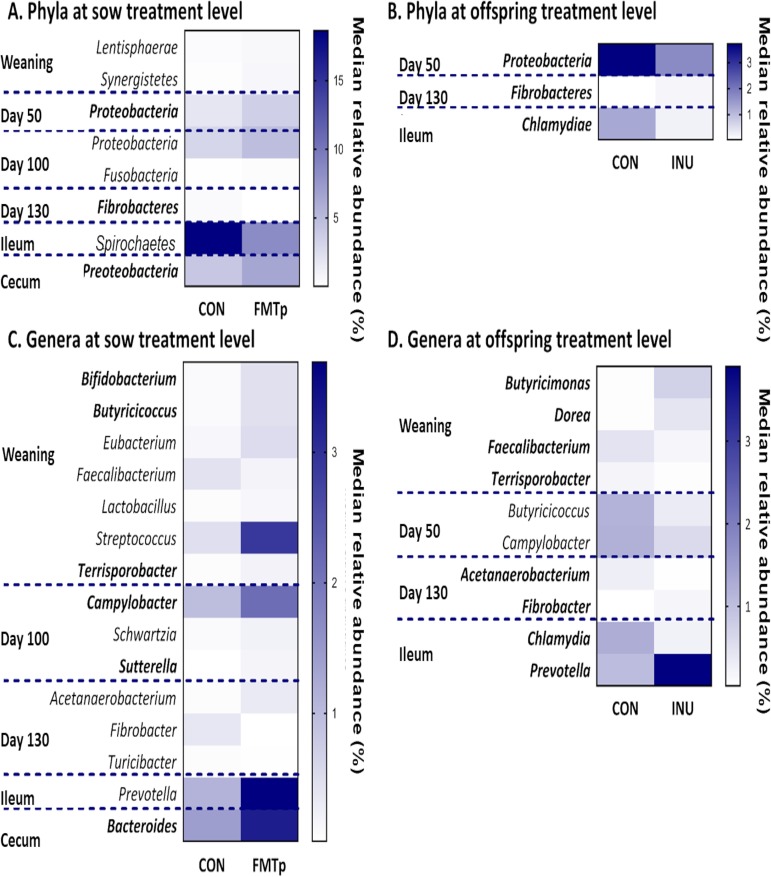
Totals of 8 phyla and 25 genera differed significantly between treatments, and these ranged in relative abundance from 0.004 to 18.6% and from 0.002 to 18.0%, respectively, but were mainly present at low relative abundances. Five phyla and 19 genera differed due to a sow × offspring treatment interaction, 6 phyla and 15 genera differed due to sow treatment, and 3 phyla and 10 genera differed due to offspring treatment. All bacterial taxa reported below are significantly different (P < 0.05) and are reported in Fig. 2 and Table S1.
FIG 2.
Effects of fecal microbiota transplantation in sows and/or dietary supplementation of inulin in offspring for 42 days postweaning on median relative abundances (percent) of bacterial phyla in feces and digesta of offspring at the sow treatment level (A) and the offspring treatment level (B) and of bacterial genera at the sow treatment level (C) and the offspring treatment level (D). Data are from 32 pigs (at the sow treatment level, n = 16 for the CON group and n = 16 for the FMTp group; at the offspring treatment level, n = 16 for the CON group and n = 16 for the INU group). Heat maps are split by relative abundance, with higher-abundance phyla/genera shown in the top heat maps and lower-abundance phyla/genera shown in the bottom heat maps. Phyla and genera in boldface type depict those also affected by a sow × offspring treatment interaction. Additional sow treatment × offspring treatment interactions not shown in either panel A, B, C, or D are shown in Table S1 in the supplemental material.
At weaning, the relative abundances of Lentisphaerae and Synergistetes were higher in offspring due to the FMTp in sows. Proteobacteria were impacted throughout the lifetime of the pigs, mainly due to sow treatment. In the feces collected at day 50, the FMTp in sows resulted in a higher relative abundance of Proteobacteria in the offspring, but the relative abundance of this phylum was lower due to inulin supplementation. This FMTp effect was also observed for the feces collected on day 100 and for the cecum as well. On day 100, the relative abundance of Fusobacteria was higher in offspring from FMTp sows, and 30 days later, Fibrobacteres were present at a lower relative abundance due to the FMTp but were present at a higher relative abundance due to inulin supplementation. In the ileum, the relative abundance of Spirochaetes was lower in offspring due to the FMTp in sows. Furthermore, the relative abundance of Chlamydiae was lower in all groups than in CON/CON offspring and was also reduced due to dietary inulin supplementation.
Most of the treatment differences at the genus level occurred in the feces at weaning or just prior to slaughter, at day 130 of age, and in the ileal digesta at slaughter. Apart from Sphaerochaeta (day 130 of age), all of the differences observed were for genera present at a <5% relative abundance. Throughout the lifetime of the pigs, several bacterial genera were impacted at more than one fecal time point as well as in the digesta collected at slaughter, with a strong effect of sow treatment observed over time.
At weaning, due to the FMTp in sows, the relative abundance of Faecalibacterium was lower in offspring, whereas that of Streptococcus was higher. Additionally, the relative abundances of Bifidobacterium, Butyricimonas, Eubacterium, Lactobacillus, and Terrisporobacter were higher due to the FMTp in sows. In the feces collected between days 28 and 130 of age, a number of bacterial genera were impacted: 10 due to an interaction effect, 6 due to sow treatment, and 4 due to offspring treatment. All impacted genera were present at a relative abundance of <5%, except for Sphaerochaeta. At 50 days of age, the relative abundances of Butyricicoccus and Campylobacter were lower due to inulin supplementation. At 100 days of age, the relative abundance of Campylobacter was higher in FMTp/CON offspring than in offspring from CON sows, and this was reflected at the sow treatment level also. The relative abundance of Sutterella was also higher due to all interventions than that in CON/CON pigs and was also higher due to the FMTp in sows. Due to the FMTp in sows, Schwartzia was present at a higher relative abundance in offspring. Thirty days later (at ∼130 days of age, just prior to slaughter), the relative abundance of Acetanaerobacterium was higher in FMTp/CON than in CON/INU pigs, and pigs from FMTp sows also had a higher relative abundance, but inulin supplementation lowered the relative abundance. In addition, the relative abundance of Fibrobacter was lower in FMTp/CON offspring than in all other groups, and offspring from FMTp sows also had a lower relative abundance of this genus, but INU pigs had a higher relative abundance. Due to the FMTp in sows, Turicibacter was present at a lower relative abundance in offspring than in those from CON sows.
In the ileum, the relative abundance of Prevotella was higher, whereas that of Chlamydia was lower, in all groups than in CON/CON pigs. Prevotella was relatively more abundant, and Chlamydia was less so, due to inulin supplementation. Additionally, the relative abundance of Prevotella was higher due to the FMTp in sows also. In the cecum, Bacteroides was relatively more abundant in the FMTp/CON group than in all other groups, and offspring born to FMTp sows also showed a higher relative abundance of this genus.
Effect of the FMTp in sows and/or supplementation of inulin in offspring on predicted functionality of the offspring intestinal microbiota.
The functionality of the intestinal microbiota was predicted for all offspring fecal and digesta samples, and significant differences are shown in Fig. S3. A total of 26 predicted bacterial pathways in offspring were significantly impacted due to an interaction (Table S1). As a result of the FMTp in sows, 10 pathways were altered in the offspring, and these were mostly related to lipid metabolism, carbohydrate metabolism, and xenobiotic degradation and metabolism (Fig. S3). Due to dietary inulin supplementation in offspring (Fig. S3), 14 predicted pathways, mostly related to carbohydrate metabolism and glycan biosynthesis and metabolism, were impacted. Overall, most of the effects were seen within the ileal microbiota. All pathways that were significantly influenced by FMTp/inulin supplementation were present at a <2.0% relative abundance.
At 70 days of age, the alpha-linolenic acid metabolism pathway was predicted to be present at a lower relative abundance due to inulin supplementation. In the ileum, the relative abundances of porphyrin and chlorophyll metabolism and seleno-compound metabolism were lower due to both intervention strategies, whereas the predicted relative abundance of the glycosphingolipid biosynthesis-ganglio series pathway was higher. The combination of the FMTp and inulin supplementation resulted in a higher predicted relative abundance of the glycosphingolipid biosynthesis-globo series pathway than in CON/INU offspring (Table S1), and inulin-supplemented offspring also had a higher relative abundance than their CON counterparts. Additionally, FMTp/INU resulted in a higher predicted relative abundance of a pathway involved in the biosynthesis of ansamycins than in CON/INU offspring, and offspring from FMTp sows also had a higher relative abundance of this pathway. The FMTp resulted in a higher predicted relative abundance of ether lipid metabolism than in offspring from CON sows. The relative abundance of the secondary bile acid biosynthesis pathway was higher due to either or both interventions. Due to the FMTp in sows, the predicted relative abundance of the phenylalanine metabolism pathway was lower, but those of the bisphenol degradation and linoleic acid metabolism pathways were higher, than in offspring from CON sows. Dietary supplementation of inulin in weaner offspring resulted in higher predicted relative abundances of two pathways related to glycan biosynthesis and phenylpropanoid biosynthesis but resulted in a lower relative abundance of the butanoate metabolism pathway.
