Key Points
Question
Is the metabolically healthy overweight phenotype associated with metabolic abnormalities?
Findings
In this cohort study of 3204 Chinese adults, metabolically healthy overweight was associated with high future risk of glucose abnormality and high blood pressure.
Meaning
These findings suggest that more attention should be given to this unique subtype of overweight and obesity if the results are replicated in additional studies.
Abstract
Importance
Whether the metabolically healthy overweight (MHO) phenotype is resistant to metabolic abnormalities remains unknown.
Objective
To evaluate the association of MHO with glucose level abnormalities and high blood pressure (BP) in Chinese adults.
Design, Setting, and Participants
This prospective cohort study was conducted from January 1, 2013, to October 31, 2018, in the Health Management Center at Ren Ji Hospital, Shanghai, China, using data from 55 155 recruited Chinese adults. Body weight, fasting blood glucose (FBG) level, hemoglobin A1c (HbA1c) level, and BP were measured annually.
Exposures
Metabolically healthy overweight was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) of 24.0 in 2013 (baseline) and 2014, no history of metabolic diseases, and normal FBG level, HbA1c level, BP, lipid profile, serum uric acid level, and liver ultrasonographic findings at baseline; the remaining participants were defined as being metabolically healthy normal.
Main Outcomes and Measures
Glucose level abnormality was confirmed if the FBG level was 101 mg/dL or greater or the HbA1c level was 5.7%, and high BP was confirmed if the systolic BP was 130 mm Hg or higher or the diastolic BP was 80 mm Hg or higher at least twice during the subsequent 4 years of follow-up.
Results
A total of 3204 metabolically healthy Chinese adults (mean [SD] age, 39.8 [10.9] years; 1940 women [60.5%]) were included in the study. The prevalence of MHO was 7.0%. A total of 146 incident cases of glucose level abnormality and 220 cases of high BP during 4 years of follow-up were identified. Compared with the metabolically healthy normal group, the MHO group had a higher risk of glucose level abnormality (adjusted hazard ratio, 2.36; 95% CI, 1.52-3.64) and high BP (adjusted hazard ratio, 1.73; 95% CI, 1.18-2.53) after adjusting for several potential confounders.
Conclusions and Relevance
Metabolically healthy overweight may be associated with a high future risk of glucose abnormality and high BP in Chinese adults. If the individuals are confirmed with MHO, early interventions, including diet and exercise, should be recommended to decrease the risk of developing abnormalities of glucose and BP.
This cohort study assesses the association of metabolically healthy overweight phenotype with the risk of glucose level abnormality and high blood pressure in Chinese adults.
Introduction
The high prevalence of obesity has become an increasing costly burden on both the Asian and the Western health care systems during the past century.1 Obesity is associated with a wide range of metabolic complications, including type 2 diabetes, hypertension, stroke, depression, and certain types of cancer, thus causing a burden to public health.2,3
However, some studies4,5 have found that obesity does not always entail metabolic abnormalities and does not necessarily increase the risk of cardiometabolic complications and mortality. The term metabolically healthy overweight (MHO) has been proposed to describe the unique phenotype of overweight and obese individuals with normal glucose level, blood pressure (BP), and lipid level.6 However, some knowledge gaps need to be addressed. Some7,8,9,10 but not all studies11,12,13,14 found a significant association between MHO and higher risk of metabolic complications. Thus, conclusive results could not be generated as to whether the MHO phenotype is a transitional stage or resistant to metabolic abnormalities based on current evidence. Furthermore, the definition of MHO differs greatly among studies.15 Even in the same population, different criteria of MHO generate mixed results.16 In addition, some metabolic abnormalities, such as hyperuricemia17 and fatty liver,18 were not taken into consideration in any of the aforementioned studies.7,8,9,10,11,12,13,14,15,16,17,18 Therefore, we aimed to evaluate the association between the MHO phenotype and the risk of incident glucose level abnormality and high BP in Chinese adults during 4 years of follow-up.
