Abstract
Hereditary breast cancer is known for its strong tendency of inheritance. Most hereditary breast cancers are related to BRCA1/BRCA2 pathogenic variants. The lifelong risk of breast cancer in pathogenic BRCA1 and BRCA2 variant carriers is approximately 65% and 45%, respectively, whereas that of ovarian cancer is estimated to be 39% and 11%, respectively. Therefore, understanding these variants and clinical knowledge on their occurrence in breast cancers and carriers are important. BRCA1 pathogenic variant breast cancer shows more aggressive clinicopathological features than the BRCA2 pathogenic variant breast cancer. Compared with sporadic breast cancer, their prognosis is still debated. Treatments of BRCA1/BRCA2 pathogenic variant breast cancer are similar to those for BRCA-negative breast cancer, mainly including surgery, radiotherapy, and chemotherapy. Recently, various clinical trials have investigated poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor treatment for advanced-stage BRCA1/BRCA2 pathogenic variant breast cancer. Among the various PARP inhibitors, olaparib and talazoparib, which reached phase III clinical trials, showed improvement of median progression-free survival around three months. Preventive and surveillance strategies for BRCA pathogenic variant breast cancer to reduce cancer recurrence and improve treatment outcomes have recently received increasing attention. In this review, we provide an information on the clinical features of BRCA1/BRCA2 pathogenic variant breast cancer and clinical recommendations for BRCA pathogenic variant carriers, with a focus on treatment and prevention strategies. With this knowledge, clinicians could manage the BRCA1/BRCA2 pathogenic variant breast cancer patients more effectively.
Keywords: BRCA1/BRCA2 pathogenic variant, Breast cancer, Ovarian cancer, Treatment, Prevention
INTRODUCTION
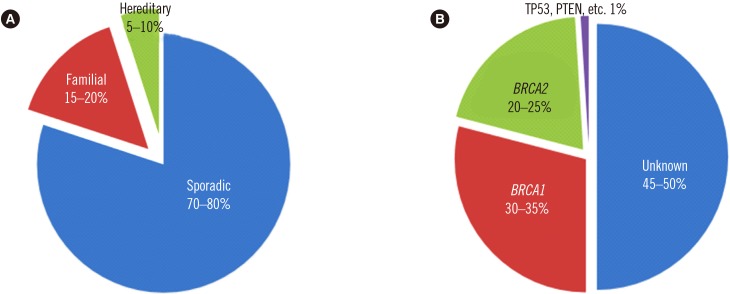
Hereditary breast cancer accounts for 5–10% of all breast cancer cases [1]. Its genesis is associated with the pathogenic variant of certain genes; in more than 90% of cases, pathogenic variants are detected in BRCA1 (MIM No. 113705)/BRCA2 (MIM No. 600185) and are inherited in an autosomal dominant fashion [2]. By the age of 70 years, pathogenic variant of BRCA1/BRCA2 augments the risk of breast cancer by 65% (44–78%) and 45% (31–56%), respectively, and that of ovarian cancer by 39% (18–54%) and 11% (2.4–19%), respectively [3]. Furthermore, BRCA pathogenic variants are known to increase the risks of fallopian tube cancer, melanoma, endometrial cancer, pancreatic cancer, prostate cancer, and colorectal cancer [4,5,6,7,8,9] (Fig. 1).
Fig. 1. Incidence of breast cancer. (A) hereditary breast cancer accounts for 5–10% of all breast cancer cases. (B) BRCA1/BRCA2 pathogenic variant breast cancer accounts for up to 60% of all hereditary breast cancer cases [97]. Copyright permission for this figure was obtained from publisher.
BRCA stands for “BReast CAncer gene,” indicating its relevance in breast cancer pathogenesis. However, the gene itself does not induce breast cancer. Instead, BRCA1/BRCA2 are involved in DNA repair of other genes that induce human cancers. BRCA1 and BRCA2 are two distinct cancer suppression genes and are essential in activating DNA repair in response to cellular stress [10,11,12]. BRCA1/BRCA2 play crucial roles in chromatin remodeling, transcription control, cell-cycle regulation, and DNA-repair processes [13], and their tumor-suppressive effects have been attributed mainly to cell-cycle checkpoint and DNA repair management. Nevertheless, the detailed mechanisms of carcinogenesis induced by BRCA1/BRCA2 germline pathogenic variants in breast and ovarian tissues are yet unrevealed [14,15]. The BRCA1 gene is located on chromosome 17q21 and has 22 exons. It encodes a 1,863-amino-acid-long nuclear protein. BRCA1 is expressed in various tissues, including breast and ovarian tissues [16]. The BRCA2 gene is located on chromosome 13q12-13 and has 27 exons [17,18,19,20]. BRCA1 and BRCA2 have similar exon structures but do not show sequence homology [21].
