Key Points
Question
Is bariatric surgery associated with health care expenditures 10 years after the procedure in veterans with severe obesity?
Findings
In this cohort study of 9954 veterans with severe obesity, total health care expenditures were higher in the 3 years before surgery and the first 2 years after surgery among patients who underwent surgery compared with those who did not. The expenditures of patients who did and did not undergo bariatric surgery converged 5 to 10 years after surgery.
Meaning
The value of bariatric surgery lies primarily in its association with improvements in health and not in its potential to decrease health care costs.
Abstract
Importance
Bariatric surgery has been associated with improvements in health in patients with severe obesity; however, it is unclear whether these health benefits translate into lower health care expenditures.
Objective
To examine 10-year health care expenditures in a large, multisite retrospective cohort study of veterans with severe obesity who did and did not undergo bariatric surgery.
Design, Setting, and Participants
A total of 9954 veterans with severe obesity between January 1, 2000, and September 30, 2011, were identified from veterans affairs (VA) electronic health records. Of those, 2498 veterans who underwent bariatric surgery were allocated to the surgery cohort. Sequential stratification was used to match each patient in the surgery cohort with up to 3 patients who had not undergone bariatric surgery but were of the same sex, race/ethnicity, diabetes status, and VA regional network and were closest in age, body mass index (calculated as weight in kilograms divided by height in meters squared), and comorbidities. A total of 7456 patients were identified and allocated to the nonsurgery (control) cohort. The VA health care expenditures among the surgery and nonsurgery cohorts were estimated using regression models. Data were analyzed from July to August 2018 and in April 2019.
Interventions
The bariatric surgical procedures (n = 2498) included in this study were Roux-en-Y gastric bypass (1842 [73.7%]), sleeve gastrectomy (381 [15.3%]), adjustable gastric banding (249 [10.0%]), and other procedures (26 [1.0%]).
Main Outcomes and Measures
The study measured total, outpatient, inpatient, and outpatient pharmacy expenditures from 3 years before surgery to 10 years after surgery, excluding expenditures associated with the initial bariatric surgical procedure.
Results
Among 9954 veterans with severe obesity, 7387 (74.2%) were men; the mean (SD) age was 52.3 (8.8) years for the surgery cohort and 52.5 (8.7) years for the nonsurgery cohort. Mean total expenditures for the surgery cohort were $5093 (95% CI, $4811-$5391) at 7 to 12 months before surgery, which increased to $7448 (95% CI, $6989-$7936) at 6 months after surgery. Postsurgical expenditures decreased to $6692 (95% CI, $6197-$7226) at 5 years after surgery, followed by a gradual increase to $8495 (95% CI, $7609-$9484) at 10 years after surgery. Total expenditures were higher in the surgery cohort than in the nonsurgery cohort during the 3 years before surgery and in the first 2 years after surgery. The expenditures of the 2 cohorts converged 5 to 10 years after surgery. Outpatient pharmacy expenditures were significantly lower among the surgery cohort in all years of follow-up ($509 lower at 3 years before surgery and $461 lower at 7 to 12 months before surgery), but these cost reductions were offset by higher inpatient and outpatient (nonpharmacy) expenditures.
Conclusions and Relevance
In this cohort study of 9954 predominantly older male veterans with severe obesity, total health care expenditures increased immediately after patients underwent bariatric surgery but converged with those of patients who had not undergone surgery at 10 years after surgery. This finding suggests that the value of bariatric surgery lies primarily in its associations with improvements in health and not in its potential to decrease health care costs.
This cohort study of 9954 US veterans with severe obesity compared the health care expenditures of veterans who did and did not undergo bariatric surgery over a 10-year period to assess whether bariatric surgery was associated with long-term expenditures.
Introduction
Bariatric surgery has been associated with substantial, sustained improvements in weight, obesity-related comorbidities, quality of life, and survival.1,2,3,4,5,6 It is unclear whether bariatric surgery is associated with lower long-term health care expenditures because, to our knowledge, few randomized clinical trials have examined health care expenditures, and most observational studies have examined expenditures only up to 2 to 3 years after surgery. The 6-year expenditures of Blue Cross and Blue Shield enrollees7 and the 3-year expenditures of patients receiving care through the Veterans Health Administration of the US Department of Veterans Affairs (VA)8 were similar between patients with obesity who underwent bariatric surgery and matched patients with obesity who did not undergo bariatric surgery, which contradicted earlier observational findings9 that the expenditure reductions among patients who underwent surgery were sufficiently large to fully offset the cost of the surgery itself within 2 to 5 years after surgery.10,11,12,13,14
Bariatric surgery procedures have been associated with improvements in long-term weight and comorbidity control, but the performance of these procedures and the management of subsequent surgical complications are costly. To date, it is unknown if the short-term costs of bariatric surgery are offset by reductions in the long-term costs. To that end, health care expenditures were compared for 2498 patients with severe obesity who underwent bariatric surgery and 7456 matched patients who had not undergone surgery with up to 10 years of follow-up.
We hypothesized that patients who underwent bariatric surgery would have lower health care costs 10 years after surgery than matched patients who did not undergo bariatric surgery. We also examined expenditures during the 3 years before surgery to understand the expenditure trajectories leading up to surgery. An evaluation of long-term health care expenditures after bariatric surgery is needed to help policy makers and health insurance providers assess the long-term value of these surgical procedures.
Methods
Study Design and Population
In this retrospective cohort study, the long-term expenditures of veterans with severe obesity (defined as a body mass index [BMI] ≥35 [calculated as weight in kilograms divided by height in meters squared]) who underwent bariatric surgery were compared with the long-term expenditures of a matched cohort of veterans with severe obesity who had not undergone bariatric surgery in the VA health care system and whose long-term survival and weight change were previously compared.15,16 We used data from the VA electronic health record to identify 2752 veterans who underwent any type of bariatric surgical procedure in a VA bariatric center between January 1, 2000, and September 30, 2011. After exclusions (eFigure 1 in the Supplement), the final surgery cohort included 2498 patients. This study was approved by the institutional review boards of the Durham VA Medical Center, the VA North Texas Health Care System, and the Kaiser Permanente Washington Health Research Institute, with a waiver of informed consent because the research could not practicably be carried out without a waiver owing to the large number of veterans sampled. Data were analyzed from July to August 2018 and in April 2019.
Potential matches for patients who underwent bariatric surgery were identified from the VA electronic health record using sequential stratification matching.17,18,19 This approach structured the data to resemble n-of-1 clinical trials (ie, single-subject clinical trials), in which each clinical trial’s start date is the same as each patient’s surgery date, which allows for the matching of treated patients with untreated patients in longitudinal studies in which the matches have multiple potential index dates and evolving comorbidity incidence. Potential matches (n = 627 547) were identified based on the patient’s sex, diabetes diagnosis, race/ethnicity, VA regional network, age, diagnostic cost group (DCG) score, and most recent BMI within 6 months of surgery. Diagnostic cost group scores aggregate diagnosis codes to generate a risk adjustment score to predict veterans’ expenditures more accurately than other measures.20,21 This method of matching patients based on their DCG scores more efficiently balances the comorbidity burden compared with matching them based on their individual health conditions; however, not all underlying health conditions will be balanced despite balances in DCG scores. All diagnosis codes were identified using the International Classification of Diseases–Ninth Revision.