Methods
Study Population
This cohort study included participants recruited from the Health Management Center at Ren Ji Hospital, Shanghai, China, from January 1, 2013, to October 31, 2018. All adults (18-100 years of age) receiving a routine health checkup at the Health Management Center from January 1 to December 31, 2013, were eligible for the study. The study protocol was approved by the Ethical Committee of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. As a deidentified secondary data analysis, patient consent was waived by the Ethical Committee. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
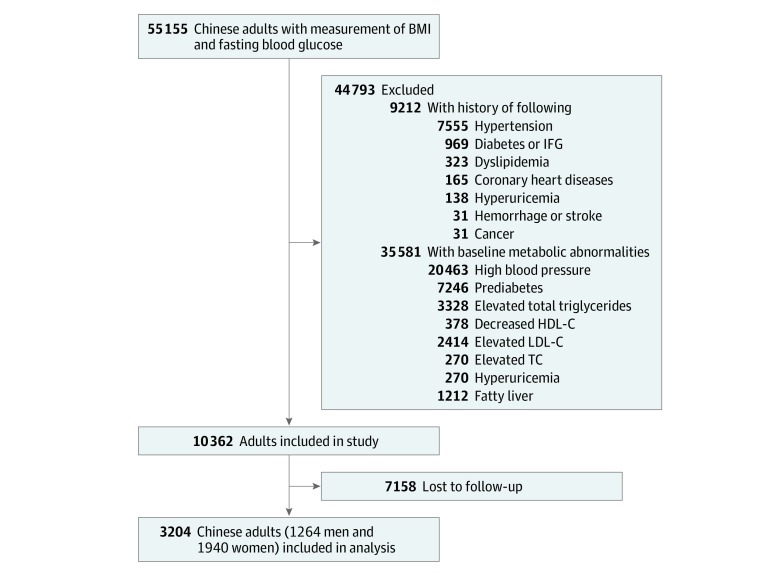
The initial recruitment resulted in the identification of 55 155 individuals. Body weight, the concentrations of hemoglobin A1c (HbA1c) and fasting blood glucose (FBG), and systolic and diastolic BP were annually measured during the subsequent 5 years. To recruit only participants who were metabolically healthy, we excluded 9212 participants with a history of chronic metabolic diseases and cancer. Then we excluded 31 415 participants with metabolic abnormalities (high BP, impaired glucose regulation, elevated concentration of triglycerides, and decreased concentration of high-density lipoprotein cholesterol) based on a joint statement.19 A total of 2414 individuals with total cholesterol level abnormalities, 270 with low-density lipoprotein level abnormalities, 270 with hyperuricemia, and 1212 with fatty liver abnormalities were also considered metabolically unhealthy18,20 and were excluded. Finally, we excluded 7158 participants who were unavailable for follow-up. The final sample size was 3204 metabolically healthy Chinese adults (Figure). Participants included in the study tended to be younger, to more likely be women, and to have lower concentrations of HbA1c and FBG and lower BP at baseline compared with those who were excluded (eTable 1 in the Supplement).
Figure. Sample Recruitment Process.
BMI indicates body mass index; HDL-C, high-density lipoprotein cholesterol; IFG, impaired fasting glucose; LDL-C, low-density lipoprotein cholesterol; and TC, triglycerides.
Assessment of FBG Levels, HbA1c Levels, and Blood Pressure
Venous blood samples were obtained and transfused into vacuum tubes containing EDTA in the morning after participants fasted for 6 hours. The whole blood samples were stored at 4 °C for further analysis. An automatic analyzer (Roche 701 Bioanalyzer) was used to measure FBG with the hexokinase/glucose-6-phosphate dehydrogenase method. The coefficient of variation using blind quality control specimens was 2.0%. The concentration of HbA1c was measured by high-performance liquid chromatography using the fully automated VARIANT II Hemoglobin Testing System (Bio-Rad). The measurement range was 2.0% to 18.0%. Glucose abnormality was confirmed if the FBG level was 101 mg/dL or higher (to convert to millimoles per liter, multiply by 0.0555) or the HbA1c level was 5.7% or higher (to convert to proportion of hemoglobin, multiply by 0.01) at least twice during the subsequent 4 years of follow-up.21
Blood pressure was measured twice using an automatic BP meter (HBP-9020, Omron Co Ltd) after participants were seated for at least 10 minutes. The mean of 2 measurements was recorded for further analysis. High BP was confirmed if systolic BP was 130 mm Hg or higher or diastolic BP was 80 mm Hg or higher at least twice during the subsequent 4 years of follow-up.22
Assessment of Body Weight and Height
Body weight (to the nearest 0.5 kg) and height (to the nearest 0.5 cm) were measured with the patient in the standing position without shoes and in light clothing by using an electronic scale (SK-CK; Shuang Jia Company). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Metabolically healthy overweight was defined as a BMI of 24.0 in 2013 (baseline) and 2014,23 and the remaining participants were considered to be metabolically healthy normal weight (MHN).