There are more than 1,600 and 1,800 known variants in BRCA1 and BRCA2, respectively, the majority of which induce frameshifts, leading to missense or non-functional proteins [22]. In addition to breast cancer, BRCA1 pathogenic variants increase the risks of ovarian cancer in women and prostate cancer in men, whereas BRCA2 pathogenic variants increase the risks of cholangiocarcinoma, gastric cancer, and melanoma [23,24]. This review provides an overview of the clinical perspectives of BRCA1/BRCA2 pathogenic variant breast cancer and clinical recommendations for BRCA pathogenic variant carriers, with a focus on treatment and prevention strategies.
Clinicopathological characteristics of BRCA1/BRCA2 pathogenic variant breast cancer
Clinicopathological characteristics of BRCA pathogenic variant and sporadic breast cancers differ from each other, and those of BRCA1- and BRCA2-related breast cancers also present distinct features from each other. In view of histological types, approximately 75% of BRCA1 pathogenic variant breast cancers are invasive ductal carcinomas, and 10% are atypical medullary cancers. In BRCA2 pathogenic variant breast cancer, lobular or ductal with lobular types are more frequent (in up to 10% of cases) [25]. Based on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) status, breast cancers can be classified into luminal A (ER+ and/or PR+ and HER2−), luminal B (ER+ and/or PR+ and HER2+), HER2-positive (ER−, PR−, and HER2+), and triple-negative (ER−, PR−, and HER2−) subtypes [26]. Triple-negative breast cancer (TNBC) shows aggressive biological behavior [27,28]. It occurs in 10–15% of sporadic breast cancers and 66–100% of BRCA1 pathogenic variant breast cancers. In contrast, 14–35% of TNBC cases carry a BRCA2 pathogenic variant, which is more similar to the proportion in sporadic breast cancer [29,30]. Moreover, BRCA1 pathogenic variant breast cancers tend to have higher histological grade than BRCA2 pathogenic variant breast cancers. Ductal carcinoma in situ (DCIS) is rare in BRCA1 pathogenic variant carriers, but rather common in BRCA2 pathogenic variant carriers [25] (Table 1).
Table 1. Clinicopathological characteristics of BRCA1/BRCA2 pathogenic variants.
| BRCA1 pathogenic variant | BRCA2 pathogenic variant | |
|---|---|---|
| Chromosome | 17q21 | 13q12-13 |
| Breast cancer | ||
| Risk of cancer (by 70 years) | 65% (44–78%) | 45% (31–56%) |
| Histological type | Invasive ductal (~75%) | Invasive ductal (~75%) |
| Atypical medullary (~10%) | Atypical medullary (<10%) | |
| Lobular or ductal with lobular feature type (up to 10%) | ||
| Histological grade | Mostly high (Grade III) | Mostly medium (Grade II) or high (Grade III) |
| TNBC | 66–100% | 14–35% |
| DCIS | Rare | Common |
| Ovarian cancer | ||
| Risk of cancer (by 70 years) | 39% (18–54%) | 11% (2.4–19%) |
Abbreviations: TNBC, triple-negative breast cancer; DCIS, ductal carcinoma in situ.
The prognosis of BRCA1/BRCA2 pathogenic variant breast cancer is debated. Some studies have indicated that BRCA pathogenic variants are related to adverse prognosis [31,32,33], whereas the predominant view is that prognoses of BRCA pathogenic variant and sporadic breast cancer do not differ [34,35,36]. A recent prospective study that compared prognoses of 338 BRCA pathogenic variant breast cancer patients (N=201 for BRCA1 variants and N=137 for BRCA2 variants) with those of 2,395 sporadic breast cancer patients revealed no difference (at two years: 97.0% vs 96.6%; at five years: 83.8% vs 85.0%; at 10 years: 73.4% vs 70.1%; hazard ratio [HR] 0.96 (95% confidence interval [CI] 0.76–1.22); P=0.76). In addition, the TNBC subgroup (N=588) of BRCA pathogenic variant patients demonstrated better 2-year survival (95% vs 91%; HR 0.59 [95% CI 0.35–0.99]; P=0.047), but no definitive difference in 5-year (81% vs 74%; HR 1.13 [0.70–1.84]; P=0.62) and 10-year survivals (72% vs 69%; HR 2.12 [0.82–5.49]; P=0.12) [35]. In a meta-analysis by Baretta, et al. [37], BRCA1/BRCA2 pathogenic variant patients had more favorable overall survival than BRCA-negative breast cancer patients (HR 0.49, 95% CI 0.26–0.92).
