Key Points
Question
Which circulating metabolites are associated with incident coronary heart disease, and what is their value for assessing the prospective incidence of coronary heart disease?
Findings
In the multinational and population-based Biomarkers for Cardiovascular Risk Assessment in Europe, the case cohort consisted of a weighted, random subcohort of an original cohort of more than 70 000 individuals, and 5 phosphatidylcholines were significantly associated with risk of incident coronary heart disease and showed comparable prognosticative values as individual classic risk factors.
Meaning
These findings underline the value of metabolomics for biomarker discovery and demonstrate the potential for use of phosphatidylcholines in coronary heart disease risk assessment.
This population-based case-cohort study combines data from 7 European databases to investigate the association of circulating metabolites in serum samples with coronary heart disease onset.
Abstract
Importance
Risk stratification for coronary heart disease (CHD) remains challenging because of the complex causative mechanism of the disease. Metabolomic profiling offers the potential to detect new biomarkers and improve CHD risk assessment.
Objective
To evaluate the association between circulating metabolites and incident CHD in a large European cohort.
Design, Setting, and Participants
This population-based study used the Biomarkers for Cardiovascular Risk Assessment in Europe (BiomarCaRE) case-cohort to measure circulating metabolites using a targeted approach in serum samples from 10 741 individuals without prevalent CHD. The cohort consisted of a weighted, random subcohort of the original cohort of more than 70 000 individuals. The case-cohort design was applied to 6 European cohorts: FINRISK97 (Finland), Monitoring of Trends and Determinants in Cardiovascular Diseases/Cooperative Health Research in the Region of Augsburg (MONICA/KORA; Germany), MONICA-Brianza and Moli-sani (Italy), DanMONICA (Denmark), and the Scottish Heart Health Extended Cohort (United Kingdom).
Main Outcomes and Measures
Associations with time to CHD onset were assessed individually by applying weighted and adjusted Cox proportional hazard models. The association of metabolites with CHD onset was examined by C indices.
Results
In 10 741 individuals (4157 women [38.7%]; median [interquartile range] age, 56.5 [49.2-62.2] years), 2166 incident CHD events (20.2%) occurred over a median (interquartile range) follow-up time of 9.2 (4.5-15.0) years. Among the 141 metabolites analyzed, 24 were significantly associated with incident CHD at a nominal P value of .05, including phosphatidylcholines (PCs), lysoPCs, amino acids, and sphingolipids. Five PCs remained significant after correction for multiple testing: acyl-alkyl-PC C40:6 (hazard ratio [HR], 1.13 [95% CI, 1.07-1.18]), diacyl-PC C40:6 (HR, 1.10 [95% CI, 1.04-1.15]), acyl-alkyl-PC C38:6 (HR, 1.11 [95% CI, 1.05-1.16]), diacyl-PC C38:6 (HR, 1.09 [95% CI, 1.04-1.14]) and diacyl-PC C38:5 (HR, 1.10 [95% CI, 1.05-1.16]). Lower levels of these metabolites were associated with increased risk of incident CHD. The strength of the associations competes with those of classic risk factors (C statistics: acyl-alkyl-PC C40:6, 0.756 [95% CI, 0.738-0.774], diacyl-PC C40:6, 0.754 [95% CI, 0.736-0.772], acyl-alkyl-PC C38:6, 0.755 [95% CI, 0.736-0.773], diacyl-PC C38:6, 0.754 [95% CI, 0.736-0.772]), diacyl-PC C38:5, 0.754 [95% CI, 0.736-0.772]). Adding metabolites to a base risk model including classic risk factors high-sensitivity C-reactive protein and high-sensitivity troponin I did not improve discrimination by C statistics.
Conclusions and Relevance
Five PCs were significantly associated with increased risk of incident CHD and showed comparable discrimination with individual classic risk factors. Although these metabolites do not improve CHD risk assessment beyond that of classic risk factors, these findings hold promise for an improved understanding of the pathophysiology of CHD.
Introduction
Coronary heart disease (CHD) constitutes an increasing public health burden worldwide.1 Improvement of cardiovascular risk stratification might advance preventive therapies. Current risk stratification is mainly based on classic risk factors, including the European Society of Cardiology or American Heart Association/American College of Cardiology recommended scores.2,3 However, these scores do not include the numerous environmental and genetic and epigenetic factors that reflect the complex and heterogenic causative mechanisms of CHD.4,5 Consequently, efforts have been undertaken to identify novel molecular markers of risk. Metabolomics, the systematic study of small molecules (<1.5 kDa), is a powerful approach to unravel metabolic disturbances linked to pathological conditions and may improve disease prognostication. The field of metabolomics and its applications to cardiovascular disease has evolved rapidly.6 Prior studies provided the first steps toward identification of metabolic disturbances in cardiovascular disease, suggesting a potential added value of metabolites as biomarkers for CHD.7,8,9,10,11,12,13,14
The aim of this study was to evaluate the association between circulating metabolites and CHD and assess the capability of metabolomics for the prognostication of incident CHD events in the general population. Using the harmonized database and biobank of the European Biomarkers for Cardiovascular Risk Assessment in Europe (BiomarCaRE) project and a large-scale approach, we evaluated the prognosticative values of 141 metabolites in more than 12 000 European individuals from the general population of Europe.
Methods
Study Cohorts and Definition of End Points
The study was conducted within the multinational BiomarCaRE project.15 Baseline variables, phenotypes, CHD events, and follow-up information from large population-based European cohorts have been harmonized in the Monica Risk, Genetics, Archiving and Monograph (MORGAM) database.16 All participating cohorts complied with the Declaration of Helsinki, and all local ethics review boards have approved the study protocol. The study protocol was approved by the local ethics review boards of all study centers, and all participants provided written informed consent.