Assessment of Other Confounders
The level of high-sensitivity C-reactive protein was measured by the immunoturbidimetric method, whereas serum insulin level was measured by the immunoassay method. Total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, creatinine, and uric acid levels were measured using an automatic biochemical analyzer (Roche 701 Bioanalyzer; Roche). All the measurements were completed in the Clinical Laboratory of Ren Ji Hospital. The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration 2-level race equation.24 The homeostasis model assessment (HOMA) index was calculated using the following equation: HOMA index = [fasting serum insulin × fasting glucose]/22.5. Participants were confirmed as having insulin resistance if their HOMA index was in the top quartile of the distribution among nondiabetic individuals.25 Data on the history of hypertension, diabetes or impaired FBG, dyslipidemia, hyperuricemia, stroke and hemorrhage, and coronary heart diseases (coronary atherosclerosis, coronary artery bypass grafting, stent surgery, and ischemic infarction) were collected using a self-report questionnaire.
Statistical Analysis
We completed all statistical analyses using SAS statistical software, version 9.4 (SAS Institute Inc). Formal hypothesis testing was 2-sided with a significance level of P < .05. Because abnormalities of glucose and BP were confirmed at least twice during the 4 years of follow-up, the person-time of follow-up for each participant was determined from January 1, 2014, to the first onset date of the outcomes (incident glucose abnormality and high BP) or the end of follow-up (December 31, 2018), whichever came first.
We used the Cox proportional hazards regression model to evaluate the association between MHO phenotype and the assessed outcomes. We adjusted the potential confounders in 2 different models: model 1 adjusted for age and sex and model 2 further adjusted for systolic BP; diastolic BP; FBG, HbA1c, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, and uric acid levels; and estimated glomerular filtration rate. We further adjusted for baseline high-sensitivity C-reactive protein and HOMA index to assess whether the potential association between MHO phenotype and the outcomes was related to inflammatory status and insulin resistance.
Likelihood ratio tests were conducted to examine statistical interactions among MHO, sex, and age (<65 vs ≥65 years) in association with abnormalities of glucose and BP by comparing −2 log likelihood χ2 between nested models with and without the cross-product terms. To test the robustness of the main results, we conducted 5 sensitivity analyses in model 2. We used the cumulative mean BMI during follow-up as the exposure. Participants were also classified into the 2 following groups based on their cumulative mean BMI: MHN (BMI, 18.5-23.9) and MHO (BMI, ≥24.0).23 Then we censored older participants (≥65 years of age), with overweight in 2013 or 2014, elevated high-sensitivity C-reactive protein level (≥1.0 mg/L [to convert to nanomoles per liter, multiply by 9.524]),26 and insulin resistance (HOMA index, ≥75th percentile).25
Results
A total of 3204 metabolically healthy Chinese adults (mean [SD] age, 39.8 [10.9] years; 1940 women [60.5%]) were included in the study. The prevalence of MHO was 7.0%. The mean (SD) participant findings were as follows: BMI, 21.8 (2.5); FBG level, 86 (7) mg/dL; HbA1c level, 5.1% (0.2%); systolic BP, 109.5 (9.6) mm Hg; and diastolic BP, 67.2 (7.0) mm Hg. Metabolically healthy overweight was associated with mean (SD) baseline age (42.5 [10.6] years for MHO vs 39.6 [10.9] years for MHN; P < .001), systolic BP (113.0 [8.9] mm Hg for MHO vs 109.2 [9.6] mm Hg for MHN; P < .001), diastolic BP (69.3 [6.7] mm Hg for MHO vs 67.0 [7.0] mm Hg for MHN; P < .001), HOMA index (1.3 [0.6] for MHO vs 1.0 [0.6] for MHN; P < .001), alanine aminotransferase (19.2 [12.0] U/L for MHO vs 16.3 [13.6] U/L for MHN; P = .002 [to convert aminotransferase to microkatals per liter, multiply by 0.0167]), high-density lipoprotein cholesterol (54 [12] mg/dL for MHO vs 62 [12] for MHN; P < .001) and low-density lipoprotein cholesterol (100 [19] mg/dL for MHO vs 97 [19] mg/dL for MHN; P = .003 [to convert high-density lipoprotein and low-density lipoprotein cholesterol to millimoles per liter, multiply by 0.0259]), and uric acid levels (5.2 [1.2] mg/dL for MHO vs 4.8 [1.2] mg/dL for MHN; P < .001 [to convert uric acid to micromoles per liter, multiply by 59.485]) (Table 1).