In the cecum, FMTp/CON offspring had higher relative abundances of fructose and mannose metabolism pathways but a lower relative abundance of the d-alanine metabolism pathway than in the other three groups, and the sow FMTp resulted in higher and lower predicted relative abundances of these pathways, respectively, whereas the opposite occurred due to inulin supplementation.
Effect of the FMTp in sows and/or supplementation of inulin in offspring on offspring intestinal volatile fatty acid concentrations.
Volatile fatty acid concentrations were measured in digesta from the ileum, cecum, and colon of the 32 selected offspring, and results are shown in Table 2 and Table S1. No differences were observed between treatments for digesta pH in any of the intestinal segments. In the ileum, offspring from the FMTp/INU group had higher concentrations of acetic acid than those of the other groups, and CON/INU offspring had lower propionic acid concentrations than those of CON/CON offspring (P < 0.05), and this VFA was also reduced in inulin-fed offspring (P < 0.05).
TABLE 2.
Effects of fecal microbiota transplantation in sows and/or dietary supplementation of inulin in offspring for 42 days postweaning on pH and volatile fatty acid concentrations in the intestinal digestaa
| Parameter | Sow effect |
Offspring effect |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean value for group |
SEM | P value | Mean value for group |
SEM | P value | |||
| Control | FMTp | Control | Inulin | |||||
| pH | ||||||||
| Ileum | 6.5 | 6.5 | 0.10 | 0.75 | 6.5 | 6.5 | 0.10 | 0.76 |
| Cecum | 5.8 | 5.8 | 0.10 | 0.67 | 5.9 | 5.7 | 0.10 | 0.32 |
| Colon | 5.9 | 5.9 | 0.10 | 0.88 | 5.8 | 6.0 | 0.10 | 0.40 |
| VFA concn (μmol/g digesta) | ||||||||
| Total | ||||||||
| Ileum | 26.1 | 28.0 | 2.05 | 0.52 | 27.5 | 26.6 | 2.08 | 0.76 |
| Cecum | 130.2 | 128.2 | 9.85 | 0.89 | 132.4 | 126.1 | 9.92 | 0.66 |
| Colon | 99.5 | 94.8 | 7.45 | 0.66 | 102.1 | 92.4 | 7.46 | 0.36 |
| Acetic acid | ||||||||
| Ileum | 12.8 | 13.1 | 1.14 | 0.85 | 12.5 | 13.5 | 1.15 | 0.52 |
| Cecum | 44.8 | 46.8 | 4.03 | 0.73 | 48.0 | 43.7 | 4.06 | 0.46 |
| Colon | 41.7 | 37.4 | 3.47 | 0.39 | 41.0 | 38.1 | 3.45 | 0.55 |
| Propionic acid | ||||||||
| Ileum | 3.15 | 3.97 | 0.563 | 0.28 | 4.7 | 2.7 | 0.58 | 0.01 |
| Cecum | 42.27 | 43.51 | 6.282 | 0.89 | 44.7 | 41.2 | 6.29 | 0.69 |
| Colon | 37.83 | 33.99 | 5.258 | 0.61 | 37.3 | 34.5 | 5.26 | 0.71 |
| Butyric acid | ||||||||
| Ileum | 4.06 | 4.29 | 0.836 | 0.85 | 3.86 | 4.52 | 0.838 | 0.57 |
| Cecum | 9.64 | 3.24 | 1.327 | <0.001 | 8.10 | 3.86 | 1.229 | 0.01 |
| Colon | 6.54 | 4.35 | 1.089 | 0.15 | 5.41 | 5.26 | 1.065 | 0.92 |
| Valeric acid | ||||||||
| Ileum | 1.78 | 1.61 | 0.168 | 0.48 | 1.74 | 1.65 | 0.170 | 0.69 |
| Cecum | 7.38 | 5.56 | 0.641 | 0.04 | 6.35 | 6.46 | 0.634 | 0.89 |
| Colon | 7.74 | 6.77 | 0.718 | 0.34 | 7.06 | 7.41 | 0.717 | 0.73 |
| Isobutyric acid | ||||||||
| Ileum | 2.18 | 2.64 | 0.509 | 0.52 | 2.67 | 2.16 | 0.511 | 0.47 |
| Cecum | 22.60 | 23.40 | 4.821 | 0.91 | 20.39 | 25.92 | 4.827 | 0.42 |
| Colon | 4.16 | 9.40 | 1.489 | 0.01 | 7.74 | 5.05 | 1.339 | 0.15 |
| Isovaleric acid | ||||||||
| Ileum | 1.32 | 1.67 | 0.176 | 0.19 | 1.47 | 1.51 | 0.175 | 0.86 |
| Cecum | 2.66 | 2.76 | 0.345 | 0.83 | 3.58 | 2.06 | 0.368 | 0.003 |
| Colon | 1.48 | 1.69 | 0.186 | 0.45 | 1.50 | 1.67 | 0.190 | 0.51 |
Data are from 32 pigs (at the sow treatment level, n = 16 for the CON group and n = 16 for the FMTp group; at the offspring treatment level, n = 16 for the CON group and n = 16 for the INU group). Standard errors of the means are depicted. The intestinal segments shown in boldface type represent those at which the indicated VFA was also impacted due to a sow × offspring interaction (details are given in Table S1 in the supplemental material).
In the cecum, butyric acid concentrations were lower for the FMTp/INU group than for all other groups and for the FMTp/CON group than for both treatment groups of offspring from control sows (P < 0.05). They were was also lower due to the FMTp in sows (P < 0.05) and inulin supplementation in offspring (P < 0.05). Moreover, cecal valeric acid concentrations were lower in the FMTp/INU group than in all other groups, but CON/INU pigs had a higher concentration than control offspring, regardless of sow treatment (P < 0.05). Due to the sow FMTp, valeric acid concentrations were also lower (P < 0.05). However, isovaleric acid concentrations were higher in the FMTp/CON group but lower in the FMTp/INU group than in all other groups (P < 0.05) and were also lower due to inulin treatment (P < 0.05). In the colon, isobutyric acid concentrations were higher in FMTp/CON pigs than in all other groups (P < 0.05) and higher due to the FMTp in sows (P < 0.05).
Influence of the FMTp in sows and/or inulin supplementation in offspring on offspring intestinal histology.
Data from histological analyses of the offspring small intestine (duodenum, jejunum, and ileum) are shown in Table 3 and Table S1. In the duodenum, none of the parameters measured differed between groups. However, FMTp offspring had a higher number of goblet cells per villus than their control counterparts (P < 0.05), and the FMTp/CON group had a higher number of jejunal goblet cells (per villus and per micrometer of villus height) than the CON/CON group; this was also observed due to the FMTp in sows (P < 0.05). The FMTp in sows resulted in shorter ileal villi and a smaller villus area than for CON sows (P < 0. 05).
TABLE 3.