Treatment of BRCA1/BRCA2 pathogenic variant breast cancer
Surgery and radiotherapy
The main treatment strategies for breast cancer are surgery and systemic treatment. One of the main concerns in surgical treatment of BRCA1/BRCA2 pathogenic variant breast cancer is whether the treatment outcome of breast-conserving surgery (BCS) combined with radiotherapy is equivalent to that of radical mastectomy. There has been only one study thus far that compared the results according to the method of operation (BCS vs mastectomy) in BRCA1/BRCA2 pathogenic variant breast cancer [38]. According to that study, no significant difference was observed between overall survival of two groups (15-year survival rate; BCS: 91.7% vs mastectomy: 92.8%, P=0.85), while BCS group showed higher ipsilateral local recurrence rate than that of mastectomy group (15-year cumulative estimated risk 23.5% vs 5.5%, P<0.0001).
Other studies have addressed this issue by comparing the outcome of BCS in BRCA1/BRCA2 pathogenic variant breast cancer to that of sporadic breast cancer [39,40,41,42]. Kirova, et al. [40] retrospectively assessed the prognosis of breast cancer patients who had BCS, by matching 131 BRCA1/BRCA2 pathogenic variant breast cancer patients with 261 sporadic breast cancer patients. The mean follow-up duration was 161 months, and there was no significant difference in overall survival between the two groups. A retrospective case-control study for the breast cancer patients who underwent BCS revealed that breast cancer-specific survival rate did not differ between BRCA pathogenic variant and sporadic breast cancer patients [41]. A recent meta-analysis of 526 BRCA1/BRCA2 pathogenic variant and 2,320 sporadic breast cancer patients revealed no difference in overall survival between these two groups [42]. However, in this study, BRCA1/BRCA2 pathogenic variant breast cancer patients showed higher ipsilateral breast cancer recurrence than sporadic breast cancer patients with a median follow-up of longer than six years (relative risk [RR] 1.51, 95% CI 1.15–1.98).
Radiation following BCS is omitted only in very exceptional cases. Given the role of BRCA in DNA repair, concerns about complications of radiation therapy in BRCA pathogenic variant breast cancer have been raised. However, a study by Pierce, et al. [41] revealed no difference in radiation complication rates between BRCA pathogenic variant and sporadic breast cancers.
Chemotherapy
On DNA damage by chemotherapy, BRCA1/BRCA2 induce a DNA damage response for repair. Thus, BRCA pathogenic variant status is considered a decisive factor in predicting chemotherapy sensitivity [43,44,45]. For instance, compared with those without, cells with BRCA1 pathogenic variant are more sensitive to platinum-based chemotherapeutic agents, which disrupt the DNA structure [46,47]. On the other hand, BRCA1 pathogenic variant cells are somewhat resistant to microtubule-inhibiting chemotherapies, such as taxanes, in vitro [48,49]. These in vitro findings have been corroborated by data from BRCA pathogenic variant breast cancer patients who underwent single taxane-based neoadjuvant or palliative chemotherapy [50,51]. However, there is insufficient evidence to exclude taxanes from adjuvant chemotherapy regimens in BRCA pathogenic variant breast cancer patients. Arun, et al. [50] reported that patients with ER− BRCA pathogenic variant breast cancer showed a better pathological complete response than sporadic breast cancer patients when treated with anthracycline- and taxane-based neoadjuvant chemotherapy compared with those who were treated with single anthracycline-based chemotherapy. Moreover, prognoses of breast cancer patients who received anthracycline- and taxane-based chemotherapy were similar, regardless of BRCA pathogenic variant status.
Poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor treatment
The promising systemic treatment drug for BRCA pathogenic variant is a PARP inhibitor [52]. PARP is essential for repairing DNA single-strand breaks through base excision repair. Inhibition of PARP leads to the accumulation of DNA single-strand breaks and eventually, replication fork damage. In normal cells, breaks are repaired by error-free homologous recombination. When there are defects in the homologous recombination pathway due to BRCA functional failure, PARP inhibitors induce synthetic lethality by hindering base excision repair [53,54,55]. Olaparib, talazoparib, rucaparib, niraparib, and veliparib are five PARP inhibitors currently available. Olaparib, rucaparib, and niraparib are approved by the United States Food and Drug Administration (FDA) for ovarian cancer treatment. Olaparib also obtained FDA approval for treatment of HER2− metastatic germline BRCA pathogenic variant breast cancer [52].
The majority of clinical trials on PARP inhibitors in BRCA pathogenic variant breast cancer patients are phase I or II trials, whereas olaparib and talazoparib have reached phase III trials [52,56,57]. A phase III trial of olaparib (OlympiAD trial) in metastatic breast cancer patients with HER2−BRCA pathogenic variants involved 302 patients, 205 of whom were treated with olaparib and 97 with conventional chemotherapy. The olaparib group showed better median progression-free survival (7.0 vs 4.2 months; HR for disease progression or death, 0.58; 95% CI, 0.43–0.80; P<0.001) [56]. A phase III trial of talazoparib (EMBRCA trial) in advanced breast cancer with BRCA pathogenic variant involved 431 patients, 287 of whom were administered talazoparib and 144 conventional chemotherapy. Talazoparib treatment resulted in more favorable median progression-free survival (8.6 vs 5.6 months; HR for disease progression or death, 0.54; 95% CI, 0.41–0.71; P<0.001) [57]. Rucaparib is a selective PARP-1 and PARP-2 inhibitor [58]. In a phase II trial in metastatic breast and ovarian BRCA pathogenic variant cancer patients, 41% of patients who received intravenous rucaparib achieved stable disease status within three months, although the objective response rate (ORR) was only up to 2%. The ORR for oral rucaparib reportedly was 15% [59]. In a phase I trial of niraparib in advanced solid organ cancer patients, four patients had BRCA pathogenic variant breast cancer, and two of them achieved a partial response [60].