Table 1. Baseline Characteristics Across Different Baseline Body Weight Status in 3204 Chinese Adultsa.
| Characteristic | MHN Group (n = 2981) | MHO Group (n = 223) | P Value |
|---|---|---|---|
| Sex, No. (%) | |||
| Men | 1133 (38.0) | 131 (58.7) | <.001 |
| Women | 1848 (62.0) | 92 (41.3) | |
| Age, y | 39.6 (10.9) | 42.5 (10.6) | <.001 |
| Blood pressure, mm Hg | |||
| Systolic | 109.2 (9.6) | 113.0 (8.9) | <.001 |
| Diastolic | 67.0 (7.0) | 69.3 (6.7) | <.001 |
| Fasting blood glucose level, mg/dL | 86 (7) | 86 (5) | .34 |
| Hemoglobin A1c level, % | 5.1 (0.2) | 5.2 (0.3) | .16 |
| HOMA indexb | 1.0 (0.6) | 1.3 (0.6) | <.001 |
| Aminotransferase level, U/L | |||
| Alanine | 16.3 (13.6) | 19.2 (12.0) | .002 |
| Aspartate | 18.2 (8.3) | 18.2 (5.7) | .91 |
| Cholesterol level, mg/dL | |||
| Total | 174 (23) | 174 (23) | .89 |
| Triglycerides | 80 (27) | 88 (27) | <.001 |
| High-density lipoprotein | 62 (12) | 54 (12) | <.001 |
| Low-density lipoprotein | 97 (19) | 100 (19) | .003 |
| Uric acid level, mg/dL | 4.8 (1.2) | 5.2 (1.2) | <.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 123.9 (26.1) | 120.7 (25.9) | .62 |
Abbreviations: HOMA, homeostasis model assessment; MHN, metabolically healthy normal weight; MHO, metabolically healthy overweight.
SI conversion factors: to convert fasting blood glucose to millimoles per liter, multiply by 0.0555; hemoglobin A1c to proportion of hemoglobin, multiply by 0.01; aminotransferase to microkatals per liter, multiply by 0.0167; total, high-density lipoprotein, and low-density lipoprotein cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113; and uric acid to micromoles per liter, multiply by 59.485.
Data are presented as mean (SD) unless otherwise indicated. Metabolically healthy overweight was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) of 24.0 in 2013 (baseline) and 2014, and the remaining participants were considered metabolically healthy normal weight.
The HOMA index was calculated as follows: HOMA index = [fasting serum insulin × fasting glucose]/22.5.
We identified 146 incident cases of glucose level abnormality and 220 cases of high BP during 4 years of follow-up. Compared with MHN, MHO was associated with a high risk of glucose level abnormality (adjusted hazard ratio [HR], 2.36; 95% CI, 1.52-3.64) and high BP (adjusted HR, 1.73; 95% CI, 1.18-2.53) after adjusting for several potential confounders (Table 2). Further adjusting for baseline high-sensitivity C-reactive protein level (for glucose abnormality: adjusted HR, 2.36; 95% CI, 1.52-3.68; for high BP: adjusted HR, 1.65; 95% CI, 1.12,-2.44) and HOMA insulin resistance index (for glucose abnormality: adjusted HR, 2.58; 95% CI, 1.65-4.04; for high BP: adjusted HR, 1.83; 95% CI, 1.23-2.71), the association between MHO and abnormalities of glucose and BP did not change (Table 2).
Table 2. Risks of Incident Glucose Abnormality and High Blood Pressure by Body Weight Status During 4-Year Follow-up Among 3204 Chinese Adultsa.