Effects of fecal microbiota transplantation in sows and/or dietary supplementation of inulin in offspring for 42 days postweaning on intestinal histologya
| Parameter | Sow effect |
Offspring effect |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean value for group |
SEM | P value | Mean value for group |
SEM | P value | |||
| Control | FMTp | Control | Inulin | |||||
| Villus height | ||||||||
| Duodenum | 469 | 483 | 10.1 | 0.34 | 472 | 480 | 10.1 | 0.56 |
| Jejunum | 192 | 191 | 10.3 | 0.93 | 192 | 190 | 10.3 | 0.91 |
| Ileum | 463 | 425 | 10.1 | 0.001 | 438 | 451 | 10.0 | 0.36 |
| Villus width | ||||||||
| Duodenum | 163 | 162 | 4.1 | 0.78 | 163 | 161 | 4.0 | 0.77 |
| Jejunum | 28 | 27 | 4.0 | 0.85 | 26 | 29 | 4.0 | 0.58 |
| Ileum | 162 | 162 | 4.2 | 0.42 | 160 | 160 | 4.1 | 0.98 |
| Villus area | ||||||||
| Duodenum | 1,024 | 1,056 | 22.1 | 0.32 | 1,031 | 1,049 | 22.1 | 0.56 |
| Jejunum | 1,201 | 1,191 | 22.5 | 0.76 | 1,199 | 1,193 | 22.4 | 0.84 |
| Ileum | 1,046 | 965 | 22.0 | 0.01 | 9,92 | 1,019 | 22.1 | 0.39 |
| Crypt depth | ||||||||
| Duodenum | 457 | 415 | 20.5 | 0.14 | 446 | 426 | 20.5 | 0.49 |
| Jejunum | 121 | 117 | 20.9 | 0.87 | 122 | 116 | 20.8 | 0.82 |
| Ileum | 329 | 332 | 20.6 | 0.91 | 353 | 308 | 20.6 | 0.12 |
| Villus height/crypt depth | ||||||||
| Duodenum | 1.09 | 1.24 | 0.097 | 0.28 | 1.16 | 1.18 | 0.098 | 0.89 |
| Jejunum | 1.64 | 1.75 | 0.100 | 0.44 | 1.64 | 1.75 | 0.100 | 0.44 |
| Ileum | 1.49 | 1.32 | 0.098 | 0.23 | 1.28 | 1.52 | 0.099 | 0.09 |
| No. of goblet cells per villus | ||||||||
| Duodenum | 36 | 37 | 1.2 | 0.51 | 37 | 36 | 1.2 | 0.71 |
| Jejunum | 26 | 31 | 1.3 | 0.01 | 29 | 28 | 1.3 | 0.55 |
| Ileum | 33 | 32 | 1.2 | 0.79 | 31 | 33 | 1.2 | 0.13 |
| No. of goblet cells/μm villus height | ||||||||
| Duodenum | 0.08 | 0.08 | 0.004 | 0.93 | 0.08 | 0.07 | 0.004 | 0.60 |
| Jejunumb | 0.13 | 0.016 | 0.004 | <0.001 | 0.15 | 0.05 | 0.004 | 0.93 |
| Ileum | 0.07 | 0.07 | 0.004 | 0.47 | 0.07 | 0.07 | 0.004 | 0.72 |
Data are from 32 pigs (at the sow treatment level, n = 16 for the CON group and n = 16 for the FMTp group; at the offspring treatment level, n = 16 for the CON group and n = 16 for the INU group). Standard errors of the means are depicted.
This was also impacted due to a sow × offspring interaction (details are given in Table S1 in the supplemental material).
Influence of the FMTp in sows and/or supplementation of inulin in offspring on offspring brush border enzyme activity and gene expression in the duodenum.
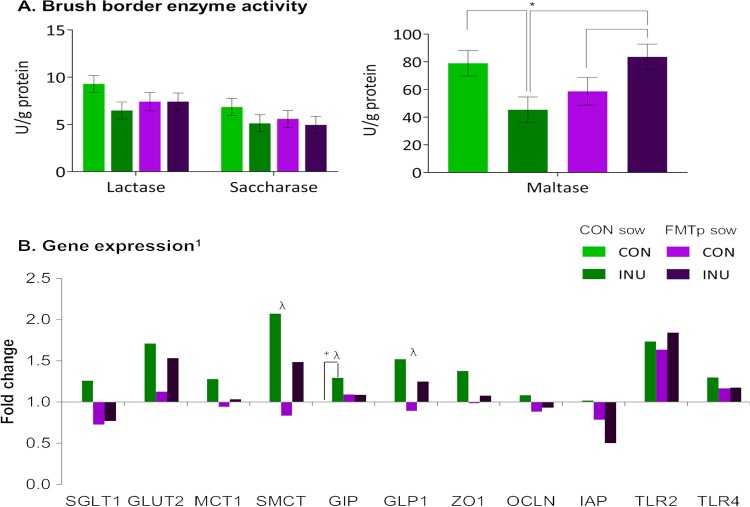
Disaccharidase activity in the duodenum of offspring at slaughter (∼140 days old) is shown in Fig. 3A. Only maltase activity was impacted by a sow-offspring treatment interaction, where CON/INU offspring had lower activity than CON/CON and FMTp/INU offspring, and the latter had higher activity than FMTp/CON offspring (P < 0.05). No differences at the sow or offspring treatment level were observed (P > 0.05).
FIG 3.
Effects of fecal microbiota transplantation in sows and/or dietary supplementation of inulin in offspring for 42 days postweaning on brush border enzyme activity (A) and expression of 11 selected genes in the duodenal mucosa of 140-day-old offspring (B). Data are from 32 pigs (at the sow treatment level, n = 16 for the CON group and n = 16 for the FMTp group; at the offspring treatment level, n = 16 for the CON group and n = 16 for the INU group). * indicates significant differences at the sow × offspring treatment level (P ≤ 0.05), and λ indicates an offspring treatment effect (P ≤ 0.05). 1, bars represent log10-fold changes relative to values for control sow × control offspring treatment after normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), beta-actin (ACTB), and beta-2-microglobulin (B2M) gene expression levels. Candidate genes measured were those encoding sodium-dependent glucose transporter 1 (SGLT1), monocarboxylate transporter 1 (MCT1), sodium-coupled monocarboxylate transporter (SMCT), intestinal alkaline phosphatase (ALPi), tight-junction proteins (zona occludens 1 [ZO1] and occludin [OCLN]), Toll-like receptor 2 (TLR2), TLR4, facilitated glucose transporter member 2 (GLUT2), glucose-dependent insulinotropic peptide (GIP), and glucagon-like peptide 1 (GLP1). Genes with expression levels affected by offspring treatment are as follows: GLP1 (CON, 0.94-fold change; INU, 1.38-fold change), GIP (CON, 1.05-fold change; INU, 1.19-fold change), SMCT (CON, 0.91-fold change; INU, 1.77-fold change), and ZO1 (CON, 0.99-fold change; INU, 1.23-fold change) (P = 0.06).
The expression levels of 3 of the 11 genes measured in the duodenum were impacted as follows (Fig. 3B). Upregulation of the glucose-dependent insulinotropic peptide gene (GIP) was observed in CON/INU compared to CON/CON offspring, and this was also observed in inulin-supplemented compared to control offspring (P < 0.05). In addition, the glucagon-like peptide 1 (GLP1) and sodium-coupled monocarboxylate transporter (SMCT) genes were upregulated in inulin-supplemented offspring compared to their control counterparts (P < 0.05).
Influence of the FMTp in sows and/or supplementation of inulin in offspring on offspring blood parameters.
The results of offspring hematological analysis at slaughter are shown in Table 4 and Table S1. White blood cell counts were lower in CON/INU than in CON/CON offspring (P < 0.05), and the hemoglobin concentration was higher in FMTp/INU than in FMTp/CON offspring (P < 0.05). Both granulocyte percentage (54 versus 64%) and number (11 versus 17) were lower in inulin-supplemented than in control offspring (P < 0.05), but the platelet volume was higher (10.3 versus 9.5 fl; P < 0.05). In addition, the mean corpuscular hemoglobin percentage was lower in offspring from FMTp sows than in their control counterparts (17.8 versus 18.8%; P < 0.05).
TABLE 4.