In the current study, a case-cohort design was applied, including 6 European cohorts: FINRISK97 (Finland), Moli-sani (Italy), Monitoring of Trends and Determinants in Cardiovascular Diseases/Cooperative Health Research in the Region of Augsburg (MONICA/KORA; Germany), MONICA-Brianza (Italy), Danish-Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (DanMONICA; Denmark), and Scottish Heart Health Extended Cohort (United Kingdom).15,17 Briefly, the present case-cohort set consisted of a weighted random subsample (subcohort) of those in the original cohort of more than 70 000 individuals who had no history of myocardial infarction, stroke, heart failure, and atrial fibrillation and who were selected independently of the definition of cases, plus all individuals of the cohort who developed CHD during the follow-up period. In SHHEC, the case-cohort selection was done from a random half of the full cohort.
Completeness of follow-up for the case cohort was 96.4% (12 462 of 12 928 individuals). Baseline serum samples from 12 928 individuals with 2166 incident CHD events were analyzed for the present study (eFigure 1 in the Supplement). Details of the individual cohorts are provided in eAppendix 1 in the Supplement. Baseline characteristics according to the individual cohorts are provided in eTable 1 and eTable 2 in the Supplement.
Incident CHD was defined as the first fatal or nonfatal coronary event (including definite or possible acute myocardial infarction, possible coronary death, or unstable angina pectoris) during the follow-up period. Details on the event classification in each cohort are provided in the description of MORGAM cohorts.18 Measurements of biomarkers high-sensitive C-reactive protein (hsCRP) and high-sensitive troponin I (hsTnI) have been described previously.19
Measurement of Metabolites and Data Processing
The AbsoluteIDQ p180 Kit (BIOCRATES Life Sciences AG) was used for sample preparation according to the manufacturer’s recommendation. A total of 184 metabolites from 5 analytic groups (amino acids, biogenic amines, acylcarnitines, glycerophospholipids, sphingolipids, and sugars) were quantified simultaneously using liquid chromatography and flow-injection analysis mass spectrometry. Measurements were performed on a TSQ Vantage (Thermo Fisher Scientific) connected to an UltiMate 3000 Pump/Autosampler (Thermo Fisher Scientific). For quantification, isotope-labeled internal standards and 7 calibrators of different concentration levels were used. In addition, 3 different levels of quality controls were included, from which quality control 2 was used for data normalization, containing metabolite levels most comparable with physiological levels. A detailed description of the sample preparation is provided in eAppendix 2 in the Supplement.
Normalization of metabolite levels was performed across the entire BiomarCaRE case-cohort set to ensure harmonization. Normalizing each metabolite level was corrected by a factor determined from the ratio of the median of all quality control level 2 values across all plates and the median of the reference samples from the corresponding plates. Within each cohort, no strong batch effects were observed. After data normalization, metabolites were excluded from all subsequent analyses if more than 30% of measurements were missing or more than 50% of measurements were at less than the level of detection (LOD). Additionally, samples were excluded from the analyses if more than 50% of metabolites were missing. A global LOD value was defined for each metabolite of the flow-injection analysis part that corresponds to the highest LOD value across all plates being measured, while the liquid chromatography LOD values were taken from the Biocrates p180 Kit manual. As a consequence, all subsequent data analyses were performed on 141 metabolites and 10 741 samples in total (eFigure 1 in the Supplement). An equal distribution of risk factors and confounding variables were observed between the CHD case-cohort set before and after normalization and quality control (12 928 individuals and 10 741 individuals, respectively) (eTable 3 in the Supplement).
Statistical Analyses
To impute missing measurements, multiple imputation was performed using multivariate imputations by chained equations.20 A total of 20 imputed data sets were created. For the metabolites, a log-normal model was used (for missing values known to be less than the LOD, the imputed values were drawn from truncated log-normal distribution). For all other variables, predictive mean matching was used. The event indicator (CHD) and the Nelson-Aalen estimate of the cumulative survival function of the time to CHD were included in the imputation model. The imputation was done separately for each center and sex.
To calculate correlations of log-transformed metabolites with each other and with cardiovascular risk factors (sex, age at examination, systolic blood pressure, total cholesterol, high-density lipoprotein [HDL] cholesterol, body mass index [BMI; calculated as weight in kilograms divided by height in meters squared], diabetes, daily smoker status, and antihypertensive treatment), Pearson correlation coefficients were calculated. For this, only the subcohort was used, and the observations were weighted by the inverse of the inclusion probability.