Recent PARP inhibitor studies have focused on combinations with platinum-based chemotherapies, such as cisplatin and carboplatin [61,62,63,64,65,66]. ER and/or PR is expressed in 21–22% of BRCA1 pathogenic variant carriers and 65–77% in BRCA2 pathogenic variant carriers. ER+ BRCA1/BRCA2 pathogenic variant cancers tend to show more adverse clinical characteristics and higher histological grades than sporadic ER+ cancers [67,68,69,70]. Some clinical trials have applied olaparib or talazoparib in ER+ metastatic BRCA1/BRCA2 pathogenic variant breast cancers and reported promising treatment outcomes [56,71,72,73]; however, the number of participants was relatively small, and thus, further investigations in larger patient cohorts are needed.
Prevention of breast cancer in BRCA1/BRCA2 pathogenic variant carriers
Prophylactic surgery
Additional considerations for surgery of pathogenic variant breast cancer are the roles of prophylactic contralateral mastectomy and salpingo-oophorectomy in patient prognosis. A study by Biglia, et al. [74] indicated that the probability of contralateral breast cancer 10 years after breast cancer surgery was 5% in sporadic breast cancer compared with 27% and 19% in BRCA1 and BRCA2 pathogenic variant breast cancer, respectively. However, contralateral mastectomy showed no survival gain in pathogenic variant carriers. According to these results, contralateral mastectomy in BRCA1/BRCA2 pathogenic variant breast cancer should be carried out for preventing contralateral breast cancer, not for improving survival. Prophylactic bilateral salpingo-oophorectomy in BRCA1/BRCA2 pathogenic variant breast cancer reduced ipsilateral and contralateral breast cancer recurrences [42]. Furthermore, it significantly decreased breast cancer mortality in BRCA1/BRCA2 pathogenic variant breast cancer patients [75]. However, breast cancer mortality was reduced only in BRCA1 pathogenic variant breast cancer patients [76]. Recent studies have indicated that BRCA1/BRCA2 pathogenic variant carriers have a higher risk of ovarian cancer, which tends to occur at a younger age in BRCA1 than in BRCA2 pathogenic variant carriers [3,77,78]. Prophylactic salpingo-oophorectomy in healthy BRCA1/BRCA2 pathogenic variant carriers reportedly reduces the risk of ovarian cancer by more than 80% [79,80]. Based on the above findings, prophylactic bilateral salpingo-oophorectomy should be considered in BRCA1/BRCA2 pathogenic variant carriers not only to reduce the risk of breast cancer but also for protection against ovarian cancer.
Chemoprevention with tamoxifen
The primary preventive effect of tamoxifen (selective ER modulator) on breast cancer in BRCA1/BRCA2 pathogenic variant carriers was examined by the National Surgical Adjuvant Breast and Bowel Project [81]. The breast cancer risk ratios in tamoxifen-treated BRCA1/BRCA2 pathogenic variant carriers were 1.67 (95% CI, 0.32–10.70) and 0.38 (95% CI, 0.06–1.56), respectively. Based on these results, the protective effect of tamoxifen in BRCA1/BRCA2 variant carriers is very limited, with a slightly better effect in BRCA2 pathogenic variant carriers. This limited effect might be related to the limited number of cases in that study (i.e., eight and 11 BRCA1 and BRCA2 pathogenic variant carriers, respectively) [81]. Regarding the secondary preventive effect of tamoxifen, a study on 1,583 BRCA1 and 881 BRCA2 pathogenic variant carriers showed a 62% breast cancer risk reduction (95% CI, 0.27–0.55) in BRCA1 pathogenic variant carriers and a 67% risk reduction (95% CI, 0.22–0.50) in BRCA2 pathogenic variant carriers [82]. In that study, there were no differences in the risk reduction rate according to the hormone receptor status of the primary breast cancer. It has been suggested that aromatase inhibitors (AIs) can prevent breast cancer in postmenopausal women [83]; however, the preventive role of AIs in BRCA1/BRCA2 pathogenic variant breast cancer has not been reported.