Effects of fecal microbiota transplantation in sows and/or dietary supplementation of inulin in offspring for 42 days postweaning on hematological and blood biochemical parameters in pigsa
| Parameter | Sow effectb |
Offspring effectc |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean value for group |
SEM | P value | Mean value for group |
SEM | P value | |||
| Control | FMTp | Control | Inulin | |||||
| No. of white blood cells (103 cells/μl) | 25.0 | 25.5 | 1.019 | 0.78 | 26.6 | 23.9 | 1.10 | 0.09 |
| Lymphocytes | ||||||||
| % | 35.7 | 33.9 | 1.93 | 0.52 | 32.5 | 37.1 | 1.93 | 0.11 |
| No. (103 cells/μl) | 8.4 | 8.5 | 0.42 | 0.95 | 8.6 | 8.3 | 0.42 | 0.64 |
| Monocytes | ||||||||
| % | 3.8 | 2.8 | 0.47 | 0.16 | 3.2 | 3.4 | 0.46 | 0.69 |
| No. (103 cells/μl) | 0.91 | 0.74 | 0.142 | 0.39 | 0.84 | 0.81 | 0.141 | 0.90 |
| Granulocytes | ||||||||
| % | 60.9 | 7.4 | 2.58 | 0.35 | 64.3 | 54.0 | 2.60 | 0.01 |
| No. (103 cells/μl) | 15.4 | 13.7 | 1.05 | 0.26 | 17.1 | 11.9 | 1.04 | 0.001 |
| No. of red blood cells (106 cells/μl) | 7.4 | 7.3 | 0.14 | 0.73 | 7.4 | 7.2 | 0.14 | 0.52 |
| Red cell distribution width (fl) | 19.1 | 20.3 | 0.57 | 0.14 | 19.7 | 19.7 | 0.57 | 0.93 |
| Hemoglobin level (g/dl) | 13.8 | 13.4 | 0.27 | 0.31 | 13.3 | 13.8 | 0.27 | 0.16 |
| Hematocrit (%) | 0.42 | 0.39 | 0.010 | 0.13 | 0.41 | 0.40 | 0.010 | 0.87 |
| Corpuscular vol (fl) | 56.6 | 54.9 | 0.71 | 0.11 | 55.3 | 56.3 | 0.71 | 0.37 |
| Corpuscular hemoglobin | ||||||||
| % | 18.8 | 17.8 | 0.32 | 0.03 | 18.0 | 18.6 | 0.31 | 0.16 |
| Concn (pg) | 32.9 | 32.1 | 0.35 | 0.09 | 32.3 | 32.8 | 0.35 | 0.34 |
| No. of platelets (106 cells/μl) | 257 | 256 | 27.9 | 0.98 | 274 | 240 | 28.0 | 0.42 |
| Platelet vol (fl) | 9.8 | 9.9 | 0.20 | 0.63 | 9.5 | 10.3 | 0.20 | 0.01 |
| Blood urea nitrogen concn (mg/dl) | 15.5 | 11.9 | 1.92 | 0.20 | 16.3 | 11.1 | 1.92 | 0.06 |
| Total protein concn (g/liter) | 66.3 | 54.9 | 6.57 | 0.23 | 58.1 | 62.9 | 6.57 | 0.60 |
| Triglyceride concn (mmol/liter) | 0.46 | 0.47 | 0.039 | 0.91 | 0.44 | 0.49 | 0.040 | 0.32 |
| Glucose concn (mmol/liter) | 4.9 | 5.0 | 0.52 | 0.91 | 5.1 | 4.9 | 0.51 | 0.79 |
| Cholesterol concn (mmol/liter) | 2.74 | 2.34 | 0.266 | 0.29 | 2.75 | 2.33 | 0.265 | 0.28 |
| Creatine concn (μmol/liter) | 147 | 129 | 11.9 | 0.28 | 142 | 135 | 11.9 | 0.66 |
| Creatinine kinase concn (μmol/liter) | 75.2 | 34.3 | 11.9 | 0.26 | 63.1 | 46.5 | 11.9 | 0.39 |
Least-square means and pooled standard errors of the mean are presented.
n = 11 for the control group, and n = 11 for the FMTp group.
n = 16 for the control group, and n = 16 for the inulin group for the first 6 weeks postweaning.
Of all the serum biochemical parameters measured in offspring at slaughter (Table 4 and Table S1), only cholesterol and urea concentrations were impacted. The cholesterol concentration tended to be lower in both treatment groups of offspring from FMTp sows than in the CON/CON group (P = 0.07), whereas the blood urea nitrogen concentration tended to be lower due to inulin supplementation in offspring (11.1 versus 16.3 mg/dl; P = 0.06).
DISCUSSION
Beneficial modulation of the intestinal microbiota may result in improved intestinal health and nutrient utilization and, ultimately, improved growth and FE in pigs. Prebiotics, most notably inulin, have been studied in pigs in order to achieve this (14, 21, 23, 24). Microbiota transplantation may also be a useful approach, as it has been shown to transfer host physiological traits, such as leanness, obesity, and immunological and gut characteristics, via “reprogramming” of the intestinal microbiota (10, 25–28). However, previous work from our group showed a depression of offspring body weight at slaughter as a result of FMT in sows and/or offspring (11). Modulation of the intestinal microbiota also occurred in pregnant sows receiving the FMTp, with some evidence of microbiome transfer from the FMT-treated sows to their offspring. However, other bacterial taxa either were not transferred to or did not colonize the offspring, and so here we tested the hypothesis that dietary prebiotic (inulin) supplementation in offspring might provide a substrate for transplanted microbiota, thereby encouraging their proliferation.
Results showed that pigs born to FMTp sows (irrespective of postweaning treatment) were ∼8.9 kg lighter at slaughter but were more feed efficient, given their lower RFI value. No improvements in weight gain or, indeed, FE were observed due to inulin inclusion in postweaning diets alone, which is contradictory to the findings of some previous studies (15) but in agreement with others (14, 29). However, FE was improved when inulin was supplemented in the diet of weaner pigs born to FMTp sows, and although body weight was reduced, it may have a role in promoting the proliferation of beneficial bacterial populations implanted as a result of modulation of the maternal microbiota. In some cases, there was an impact of the FMTp and/or inulin supplementation on offspring bacterial diversity, with a significant clustering effect occurring within sample time points. However, although significant, the R2 values are low, and therefore, these findings should be interpreted with caution. Due to the complexity of the FMTp employed in the present study, it could be argued that the negative effects on pig weight were due to in utero effects of the antibiotic, the purgative, and/or the proton pump inhibitor administered to sows as part of the regimen or due to the fasting period, as control animals were not given the same regimen. However, none of these interventions were applied to FMT-treated offspring in a related study of ours (11), and similar FMT-associated weight reductions were observed. Nonetheless, further studies with additional control groups are warranted in order to fully elucidate the potential impact of these confounding factors on the offspring microbiome.
Higher relative abundances of bacteria deemed “beneficial” for host health were observed in offspring feces due to either the FMTp in sows (most pronounced) or inulin supplementation in offspring. However, for inulin treatment, all of these were at weaning, which is meaningless, as inulin supplementation only commenced at that point, highlighting the importance of biological versus statistical significance. However, in later life, some bacterial populations considered potentially beneficial were relatively more abundant in offspring from FMTp sows supplemented with inulin than in their unsupplemented counterparts, for example, Fibrobacter, which is a fiber degrader (30). In the ileum, Prevotella relative abundance was increased by both FMT in sows and inulin supplementation in offspring, and this is a key genus in pigs, previously associated with weight gain (30) but also with poor FE (3). However, weight gain was not observed in the present study, and FE was improved in offspring, highlighting the difficulty in relating shifts in taxonomic composition to true functional differences. In general, treatment effects were more evident within the fecal microbiome of pigs at the end of the finishing period, at 100 and 130 days of age, even though inulin was removed from the diet 30 to 60 days prior to this and the FMTp was performed on the sows only, demonstrating that the effects of both treatments persisted throughout the productive life of the pig. While the exact mechanism by which the sow FMT influences the offspring gut microbiome is unknown, it is most likely through altered microbiome exposure both pre- and postbirth. Evidence of the effects of prebirth exposure comes from the fact that the microbiome of the offspring of sows administered FMT during gestation only (FMT/CON) differs from that of the offspring of sows not administered FMT (CON/CON). There will also likely be residual effects on the microbiome of these pigs postbirth as a result of exposure to the altered intestinal and colostrum microbiome of FMTp-treated sows (information on the sow microbiome is presented in our related publication [11]). Indeed, there was some evidence of microbiome transfer from the FMT-treated sows to their offspring. Additional evidence of the influence of postbirth effects on the microbiome also comes from this associated study, which showed that offspring from untreated mothers administered FMT themselves have an altered microbiome (11).
At the genus level, the cellulolytic genus Fibrobacter was present at a lower relative abundance in offspring due to maternal FMT but was more abundant due to offspring inulin supplementation in the feces just prior to slaughter. However, the opposite was true for Bacteroides, a genus known to be hemicellulolytic, for which the relative abundance increased in the cecum of offspring as a result of FMT in sows. Interestingly, Bacteroides was previously found to be associated with better FE in finisher pigs (4); thus, the higher relative abundance of Bacteroides in the cecum of offspring from FMTp sows may explain the improved FE observed in these animals in the present study.
The effect of the combination of maternal FMT and inulin supplementation on offspring microbiota was evident throughout this study, in terms of not only composition but also potential function, most notably carbohydrate and lipid metabolism. In agreement with the fact that inulin is a plant storage glycan, the microbiota of inulin-supplemented offspring had enhanced predicted relative abundances of glycan biosynthesis and metabolism pathways and lower relative abundances of other carbohydrate metabolism pathways. However, a concomitant increase in VFA concentrations was not observed, in contrast to previous findings in humans (31).
Genes involved in glucose homeostasis, in particular the secretion of insulin, such as GIP and GLP1, were present at a higher relative abundance in the duodenum of inulin-supplemented pigs. This is likely indicative of inulin fermentation in the upper gastrointestinal tract (GIT), or perhaps a compensatory mechanism for nutrient digestion in the small intestine, potentially leading to a better metabolic capability of inulin-supplemented pigs. Furthermore, greater utilization of protein/nitrogen by the microbiota may have occurred, as indicated by lower serum urea concentrations in inulin-fed offspring (32). Inulin has also been linked with possible lipid-modulatory effects in humans and piglets (15, 33), which is in accordance with the reduced serum cholesterol concentrations found in the present study. Furthermore, the reduced cholesterol concentration observed may be due to the higher ileal concentrations of acetic acid, as dietary acetic acid has been found to reduce serum cholesterol in rats (34).