Associations with time to CHD were assessed for each of the 141 log-transformed metabolites individually by applying weighted Cox proportional hazard models adjusted for BMI, systolic blood pressure, antihypertensive treatment, diabetes, total cholesterol, sex, daily smoker status, study center, and age at examination. Adjustment for age, sex, daily smoker status, and study center was performed without modeling their effects; age was used as the time scale, and the other 3 factors were used as stratification variables in the Cox models. For all Cox regression analyses, the case-cohort design was taken into account using methods described by Kulathinal et al.17 More specifically, in Cox regressions, individuals were weighted with inverse subcohort-sampling probability weights, and robust standard errors were used. Nominal significance was met at a P value of .05 or lower. After Bonferroni correction for multiple testing, significance was met if P values were less than 4.43 × 10−4 (.05 divided into 113). The number of tests used in the Bonferroni correction (n = 113) was estimated following the methods described in Gao et al.21 This method estimates the effective number of tests as the number of principal components of the log-metabolites that explain 99.5% of the variation. To further assess the effect of renal function and lipid metabolism, any significant findings after Bonferroni correction were additionally adjusted for estimated glomerular filtration rate and non-HDL cholesterol instead of total cholesterol. Hazard ratios (HRs) for the log-metabolites were reported per each 1-SD increase, in which the SD was calculated in the subcohort. For a direct comparison of HRs of metabolites and risk factors, log-metabolite levels were multiplied by −1. Sex-specific HRs for the log-metabolites were estimated by adding sex–log-metabolite interactions to the Cox models. Similarly, log-metabolite HRs for those using vs not using lipid-lowering medication were computed by including the interactions of lipid-lowering medication status and log-metabolites in the Cox models. This analysis was performed only on the Moli-sani and FINRISK97 cohorts because of the availability of information on lipid-lowering medications. Cox regressions in each study center were also performed, and the I2 statistic and prediction intervals for the HRs were calculated.22,23
Survival curves were estimated by the Kaplan-Meier method. Methods previously described17 were used to account for the case-cohort design. Age was used as the time scale. For each of the significant metabolites after Bonferroni correction, HRs according to tertiles of the metabolite levels were calculated using Cox models. The tertiles were defined using the subcohort, and analyses were weighted by the inverse of the inclusion probabilities. Two models of adjustment were used: (1) basic adjustment for age at examination, sex, and study center and (2) full adjustment using BMI, systolic blood pressure, antihypertensive treatment, diabetes, total cholesterol, sex, daily smoker status, study center, and examination age. As before, age was used as the time scale and sex, daily smoker status, and study center were used as the strata.
The prognosticative value of the metabolites was examined in terms of discrimination, as assessed by C indices. Probabilities of CHD within the next 10 years were estimated for each combination of sex, daily smoking status, and study center by fitting a Weibull model with age as the time scale and the linear predictor in the corresponding Cox model. In the estimation of these probabilities, 10-fold cross-validation was used. We computed the 95% CIs and P values for differences of the C indices using the methods described in Antolini et al.24 All statistical analyses were performed using R version 3.4.4 (R Development Core team).
Results
The analysis included 10 741 individuals (FINRISK, n = 1771; Brianza, n = 511; Moli-sani, n = 1364; MONICA/KORA, n = 1085; DanMONICA, n = 3432; and SHHEC, n = 2578) without CHD at baseline, including 4157 women (38.7%) and 6584 men, with a median (interquartile range) age of 56.5 (49.2-62.2) years. During a median (interquartile range) follow-up time of 9.2 (4.5-15.0) years, 2166 individuals (20.2%) experienced an incident CHD event, including 677 fatal CHD events (6.3% of participants) and 1489 nonfatal CHD events (13.9% of participants). The prevalence of hypertension, diabetes, and daily smoking at baseline was 48.1% (5169 participants), 5.2% (562 participants), and 31.8% (3416 participants), respectively. The baseline characteristics and CHD event numbers for the overall cohort, as well as individuals with and without incident CHD events, are summarized in Table 1.
Table 1. Baseline Characteristics.
| Characteristic | Overall (N = 10 741) | Individuals With Incident Coronary Heart Disease (n = 2166) | Individuals Without Incident Coronary Heart Disease (n = 8575) |
|---|---|---|---|
| Age at baseline examination, median (IQR), y | 56.5 (49.2-62.2) | 56.6 (49.9-61.2) | 56.5 (49.0-62.6) |
| Cardiovascular risk factors, median (IQR) | |||
| BMI | 26.2 (23.7-29.1) | 26.6 (24.2-29.4) | 26.1 (23.6-29.0) |
| Systolic blood pressure, mm Hg | 135 (121-150) | 137 (125-153) | 134 (121-150) |
| Diastolic blood pressure, mm Hg | 82 (75-90) | 84 (76-92) | 82 (74-89) |
| Total cholesterol, mg/dL | 232 (201-263) | 244 (217-278) | 228 (201-259) |
| High-density lipoprotein cholesterol, mg/dL | 53.0 (44.1-64.2) | 49.4 (41.0-60.3) | 54.1 (45.2-65.0) |
| Low-density lipoprotein cholesterol, mg/dL | 146 (122-175) | 159 (134-188) | 143 (119-171) |
| Triglycerides, mg/dL | 123 (89-180) | 143 (103-212) | 118 (85-172) |
| Cardiovascular risk factors, No. (%) | |||
| Daily smoking status | 3416 (31.8) | 958 (44.2) | 2458 (28.7) |
| Diabetes mellitus | 562 (5.2) | 187 (8.6) | 376 (4.4) |
| Hypertension | 5169 (48.1) | 1151 (53.2) | 4018 (46.9) |
| Antihypertensive medication | 1795 (16.7) | 429 (19.8) | 1366 (15.9) |
| Coronary heart disease end points, No. (%) | |||
| Total cases | 2166 (20.2) | 2166 (100) | NA |
| Fatal cases | 677 (6.3) | 677 (31.3) | NA |
| Nonfatal cases | 1489 (13.9) | 1489 (68.7) | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NA, not applicable.
SI conversion factors: To convert total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113.
The baseline characteristics, weighted to represent the full cohort according to the case-cohort design,17 as well as according to sex, are provided in eTable 4 and eTable 5 in the Supplement. Levels of 141 serum metabolites were analyzed, and mean metabolite levels are presented in eTable 6 in the Supplement.