Up to 3 matched patients who had not undergone bariatric surgery were selected for each patient who had undergone surgery based on the smallest caliper of the Mahalanobis distance function22 that preserved the covariate balance and minimized the loss of surgical patients who lacked comparable matches. Some potential nonsurgical patient matches had many BMI measurements available, so these patients could be matched to multiple patients who underwent surgery. The final nonsurgery (control) cohort comprised 7110 individual patients, representing 7456 matches. The matching process was not contingent on future information, so matched patients in the nonsurgery cohort who later received bariatric surgery (142 patients representing 152 matches) contributed untreated person-time until 6 months before their surgery date, when they were censored to minimize contamination of the expenditure data in the nonsurgery cohort (eTable in the Supplement).
Health Care Expenditures
We examined VA outpatient, inpatient, and outpatient pharmacy expenditures and their aggregate data as total expenditures from 3 years before surgery to 10 years after the surgical discharge date. The unit of analysis was a person half–year. Outpatient and inpatient expenditures were obtained from the outpatient and inpatient average cost data of the VA Health Economics Resource Center and fee basis files.23 These data sets include national average costs for VA-provided outpatient visits and inpatient stays, providing comparable expenditures across VA facilities. Outpatient pharmacy expenditures were obtained from the VA pharmacy benefits management database, which tracks every outpatient medication prescription filled in the VA health care system.
Expenditures were obtained from January 1, 2000, through December 31, 2016. Person half–years were excluded if they occurred entirely outside of this period, after a patient’s date of death, or after censoring a patient in the nonsurgery cohort who later had bariatric surgery. Expenditures for the index hospitalization and the 6 months of care before surgery were excluded from all models so that spikes in expenditures among the surgery cohort (associated with the surgery itself and the presurgical workup) would not affect results. This analysis was performed from a payer’s perspective, so any person half–years that did not have observed expenditures were considered equal to $0. For each bariatric procedure, we also estimated the median expenditures to the health care system for the entire hospital stay during which the surgical procedure was performed. Expenditures were adjusted for inflation in 2016 dollars using the general consumer price index from the Bureau of Labor Statistics, US Department of Labor.
Statistical Analysis
The covariate balance between cohorts was evaluated using standardized differences.24 Specification testing25 for total and outpatient expenditures indicated that the data should be analyzed using generalized linear models with generalized estimating equations that included log links, SDs proportional to the means, unstructured covariance, and empirical sandwich standard errors for SEs. For outpatient expenditures, time was specified as linear, with a discontinuity at the time of surgery and separate slopes before and after surgery. For total expenditures, time was specified as quadratic, with a similar discontinuity and change in slopes. A surgical patient indicator was used to interact with all of the time values in the models.
The VA outpatient pharmacy and inpatient expenditures were estimated using marginalized 2-part models through a Bayesian Markov chain Monte Carlo (MCMC) sampling method because of the large proportion of $0 values26,27; noninformative prior distributions were specified using the PROC MCMC procedure in SAS, version 9.4 (SAS Institute).28 The first part of the model estimated the probability of incurring positive expenditures, and the second part estimated overall mean expenditures while accounting for the portion with $0 expenditures. Linear time trends in each component allowed for the calculation of different slopes before and after surgery, with specification testing through deviance information criterion. All time values were interacted with the surgical patient indicator, allowing for the calculation of each group’s distinct intercepts and slopes before and after surgery. Associated random intercepts and slopes were included in both components for outpatient pharmacy and inpatient expenditures, which accounted for the association among repeated measures and allowed dependence between the 2 parts of the model.29
The patient’s age, BMI, DCG score, marital status, copayment status, and prevalence of 12 comorbidities (hypertension, dyslipidemia, arthritis, depression, coronary artery disease, gastroesophageal reflux disease, asthma, fatty liver disease, posttraumatic stress disorder, alcohol abuse, substance abuse, and schizophrenia) at baseline were adjusted for in all models. Three sensitivity analyses were conducted. To assess the association of baseline covariates with results, expenditure estimates without covariate adjustments were generated. To assess the extent to which expenditure differences before surgery were associated with results after surgery, we estimated total expenditures in a subset (1:1 match) of the current cohort by restricting nonsurgical controls to the single patient in the nonsurgery cohort with outpatient expenditures closest to those of each patient in the surgery cohort at 13 to 24 months before surgery (n = 2329 patients and 2329 matches, with expenditures from 13-24 months before surgery included in the analysis). In addition, we estimated total postsurgical expenditures in the 3:1 cohort using a model that adjusted for the same covariates used in the main analysis plus total expenditures from 13 to 24 months before surgery. All tests were 2-sided with a significance threshold of P = .05, and analyses were conducted using SAS, version 9.4 (SAS Institute).
Results
The surgery cohort (n = 2498) and the matched nonsurgery cohort (n = 7456) were similar in all observed characteristics on which they were matched and in most covariates on which they were not matched (Table). The surgery and nonsurgery cohorts had mean (SD) ages of 52.3 (8.8) years and 52.5 (8.7) years, mean (SD) BMIs of 47 (7.9) and 46 (7.3), and mean (SD) DCG scores of 0.89 (0.76) and 0.82 (0.70), respectively. Most patients in the 2 cohorts (N = 9954) were men (7387 [74.2%]), white (8097 [81.3%]), and had received a diagnosis of diabetes at baseline (5440 [54.7%]). Several unmatched comorbidities were more prevalent among patients in the surgery cohort than the nonsurgery cohort. These comorbidities included hypertension (2010 [80.5%] vs 5196 [69.7%]), dyslipidemia (1513 [60.6%] vs 3902 [52.3%]), arthritis (685 [27.4%] vs 1116 [15.0%]), gastroesophageal reflux disease (870 [34.8%] vs 1428 [19.2%]), and depression (1102 [44.1%] vs 2409 [32.3%]). In the surgery cohort, 1842 patients (73.7%) underwent Roux-en-Y gastric bypass (RYGB) surgery (1318 [52.8%] received open RYGB and 524 [21.0%] received laparoscopic RYGB), 381 patients (15.3%) underwent sleeve gastrectomy, 249 patients (10.0%) underwent adjustable gastric banding, and 26 patients (1.0%) underwent biliopancreatic diversion or vertical banded gastroplasty.