Inulin has been shown to modulate not only growth and FE but also immunological features in pigs (15). Interestingly, white blood cell and granulocyte counts decreased due to the FMTp in sows and/or inulin supplementation in offspring, and the lower counts of these immune cells may be linked to the lower relative abundance of potential pathogens (Campylobacter and Chlamydia) observed in the feces and digesta of these pigs. This in turn may be linked to the higher relative abundance of lactic acid bacteria in these animals, as these are known to reduce pathogens in the GIT (35). Moreover, offspring from FMT sows may have overenhanced mucin production in the small intestine, as more goblet cells were present in the jejunum, and mucin is a physical barrier that prevents pathogen adherence to the epithelial lining (36).
Conclusions.
We provide evidence that maternal FMT alone or in combination with dietary inulin supplementation in offspring, as a strategy to modulate the intestinal microbiota of pigs, has a beneficial impact on FE but a detrimental effect on body weight throughout the pigs’ productive lifetime. These effects were accompanied by influences on both intestinal microbiota composition and predicted functionality in the offspring. Although dietary supplementation with inulin alone had a similar impact on the intestinal microbiota, the effects were not as pronounced, and improvements in offspring growth or FE were not observed. Bacterial taxa considered potentially beneficial, such as Prevotella, albeit mainly present at a low relative abundance, were increased in the offspring, mainly due to FMT. Dietary inulin supplementation in offspring from FMTp sows led to a higher relative abundance of Fibrobacter than in their nonsupplemented counterparts, suggesting a possible role of inulin in supporting maternally derived microbiota in the offspring. Pigs supplemented with inulin had lower levels of blood urea nitrogen and granulocytes, indicating an improved health status. Taken together, the hematological, biochemical, and gene expression data suggest improved health in offspring from FMT-treated sows and/or those supplemented with inulin. Overall, the results from this study show that the maternal FMT regimen used in the present study, either alone or in combination with postweaning inulin supplementation, is not suitable for use in pig production, due to the detrimental impact on lifetime growth. However, possible in utero effects of the antibiotic and other interventions used as part of the FMT regimen cannot be ruled out, and further work with additional control groups is needed to unravel the influence of the different components used. Additional research based on the findings from this study may also identify specific prebiotic or other dietary supplements for the promotion/maintenance of the microbiota transferred via the maternal FMTp, thereby optimizing pig growth and FE. Further studies on the exact mechanism(s) of action of FMT are also warranted.
MATERIALS AND METHODS
Ethics approval.
The pig study was approved by the animal ethics committees of Teagasc (TAEC9/2013) and the Waterford Institute of Technology (13/CLS/02) and performed according to European Union regulations outlining minimum standards for the protection of pigs (91/630/EEC) and concerning the protection of animals kept for farming purposes (98/58/EC). An experimental license (no. AE1932/P032) was obtained from the Irish Health Products Regulatory Authority.
Animal management, recording, and sampling.
Feces were collected from four highly feed-efficient finisher pigs and anaerobically processed, and the resultant fecal extracts were prepared for use as an FMT inoculum as previously described by McCormack et al. (11). The same 22 sows used in the study by McCormack et al. were used here; on day 60 of gestation, sows were assigned to one of two treatment groups: (i) control (CON) (n = 11) and (ii) antibiotic treatment, purgative, and FMT on days 70 and 100 of gestation (FMTp) (n = 11). On day 61 of gestation, FMTp sows received a 7-day course of a broad-spectrum antibiotic cocktail (20 mg/kg of body weight/day amoxicillin trihydrate [Amoxinsol; Vetoquinol UK Ltd., Buckingham, UK], 10 mg/kg/day lincomycin-spectinomycin [Linco-Spectin 100; Pfizer, Cork, Ireland], and 100,000 IU/kg/day of colistin [Coliscour; Ceva Sante Animale, Libourne, France]), followed by 2 doses of a purgative (sodium picosulfate, magnesium oxide, and citric acid) (Picolax powder; Ferring Ltd., Dublin, Ireland) to clear the GIT of resident microbiota and by a fasting period of 36 h. On days 70 and 100 of gestation, sows received FMT (200 ml, which delivered a dose of ∼2.6 × 1011 CFU) via gastric intubation along with a proton pump inhibitor (omeprazole) (Romep; Rowex Ltd., Cork, Ireland) to prevent possible inhibition of the bacteria in the inoculum by the acidity of the stomach.
A schematic depicting sow and offspring treatments and details of sampling is shown in Fig. S4 in the supplemental material. At farrowing, the numbers of pigs born alive, stillbirths, and mummies were recorded, as were individual piglet birth weights and genders. All viable piglets were tagged for identification purposes, and litters remained intact insofar as possible between farrowing and weaning. A commercial nonmedicated starter diet (Table S3) was creep fed between day 12 and weaning at day ∼28 of age.
At weaning, 132 pigs were selected across all litters, blocked by sow treatment, piglet gender, and body weight, and randomly assigned to single-gender pens, with 8 to 12 pigs per pen. Within sow treatments, pens of pigs were randomly assigned to the following groups: (i) control (CON) (6 pens; n = 67 pigs) or (ii) inulin for the first 6 weeks postweaning (INU) (6 pens; n = 65 pigs). Once weaned, piglets in both the CON and INU groups were provided with the same sequence of diets (Table S3) (starter diet for 1 week, followed by link diet for 2 weeks, weaner diet for 3 weeks, and finisher diet until slaughter at ∼140 days of age), except that for the INU group, starter and link diets contained 2% inulin (Orafti Synergy 1, 50:50 chain length; Beneo Animal Nutrition, Belgium) and the weaner diet contained 3% inulin. Pigs were provided with ad libitum access to feed using a feed intake recording equipment (FIRE) feeding system (Schauer Agrotronic, Wels, Austria). The first week on the trial diets was regarded as a training period for the piglets, so feed intake for this period was not included in the data analysis.
From weaning to ∼78 days of age, pigs were housed in 12 fully slatted concrete (80-mm solid width; 18-mm slots) pens (2.4 m by 2.0 m). A canopy (2.4 m by 1.2 m) with 2 heat lamps was placed at the back of each pen to create a microclimate, and a suitable lying area was created using a solid rubber mat (2.4 m by 1.2 m) under the canopy. From ∼78 days of age, the size of each pen was increased to 2.4 m by 4.8 m, and the canopy and rubber mat were removed.
Body weight was recorded weekly, as was feed disappearance daily between ∼35 and ∼140 days of age, from which ADFI, ADG, and FCE were determined and used to calculate RFI, as previously outlined (11). A total of 11 pigs were removed due to the following health issues: rectal prolapse (n = 1) in the CON/CON group, shoulder injury (n = 1) and navel rupture (n = 2) in the CON/INU group, lameness (n = 1) and navel rupture (n = 3) in the FMTp/CON group, and lameness (n = 1) and navel rupture (n = 2) in the FMTp/INU group.
At ∼140 days of age, all pigs were slaughtered by CO2 stunning followed by exsanguination. Following evisceration, hot-carcass weight was recorded and multiplied by 0.98 to obtain the cold-carcass weight. The kill-out percentage was calculated as (carcass weight/body weight at slaughter) × 100, and back fat and muscle depth were measured 6 cm from the edge of the split back at the third and fourth last ribs using a Hennessy grading probe (Hennessy and Chong, Auckland, New Zealand). The lean meat yield was estimated according to the following formula: lean meat yield = 60.30 − 0.847 X1 + 0.147 X2 (where X1 is back fat depth [millimeters] and X2 is muscle depth [millimeters]).
Fecal sampling was conducted by rectal stimulation at 28 days (weaning) and at 50, 65, 100, and 130 days of age on the same subsample of 32 pigs (n = 16 per sow treatment and n = 16 per offspring treatment) (Fig. S4). Digesta samples from the ileum, cecum, and colon were also collected at slaughter from the same 32 selected pigs, as previously described (11). All samples were snap-frozen in liquid nitrogen and stored at −80°C for microbiota and VFA analyses. Additionally, tissues from the duodenum, jejunum, and ileum were collected from the same 32 selected pigs for histological analysis, and duodenal tissue scrapings were taken for both brush border enzyme and gene expression analyses, as previously described (11).
DNA extraction, 16S rRNA gene sequencing, and data analysis.
Total DNA was extracted from fecal, ileal, cecal, and colonic samples using the QIAamp DNA stool minikit (Qiagen, Crawley, United Kingdom) according to the manufacturer’s instructions, apart from adding a bead-beating step and increasing the lysis temperature to 95°C to increase the DNA yield (37).