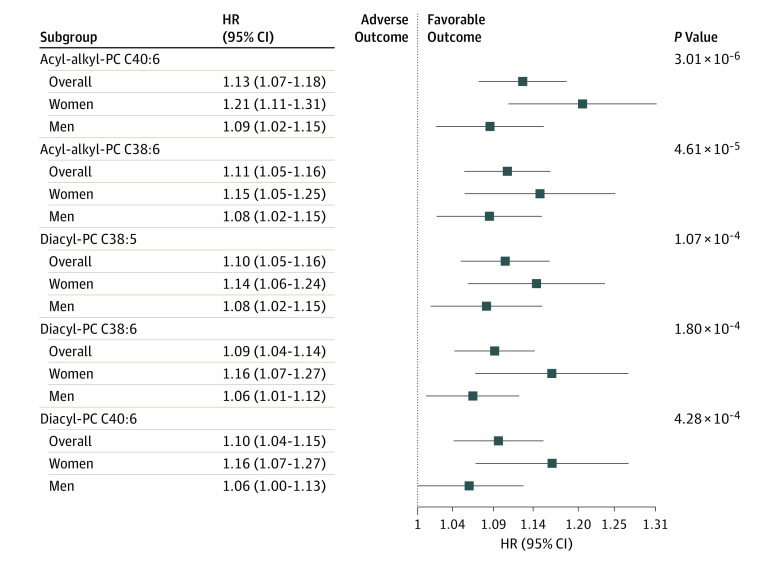
In this analysis, 24 individual metabolites were nominally associated with CHD at a P value of .05 or less. These 24 metabolites belonged to 3 different metabolite classes (phosphatidylcholines [PC]), amino acids, and sphingolipids; Table 2). Pearson correlations of individual significant metabolites are shown in eFigure 2 in the Supplement. When controlling for multiple testing, 5 PCs remained significant (HRs: acyl-alkyl-PC C40:6, 1.13 [95% CI, 1.07-1.18]; P = 3.01 × 10−6; diacyl-PC C40:6, 1.10 [95% CI, 1.04-1.15]; P = 4.28 × 10−4; acyl-alkyl-PC C38:6, 1.11 [95% CI, 1.05-1.16]; P = 4.61 × 10−5; diacyl-PC C38:6, 1.09 [95% CI, 1.04-1.14]; P = 1.8 × 10−4; diacyl-PC C38:5, 1.10 [95% CI, 1.05-1.16]; P = 1.07 × 10−4; Figure 1; Table 2). All significant metabolites were negatively associated with CHD. The associations were consistent between men and women, with slightly higher HRs in women; all were nonsignificantly different, with the exception of acyl-alkyl-PC C40:6 (men: HR, 1.09 [95% CI, 1.02-1.15], P = .009; women: HR, 1.21 [95% CI, 1.11-1.31]; P = 1.3 × 10−5; interaction P = .04). Associations of the 5 significant PCs with CHD per study center are illustrated in eFigure 3 in the Supplement, including for acyl-alkyl-PC C40:6 (FINRISK: HR, 1.31 [95% CI, 1.15-1.50]; P = 4.77 × 10−5; SHHEC: HR, 1.29 [95% CI, 1.17-1.43]; P = 3.11 × 10−7), acyl-alkyl-PC C38:6 (FINRISK: HR, 1.26 [95% CI, 1.11-1.44]; P = 3.55 × 10−4; SHHEC: HR, 1.22 [95% CI, 1.13-1.32]; P = 1.42 × 10−7), diacyl-5 PC C38:5 (FINRISK: HR, 1.21 [95% CI, 1.06-1.37]; P = .004; SHHEC: HR, 1.23 [95% CI, 1.14-1.34]; P = 5.92×10−7), diacyl-PC C38:6 (FINRISK: HR, 1.30 [95% CI, 1.15-1.47]; P = 3.62×10−5; SHHEC: HR, 1.11 [95% CI, 1.04-1.19]; P = .003), and diacyl-PC C40:6 (FINRISK: HR, 1.25 [95% CI, 1.10-1.43]; P = 8.67×10−4; SHHEC: HR, 1.27 [95% CI, 1.15-1.40]; P = 1.32×10−6). Importantly, the associations of these 5 significant metabolites were not attenuated after additional adjustments for estimated glomerular filtration rate and non-HDL cholesterol instead of total cholesterol (eTable 7 in the Supplement).
Table 2. Metabolite Associations With Future Coronary Heart Diseasea.
| Metabolite | Hazard Ratio per SD (95% CI) | P Value |
|---|---|---|
| Acyl-alkyl-PC C40:6 | 1.13 (1.07-1.18) | 3.01 × 10−6 |
| Acyl-alkyl-PC C38:6 | 1.11 (1.05-1.16) | 4.61 × 10−5 |
| Diacyl-PC C38:5 | 1.10 (1.05-1.16) | 1.07 × 10−4 |
| Diacyl-PC C38:6 | 1.09 (1.04-1.14) | 1.80 × 10−4 |
| Diacyl-PC C40:6 | 1.10 (1.04-1.15) | 4.28 × 10−4 |
| Acyl-alkyl-PC C34:3 | 1.08 (1.03-1.13) | 7.27 × 10−4 |
| Acyl-alkyl-PC C36:5 | 1.08 (1.03-1.13) | .002 |
| Acyl-alkyl-PC C44:5 | 1.09 (1.03-1.16) | .002 |
| Acyl-alkyl-PC C38:5 | 1.09 (1.03-1.15) | .003 |
| Acyl-alkyl-PC C40:5 | 1.12 (1.04-1.21) | .003 |
| Diacyl-PC C40:5 | 1.08 (1.03-1.14) | .003 |
| Acyl-alkyl-PC C38:0 | 1.12 (1.04-1.20) | .004 |
| Diacyl-PC C36:5 | 1.07 (1.02-1.13) | .004 |
| Methionine | 1.10 (1.03-1.18) | .005 |
| Diacyl-PC C36:4 | 1.08 (1.02-1.14) | .006 |
| Acyl-alkyl-PC C44:6 | 1.07 (1.02-1.12) | .009 |
| Acyl-alkyl-PC C36:4 | 1.07 (1.02-1.13) | .01 |
| Diacyl-PC C36:6 | 1.10 (1.02-1.19) | .01 |
| LysoPC a C18:2 | 1.08 (1.02-1.15) | .02 |
| Acyl-alkyl-PC C42:5 | 1.07 (1.01-1.14) | .02 |
| Histidine | 1.08 (1.01-1.15) | .02 |
| Sphingomyelin C18:1 | 1.04 (1.00-1.07) | .03 |
| LysoPC a C20:4 | 1.07 (1.01-1.13) | .03 |
| Glutamine | 1.10 (1.00-1.19) | .04 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); PC, phosphatidylcholine.