Table. Baseline Characteristics of Surgery and Nonsurgery Cohorts .
| Characteristic | Surgery Cohort, No. (%) (n = 2498) | Nonsurgery Cohort, No. (%) (n = 7456) | Standardized Differences, %a |
|---|---|---|---|
| Variables included in match | |||
| Men | 1848 (74.0) | 5539 (74.3) | 0 |
| Diagnosed with diabetes | 1366 (54.7) | 4074 (54.6) | 0 |
| White | 2031 (81.3) | 6066 (81.4) | 0 |
| Nonwhite/unknown | 467 (18.7) | 1390 (18.6) | 0 |
| Age, mean (SD), y | 52.3 (8.8) | 52.5 (8.7) | −1.8 |
| BMI, mean (SD) | 47 (7.9) | 46 (7.3) | 6.8 |
| Super obese (BMI ≥50) | 729 (29.2) | 2127 (28.5) | 0 |
| DCG score, mean (SD) | 0.89 (0.76) | 0.82 (0.70) | 9.1 |
| DCG score, median (25th-75th percentile) | 0.69 (0.38-1.17) | 0.63 (0.35-1.11) | NA |
| VA regional network | |||
| New England, VISN1 | 18 (0.7) | 54 (0.7) | 0 |
| Upstate New York, VISN2 | 9 (0.4) | 27 (0.4) | 0 |
| New York/New Jersey, VISN3 | 57 (2.3) | 171 (2.3) | 0 |
| VISN4 Network, VISN4 | 251 (10.0) | 746 (10.0) | 0 |
| Capitol, VISN5 | 14 (0.6) | 40 (0.5) | 0 |
| Mid-Atlantic, VISN6 | 45 (1.8) | 135 (1.8) | 0 |
| Southeast, VISN7 | 52 (2.1) | 155 (2.1) | 0 |
| Sunshine, VISN8 | 150 (6.0) | 449 (6.0) | 0 |
| Mid-South, VISN9 | 293 (11.7) | 879 (11.8) | 0 |
| Ohio, VISN10 | 78 (3.1) | 228 (3.1) | 0 |
| Veterans in Partnership, VISN11 | 9 (0.4) | 27 (0.4) | 0 |
| Great Lakes, VISN12 | 66 (2.6) | 193 (2.6) | 0 |
| Heartland, VISN15 | 36 (1.4) | 108 (1.4) | 0 |
| South Central, VISN16 | 154 (6.2) | 462 (6.2) | 0 |
| Texas, VISN17 | 285 (11.4) | 852 (11.4) | 0 |
| Southwest, VISN18 | 31 (1.2) | 93 (1.2) | 0 |
| Rocky Mountain, VISN19 | 18 (0.7) | 54 (0.7) | 0 |
| Northwest, VISN20 | 171 (6.8) | 513 (6.9) | 0 |
| Sierra Pacific, VISN21 | 324 (13.0) | 963 (12.9) | 0 |
| Desert Pacific, VISN22 | 330 (13.2) | 987 (13.2) | 0 |
| Midwest, VISN23 | 107 (4.3) | 320 (4.3) | 0 |
| Variables not included in match | |||
| Married | 1335 (53.4) | 3649 (48.9) | 9.0 |
| Required to pay VA copayments | 278 (11.1) | 715 (9.6) | 5.1 |
| Exempt from VA copayments owing to disability | 1380 (55.2) | 3868 (51.9) | 6.8 |
| Exempt from VA copayments owing to low income | 650 (26.0) | 2168 (29.1) | -6.8 |
| Diagnoses not included in matchb | |||
| Hypertension | 2010 (80.5) | 5196 (69.7) | 25.1 |
| Dyslipidemia | 1513 (60.6) | 3902 (52.3) | 16.7 |
| Arthritis | 685 (27.4) | 1116 (15.0) | 30.8 |
| Depression | 1102 (44.1) | 2409 (32.3) | 24.5 |
| Coronary artery disease | 493 (19.7) | 1380 (18.5) | 3.1 |
| GERD | 870 (34.8) | 1428 (19.2) | 35.9 |
| Asthma | 291 (11.6) | 713 (9.6) | 6.8 |
| Fatty liver disease | 164 (6.6) | 43 (0.6) | 32.7 |
| PTSD | 450 (18.0) | 1198 (16.1) | 5.2 |
| Alcohol abuse | 97 (3.9) | 462 (6.2) | −10.6 |
| Substance abuse | 87 (3.5) | 316 (4.2) | −3.9 |
| Schizophrenia | 44 (1.8) | 365 (4.9) | −17.5 |
| Surgical procedure | |||
| AGB | 249 (10.0) | NA | NA |
| BPD | 18 (0.7) | NA | NA |
| RYGB lap | 524 (21.0) | NA | NA |
| RYGB open | 1318 (52.8) | NA | NA |
| Sleeve | 381 (15.3) | NA | NA |
| VBG | 8 (0.3) | NA | NA |
Abbreviations: AGB, adjustable gastric banding; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BPD, biliopancreatic diversion; DCG, diagnostic cost group; GERD, gastroesophageal reflux disease; NA, not applicable; PTSD, posttraumatic stress disorder; RYGB lap, laparoscopic Roux-en-Y gastric bypass; RYGB open, open Roux-en-Y gastric bypass; sleeve, sleeve gastrectomy; VA, US Department of Veterans Affairs; VBG, vertical banded gastroplasty; VISN, veterans integrated service network.
Standardized differences were calculated by comparing each covariate’s mean or proportion between the surgery and nonsurgery cohorts in units of the pooled SD, and the difference was then multiplied by 100.24
All diagnoses were identified from inpatient and outpatient visit records using diagnosis codes from the International Classification of Diseases, Ninth Revision.
Expenditure Trends of Surgical Patients
The median VA bariatric procedure expenditures (eFigure 2 in the Supplement) were lowest for adjustable gastric banding ($15 546), laparoscopic RYGB ($20 046), and vertical banded gastroplasty ($20 143); the median expenditures were highest for open RYGB ($22 191) and biliopancreatic diversion ($36 513).