RECOMMENDATION OF BRCA PATHOGENIC VARIANT SCREENING IN BREAST CANCER PATIENTS
If a BRCA1/BRCA2 pathogenic variant is suspected, genetic testing is required. The American Society of Clinical Oncology and National Comprehensive Cancer Network (NCCN) recommend genetic testing if the BRCA pathogenic variant risk is high, with risk factors including family history of BRCA pathogenic variant, family history of breast or ovarian cancer, diagnosis of breast cancer or ovarian cancer, male breast cancer, onset of breast cancer at young age, and diagnosis of TNBC at age 60 years or younger [84,85]. More detailed BRCA genetic testing criteria are as follows: there are more than three breast cancer patients in the family other than the patient, regardless of the age at diagnosis; there are two breast cancer patients in the family and at least one was diagnosed at an age under 50 years; the patient has breast cancer and another family member is diagnosed as having squamous ovarian cancer; the patient has bilateral breast cancer; the patient is diagnosed as having breast cancer at an age under 45 years; and the patient is also diagnosed as having squamous ovarian cancer. The NCCN guidelines recommend BRCA genetic testing if a breast cancer patient has two or more family members with a history of pancreas cancer or has more than two third-degree relatives diagnosed as having prostate cancer (Gleason score ≥7) [85].
Recently, US Preventive Services Task Force updated its recommendation for genetic testing for BRCA1 and BRCA2 pathogenic variants. It suggests assessment for women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer or who have an ancestry associated with BRCA1/2 pathogenic variants with an appropriate brief familial risk assessment tool [86].
SURVEILLANCE OF BRCA PATHOGENIC VARIANT CARRIERS
The most frequent cancers in BRCA pathogenic variant carriers are breast and ovarian cancers in women and prostate cancer in men [2,3,87]. Regular screening for these cancers is recommended. Breast cancer screening in women should start at the age of 18 years with monthly self-examination, followed by regular breast examination by a physician from the age of 25 years. Annual breast magnetic resonance imaging is recommended for women of 25–29 years of age, and mammography should be added from the age of 30 years. Ovarian cancer screening by vaginal sonography and CA125 blood testing is suggested from the age of 30 years, despite the lack of evidence of its superiority over prophylactic salpingo-oophorectomy [88,89,90,91]. For men, monthly self-examination and annual breast examination by a physician should be started at the age of 35 years. Men are recommended to start prostate cancer screening at the age of 45 years [87,92,93]. BRCA pathogenic variants also increase colorectal and pancreatic cancer risks [93,94,95,96]; screening tests for these cancers should be performed according to general cancer examination principles [92].
CONCLUSIONS
We described clinical aspects of BRCA1/BRCA2 pathogenic variant breast cancers. In BRCA1/BRCA2 pathogenic variant breast cancer patients, surgical and radiation treatment outcomes were not inferior to those in sporadic breast cancer patients. For systemic treatment, platinum-based chemotherapy is thought to be effective. PARP inhibitors have been introduced recently and are increasingly used in metastatic breast cancer patients with BRCA1/BRCA2 pathogenic variants. To inhibit secondary breast cancer in BRCA1/BRCA2 pathogenic variants patients, prophylactic contralateral mastectomy and salpingo-oophorectomy could be considered. Chemoprevention using tamoxifen has shown effectiveness in secondary breast cancer. However, its role in prevention of primary breast cancer in healthy BRCA1/BRCA2 pathogenic variants carriers is not confirmed. If BRCA1/BRCA2 pathogenic variants are suspected, BRCA genetic testing is required, and for carriers of these variants, genetic counseling is indispensable. Additionally, for the better treatment and genetic counselling of BRCA1/BRCA2 pathogenic variants carriers, further studies on BRCA variants of uncertain significance, which account for 10–20% of BRCA genetic testing results, should be performed.
Footnotes
Conflicts of Interest: The authors have declared that there are no competing interests.
- Conceptualization and writing: Anbok Lee.
- Formal analysis: Byung-In Moon.
- Data curation: Tae Hyun Kim.