24 Metabolites associated at a nominal P value less than .05 are shown. Hazard ratios are per SD of log-transformed metabolite level and are adjusted for body mass index, systolic blood pressure, antihypertensive treatment, diabetes, total cholesterol, sex, daily smoking, study center, and examination age. The log-metabolites levels were multiplied by −1 to obtain hazard ratios greater or equal than 1. Thus, these are described as hazard ratios per 1-SD decrease.
Figure 1. Metabolite Associations With Future Coronary Heart Disease.
Five phosphatidylcholine metabolites were associated with future coronary heart disease after Bonferroni correction. Hazard ratios (HR) are per SD of log-transformed metabolite level and are adjusted for body mass index, systolic blood pressure, antihypertensive treatment, diabetes, total cholesterol, sex, daily smoker status, study center, and examination age. Error bars indicate 95% CIs. The log-metabolite levels were multiplied by −1 to obtain HRs greater or equal to 1. Thus, HRs are described as HRs per a 1-SD decrease. A logarithmic scale is used on the x-axis. PC indicates phosphatidylcholine.
Because medications, such as lipid-lowering drugs, might affect the levels of metabolites, an interaction analysis was performed in the subset of the case-cohort set in which data on lipid-lowering medications were available. As shown in eTable 8 in the Supplement, lipid-lowering medication use had no significant association with the prognosticative values of the PCs.
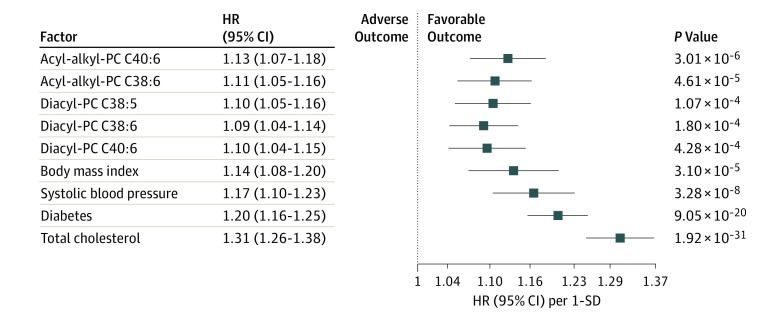
Comparing the association between the 5 significant CHD-associated PCs and classic cardiovascular risk factors (BMI, systolic blood pressure, diabetes, and total cholesterol), showed only a slightly weaker association for metabolites than some of the classic risk factors. For instance, for acyl-alkyl-PCs C40:6 (HR, 1.13 [95% CI, 1.07-1.18]; P = 3.01 × 10−6) and C38:6 (HR, 1.11 [95% CI, 1.05-1.16]; P = 4.61 × 10−5), the strength of association was similar to those of BMI (HR, 1.14 [95% CI, 1.07-1.20]; P = 3.10 × 10−5) and systolic blood pressure (HR, 1.17 [95% CI, 1.10-1.23]; P = 3.28 × 10−8) (Figure 2).
Figure 2. Comparison of Hazard Ratios (HRs) for Incident Coronary Heart Disease of Classic Risk Factors and Metabolites.
For each metabolite, a Cox model, adjusted for examination age, sex, systolic blood pressure, total cholesterol, body mass index, diabetes, daily smoker status, antihypertensive medication, and study center, was computed. The HRs for the risk factors were computed from a model that included only the aforementioned adjusting variables. No association could be estimated for age, since it was used as the time scale, nor for sex and daily smoker status, since they were used as strata in the Cox models. All HRs are per 1-SD increase. The log-metabolite levels were multiplied by −1 to obtain HRs greater or equal to 1. Thus, HRs are per a 1-SD decrease. A logarithmic scale is used on the x-axis. PC indicates phosphatidylcholine.
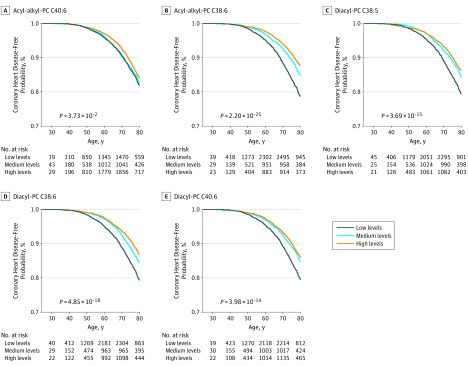
To assess the ability of the 5 PC metabolites to estimate CHD risk, Kaplan-Meier survival analyses according to tertiles of metabolite levels using age as the time scale were performed. Figure 3 shows unadjusted Kaplan-Meier curves of CHD event-free time for each of the 5 PC metabolites, while eFigure 4 in the Supplement shows HRs across metabolite tertiles. A decrease in CHD risk was observed across increasing tertiles. Individuals in the highest tertile of metabolite distributions had a decreased risk of developing future CHD compared with those in the lowest tertile (acyl-alkyl-PC C40:6: HR, 1.21 [95% CI, 1.06-1.37]; P = .004; acyl-alkyl-PC C38:6: HR, 1.29 [95% CI, 1.12-1.47], P = 2.29 × 10−4; diacyl-PC C38:5: HR, 1.14 [95% CI, 1.00-1.29], P = .05; diacyl-PC C38:6: HR, 1.21 [95% CI, 1.06-1.38], P = .004; diacyl-PC C40:6: HR, 1.09 [95% CI, 0.96-1.24], P = .17). These findings were similar after full adjustment.