The V3-V4 region of the 16S rRNA gene (∼460 bp) was sequenced (2- by 250-bp paired end reads) on an Illumina MiSeq platform according to standard protocols, with alterations, as previously outlined (4). Sequence reads were checked for quality using FastQC and trimmed to a length of 240 bp at the end of the sequence using Trimmomatic version 0.36 (38), with adapters removed (Illumina CLIP software). Forward and reverse reads were merged using Flash version 1.2.11 (39), and quality checks were performed to guarantee maximum read coverage. Reads were then clustered into operational taxonomic units (OTUs) using a 97% sequence identity threshold, chimeras were removed, reads were aligned to sequences in the CD-HIT-OTU specific database (version 11), and the Ribosomal Database Project (RDP) classifier database (version 11.5) was then used for taxonomy assignments (40), with any samples containing reads with a sequence identity of <80% labeled “unclassified.” Samples with <1,000 total reads were excluded from the analysis. The OTU data were scaled to the minimum number of total reads for each sample type (feces at weaning, 67,236; day 50, 51,458; day 65, 38,887; day 100, 70,095; ileum, 4,242; cecum, 78,276; colon, 41,924) and filtered to remove OTUs present at <100 reads. As an alternative to rarefaction of the data, data were scaled by dividing each OTU count by the sample total OTU count and by the minimum total OTU counts across samples in order to normalize counts to equal depths. Alpha-diversity indices, i.e., Shannon’s and Simpson’s diversity indices (which measure OTU richness and evenness), and beta-dispersion estimates were then calculated by using the Adonis2 and beta permutation functions of the Vegan package in R, each with 999 permutations. The Adonis2 function performs permutational multivariate analysis of variance (PERMANOVA) in Vegan on a Bray-Curtis dissimilarity/distance matrix, and the betadisper function assesses the homogeneity of dispersion among the groups. The PCA plots were generated using the OTU data and calculated on the intersample distance in the distance/dissimilarity matrix, with the bioconductor package DESeq2 version 1.24.0 (41) and ggplot in R version 3.4.0. Heat maps depicting relative abundances were generated in GraphPad Prism7.
Prediction of microbial function.
The functionality of the microbiota for each sample based on 16S rRNA gene sequences and version 13_5 of the Greengenes database for taxonomy and OTU assignments was predicted in silico using Phylogenetic Investigation of Communities by Reconstruction of Unobserved Species (PICRUSt) software (42) version 1.1.0. Prediction of functions was inferred based on Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations, using level 3 pathways from the KEGG database. Pathways not related to bacteria, not relevant to porcine studies, and for which the relative abundance was <0.001% in samples were dismissed.
Volatile fatty acid concentrations and pH.
Concentrations of acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids were measured in the ileal, cecal, and colonic digesta as previously described (4). Briefly, ∼8 g of sample was weighed, the pH was recorded, and the sample was then diluted with trichloroacetic acid (2.5 times the sample weight) and centrifuged (1,800 × g at 4°C for 10 min). The resultant supernatants were mixed with equal volumes of an internal standard (1.5 ml) filtered into vials and stored at −80°C until analysis by gas chromatography (Agilent 5890 gas chromatograph) using hydrogen (30 lb/in2) and helium (50 lb/in2) as carrier gases and temperatures of 80°C (oven), 280°C (detector), and 250°C (injector).
Intestinal histology, disaccharidase activity, and gene expression analysis.
Intestinal tissues from the duodenum, jejunum, and ileum (∼3-cm sections) collected at slaughter were rinsed in phosphate-buffered saline (PBS), placed in No-Tox fixative (Scientific Device Lab, Des Plaines, IL, USA), and put on a shaker for 48 h. Samples were then removed from the shaker and stored at room temperature in the fixative until processing, which was performed as outlined previously (11). Ten villi were examined per sample slide for villus height and width, crypt depth, and goblet cell number using a light microscope at a ×400 magnification.
Duodenal mucosal scrapings were collected over a length of 10 cm for the analysis of disaccharidase activity and relative gene expression. Preparation of duodenal homogenates (20%, wt/vol) and mucosal enzyme activity measurements were performed as previously described by Metzler-Zebeli et al. (43). Target genes included those encoding intestinal alkaline phosphatase (IAP), facilitated glucose transporter member 2 (GLUT2), GIP, GLP1, monocarboxylate transporter 1 (MCT1), SMCT, sodium/glucose cotransporter member 1 (SGLT1), tight junction proteins (occludin [OCLN] and zonula occludens 1 [ZO1]), and Toll-like receptors (TLR2 and TLR4). Total RNA was isolated from 20-mg duodenal mucosal scrapings using mechanical homogenization and the RNeasy minikit (Qiagen, Hilden, Germany). Samples were homogenized using the FastPrep-24 instrument (MP Biomedicals, Santa Ana, CA, USA) (three times for 60 s [speed, 6.5 m/s], with cooling on ice for 1 min between runs). After isolation, genomic DNA was removed by treating samples with the Turbo DNA kit (Life Technologies Ltd., Vienna, Austria). The RNA was quantified using the Qubit HS RNA assay kit on the Qubit 2.0 fluorometer (Life Technologies Ltd., Vienna, Austria), and the quality of extracted RNA was evaluated with the Agilent 2100 bioanalyzer (Agilent RNA 6000 nano assay; Agilent Technologies, Waghaeusel-Wiesental, Germany). cDNA was synthesized from 2 μg RNA using the high-capacity cDNA reverse transcription (RT) kit (Life Technologies Ltd.), and 1 μl of an RNase inhibitor (Biozym, Hessisch Oldendorf, Germany) was added to each reaction mixture. Primers used for quantitative PCR (qPCR) are listed in Table 5.
TABLE 5.
Forward and reverse primers used for quantitative PCR and for determination of PCR efficiency and coefficients of correlation of standard curves used in gene expression analysis
| Genea | GenBank accession no.b | Encoded protein | Forward primer (5′–3′) | Reverse primer (5′–3′) | Amplicon size (bp) | Reference | Eff (%)c | Corrd |
|---|---|---|---|---|---|---|---|---|
| ACTB | XM_003357928.2 | Beta-actin | GGGCATCCTGACCCTCAAG | TGTAGAAGGTGTGATGCCAGATCT | 89 | 46 | 97.3 | 0.99 |
| B2M | NM_213978.1 | Beta-2-microglobulin | CCCCCGAAGGTTCAGGTT | GCAGTTCAGGTAATTTGGCTTTC | 66 | 46 | 102.2 | 0.99 |
| GAPDH | NM_001206359.1 | Glyceraldehyde-3-phosphate dehydrogenase | GGCGTGAACCATGAGAAGTATG | GGTGCAGGAGGCATTGCT | 60 | 46 | 96.5 | 0.99 |
| HPRT1 | NM_001032376.2 | Hypoxanthine guanine phosphoribosyl transferase | AGAAAAGTAAGCAGTCAGTTTCATATCAGT | ATCTGAACAAGAGAGAAAATACAGTCAATAG | 131 | 46 | 92.1 | 0.99 |
| OAZ1 | NM_001122994.2 | Ornithine decarboxylase antizyme 1 | TCGGCTGAATGTAACAGAGGAA | GAGCCTGGATTGGACGTTTAAA | 70 | 46 | 99.2 | 0.99 |
| OCLN | NM_001163647.2 | Occludin | TTGTGGGACAAGGAACGTATTTA | TGCCTGCCGACACGTTT | 76 | 46 | 95.4 | 0.98 |
| ZO1 | XM_013993251.1 | Zona occludens 1 | AAGCCCTAAGTTCAATCACAATCT | ATCAAACTCAGGAGGCGGC | 131 | 46 | 109.2 | 0.98 |
| SGLT1 (SLC5A1) | NM_001164021.1 | Sodium-dependent glucose transporter 1 | TGTCTTCCTCATGGTGCCAA | AGGAGGGTCTCAGGCCAAA | 149 | 46 | 108.0 | 0.99 |
| GLUT2 (SLC2A2) | NM_001097417.1 | Facilitated glucose transporter member 2 | TACGGCATCTGCTAGCCTCAT | CCACCAATTGCAAAGATGGAC | 66 | 47 | 89.3 | 1.00 |
| MCT1 (SLC16A1) | AM286425.1 | Monocarboxylate transporter 1 | GGTGGAGGTCCTATCAGCAG | AAGCAGCCGCCAATAATCAT | 74 | 46 | 96.4 | 1.00 |
| SMCT (SLC5A12) | XM_003122908.1 | Sodium-coupled monocarboxylate cotransporter | AGGTCTACCGCTTTGGAGCAT | GAGCTCTGATGTGAAGATGATGACA | 77 | 47 | 82.3 | 0.99 |
| GIP | NM_001287408.1 | Glucose-dependent insulinotropic peptide | GGATGGTGGAGCAGTTGGA | CCAATCCTGAGCTGGGTTTG | 71 | 47 | 98.1 | 0.99 |
| GLP1 | NM_001256594.1 | Glucagon-like peptide 1 | GCTGATGGTGGCGATCTTGT | TCCCAGCTCTTCCGAAACTC | 69 | 47 | 98.1 | 0.99 |
| TRL2 | NM_213761.1 | Toll-like receptor 2 | AATAAGTTGAAGACGCTCCCAGAT | GTTGCTCCTTAGAGAAAGTATTGATCGT | 97 | 46 | 92.7 | 0.99 |
| TRL4 | AB188301.2 | Toll-like receptor 4 | TGTGGCCATCGCTGCTAAC | GGTCTGGGCAATCTCATACTCA | 124 | 46 | 105.8 | 0.98 |
| ALPI | XM_003133729.3 | Intestinal alkaline phosphatase | AGGAACCCAGAGGGACCATTC | CACAGTGGCTGAGGGACTTAGG | 83 | 47 | 97.1 | 0.99 |
Alternate gene names are shown in parentheses.