References
- 1.Mahdavi M, Nassiri M, Kooshyar MM, Vakili-Azghandi M, Avan A, Sandry R, et al. Hereditary breast cancer; genetic penetrance and current status with BRCA. J Cell Physiol. 2019;234:5741–5750. doi: 10.1002/jcp.27464. [DOI] [PubMed] [Google Scholar]
- 2.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson D, Easton DF Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi A, de Giacomi C, Magri MD, Lombardi D, Zanetti M, Scuderi C, et al. Familial breast cancer: characteristics and outcome of BRCA 1-2 positive and negative cases. BMC Cancer. 2005;5:70. doi: 10.1186/1471-2407-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debniak T, Scott RJ, Górski B, Cybulski C, van de Wetering T, Serrano-Fernandez P, et al. Common variants of DNA repair genes and malignant melanoma. Eur J Cancer. 2008;44:110–114. doi: 10.1016/j.ejca.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Kadouri L, Hubert A, Rotenberg Y, Hamburger T, Sagi M, Nechushtan C, et al. Cancer risks in carriers of the BRCA1/2 Ashkenazi founder mutations. J Med Genet. 2007;44:467–471. doi: 10.1136/jmg.2006.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cybulski C, Górski B, Gronwald J, Huzarski T, Byrski T, Debniak T, et al. BRCA1 mutations and prostate cancer in Poland. Eur J Cancer Prev. 2008;17:62–66. doi: 10.1097/CEJ.0b013e32809b4d20. [DOI] [PubMed] [Google Scholar]
- 9.Suchy J, Cybulski C, Górski B, Huzarski T, Byrski T, Dębniak T, et al. BRCA1 mutations and colorectal cancer in Poland. Fam Cancer. 2010;9:541–544. doi: 10.1007/s10689-010-9378-x. [DOI] [PubMed] [Google Scholar]
- 10.Moynahan ME, Chiu JW, Koller BH, Jasin M. BRCA1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 11.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 12.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 13.Orban TI. Emerging roles of BRCA1 alternative splicing. Mol Pathol. 2003;56:191–197. doi: 10.1136/mp.56.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stecklein SR, Jensen RA, Pal A. Genetic and epigenetic signatures of breast cancer subtypes. Front Biosci (Elite Ed) 2012;4:934–949. doi: 10.2741/E431. [DOI] [PubMed] [Google Scholar]
- 15.D'Andrea AD. The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 16.Albertsen H, Plaetke R, Ballard L, Fujimoto E, Connolly J, Lawrence E, et al. Genetic mapping of the BRCA1 region on chromosome 17q21. Am J Hum Genet. 1994;54:516–525. [PMC free article] [PubMed] [Google Scholar]
- 17.Wooster R, Neuhausen SL, Mangion J, Quirk Y, Ford D, Collins N, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265:2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 18.Bork P, Blomberg N, Nilges M. Internal repeats in the BRCA2 protein sequence. Nat Genet. 1996;13:22–23. doi: 10.1038/ng0596-22. [DOI] [PubMed] [Google Scholar]
- 19.Chen PL, Chen CF, Chen Y, Xiao J, Sharp ZD, Lee WH. The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc Natl Acad Sci U S A. 1998;95:5287–5292. doi: 10.1073/pnas.95.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong AK, Pero R, Ormonde PA, Tavtigian SV, Bartel PL. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene BRCA2. J Biol Chem. 1997;272:31941–31944. doi: 10.1074/jbc.272.51.31941. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Silver DP, Walpita D, Cantor SB, Gazdar AF, Tomlinson G, et al. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998;2:317–328. doi: 10.1016/s1097-2765(00)80276-2. [DOI] [PubMed] [Google Scholar]
- 22.Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med. 2010;12:245–259. doi: 10.1097/GIM.0b013e3181d38f2f. [DOI] [PubMed] [Google Scholar]
- 23.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 24.Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 25.Kim S. Korean hereditary breast cancer. J Korean Med Assoc. 2009;52:952–962. [Google Scholar]
- 26.Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8:3131–3141. doi: 10.7150/jca.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 28.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 29.Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15:e625–e634. doi: 10.1016/S1470-2045(14)70364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peshkin BN, Alabek ML, Isaacs C. BRCA1/2 mutations and triple negative breast cancers. Breast Dis. 2010;32:25–33. doi: 10.3233/BD-2010-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansquer Y, Gautier C, Fourquet A, Asselain B, Stoppa-Lyonnet D. Survival in early-onset BRCA1 breast-cancer patients. Institut Curie Breast Cancer Group. Lancet. 1998;352:541. doi: 10.1016/s0140-6736(05)79248-5. [DOI] [PubMed] [Google Scholar]
- 32.Chappuis PO, Kapusta L, Bégin LR, Wong N, Brunet JS, Narod SA, et al. Germline BRCA1/2 mutations and p27(Kip1) protein levels independently predict outcome after breast cancer. J Clin Oncol. 2000;18:4045–4052. doi: 10.1200/JCO.2000.18.24.4045. [DOI] [PubMed] [Google Scholar]
- 33.Stoppa-Lyonnet D, Ansquer Y, Dreyfus H, Gautier C, Gauthier-Villars M, Bourstyn E, et al. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol. 2000;18:4053–4059. doi: 10.1200/JCO.2000.18.24.4053. [DOI] [PubMed] [Google Scholar]