Figure 3. Survival Curves According to Tertiles of the Metabolite Distributions in the Case-Cohort Set.
Survival curves are shown separately for each of the metabolites with significant associations with coronary heart disease. The P values given in the respective survival curves are for the log-rank test. The number of persons at risk during the follow-up period is given per one-third in the table directly below the survival curves. Tertiles were used to categorize the metabolite distributions into thirds, and these values were 3.28μM (first tertile) and 4.54μM (second tertile) for acyl-alkyl-PC C40:6, 4.72μM (first tertile) and 6.61μM (second tertile) for acyl-alkyl-PC C38:6, 33.46μM (first tertile) and 46.37μM (second tertile) for diacyl-PC C38:5, and 46.98μM (first tertile) and 69.87μM (diacyl-PC C38:6) and 15.80μM (first tertile) and 23.58μM (second tertile) for diacyl-PC C40:6. PC indicates phosphatidylcholine.
To assess the discriminatory potential of the 5 significant metabolites, C indices were calculated for the 10-year prognostication of CHD. Acyl-alkyl-PC C40:6 performed best in the discrimination of CHD, with a C index of 0.756 (95% CI, 0.738-0.774), followed by acyl-alkyl-PC C38:6 (0.755 [95% CI, 0.736-0.773]). Diacyl-PC C38:5, C38:6, and C40:6 all performed with a C index of 0.754 (95% CI, 0.736-0.772). The C indices of the metabolites were comparable with those of classic cardiovascular risk factors and the 2 clinically established biomarkers, hsCRP, and hsTnI. However, the addition of the metabolites, either individually or as a combination to a base risk model (including sex, examination age, systolic blood pressure, total cholesterol, HDL cholesterol, BMI, diabetes, daily smoker status, antihypertensive treatment, hsCRP, hsTnI, and study center) did not improve discrimination by C statistics (C index base model, 0.828 [95% CI, 0.809-0.846]; C index plus metabolites, 0.828 [95% CI, 0.810-0.846]; eTable 9 in the Supplement).
Discussion
Using a comprehensive targeted metabolomics approach, we analyzed the association of 141 serum metabolites with risk of incident CHD in a population based case-cohort sample of more than 70 000 European individuals. We report 3 main findings: first, we identified 24 metabolites that were significantly and independently associated with incident CHD. These metabolites are mainly acyl-alkyl-PCs and diacyl-PCs. Five of these 24 metabolites remained significantly associated after correcting for multiple testing. Second, these 5 significant metabolites showed an inverse association with CHD risk, because lower levels were accompanied with higher risk. Third, the discriminatory potential of these metabolites for CHD events was comparable with those of classic risk factors and established biomarkers hsCRP and hsTnI. However, the metabolites did not relevantly improve the assessment of prospective incident CHD compared with existing conventional risk factors.
Data from prospective studies are sparse, and previous smaller studies report conflicting results. Most recent data suggest that beyond the established association between low-density lipoprotein (LDL) cholesterol, and future cardiovascular events PCs might also contribute to cardiovascular disease.25,26 In cross-sectional analyses, PCs were identified in plasma lipoproteins and atherosclerotic plaques, and alterations in PC levels were described in patients with stable coronary artery disease, myocardial infarction, and atherosclerosis as compared with healthy participants.25,26 Other prospective analyses in primary and secondary cardiovascular prevention identified PCs as markers for cardiovascular mortality in patients with established coronary artery disease,27 in the population-based Bruneck study12 and as markers for risk of myocardial infarction or CHD in healthy adults in prospective cohorts.10,28 The group of PCs presents a heterogeneous group, and different subtypes have different associations and thereby confer different risks. While the PC subtypes identified by this study associated inversely with incident disease, Paytner et al28 identified different PC subtypes (C34:2 and C36:4) that were positively associated with increased risk of CHD.
The growing discipline of metabolomics and lipidomics provides new insights into the pathogenesis of cardiovascular disease. In this study, increasing levels of PCs were protective against incident CHD. This is in line with a previous interventional study29: using lipidomics, the Rosuvastatin and Atorvastatin in Different Dosages and Reverse Cholesterol Transport (RADAR) study analyzed in depth the lipid changes induced by rosuvastatin at different dosages. Liquid chromatography–mass spectrometry was applied to obtain the metabolite profiles of plasma samples, for which a randomly assigned treatment with rosuvastatin in increasing dosages was administered during an 18-week period. In result, rosuvastatin significantly increased the plasma levels of PCs after 6 and 18 weeks. In accordance, Paapstel et al25 observed inverse associations between PC and lysoPC profiles and inflammation as well as arterial function in patients with atherosclerosis using the same metabolomics approach as in this study. The authors investigated serum PC and lysoPC species in association with arterial stiffness, hemodynamics, and endothelial dysfunction in 32 patients with peripheral arterial disease, 52 patients with coronary artery disease, and in 40 healthy control participants. Contrarily, Floegel et al10 identified PC classes that were positively associated with risk of myocardial infarction in healthy adults. Furthermore, Tang et al30 demonstrated that higher levels of trimethylamine N-oxide, which is a product of PCs, are positively associated with cardiovascular event rates. Interestingly, trimethylamine N-oxide levels depend on gut flora metabolism, suggesting a possible therapy target.31 Notably, newly identified associations of PC classes with CHD need to be considered separately, because different pathophysiological pathways could underlie disease development.