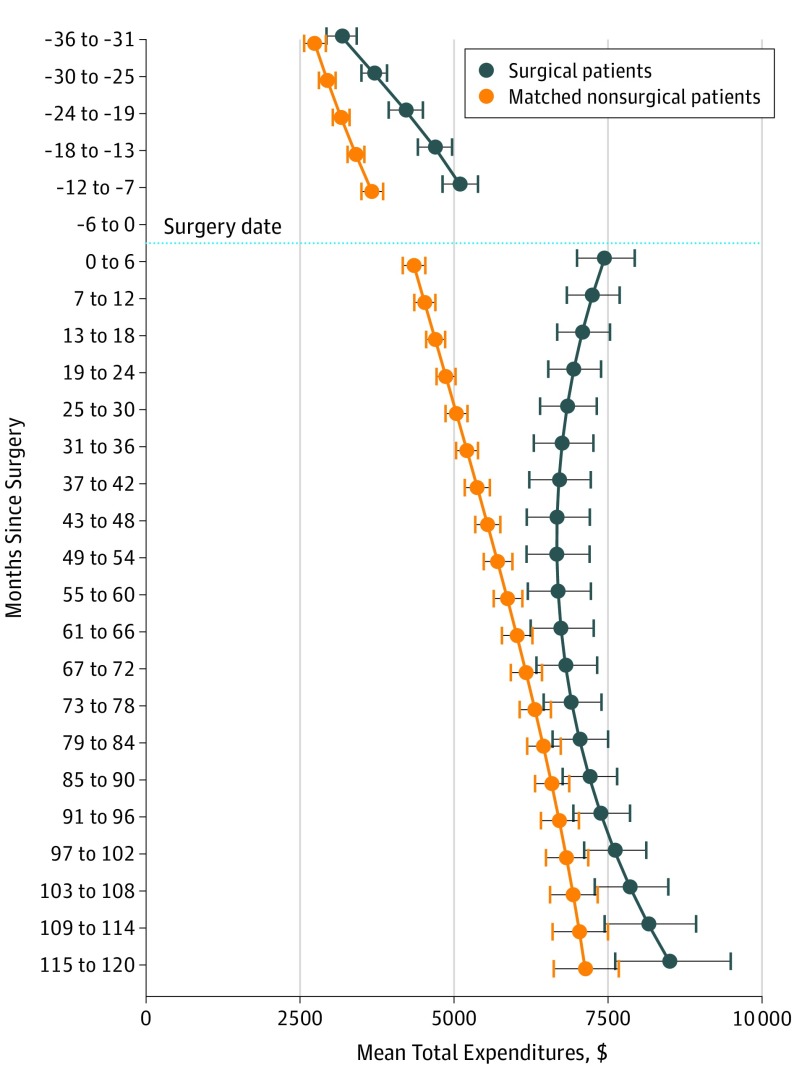
Three years before surgery, the model-estimated total expenditures were $3167 (95% CI, $2931-$3421) for patients in the surgery cohort, increasing to $5093 (95% CI, $4811-$5391) at 7 to 12 months before surgery and $7448 (95% CI, $6989-$7936) at 0 to 6 months after surgery (Figure 1). Thereafter, total expenditures decreased to $6671 (95% CI, $6174-$7207) at 4.5 years after surgery before increasing to $8495 (95% CI, $7609-$9484) at 10 years after surgery.
Figure 1. Mean Total Expenditures for Surgery and Nonsurgery Cohorts.
Mean total expenditures were estimated using a generalized linear model with a log link and SD proportional to the mean. The model adjusted for the following baseline covariates: age, body mass index (calculated as weight in kilograms divided by height in meters squared), diagnostic cost group score, marital status, copayment status, and prevalence of 12 comorbidities (hypertension, dyslipidemia, arthritis, depression, coronary artery disease, gastroesophageal reflux disease, asthma, fatty liver disease, posttraumatic stress disorder, alcohol abuse, substance abuse, and schizophrenia) at baseline. Cumulative model-estimated total expenditures at 10 years after surgery were $25 870 greater for the surgery cohort ($143 248) than the matched nonsurgery cohort ($117 378). The whiskers indicate the 95% CIs for the mean expenditure estimates at each time period.
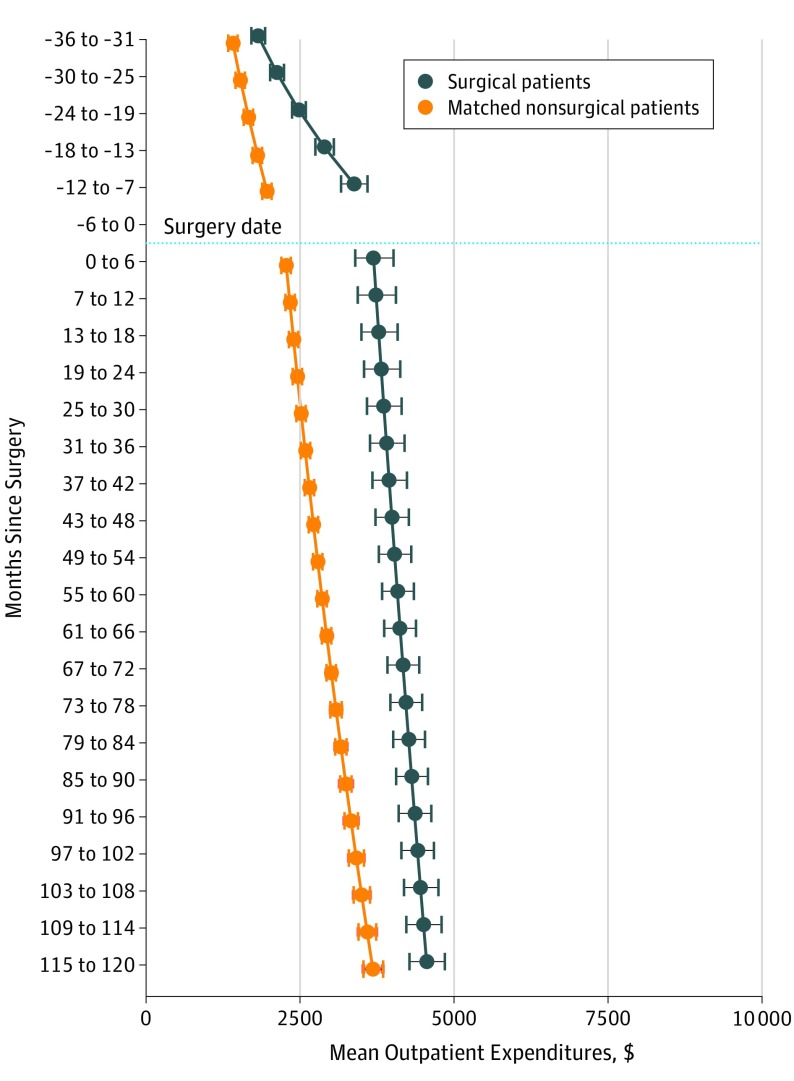
The model-estimated outpatient expenditures for the surgery cohort were $1818 (95% CI, $1715-$1926) at 3 years before surgery (Figure 2), which nearly doubled to $3372 (95% CI, $3164-$3593) at 7 to 12 months before surgery. These expenditures continued to increase during the postsurgical period, from $3691 (95% CI, $3393-$4014) at 0 to 6 months after surgery to a high of $4551 (95% CI, $4268-$4852) at 10 years after surgery.
Figure 2. Mean Outpatient Expenditures for Surgery and Nonsurgery Cohorts .
Mean outpatient expenditures were estimated using a generalized linear model with a log link and SD proportional to the mean. The model adjusted for the following baseline covariates: age, body mass index (calculated as weight in kilograms divided by height in meters squared), diagnostic cost group score, marital status, copayment status, and prevalence of 12 comorbidities (hypertension, dyslipidemia, arthritis, depression, coronary artery disease, gastroesophageal reflux disease, asthma, fatty liver disease, posttraumatic stress disorder, alcohol abuse, substance abuse, and schizophrenia) at baseline. The whiskers indicate the 95% CIs for the mean expenditure estimates at each time period.