- 34.El-Tamer M, Russo D, Troxel A, Bernardino LP, Mazziotta R, Estabrook A, et al. Survival and recurrence after breast cancer in BRCA1/2 mutation carriers. Ann Surg Oncol. 2004;11:157–164. doi: 10.1245/aso.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 35.van den Broek AJ, Schmidt MK, van't Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what's the evidence? A systematic review with meta-analysis. PLoS One. 2015;10:e0120189. doi: 10.1371/journal.pone.0120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19:169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4975. doi: 10.1097/MD.0000000000004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce LJ, Phillips KA, Griffith KA, Buys S, Gaffney DK, Moran MS, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010;121:389–398. doi: 10.1007/s10549-010-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallard A, Magné N, Guy JB, Espenel S, Rancoule C, Diao P, et al. Is breast-conserving therapy adequate in BRCA 1/2 mutation carriers? The radiation oncologist's point of view. Br J Radiol. 2019;92:20170657. doi: 10.1259/bjr.20170657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirova YM, Savignoni A, Sigal-Zafrani B, de La Rochefordiere A, Salmon RJ, This P, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat. 2010;120:119–126. doi: 10.1007/s10549-009-0685-6. [DOI] [PubMed] [Google Scholar]
- 41.Pierce LJ, Strawderman M, Narod SA, Oliviotto I, Eisen A, Dawson L, et al. Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol. 2000;18:3360–3369. doi: 10.1200/JCO.2000.18.19.3360. [DOI] [PubMed] [Google Scholar]
- 42.Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144:443–455. doi: 10.1007/s10549-014-2890-1. [DOI] [PubMed] [Google Scholar]
- 43.Iglehart JD. Synthetic lethality--a new direction in cancer-drug development. N Engl J Med. 2009;361:189–191. doi: 10.1056/NEJMe0903044. [DOI] [PubMed] [Google Scholar]
- 44.Carey LA, Sharpless NE. PARP and cancer--if it's broke, don't fix it. N Engl J Med. 2011;364:277–279. doi: 10.1056/NEJMe1012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96:1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA crosslinking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 47.Fedier A, Steiner RA, Schwarz VA, Lenherr L, Haller U, Fink D. The effect of loss of BRCA1 on the sensitivity to anticancer agents in p53-deficient cells. Int J Oncol. 2003;22:1169–1173. [PubMed] [Google Scholar]
- 48.Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88:1285–1291. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chabalier C, Lamare C, Racca C, Privat M, Valette A, Larminat F. BRCA1 downregulation leads to premature inactivation of spindle checkpoint and confers paclitaxel resistance. Cell Cycle. 2006;5:1001–1007. doi: 10.4161/cc.5.9.2726. [DOI] [PubMed] [Google Scholar]
- 50.Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol. 2011;29:3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kriege M, Jager A, Hooning MJ, Huijskens E, Blom J, van Deurzen CH, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. 2012;118:899–907. doi: 10.1002/cncr.26351. [DOI] [PubMed] [Google Scholar]
- 52.Zimmer AS, Gillard M, Lipkowitz S, Lee JM. Update on PARP inhibitors in breast cancer. Curr Treat Options Oncol. 2018;19:21. doi: 10.1007/s11864-018-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3:257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 57.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas HD, Calabrese CR, Batey MA, Canan S, Hostomsky Z, Kyle S, et al. Preclinical selection of a novel poly (ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther. 2007;6:945–956. doi: 10.1158/1535-7163.MCT-06-0552. [DOI] [PubMed] [Google Scholar]
- 59.Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer. 2016;114:723–730. doi: 10.1038/bjc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 61.Lee JM, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106:dju089. doi: 10.1093/jnci/dju089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balmaña J, Tung NM, Isakoff SJ, Graña B, Ryan PD, Saura C, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol. 2014;25:1656–1663. doi: 10.1093/annonc/mdu187. [DOI] [PubMed] [Google Scholar]
- 63.Miller K, Tong Y, Jones DR, Walsh T, Danso MA, Ma CX, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple negative breast cancer: final efficacy results of Hoosier Oncology Group BRE09-146. J Clin Oncol. 2015;33(S15):1082. [Google Scholar]
- 64.Wilson RH, Evans TRJ, Middleton MR, Molife LR, Spicer J, Dieras V, et al. A phase I study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br J Cancer. 2017;116:884–892. doi: 10.1038/bjc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Somlo G, Frankel PH, Luu TH, Ma CX, Arun B, Garcia AA, et al. Efficacy of the PARP inhibitor (PI) ABT- 888 (veliparib [vel]) either with carboplatin (carb) or as a single agent followed by post-progression therapy in combination with carb in patients (pts) with BRCA1- or BRCA2-(BRCA)-associated metastatic breast cancer (MBC) J Clin Oncol. 2015;33:520. [Google Scholar]