The molecular bases of these findings still need to be elucidated. The 3 major phospholipid classes (sphingomyelins, PCs, and lysoPCs) are important structural components of plasma lipoproteins and cell membranes and are involved in regulation of cell function, membrane protein trafficking, and inflammation.32 In human plasma, PCs and lysoPCs make up about 60% to 70% and 10% to 20%, respectively, of circulating phospholipids. Production of lysoPCs is the result of partial hydrolysis of PCs, lecithin-cholesterol acyltransferase activity, or hepatic secretion.25 These lipid species are important components of oxidized LDL. Oxidized LDL has been hypothesized to induce foam-cell formation (an early yet critical step in the development of atherosclerosis); it downregulates endothelial nitric oxide synthase, increases the formation of metalloproteinases, and induces apoptosis in human coronary endothelial cells.33 One may hypothesize that the protective effects of increased PC levels are attributable to modification of oxidized LDL. However, this study is not capable of deciphering underlying molecular mechanisms, and potential effects on oxidized LDL and other molecules in this setting remain speculative.
Strengths
In these analyses, we used the harmonized database of the BiomarCaRE project, providing, to our knowledge, the best possible end point validation across Europe and including a large sample size and many incident CHD events. In addition to the use of a harmonized database, all metabolite measurements were performed in 1 central laboratory using a well-validated assay platform and stringent quality control was applied. Furthermore, we included study cohorts from different European regions. Most importantly, the identification of new and solid factors associated with CHD, which have not been reported yet in the context of CHD, might boost the way to new and unknown pathopysiologic pathways in cardiovascular disease.
The strength of the association of the 5 PCs with the risk of future CHD was comparable with that of classic risk factors, in particular BMI and systolic blood pressure. However, contrary to previous reports,12,34 the metabolites identified in this study did not appreciably improve the association of incident CHD compared with existing conventional risk factors. In contrast to this study, in which only PCs were identified and assessed in detail, the previous studies used combinations of several metabolite classes in their discrimination analyses. An explanation for the differences in the ability to improve risk assessment might be that the various metabolites classes contribute to different pathophysiological pathways based on their composition,25,35 thus leading to an improved risk assessment when combined.
Limitations
A limitation of our study is the use of a targeted metabolite approach and thereby the measurement of formally selected metabolites. The currently known and quantifiable metabolome consists of more than 100 000 metabolites.36,37 Thus, we cannot exclude the possibility that by using a nontargeted approach with a broad coverage of the entire metabolome, additional metabolite classes might have been identified. Furthermore, we were unable to control for dietary and lifestyle parameters in general, which have obviously changed during the last 3 decades of study time, or for medication, which may have affected our results of associated metabolites.3,38 Therefore, future studies with information on dietary intake and fasting status are needed to validate these data. Additionally, basic research is crucial to provide answers as to whether the identified metabolite markers are central to the pathogenesis of CHD.
Conclusions
In conclusion, by quantifying a large set of metabolites in 12 928 European individuals, we identified 5 PCs that were associated with increased risk of incident CHD. The strength of the association of metabolites was similar to that of individual classic cardiovascular risk factors, and C indices were comparable with those obtained from classic risk factors and established biomarkers hsCRP and hsTnI. However, these metabolites did not appreciably improve the prognostication of incident CHD compared with existing conventional risk factors. The present study demonstrates the value of metabolomics for biomarker discovery and holds promise for improved understanding of pathophysiology of CHD. Further studies to investigate the link between newly detected PCs and CHD development are needed.
eAppendix 1. Description of individual study cohorts of the BiomarCaRE case-cohort
eAppendix 2. Samples preparation and measurement of metabolites
eTable 1. Study characteristics according to center
eTable 2. Weighted study characteristics according to center
eTable 3. Comparative study characteristics of study participants before and after quality control
eTable 4. Weighted study characteristics of the overall case-cohort set
eTable 5. Baseline characteristics of the overall case-cohort set, and males and females, separately
eTable 6. Levels of metabolites after normalization
eTable 7. Association of significant metabolites with future coronary heart disease adjusted for eGFR and non-HDL
eTable 8. Results of interaction analyses for cholesterol-lowering medication
eTable 9. C-indices for 10-year risk prediction of coronary heart disease
eFigure 1. Flow diagram of BiomarCaRE case cohort set, metabolite measurement and data processing
eFigure 2. Pearson correlation matrix for serum metabolite levels
eFigure 3. Metabolite associations with future coronary heart disease per individuals study center
eFigure 4. Hazard ratios across thirds (low, medium, high) of the significant phosphatidylcholine metabolites
eReferences.