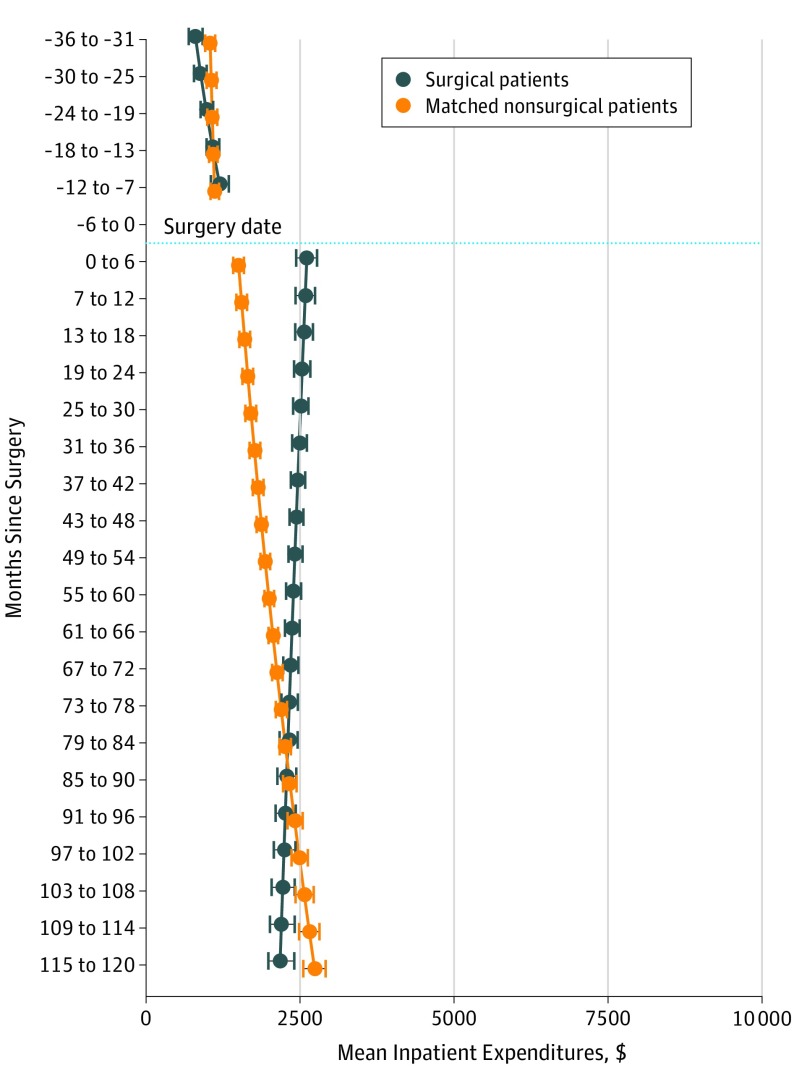
The model-estimated inpatient expenditures were $804 (95% CI, $703-$917) at 3 years before surgery (Figure 3), increasing to $1196 (95% CI, $1056-$1343) at 7 to 12 months before surgery. These expenditures increased to $2612 (95% CI, $2438-$2777) at 0 to 6 months after surgery before a linear decrease to $2179 (95% CI, $1987-$2407) at 10 years after surgery.
Figure 3. Mean Inpatient Expenditures for Surgery and Nonsurgery Cohorts .
Mean inpatient expenditures were estimated using a marginalized 2-part model. The model adjusted for the following baseline covariates: age, body mass index (calculated as weight in kilograms divided by height in meters squared), diagnostic cost group score, marital status, copayment status, and prevalence of 12 comorbidities (hypertension, dyslipidemia, arthritis, depression, coronary artery disease, gastroesophageal reflux disease, asthma, fatty liver disease, posttraumatic stress disorder, alcohol abuse, substance abuse, and schizophrenia) at baseline. The whiskers indicate the highest posterior density interval, a Bayesian analog to CIs, for the mean expenditure estimates at each time period.
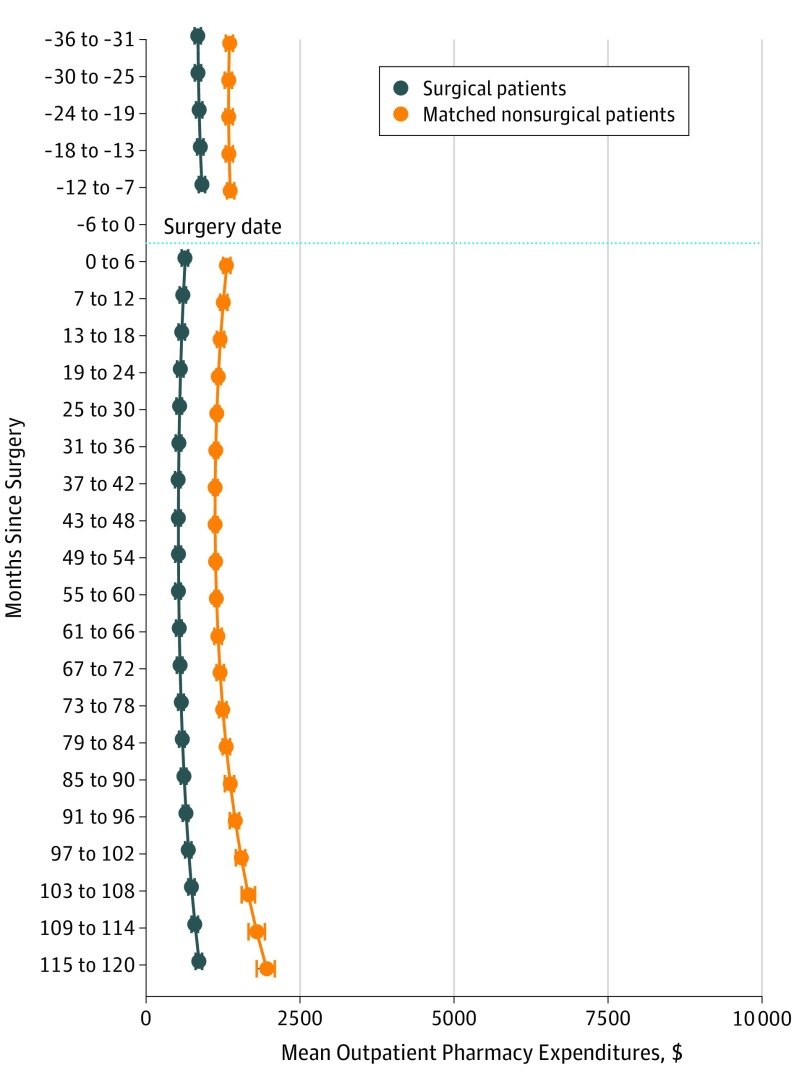
The model-estimated outpatient pharmacy expenditures were $845 (95% CI, $803-$886) at 3 years before surgery (Figure 4), increasing to $907 (95% CI, $869-$948) at 7 to 12 months before surgery. These expenditures decreased to $639 (95% CI, $611-$666) at 0 to 6 months after surgery and increased to $863 (95% CI, $791-$926) at 10 years after surgery.