| Outcome | Adjusted HR (95% CI) | |
|---|---|---|
| MHN Group (n = 2981) | MHO Group (n = 223) | |
| Glucose Level Abnormality | ||
| Participants, No. (%) | 2981 (93.0) | 223 (7.0) |
| Incident cases, No. (%) | 119 (81.5) | 27 (18.5) |
| Age- and sex-adjusted model | 1 [Reference] | 2.47 (1.62-3.77) |
| Multiple-adjusted modelb | 1 [Reference] | 2.36 (1.52-3.64) |
| Further adjusting for baseline hs-CRP | 1 [Reference] | 2.36 (1.52-3.68) |
| Further adjusting for baseline HOMA indexc | 1 [Reference] | 2.58 (1.65-4.04) |
| High Blood Pressure | ||
| Participants, No. (%) | 2981 (93.0) | 223 (7.0) |
| Incident cases, No. (%) | 185 (84.1) | 35 (15.9) |
| Age- and sex-adjusted model | 1 [Reference] | 2.18 (1.52-3.14) |
| Multiple-adjusted modelb | 1 [Reference] | 1.73 (1.18-2.53) |
| Further adjusting for baseline hs-CRP | 1 [Reference] | 1.65 (1.12-2.44) |
| Further adjusting for baseline HOMA indexc | 1 [Reference] | 1.83 (1.23-2.71) |
Abbreviations: HOMA, homeostasis model assessment; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; MHN, metabolically healthy normal weight; MHO, metabolically healthy overweight.
Metabolically healthy overweight was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) of 24.0 in 2013 (baseline) and 2014, and the remaining participants were considered metabolically healthy normal weight.
Multiple-adjusted model was adjusted for the following: age; sex; systolic blood pressure; diastolic blood pressure; fasting blood glucose, hemoglobin A1c, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, and uric acid levels; and estimated glomerular filtration rate.
The HOMA index was calculated as follows: HOMA index = [fasting serum insulin × fasting glucose]/22.5.
Sex and age modified the association between MHO and abnormality of glucose levels, whereas sex but not age modified the association between MHO and high BP (eTable 2 in the Supplement). Use of the cumulative mean BMI as the exposure (for glucose abnormality: adjusted HR, 2.33; 95% CI, 1.60-3.38; for high BP: adjusted HR, 2.50; 95% CI, 1.83-3.41) or excluding older participants (for glucose abnormality: adjusted HR, 2.43; 95% CI, 1.54-3.85; for high BP: adjusted HR, 1.88; 95% CI, 1.27-2.77), with elevated high-sensitivity C-reactive protein levels (for glucose abnormality: adjusted HR, 2.14; 95% CI, 1.21-3.78; for high BP: adjusted HR, 1.85; 95% CI, 1.18-2.92), insulin resistance (for glucose abnormality: adjusted HR, 2.11; 95% CI, 1.21-3.67), and overweight in 2013 (baseline) or 2014 (for glucose abnormality: adjusted HR, 2.20; 95% CI, 1.41-3.42; for high BP: adjusted HR, 1.60; 95% CI, 1.09-2.36) generated similar results except for an association between MHO and high BP (HR, adjusted 1.61; 95% CI, 0.99-2.62) after excluding those with insulin resistance (Table 3 and Table 4).
Table 3. Sensitivity Analyses of Risks of Incident Glucose Abnormality by Body Weight Status During 4-Year Follow-up Among 3204 Chinese Adultsa.
| Sensitivity Analysis | MHN Group (n = 2981) | MHO Group (n = 223) |
|---|---|---|
| Sensitivity Analysis 1b | ||
| Participants, No. (%) | 2797 (87.3) | 407 (12.7) |
| Cases of glucose level abnormality, No. (%) | 97 (66.4) | 49 (33.6) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 2.33 (1.60-3.38) |
| Sensitivity Analysis 2d | ||
| Participants, No. (%) | 2855 (92.9) | 217 (7.1) |
| Cases of glucose level abnormality, No. (%) | 106 (80.9) | 25 (19.1) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 2.43 (1.54-3.85) |
| Sensitivity Analysis 3e | ||
| Participants, No. (%) | 2505 (94.2) | 153 (5.8) |
| Cases of glucose level abnormality, No. (%) | 93 (86.1) | 15 (13.9) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 2.14 (1.21-3.78) |
| Sensitivity Analysis 4f | ||
| Participants, No. (%) | 2295 (94.5) | 133 (5.5) |
| Cases of glucose level abnormality, No. (%) | 94 (85.5) | 16 (14.5) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 2.11 (1.21-3.67) |
| Sensitivity Analysis 5g | ||
| Participants, No. (%) | 2711 (92.4) | 223 (7.6) |
| Cases of glucose level abnormality, No. (%) | 110 (80.3) | 27 (19.7) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 2.20 (1.41-3.42) |
Abbreviations: MHN, metabolically healthy normal weight; MHO, metabolically healthy overweight.