References
- 1.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232-3245. doi: 10.1093/eurheartj/ehw334 [DOI] [PubMed] [Google Scholar]
- 2.Piepoli MF, Hoes AW, Agewall S, et al. ; ESC Scientific Document Group . 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts), developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S49-S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 4.Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349-2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet. 2017;18(6):331-344. doi: 10.1038/nrg.2016.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGarrah RW, Crown SB, Zhang GF, Shah SH, Newgard CB. Cardiovascular metabolomics. Circ Res. 2018;122(9):1238-1258. doi: 10.1161/CIRCRESAHA.117.311002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brindle JT, Antti H, Holmes E, et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8(12):1439-1444. doi: 10.1038/nm1202-802 [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448-453. doi: 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee EP, Gerszten RE. Metabolomics and cardiovascular biomarker discovery. Clin Chem. 2012;58(1):139-147. doi: 10.1373/clinchem.2011.169573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floegel A, Kühn T, Sookthai D, et al. Serum metabolites and risk of myocardial infarction and ischemic stroke: a targeted metabolomic approach in two German prospective cohorts. Eur J Epidemiol. 2018;33(1):55-66. doi: 10.1007/s10654-017-0333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Würtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774-785. doi: 10.1161/CIRCULATIONAHA.114.013116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stegemann C, Pechlaner R, Willeit P, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129(18):1821-1831. doi: 10.1161/CIRCULATIONAHA.113.002500 [DOI] [PubMed] [Google Scholar]
- 13.Ward-Caviness CK, Xu T, Aspelund T, et al. Improvement of myocardial infarction risk prediction via inflammation-associated metabolite biomarkers. Heart. 2017;103(16):1278-1285. doi: 10.1136/heartjnl-2016-310789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhang D, He Y, et al. Investigation of novel metabolites potentially involved in the pathogenesis of coronary heart disease using a UHPLC-QTOF/MS-based metabolomics approach. Sci Rep. 2017;7(1):15357. doi: 10.1038/s41598-017-15737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeller T, Hughes M, Tuovinen T, et al. BiomarCaRE: rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur J Epidemiol. 2014;29(10):777-790. doi: 10.1007/s10654-014-9952-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The MORGAM Project MORGAM manual. http://www.thl.fi/publications/morgam/manual/contents.htm. Published 2001. Accessed September 18, 2019.
- 17.Kulathinal S, Karvanen J, Saarela O, Kuulasmaa K. Case-cohort design in practice—experiences from the MORGAM Project. Epidemiol Perspect Innov. 2007;4:15. doi: 10.1186/1742-5573-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulathinal S, Niemelä M, Niiranen T, et al. Description of MORGAM cohorts. https://www.thl.fi/publications/morgam/cohorts/index.html. 2005.
- 19.Blankenberg S, Salomaa V, Makarova N, et al. ; BiomarCaRE Investigators . Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J. 2016;37(30):2428-2437. doi: 10.1093/eurheartj/ehw172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3). doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 21.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32(4):361-369. doi: 10.1002/gepi.20310 [DOI] [PubMed] [Google Scholar]
- 22.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5-18. doi: 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 24.Antolini L, Nam B-H, D’Agostino RB. Inference on correlated discrimination measures in survival analysis: a nonparametric approach. Commun Stat Theory Methods. 2004;33(9):2117-2135. doi: 10.1081/STA-200026579 [DOI] [Google Scholar]
- 25.Paapstel K, Kals J, Eha J, et al. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr Metab Cardiovasc Dis. 2018;28(1):44-52. doi: 10.1016/j.numecd.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 26.Moxon JV, Jones RE, Wong G, et al. Baseline serum phosphatidylcholine plasmalogen concentrations are inversely associated with incident myocardial infarction in patients with mixed peripheral artery disease presentations. Atherosclerosis. 2017;263:301-308. doi: 10.1016/j.atherosclerosis.2017.06.925 [DOI] [PubMed] [Google Scholar]
- 27.Sigruener A, Kleber ME, Heimerl S, Liebisch G, Schmitz G, Maerz W. Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS One. 2014;9(1):e85724. doi: 10.1371/journal.pone.0085724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137(8):841-853. doi: 10.1161/CIRCULATIONAHA.117.029468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergheanu SC, Reijmers T, Zwinderman AH, et al. Lipidomic approach to evaluate rosuvastatin and atorvastatin at various dosages: investigating differential effects among statins. Curr Med Res Opin. 2008;24(9):2477-2487. doi: 10.1185/03007990802321709 [DOI] [PubMed] [Google Scholar]
- 30.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575-1584. doi: 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57-63. doi: 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1-23. doi: 10.1007/978-1-4419-6741-1_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenig W, Karakas M, Zierer A, et al. Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study. Clin Chem. 2011;57(8):1196-1200. doi: 10.1373/clinchem.2011.165134 [DOI] [PubMed] [Google Scholar]
- 34.Ganna A, Salihovic S, Sundström J, et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014;10(12):e1004801. doi: 10.1371/journal.pgen.1004801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859(9 pt B):1558-1572. doi: 10.1016/j.bbamem.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 36.Noronha A, Modamio J, Jarosz Y, et al. The virtual metabolic human database: integrating human and gut microbiome metabolism with nutrition and disease. Nucleic Acids Res. 2018;47(1):614-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608-D617. doi: 10.1093/nar/gkx1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geidenstam N, Magnusson M, Danielsson APH, et al. Amino acid signatures to evaluate the beneficial effects of weight loss. Int J Endocrinol. 2017;2017:6490473. doi: 10.1155/2017/6490473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Description of individual study cohorts of the BiomarCaRE case-cohort
eAppendix 2. Samples preparation and measurement of metabolites
eTable 1. Study characteristics according to center
eTable 2. Weighted study characteristics according to center
eTable 3. Comparative study characteristics of study participants before and after quality control
eTable 4. Weighted study characteristics of the overall case-cohort set
eTable 5. Baseline characteristics of the overall case-cohort set, and males and females, separately
eTable 6. Levels of metabolites after normalization
eTable 7. Association of significant metabolites with future coronary heart disease adjusted for eGFR and non-HDL
eTable 8. Results of interaction analyses for cholesterol-lowering medication
eTable 9. C-indices for 10-year risk prediction of coronary heart disease
eFigure 1. Flow diagram of BiomarCaRE case cohort set, metabolite measurement and data processing
eFigure 2. Pearson correlation matrix for serum metabolite levels
eFigure 3. Metabolite associations with future coronary heart disease per individuals study center
eFigure 4. Hazard ratios across thirds (low, medium, high) of the significant phosphatidylcholine metabolites
eReferences.