Shown are National Center for Biotechnology Information (NCBI) Entrez gene accession numbers (https://www.ncbi.nlm.nih.gov/sites/entrez?db=gene).
Eff, PCR efficiency [E = 10(−1/slope) − 1].
Corr, correlation coefficient of the standard curve.
The primers were verified with PrimerBLAST (www.ncbi.nlm.nih.gov/tools/primer-blast/) and tested for efficiencies and specificities using melting-curve analysis. Amplifications were performed on a real-time PCR Mx3000P thermocycler (Agilent Technologies) under the following conditions: 95°C for 5 min followed by 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s for 40 cycles, followed by the generation of melting curves. Negative controls and reverse transcription controls (RT minus) were included in order to control for residual DNA contamination. Each 20-μl reaction mixture consisted of 50 ng cDNA, 10 μl Fast Plus Eva green master mix with low ROX (Biotium, Hayward, CA, USA), 100 nM (each) forward and reverse primers, and 10 μl diethyl pyrocarbonate (DEPC)-treated water in a 96-well plate (VWR, Vienna, Austria). All reactions were performed in duplicate as previously described by Metzler-Zebeli et al. (43).
Hematology and blood biochemistry analyses.
Blood was collected during exsanguination at slaughter for hematology and biochemistry analyses from the same 32 selected pigs. For hematological analysis, blood was collected in Vacuette tubes (Labstock, Dublin, Ireland) containing EDTA to prevent clotting and analyzed within 4 h using a Beckman Coulter Ac T Diff analyzer (Beckman Coulter Ltd., High Wycombe, UK).
For biochemical analysis, blood was collected in Vacuette tubes (Labstock) and allowed to clot at room temperature, followed by centrifugation at 1,500 × g for 10 min. The serum was then collected and stored at −80°C for subsequent analysis. Concentrations of total protein, blood urea nitrogen, glucose, triglycerides, cholesterol, creatinine, and creatine kinase were measured using an ABS Pentra 400 clinical chemistry analyzer (Horiba, ABX, North Hampton, UK). The analyzer was calibrated according to the manufacturer’s instructions, and every fifth sample was analyzed in duplicate to determine analyzer accuracy.
Statistical analysis.
Growth performance parameters recorded throughout the study were analyzed for repeated measures using PROC MIXED in SAS 9.3 (44), with gender, boar, and treatment (sow/offspring) used as fixed effects. Pig nested within pen was used as a random effect to account for variability regarding pen assignment. The RFI was calculated between days 35 and ∼140 of age (at slaughter) as the residuals from a least-squares regression model of ADFI on ADG, metabolic live weight, gender, and all relevant two-way interactions, as well as the effects of back fat and muscle depth, which were recorded at slaughter.
Intestinal histology, gene expression, brush border enzymatic activity, and blood parameters (hematology and serum biochemistry) were also analyzed using the MIXED procedure in SAS 9.3, with models similar to the ones used for growth performance. A generalized linear mixed model using PROC GLIMMIX in SAS 9.3 was used to analyze VFA concentrations, which were deemed “not normal,” following log transformation.
Microbial composition and predicted functionality data were analyzed using generalized linear mixed model equation methods in PROC GLIMMIX of SAS 9.3. A gamma distribution was assumed for all data. Models for offspring bacterial relative abundance for the fecal time points and digesta included sow treatment, offspring treatment, fecal sampling time point, and their interactions as fixed effects. Additionally, a random intercept for each fecal time point was included to account for the repeated measurements. Microbial composition and predicted functionality for which the relative abundance was <0.001% were dismissed. The PCA plots were calculated from regularized log-transformed counts and plotted using ggplot 2, and the DESeq2 package was used to calculate the differential abundance, which used negative binomial generalized liner models. In all models, data were back-transformed to the original distribution using the ilink option in PROC GLIMMIX. Multiple comparisons were corrected for by using the Benjamini-Hochberg method in SAS also (45).
For all analyses, statistical significance was set at a P value of <0.05. Heat maps used to depict differences in relative abundances between treatments (for microbial composition and predicted functionality) were generated in GraphPad Prism 7.
Accession number(s).
The raw 16S rRNA gene sequence data generated from this study are available at the European Nucleotide Archive under accession no. PRJEB22233.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stefan Buzoianu, Tomas Ryan, the farm staff, and work placement students in the Pig Development Department at Teagasc Moorepark for assistance with the pig study. We also acknowledge Orla O’Sullivan (Teagasc and the APC Microbiome Institute) for assistance with sequence data; Donagh Berry (Teagasc) for help with RFI calculations; Maria Luz Prieto, Orla O’Donovan, Michael Harrison, and Stuart Graham (Waterford Institute of Technology), Vicki Murray and Laura Finnegan (Teagasc), David Clarke (Teagasc), and Arife Sener (University of Veterinary Medicine Vienna) for technical assistance; and Peter White (Department of Agriculture, Food and the Marine [DAFM], Back Weston, Co. Kildare, UK) for preparation of histology slides.
P.G.L., G.E.G., B.U.M.-Z., and P.D.C. conceived and designed the study. U.M.M. and P.G.L. conducted the animal study and, together with G.E.G. and H.R., collected intestinal samples. U.M.M., H.R., B.U.M.-Z., and F.C. performed laboratory analysis. C.J.C., T.W., and T.C. performed bioinformatics analyses. U.M.M. statistically analyzed all the data and, together with T.C., P.G.L., and G.E.G., interpreted the data and drafted the manuscript. H.R., F.C., P.D.C., B.U.M.-Z., G.E.G., and P.G.L. revised the manuscript. All authors read and approved the final version of the manuscript.
We declare that we have no competing interests.
The research leading to these results received funding from the European Union’s Seventh Framework Programme (ECO-FCE project no. 311794) for research, technological development, and demonstration independently of any commercial input, financial or otherwise. U.M.M. was funded by the Teagasc Walsh fellowship program.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01255-19.
REFERENCES
- 1.Teagasc. 2017. National pig herd performance report, p 8–9. Pig Development Department, Teagasc Moorepark, Fermoy, Co. Cork, Ireland. [Google Scholar]
- 2.Tan Z, Yang T, Wang Y, Xing K, Zhang F, Zhao X, Ao H, Chen S, Liu J, Wang C. 2017. Metagenomic analysis of cecal microbiome identified microbiota and functional capacities associated with feed efficiency in Landrace finishing pigs. Front Microbiol 8:1546. doi: 10.3389/fmicb.2017.01546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Huang X, Fang S, He M, Zhao Y, Wu Z, Yang M, Zhang Z, Chen C, Huang L. 2017. Unraveling the fecal microbiota and metagenomic functional capacity associated with feed efficiency in pigs. Front Microbiol 8:1555. doi: 10.3389/fmicb.2017.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack UM, Curião T, Buzoianu SG, Prieto ML, Ryan T, Varley P, Crispie F, Magowan E, Metzler-Zebeli BU, Berry D, O’Sullivan O, Cotter PD, Gardiner GE, Lawlor PG. 2017. Exploring a possible link between the intestinal microbiota and feed efficiency in pigs. Appl Environ Microbiol 83:e00380-17. doi: 10.1128/AEM.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack UM, Curiao T, Metzler-Zebeli BU, Magowan E, Berry DP, Reyer H, Prieto ML, Buzoianu SG, Harrison M, Rebeiz N, Crispie F, Cotter PD, O’Sullivan O, Gardiner GE, Lawlor PG. 2019. Porcine feed efficiency-associated intestinal microbiota and physiological traits: finding consistent cross-locational biomarkers for residual feed intake. mSystems 4:e00324-18. doi: 10.1128/mSystems.00324-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang X, Hua X, Yang Q, Ding D, Che C, Cui L, Jia W, Bucheli P, Zhao L. 2007. Inter-species transplantation of gut microbiota from humans to pigs. ISME J 1:156–162. doi: 10.1038/ismej.2007.23. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Widmer G, Tzipori S. 2013. A pig model of the human gastrointestinal tract. Gut Microbes 4:193–200. doi: 10.4161/gmic.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirayama K. 1999. Ex-germfree mice harbouring intestinal microbiota derived from other animal species as an experimental model for ecology and metabolism of intestinal bacteria. Exp Anim 48:219–227. doi: 10.1538/expanim.48.219. [DOI] [PubMed] [Google Scholar]
- 9.Martin L, Cilieborg MS, Birck M, Thymann T, Sangild PT. 2015. Fecal microbiota transplantation decreases necrotizing enterocolitis but is associated with increased neonatal mortality in preterm pigs. Abstr jENS 2015, Budapest, Hungary.