Metabolically healthy overweight was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) of 24.0 in 2013 (baseline) and 2014, and the remaining participants were considered metabolically healthy normal weight.
Sensitivity analysis 1 used the cumulative mean body mass index (2013-2018) as the exposure.
Multiple-adjusted model was adjusted for the following: age; sex; systolic blood pressure; diastolic blood pressure; fasting blood glucose, hemoglobin A1c, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, and uric acid levels; and estimated glomerular filtration rate.
Sensitivity analysis 2 excluded older adults (n = 132).
Sensitivity analysis 3 excluded participants with high C-reactive protein levels (≥1 mg/L) at baseline (n = 546).
Sensitivity analysis 4 excluded participants with insulin resistance (homeostasis assessment model index ≥75th percentile) at baseline (n = 776).
Sensitivity analysis 5 excluded participants with overweight in 2013 (baseline) or 2014 (n = 270).
Table 4. Sensitivity Analyses of Risks of Incident High Blood Pressure by Body Weight Status During 4-Year Follow-up Among 3204 Chinese Adultsa.
| Sensitivity Analysis | MHN Group (n = 2981) | MHO Group (n = 223) |
|---|---|---|
| Sensitivity Analysis 1b | ||
| Participants, No. (%) | 2797 (87.3) | 407 (12.7) |
| Cases of high blood pressure, No. (%) | 147 (66.8) | 73 (33.2) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 2.50 (1.83-3.41) |
| Sensitivity Analysis 2d | ||
| Participants, No. (%) | 2855 (92.9) | 217 (7.1) |
| Cases of high blood pressure, No. (%) | 163 (82.7) | 34 (17.3) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 1.88 (1.27-2.77) |
| Sensitivity Analysis 3e | ||
| Participants, No. (%) | 2505 (94.2) | 153 (5.8) |
| Cases of high blood pressure, No. (%) | 154 (86.5) | 24 (13.5) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 1.85 (1.18-2.92) |
| Sensitivity Analysis 4f | ||
| Participants, No. (%) | 2295 (94.5) | 133 (5.5) |
| Cases of high blood pressure, No. (%) | 141 (87. 0) | 21 (13.0) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 1.61 (0.99-2.62) |
| Sensitivity Analysis 5g | ||
| Participants, No. (%) | 2711 (92.4) | 223 (7.6) |
| Cases of high blood pressure, No. (%) | 167 (80.3) | 35 (19.7) |
| Multiple-adjusted model adjusted hazard ratio (95% CI)c | 1 [Reference] | 1.60 (1.09-2.36) |
Abbreviations: MHN, metabolically healthy normal weight; MHO, metabolically healthy overweight.
Metabolically healthy overweight was defined as a body mass index (calculated as weight in kilograms divided by height in meters squared) of 24.0 in 2013 (baseline) and 2014, and the remaining participants were considered metabolically healthy normal weight.
Sensitivity analysis 1 used the cumulative mean body mass index (2013-2018) as the exposure.
Multiple-adjusted model was adjusted for the following: age; sex; systolic blood pressure; diastolic blood pressure; fasting blood glucose, hemoglobin A1c, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, alanine aminotransferase, aspartate aminotransferase, and uric acid levels; and estimated glomerular filtration rate.
Sensitivity analysis 2 excluded older adults (n = 132).
Sensitivity analysis 3 excluded participants with high C-reactive protein levels (≥1 mg/L) at baseline (n = 546).
Sensitivity analysis 4 excluded participants with insulin resistance (homeostasis assessment model index ≥75th percentile) at baseline (n = 776).
Sensitivity analysis 5 excluded participants with overweight in 2013 (baseline) and 2014 (n = 270).
Discussion
In the current prospective cohort study, we found that MHO was associated with glucose level abnormality and high BP in 3204 Chinese adults without a history of major metabolic diseases and whose blood glucose level, HbA1c level, BP, lipid profile, uric acid level, and liver ultrasonographic findings were normal at baseline. The key strength of our research was the strict exclusion of people with a range of metabolic disorders, which provided greater breadth of insights regarding accurate estimates of metabolic outcomes of the MHO phenotype.