- 66.Han HS, Diéras V, Robson M, Palácová M, Marcom PK, Jager A, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/ metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29:154–161. doi: 10.1093/annonc/mdx505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tung N, Wang Y, Collins LC, Kaplan J, Li H, Gelman R, et al. Estrogen receptor positive breast cancers in BRCA1 mutation carriers: clinical risk factors and pathologic features. Breast Cancer Res. 2010;12:R12. doi: 10.1186/bcr2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomarkers Prev. 2012;21:134–147. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larsen MJ, Kruse TA, Tan Q, Lænkholm AV, Bak M, Lykkesfeldt AE, et al. Classifications within molecular subtypes enables identification of BRCA1/BRCA2 mutation carriers by RNA tumor profiling. PLoS One. 2013;8:e64268. doi: 10.1371/journal.pone.0064268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah PD, Patil S, Dickler MN, Offit K, Hudis CA, Robson ME. Twenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: differences based on germline mutation status. Cancer. 2016;122:1178–1184. doi: 10.1002/cncr.29903. [DOI] [PubMed] [Google Scholar]
- 71.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 72.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner NC, Telli ML, Rugo HS, Mailliez A, Ettl J, Grischke EM, et al. A Phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations (ABRAZO) Clin Cancer Res. 2019;25:2717–2724. doi: 10.1158/1078-0432.CCR-18-1891. [DOI] [PubMed] [Google Scholar]
- 74.Biglia N, D'Alonzo M, Sgro LG, Tomasi Cont N, Bounous V, Robba E. Breast cancer treatment in mutation carriers: surgical treatment. Minerva Ginecol. 2016;68:548–556. [PubMed] [Google Scholar]
- 75.Li X, You R, Wang X, Liu C, Xu Z, Zhou J, et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: a meta-analysis and systematic review. Clin Cancer Res. 2016;22:3971–3981. doi: 10.1158/1078-0432.CCR-15-1465. [DOI] [PubMed] [Google Scholar]
- 76.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Satagopan JM, Boyd J, Kauff ND, Robson M, Scheuer L, Narod S, et al. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res. 2002;8:3776–3781. [PubMed] [Google Scholar]
- 78.King MC, Marks JH, Mandell JB New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 79.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.King MC, Wieand S, Hale K, Lee M, Walsh T, Owens K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: national Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 82.Phillips KA, Milne RL, Rookus MA, Daly MB, Antoniou AC, Peock S, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091–3099. doi: 10.1200/JCO.2012.47.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Behan LA, Amir E, Casper RF. Aromatase inhibitors for prevention of breast cancer in postmenopausal women: a narrative review. Menopause. 2015;22:342–350. doi: 10.1097/GME.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 84.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66:43–73. doi: 10.3322/caac.21319. [DOI] [PubMed] [Google Scholar]
- 85.NCCN. The NCCN Clinical Practice Guidelines in Oncology, genetic/familial high-risk assessment: breast and ovarian. 3.2019 version. 2019. [Google Scholar]
- 86.US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:652–665. doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- 87.Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 88.Burke W, Daly M, Garber J, Botkin J, Kahn MJ, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer II BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997–1003. [PubMed] [Google Scholar]
- 89.Scheuer L, Kauff N, Robson M, Kelly B, Barakat R, Satagopan J, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260–1268. doi: 10.1200/JCO.2002.20.5.1260. [DOI] [PubMed] [Google Scholar]
- 90.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 91.Woodward ER, Sleightholme HV, Considine AM, Williamson S, McHugo JM, Cruger DG. Annual surveillance by CA125 and transvaginal ultrasound for ovarian cancer in both high-risk and population risk women is ineffective. BJOG. 2007;114:1500–1509. doi: 10.1111/j.1471-0528.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 92.Baumiller RC, Cunningham G, Fisher N, Fox L, Henderson M, Lebel R, et al. Code of ethical principles for genetics professionals: an explication. Am J Med Genet. 1996;65:179–183. doi: 10.1002/(SICI)1096-8628(19961028)65:3<179::AID-AJMG2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 93.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22:735–742. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 94.Kirchhoff T, Satagopan JM, Kauff ND, Huang H, Kolachana P, Palmer C, et al. Frequency of BRCA1 and BRCA2 mutations in unselected Ashkenazi Jewish patients with colorectal cancer. J Natl Cancer Inst. 2004;96:68–70. doi: 10.1093/jnci/djh006. [DOI] [PubMed] [Google Scholar]
- 95.Niell BL, Rennert G, Bonner JD, Almog R, Tomsho LP, Gruber SB. BRCA1 and BRCA2 founder mutations and the risk of colorectal cancer. J Natl Cancer Inst. 2004;96:15–21. doi: 10.1093/jnci/djh008. [DOI] [PubMed] [Google Scholar]
- 96.Garber JE, Syngal S. One less thing to worry about: the shrinking spectrum of tumors in BRCA founder mutation carriers. J Natl Cancer Inst. 2004;96:2–3. doi: 10.1093/jnci/djh021. [DOI] [PubMed] [Google Scholar]
- 97.Korean Hereditary Breast Cancer Study group. Hereditary Breast Cancer. 1st ed. Gyeonggi-do: KOONJA publishing; 2012. pp. 393–394. [Google Scholar]