Figure 4. Mean Outpatient Pharmacy Expenditures for Surgery and Nonsurgery Cohorts .
Mean outpatient pharmacy expenditures were estimated using a marginalized 2-part model. The model adjusted for the following baseline covariates: age, body mass index (calculated as weight in kilograms divided by height in meters squared), diagnostic cost group score, marital status, copayment status, and prevalence of 12 comorbidities (hypertension, dyslipidemia, arthritis, depression, coronary artery disease, gastroesophageal reflux disease, asthma, fatty liver disease, posttraumatic stress disorder, alcohol abuse, substance abuse, and schizophrenia) at baseline. The whiskers indicate the highest posterior density interval, a Bayesian analog to CIs, for the mean expenditure estimates at each time period.
Expenditure Comparison of Matched Cohorts
The model-estimated total expenditures were $427 higher for the surgery cohort than the nonsurgery cohort at 3 years before surgery. This difference increased to $1426 at 7 to 12 months before surgery (Figure 1). The between-group difference in model-estimated total expenditures increased to $3097 at 0 to 6 months after surgery but decreased rapidly over the next 5 years. By 5.5 years after surgery, the between-group difference in the model-estimated total expenditures decreased. The between-group difference remained consistently smaller in years 6 to 10 of follow-up than in earlier years. The aggregated model-estimated mean total expenditures in the 10 years after surgery indicated that cumulative expenditures were $25 870 higher among patients in the surgery cohort ($143 248) than patients in the nonsurgery cohort ($117 378).
The model-estimated outpatient expenditures were $409 higher in the surgery cohort than the nonsurgery cohort at 3 years before surgery (Figure 2), which increased to $1408 higher at 7 to 12 months before surgery. These expenditures were $1416 higher among the surgery cohort at 0 to 6 months after surgery. The expenditures remained higher throughout follow-up, although expenditures in the surgery cohort increased less than those of the nonsurgery cohort through the 10 years after surgery. The between-group difference was $872 at 10 years.
The model-estimated inpatient expenditures were similar for the surgery and nonsurgery cohorts at 3 years before surgery (Figure 3); these expenditures were $1106 higher for the surgery cohort at 0 to 6 months after surgery but gradually decreased thereafter. There was no difference in inpatient expenditures between the cohorts at 6 years after surgery. The model-estimated inpatient expenditures for the surgery cohort continued to decrease after surgery; they were $559 lower than those of the nonsurgery cohort 10 years after surgery.
Among the surgery cohort, the model-estimated outpatient pharmacy expenditures were $509 lower at 3 years before surgery and $461 lower at 7 to 12 months before surgery than those of the nonsurgery cohort (Figure 4); these expenditures decreased immediately after surgery and remained lower than those of the nonsurgery cohort throughout follow-up, decreasing to $1096 lower than the nonsurgery cohort at 10 years after surgery.
Sensitivity Analyses
The matched unadjusted regression analysis suggested similar overall results in presurgical and postsurgical expenditures (eFigure 3, eFigure 4, eFigure 5, and eFigure 6 in the Supplement). In general, the regression models without covariate adjustments resulted in slightly higher mean expenditures, with slightly larger differences between the surgery and nonsurgery cohorts for total and outpatient expenditures.
The unadjusted results suggested slightly smaller differences in outpatient pharmacy expenditures. In 2 additional sensitivity analyses, the results were consistent with results from the original analysis, suggesting that the main results were robust. eFigure 7 and eFigure 8 in the Supplement show the 1:1 expenditure match before surgery and the 3:1 baseline adjustment for expenditures before surgery.
Discussion
In this study of predominantly older male veterans with severe obesity, total health care expenditures were increasing 3 years before bariatric surgery, largely owing to increased outpatient expenditures. Patients in the surgery cohort had a greater increase in total expenditures in the 2 years after surgery compared with matched patients in the nonsurgery cohort. Thereafter, significant reductions in the outpatient pharmacy expenditures of the surgery cohort were offset by higher inpatient and outpatient (nonpharmacy) expenditures. The total expenditures of the surgery cohort converged with but never fell below the gradually increasing expenditures of the nonsurgery cohort 10 years after surgery.
The outpatient expenditures of the surgery cohort indicated a linear increase during the 10 years after surgery, and they were $1000 to $1500 higher than the outpatient expenditures of the nonsurgery cohort, which was consistent with the 20-year expenditure results from the Swedish Obese Subjects (SOS) study.30 These findings may be explained by the persistence of some obesity-related conditions (eg, not all patients with diabetes experience remission after bariatric surgery, and even those who experience remission may continue to be monitored for the complications of diabetes) and by the care needed to treat the long-term adverse effects of bariatric surgery (eg, the treatment of anemia and vitamin deficiencies).
The inpatient expenditures of the surgery cohort increased sharply after bariatric surgery, likely owing to subsequent surgical or intervention procedures or other complications (eg, nausea, vomiting, and dehydration).31,32 Weight loss among the surgery cohort may have also required the removal of excess skin or have qualified those with severe osteoarthritis for hip or knee replacements.33,34 Although inpatient expenditures in the surgery cohort were higher after surgery than before surgery, these expenditures gradually decreased to become lower than the expenditures of the matched nonsurgery cohort 7.5 years after surgery. Medication expenditures decreased immediately after surgery, which is a finding consistent with those of previous studies,13,30,35,36,37,38 particularly the SOS study, in which annual drug expenditures were $228 lower in the 7 to 20 years after surgery.30
Although no previous US-based cohort study, to our knowledge, has examined 10-year postsurgery expenditures, our finding of similar long-term total expenditures between patients who did and did not undergo bariatric surgery is consistent with the results of the SOS and other shorter-term studies.7,8,30,39 The SOS study reported that patients who underwent surgery had more hospital days and more nonprimary care outpatient visits in the first 2 to 6 years after surgery; however, the use of inpatient health care services was similar thereafter among patients who did and did not undergo surgery.30 Our results counter other studies that suggested expenditure reductions after bariatric surgery were sufficiently large to offset the cost of surgery.10,11,12,13,14
The differences in findings between this study and previous studies may be attributable to 3 factors. First, this cohort study included predominantly men who were older than 50 years and had significant comorbidity burdens, while previous cohort studies comprised predominantly women aged 35 to 55 years who had relatively lower comorbidity burdens. The patients who underwent bariatric surgery in this study had a higher prevalence of hypertension (80% vs 44%-55%),7,12,14 asthma (12% vs 3%-10%),12,13,14 and depression (44% vs 9%-15%)12,13,14 compared with patients in other cohort studies. Second, our nonsurgery cohort was identified based on BMI measurements rather than obesity diagnosis codes, which have been used in most previous studies.7,11,12 Bariatric surgery may appear to save costs if matched patients with obesity who do not undergo surgery have greater overall comorbidity than patients with obesity who undergo surgery, a supposition supported by Finkelstein et al.14 Third, unlike some previous studies that demonstrated cost savings, our cohorts were not matched based on presurgical expenditures. We assumed that patients who did not undergo surgery would not have a similar expenditure escalation at 6 to 12 months before surgery as those who underwent surgery, partly owing to the administration of additional tests and procedures related to presurgical medical evaluations. The high postsurgical expenditures of nonsurgery control cohorts that were matched based on presurgical expenditures in previous studies may not represent the expected expenditure trajectory of patients who underwent surgery if they had not received that surgery, which raises questions about the validity of the data from those cohorts.40
These 10-year results may inform future studies of the cost-effectiveness of bariatric surgery, which likely varies by procedure and patient subgroups.9,41 An expanding body of evidence from modeling studies suggests that RYGB surgery may be cost-effective for patients with severe obesity and for those with diabetes and class 1 (BMI of 30.0-34.9) or class 2 (BMI of 35.0-39.9) obesity.42,43,44 The cost-effectiveness of sleeve gastrectomy remains uncertain because long-term costs and clinical outcomes have not yet been accrued and reported. It is possible that bariatric surgery is more cost-effective for younger patients who have fewer comorbidities than those in this cohort study. Our results do not imply cost-effectiveness, but future work is needed to understand how cost-effectiveness varies by age, comorbidity, and disease severity for patients undergoing sleeve gastrectomy and RYGB surgery.