The lack of a consensus definition of metabolically healthy was often given as a reason for the discrepancies of the associations between MHO and metabolic abnormalities among previous studies. Even in the same study population, different criteria of MHO could generate mixed results.16 Different criteria have been used depending on the number of abnormalities (eg, 0-1, ≤2, and ≤3), HOMA index, and the combination.15,27 Usually, metabolic abnormalities have been defined based on the FBG level, BP, and lipid profile. However, determinants of MHO should not be limited to these factors only. For example, hyperuricemia17 and fatty liver18 have also been associated with metabolic abnormalities. Furthermore, it is doubtful whether an individual with 1 type of metabolic abnormality, such as high BP, could be considered as metabolically healthy.
To eliminate the potential bias associated with the disagreement of the definition, we defined metabolically healthy as follows: no history of diabetes or impaired FBG, hypertension, dyslipidemia, cardiovascular heart diseases, stroke or hemorrhage, hyperuricemia, or cancer and no metabolic abnormalities based on FBG level, HbA1c level, BP, lipid profile, serum uric acid level, and liver ultrasonographic findings at baseline. To lower the possibility of misclassification, we used repeated measurements (at baseline and in 2014) to define MHO. To our knowledge, the criterion might be the strictest one compared with those in the previous studies.6,7,15 We found that MHO was associated with glucose level abnormality and high BP after adjusting for conventional risk factors and further adjusting for HOMA index and high-sensitivity C-reactive protein level. Our results are supported by another large cohort study10 (3.5 million adults without a history of cardiovascular vascular disease), which also defined metabolically healthy as having no metabolic abnormalities. The authors of that study10 reported that the MHO phenotype was associated with cardiovascular disease events during a mean follow-up of 5.4 years compared with the normal-weight phenotype. Our results, together with those of the aforementioned cohort study10 and a previous review,28 suggest that overweight individuals who are metabolically healthy may have a high risk of developing metabolic abnormalities in the future.
Because BMI is a protective factor against mortality in the elderly population,5 age can be a significant confounder in developing BMI-associated metabolic abnormalities. Thus, to avoid the age interaction, a sensitivity analysis was conducted by stratifying MHO outcomes by age. The results indicated that the association between MHO and glucose abnormality was only significant for those younger than 65 years. The mechanism underlying the complexity of BMI-related risk remained unclear; however, the reason might be associated with BMI having limited value in distinguishing between lean and fat mass.29
Limitations
This study has several limitations. First, the use of medication was scarce. However, we excluded participants with a self-reported history of a series of metabolic diseases, which might mitigate the association with medication. Second, behavior habit, such as diet and physical activities, were not collected in the current analysis. We thus could not analyze the extent to which adjustment for diet and physical activity would have modified the association between MHO and the outcomes. Moreover, the duration of follow-up was relatively short. Also, waist circumference measurements were not collected. A single use of BMI might lead to controversial results.30 Prospective studies with a representative population and deliberate collection of information about potential confounders are warranted to confirm the association of MHO with health.
Conclusions
Metabolically healthy overweight was associated with a high future risk of glucose level abnormality and high BP in Chinese adults. These findings suggest that more attention should be given to this unique subtype of overweight and obesity if the results are replicated in additional studies.
eTable 1. Baseline Characteristics of Included and Excluded Study Participants
eTable 2. Adjusted Hazard Ratios and 95% Confidence Intervals for Risks of Incident Abnormalities of Glucose and Blood Pressure Across Different Body Weight Status During Four-Year Follow-up Among 3204 Chinese Adults: Subgroup Analysis
References
- 1.Xi B, Liang Y, He T, et al. . Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993-2009. Obes Rev. 2012;13(3):-. doi: 10.1111/j.1467-789X.2011.00944.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radwan H, Ballout RA, Hasan H, Lessan N, Karavetian M, Rizk R. The epidemiology and economic burden of obesity and related cardiometabolic disorders in the United Arab Emirates: a systematic review and qualitative synthesis. J Obes. 2018;2018:2185942. doi: 10.1155/2018/2185942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansur RB, Brietzke E, McIntyre RS. Is there a “metabolic-mood syndrome”? a review of the relationship between obesity and mood disorders. Neurosci Biobehav Rev. 2015;52:89-104. doi: 10.1016/j.neubiorev.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71-82. doi: 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng FW, Gao X, Mitchell DC, et al. . Metabolic health status and the obesity paradox in older adults. J Nutr Gerontol Geriatr. 2016;35(3):161-176. doi: 10.1080/21551197.2016.1199004 [DOI] [PubMed] [Google Scholar]