- 10.Xiao Y, Yan H, Diao H, Yu B, He J, Yu J, Zheng P, Mao X, Luo Y, Chen D. 2017. Early gut microbiota intervention suppresses DSS-induced inflammatory responses by deactivating TLR/NLR signalling in pigs. Sci Rep 7:3224. doi: 10.1038/s41598-017-03161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack UM, Curião T, Wilkinson T, Metzler-Zebeli BU, Reyer H, Ryan T, Calderon-Diaz JA, Crispie F, Cotter PD, Creevey CJ, Gardiner GE, Lawlor PG. 2018. Fecal microbiota transplantation in gestating sows and neonatal offspring alters lifetime intestinal microbiota and growth in offspring. mSystems 3:e00134-17. doi: 10.1128/mSystems.00134-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. 2017. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 13.Roberfroid M. 2007. Prebiotics: the concept revisited. J Nutr 137:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 14.Metzler-Zebeli BU, Trevisi P, Prates JAM, Tanghe S, Bosi P, Canibe N, Montagne L, Freire J, Zebeli Q. 2017. Assessing the effect of dietary inulin supplementation on gastrointestinal fermentation, digestibility and growth in pigs: a meta-analysis. Anim Feed Sci Technol 10:120–132. doi: 10.1016/j.anifeedsci.2017.05.010. [DOI] [Google Scholar]
- 15.Grela ER, Sobolewska S, Kowalczuk-Vasilev E, Krasucki W. 2014. Effect of dietary inulin source on piglet performance, immunoglobulin concentration, and plasma lipid profile. Bull Vet Inst Pulawy 58:453–458. doi: 10.2478/bvip-2014-0069. [DOI] [Google Scholar]
- 16.Patterson JK, Yasuda K, Welch RM, Miller DD, Lei XG. 2010. Supplemental dietary inulin of variable chain lengths alters intestinal bacterial populations in young pigs. J Nutr 140:2158–2161. doi: 10.3945/jn.110.130302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loh G, Eberhard M, Brunner RM, Hennig U, Kuhla S, Kleessen B, Metges CC. 2006. Inulin alters the intestinal microbiota and short-chain fatty acid concentrations in growing pigs regardless of their basal diet. J Nutr 136:1198–1202. doi: 10.1093/jn/136.5.1198. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinov SR, Awati A, Smidt H, Williams BA, Akkermans ADL, de Vos WM. 2004. Specific response of a novel and abundant Lactobacillus amylovorus-like phylotype to dietary prebiotics in the guts of weaning piglets. Appl Environ Microbiol 70:3821–3830. doi: 10.1128/AEM.70.7.3821-3830.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awad WA, Ghareeb K, Paßlack N, Zentek J. 2013. Dietary inulin alters the intestinal absorptive and barrier function of piglet intestine after weaning. Res Vet Sci 95:249–254. doi: 10.1016/j.rvsc.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Kelly G. 2008. Inulin-type prebiotics—a review: part 1. Altern Med Rev 13:315–329. [PubMed] [Google Scholar]
- 21.Kozłowska I, Marć-Pieńkowska J, Bednarczyk M. 2016. Beneficial aspects of inulin supplementation as a fructooligosaccharide prebiotic in monogastric animal nutrition—a review. Ann Anim Sci 16:315–331. doi: 10.1515/aoas-2015-0090. [DOI] [Google Scholar]
- 22.McCormack UM. 2017. Investigation and subsequent manipulation of the intestinal microbiota of pigs, with a view to optimising feed efficiency. PhD thesis. Waterford Institute of Technology, Waterford, United Kingdom. [Google Scholar]
- 23.van der Aar PJ, Molist F, van der Klis JD. 2017. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim Feed Sci Technol 233:64–75. doi: 10.1016/j.anifeedsci.2016.07.019. [DOI] [Google Scholar]
- 24.Grela ER, Kowalczyk-Pecka D, Hanczakowska E, Matras J. 2016. Effect of inulin and a probiotic supplement in the diet of pigs on selected traits of the gastrointestinal microbiome. Med Weter 72:448–452. doi: 10.21521/mw.5532. [DOI] [Google Scholar]
- 25.Diao H, Yan HL, Xiao Y, Yu B, Yu J, He J, Zheng P, Zeng BH, Wei H, Mao XB, Chen DW. 2016. Intestinal microbiota could transfer host gut characteristics from pigs to mice. BMC Microbiol 16:238. doi: 10.1186/s12866-016-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan H, Diao H, Xiao Y, Li W, Yu B, He J, Yu J, Zheng P, Mao X, Luo Y, Zeng B, Wei H, Chen D. 2016. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci Rep 6:31786. doi: 10.1038/srep31786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. 2013. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellekilde M, Selfjord E, Larsen CS, Jakesevic M, Rune I, Tranberg B, Vogensen FK, Nielsen DS, Bahl MI, Licht TR, Hansen AK, Hansen CHF. 2014. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci Rep 4:5922. doi: 10.1038/srep05922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frantz N, Nelssen JL, DeRouchey JM, Goodband RD, Tokach MD, Dritz SS. 2003. Effects of a prebiotic, inulin, and a direct fed microbial on growth performance of weanling pigs. Kansas Agric Exp Station Res Rep 2003:123–127. doi: 10.4148/2378-5977.6818. [DOI] [Google Scholar]
- 30.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koropatkin NM, Cameron EA, Martens EC. 2012. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halas D, Hansen CF, Hampson DJ, Kim J-C, Mullan BP, Wilson RH, Pluske JR. 2010. Effects of benzoic acid and inulin on ammonia-nitrogen excretion, plasma urea levels, and the pH in faeces and urine of weaner pigs. Livest Sci 134:243–245. doi: 10.1016/j.livsci.2010.06.153. [DOI] [Google Scholar]
- 33.Davidson MH, Maki KC. 1999. Effects of dietary inulin on serum lipids. J Nutr 129:1474S–1477S. doi: 10.1093/jn/129.7.1474S. [DOI] [PubMed] [Google Scholar]
- 34.Fushimi T, Suruga K, Oshima Y, Fukiharu M, Tsukamoto Y, Goda T. 2006. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr 95:916–924. doi: 10.1079/bjn20061740. [DOI] [PubMed] [Google Scholar]
- 35.Naidu A, Bidlack W, Clemens R. 1999. Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr 39:13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- 36.Kim YS, Ho SB. 2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buzoianu SG, Walsh MC, Rea MC, O’Sullivan O, Cotter PD, Ross RP, Gardiner GE, Lawlor PG. 2012. High-throughput sequence-based analysis of the intestinal microbiota of weanling pigs fed genetically modified MON810 maize expressing Bacillus thuringiensis Cry1Ab (Bt maize) for 31 days. Appl Environ Microbiol 78:4217–4224. doi: 10.1128/AEM.00307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metzler-Zebeli BU, Lawlor PG, Magowan E, McCormack UM, Curiao T, Hollmann M, Ertl R, Aschenbach JR, Zebeli Q. 2017. Finishing pigs that are divergent in feed efficiency show small differences in intestinal functionality and structure. PLoS One 12:e0174917. doi: 10.1371/journal.pone.0174917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SAS. 2011. SAS 9.3. Statistical analysis system. SAS Institute Inc, Cary, NC. [Google Scholar]
- 45.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300. [Google Scholar]
- 46.Metzler-Zebeli BU, Mann E, Ertl R, Schmitz-Esser S, Wagner M, Klein D, Ritzmann M, Zebeli Q. 2015. Dietary calcium concentration and cereals differentially affect mineral balance and tight junction proteins expression in jejunum of weaned pigs. Br J Nutr 113:1019–1031. doi: 10.1017/S0007114515000380. [DOI] [PubMed] [Google Scholar]
- 47.Metzler-Zebeli BU, Ertl R, Grüll D, Molnar T, Zebeli Q. 2016. Enzymatically modified starch up-regulates expression of incretins and sodium-coupled monocarboxylate transporter in jejunum of growing pigs. Animal 11:1180–1188. doi: 10.1017/S1751731116002615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.