Limitations
This study had several limitations. The analyses may have been subject to unobserved confounding because patients were not randomized, and we could not match patients on every available characteristic. The estimates represent associations and not the causal effects of bariatric surgery on expenditures. Further, the presurgical expenditure estimates differed between the surgery and nonsurgery cohorts. We chose not to match patients based on presurgical expenditures because patients typically undergo lengthy workups before surgery. If some of the escalating costs observed were associated with the patient’s knowledge of future surgery, matching patients based on presurgical expenditures would have produced biased results in favor of the surgery cohort. Therefore, we presented presurgical and postsurgical expenditure data to provide important context.
This study used data from the VA only. Medicare, Medicaid, and private insurance expenditures were not considered nor were the social costs associated with changes in employment status, attendance, and absenteeism that have been reported in previous work.45,46,47,48,49 In future studies based on a social perspective, if productivity costs are included and shown to decline (eg, lower absenteeism, higher attendance, and fewer disability payments), bariatric surgery may be found to be associated with increased cost-effectiveness or even cost savings. In addition, most patients who underwent RYGB surgery received open rather than laparoscopic RYGB, potentially limiting the generalizability of our estimates to the current most commonly performed procedures.
Future research should also examine whether newer bariatric procedures (eg, sleeve gastrectomy, which represented only 15.3% of the surgical procedures in our study) have greater potential for cost savings given their lower up-front costs and lower risk of short-term and long-term complications compared with RYGB surgery. In addition, results from these older, predominantly male patients with greater comorbidity burdens may not be generalizable to nonveteran, younger, female, or healthier populations.
Conclusions
In this study, bariatric surgery did not appear to be associated with lower health care expenditures 10 years after the procedure in a cohort study of predominantly older male veterans with severe obesity. Lower outpatient pharmacy expenditures were offset by higher inpatient and outpatient (nonpharmacy) expenditures. Given the evidence that bariatric surgery is associated with long-term, durable weight loss and improvements in long-term survival, the value of bariatric surgery may be primarily in its association with substantial improvements in health and quality of life, not in its potential to achieve cost savings.
eTable. Number of Patients Remaining in 4 Cost Analyses
eFigure 1. Flow of Surgical Patients and Matched Patients from January 1, 2000, to September 30, 2011
eFigure 2. Unadjusted Median VA Inpatient Expenditures for Bariatric Surgery, By Procedure Type
eFigure 3. Model-Estimated Trends Without Covariate Adjustment in Estimated Total Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 4. Model-Estimated Trends Without Covariate Adjustment in Estimated Outpatient Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 5. Model-Estimated Trends Without Covariate Adjustment in Estimated Inpatient Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 6. Model-Estimated Trends Without Covariate Adjustment in Estimated Outpatient Pharmacy Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 7. Model-Estimated Trends in Estimated Postsurgical Total Expenditures for Surgical Patients and Nonsurgical Matches From a Model That Adjusts for Outpatient Expenditures 13-24 Months Prior to Surgery
eFigure 8. Model-Estimated Trends in Estimated Total Expenditures for Surgical Patients and the Single Nonsurgical Match With the Closest Outpatient Expenditures 13-24 Months Prior to Surgery
References
- 1.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):-. doi: 10.1001/jamasurg.2013.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219-234. doi: 10.1111/joim.12012 [DOI] [PubMed] [Google Scholar]
- 3.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond). 2007;31(8):1248-1261. doi: 10.1038/sj.ijo.0803573 [DOI] [PubMed] [Google Scholar]
- 4.Kolotkin RL, Davidson LE, Crosby RD, Hunt SC, Adams TD. Six-year changes in health-related quality of life in gastric bypass patients versus obese comparison groups. Surg Obes Relat Dis. 2012;8(5):625-633. doi: 10.1016/j.soard.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjostrom L, Narbro K, Sjostrom CD, et al. ; Swedish Obese Subjects Study . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741-752. doi: 10.1056/NEJMoa066254 [DOI] [PubMed] [Google Scholar]
- 6.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-761. doi: 10.1056/NEJMoa066603 [DOI] [PubMed] [Google Scholar]
- 7.Weiner JP, Goodwin SM, Chang HY, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg. 2013;148(6):555-562. doi: 10.1001/jamasurg.2013.1504 [DOI] [PubMed] [Google Scholar]
- 8.Maciejewski ML, Livingston EH, Smith VA, Kahwati LC, Henderson WG, Arterburn DE. Health expenditures among high-risk patients after gastric bypass and matched controls. Arch Surg. 2012;147(7):633-640. doi: 10.1001/archsurg.2012.818 [DOI] [PubMed] [Google Scholar]
- 9.Maciejewski ML, Arterburn DE. Cost-effectiveness of bariatric surgery. JAMA. 2013;310(7):742-743. doi: 10.1001/jama.2013.276131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremieux PY, Buchwald H, Shikora SA, Ghosh A, Yang HE, Buessing M. A study on the economic impact of bariatric surgery. Am J Manag Care. 2008;14(9):589-596. [PubMed] [Google Scholar]
- 11.Finkelstein EA, Brown DS. A cost-benefit simulation model of coverage for bariatric surgery among full-time employees. Am J Manag Care. 2005;11(10):641-646. [PubMed] [Google Scholar]
- 12.Finkelstein EA, Allaire BT, Burgess SM, Hale BC. Financial implications of coverage for laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2011;7(3):295-303. doi: 10.1016/j.soard.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 13.Klein S, Ghosh A, Cremieux PY, Eapen S, McGavock TJ. Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI ≥35 kg/m2. Obesity (Silver Spring). 2011;19(3):581-587. doi: 10.1038/oby.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelstein EA, Allaire BT, Globe D, Dixon JB. The business case for bariatric surgery revisited: a non-randomized case-control study. PLoS One. 2013;8(9):e75498. doi: 10.1371/journal.pone.0075498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arterburn DE, Eid G, Maciejewski ML. Long-term survival following bariatric surgery in the VA health system—reply. JAMA. 2015;313(14):1474-1475. doi: 10.1001/jama.2015.2586 [DOI] [PubMed] [Google Scholar]