- 6.Stefan N, Kantartzis K, Machann J, et al. . Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609-1616. doi: 10.1001/archinte.168.15.1609 [DOI] [PubMed] [Google Scholar]
- 7.Espinosa De Ycaza AE, Donegan D, Jensen MD. Long-term metabolic risk for the metabolically healthy overweight/obese phenotype. Int J Obes (Lond). 2018;42(3):302-309. doi: 10.1038/ijo.2017.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121(2):230-236. doi: 10.1161/CIRCULATIONAHA.109.887521 [DOI] [PubMed] [Google Scholar]
- 9.Lassale C, Tzoulaki I, Moons KGM, et al. . Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J. 2018;39(5):397-406. doi: 10.1093/eurheartj/ehx448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caleyachetty R, Thomas GN, Toulis KA, et al. . Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70(12):1429-1437. doi: 10.1016/j.jacc.2017.07.763 [DOI] [PubMed] [Google Scholar]
- 11.Meigs JB, Wilson PW, Fox CS, et al. . Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906-2912. doi: 10.1210/jc.2006-0594 [DOI] [PubMed] [Google Scholar]
- 12.Durward CM, Hartman TJ, Nickols-Richardson SM. All-cause mortality risk of metabolically healthy obese individuals in NHANES III. J Obes. 2012;2012:460321. doi: 10.1155/2012/460321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97(7):2482-2488. doi: 10.1210/jc.2011-3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St-Pierre AC, Cantin B, Mauriège P, et al. . Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172(10):1301-1305. doi: 10.1503/cmaj.1040834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Primeau V, Coderre L, Karelis AD, et al. . Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond). 2011;35(7):971-981. doi: 10.1038/ijo.2010.216 [DOI] [PubMed] [Google Scholar]
- 16.Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring). 2012;20(3):651-659. doi: 10.1038/oby.2011.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metabolic Syndrome Study Cooperation Group of Chinese Diabetes Society Suggestions about metabolic syndrome of Chinese Diabetes Society. Chin J Diabetes. 2004;12:156-161. [Google Scholar]
- 18.Sookoian S, Pirola CJ. Review article: shared disease mechanisms between non-alcoholic fatty liver disease and metabolic syndrome: translating knowledge from systems biology to the bedside. Aliment Pharmacol Ther. 2019;49(5):516-527. doi: 10.1111/apt.15163 [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 20.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1-17. doi: 10.1007/978-3-319-48382-5_1 [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81-S90. doi: 10.2337/dc14-S081 [DOI] [PubMed] [Google Scholar]
- 22.Whelton PK, Carey RM, Aronow WS, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115.doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 23.Zhou B; Cooperative Meta-Analysis Group of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:5-10. [PubMed] [Google Scholar]
- 24.Kong X, Ma Y, Chen J, et al. ; Chinese eGFR Investigation Collaboration . Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant. 2013;28(3):641-651. doi: 10.1093/ndt/gfs491 [DOI] [PubMed] [Google Scholar]
- 25.Balkau B, Charles MA; European Group for the Study of Insulin Resistance (EGIR) . Comment on the provisional report from the WHO consultation. Diabet Med. 1999;16(5):442-443. doi: 10.1046/j.1464-5491.1999.00059.x [DOI] [PubMed] [Google Scholar]
- 26.Pearson TA, Mensah GA, Alexander RW, et al. ; Centers for Disease Control and Prevention; American Heart Association . Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499-511. doi: 10.1161/01.CIR.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Wang C, Guan S, et al. . The prevalence of metabolically healthy and unhealthy obesity according to different criteria. Obes Facts. 2019;12(1):78-90. doi: 10.1159/000495852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beh S. Is metabolically healthy obesity a useful concept? Diabet Med. 2019;36(5):539-545. doi: 10.1111/dme.13869 [DOI] [PubMed] [Google Scholar]
- 29.Iliodromiti S, Celis-Morales CA, Lyall DM, et al. . The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J. 2018;39(17):1514-1520. doi: 10.1093/eurheartj/ehy057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chrysant SG, Chrysant GS. The single use of body mass index for the obesity paradox is misleading and should be used in conjunction with other obesity indices. Postgrad Med. 2019;131(2):96-102. doi: 10.1080/00325481.2019.1568019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of Included and Excluded Study Participants
eTable 2. Adjusted Hazard Ratios and 95% Confidence Intervals for Risks of Incident Abnormalities of Glucose and Blood Pressure Across Different Body Weight Status During Four-Year Follow-up Among 3204 Chinese Adults: Subgroup Analysis