- 16.Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151(11):1046-1055. doi: 10.1001/jamasurg.2016.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy EH, Taylor JM, Schaubel DE, Williams S. The effect of salvage therapy on survival in a longitudinal study with treatment by indication. Stat Med. 2010;29(25):2569-2580. doi: 10.1002/sim.4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YP, Propert KJ, Rosenbaum PR. Balanced risk set matching. J Am Stat Assoc. 2001;96(455):870-882. doi: 10.1198/016214501753208573 [DOI] [Google Scholar]
- 19.Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005;61(3):721-728. doi: 10.1111/j.1541-0420.2005.00356.x [DOI] [PubMed] [Google Scholar]
- 20.Maciejewski ML, Liu CF, Derleth A, McDonell M, Anderson S, Fihn SD. The performance of administrative and self-reported measures for risk adjustment of Veterans Affairs expenditures. Health Serv Res. 2005;40(3):887-904. doi: 10.1111/j.1475-6773.2005.00390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maciejewski ML, Liu CF, Fihn SD. Performance of comorbidity, risk adjustment, and functional status measures in expenditure prediction for patients with diabetes. Diabetes Care. 2009;32(1):75-80. doi: 10.2337/dc08-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu XS, Rosenbaum PR. Comparison of multivariate matching methods: structures, distances and algorithms. J Comput Graph Stat. 1993;2(4):405-420. [Google Scholar]
- 23.Wagner TH, Chen S, Barnett PG. Using average cost methods to estimate encounter-level costs for medical-surgical stays in the VA. Med Care Res Rev. 2003;60(3 suppl):15S-36S. doi: 10.1177/1077558703256485 [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461-494. doi: 10.1016/S0167-6296(01)00086-8 [DOI] [PubMed] [Google Scholar]
- 26.Smith VA, Neelon B, Maciejewski ML, Preisser JS. Two parts are better than one: modeling marginal means of semicontinuous data. Health Serv Outcomes Res Methodol. 2017;17(3-4):198-218. doi: 10.1007/s10742-017-0169-9 [DOI] [Google Scholar]
- 27.Smith VA, Maciejewski ML, Olsen MK. Modeling semicontinuous longitudinal expenditures: a practical guide. Health Serv Res. 2018;53(suppl 1):3125-3147. doi: 10.1111/1475-6773.12815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith VA, Neelon B, Preisser JS, Maciejewski ML. A marginalized two-part model for longitudinal semicontinuous data. Stat Methods Med Res. 2017;26(4):1949-1968. doi: 10.1177/0962280215592908 [DOI] [PubMed] [Google Scholar]
- 29.Su L, Tom BD, Farewell VT. Bias in 2-part mixed models for longitudinal semicontinuous data. Biostatistics. 2009;10(2):374-389. doi: 10.1093/biostatistics/kxn044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. JAMA. 2012;308(11):1132-1141. doi: 10.1001/2012.jama.11792 [DOI] [PubMed] [Google Scholar]
- 31.Dorman RB, Miller CJ, Leslie DB, et al. Risk for hospital readmission following bariatric surgery. PLoS One. 2012;7(3):e32506. doi: 10.1371/journal.pone.0032506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruze G, Ottosson J, Neovius M, Naslund I, Marsk R. Hospital admission after gastric bypass: a nationwide cohort study with up to 6 years follow-up. Surg Obes Relat Dis. 2017;13(6):962-969. doi: 10.1016/j.soard.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inacio MC, Paxton EW, Fisher D, Li RA, Barber TC, Singh JA. Bariatric surgery prior to total joint arthroplasty may not provide dramatic improvements in post-arthroplasty surgical outcomes. J Arthroplasty. 2014;29(7):1359-1364. doi: 10.1016/j.arth.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin JR, Watts CD, Taunton MJ. Bariatric surgery does not improve outcomes in patients undergoing primary total knee arthroplasty. Bone Joint J. 2015;97-B(11):1501-1505. doi: 10.1302/0301-620X.97B11.36477 [DOI] [PubMed] [Google Scholar]
- 35.Nguyen NT, Varela JE, Sabio A, Naim J, Stamos M, Wilson SE. Reduction in prescription medication costs after laparoscopic gastric bypass. Am Surg. 2006;72(10):853-856. [PubMed] [Google Scholar]
- 36.Makary MA, Clark JM, Shore AD, et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg. 2010;145(8):726-731. doi: 10.1001/archsurg.2010.150 [DOI] [PubMed] [Google Scholar]
- 37.Keating CL, Peeters A, Swinburn BA, Carter R, Moodie ML. Pharmaceutical utilisation and costs before and after bariatric surgery. Int J Obes (Lond). 2013;37(11):1467-1472. doi: 10.1038/ijo.2013.24 [DOI] [PubMed] [Google Scholar]
- 38.Lewis KH, Zhang F, Arterburn DE, Ross-Degnan D, Gillman MW, Wharam JF. Comparing medical costs and use after laparoscopic adjustable gastric banding and Roux-en-Y gastric bypass. JAMA Surg. 2015;150(8):787-794. doi: 10.1001/jamasurg.2015.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerjee S, Garrison LP Jr, Flum DR, Arterburn DE. Cost and health care utilization implications of bariatric surgery versus intensive lifestyle and medical intervention for type 2 diabetes. Obesity (Silver Spring). 2017;25(9):1499-1508. doi: 10.1002/oby.21927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daw JR, Hatfield LA. Matching and regression to the mean in difference-in-differences analysis. Health Serv Res. 2018;53(6):4138-4156. doi: 10.1111/1475-6773.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang BC, Wong ES, Alfonso-Cristancho R, et al. Cost-effectiveness of bariatric surgical procedures for the treatment of severe obesity. Eur J Health Econ. 2014;15(3):253-263. doi: 10.1007/s10198-013-0472-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keating CL, Dixon JB, Moodie ML, et al. Cost-effectiveness of surgically induced weight loss for the management of type 2 diabetes: modeled lifetime analysis. Diabetes Care. 2009;32(4):567-574. doi: 10.2337/dc08-1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikramuddin S, Klingman D, Swan T, Minshall ME. Cost-effectiveness of Roux-en-Y gastric bypass in type 2 diabetes patients. Am J Manag Care. 2009;15(9):607-615. [PubMed] [Google Scholar]
- 44.Hoerger TJ, Zhang P, Segel JE, Kahn HS, Barker LE, Couper S. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care. 2010;33(9):1933-1939. doi: 10.2337/dc10-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narbro K, Agren G, Jonsson E, et al. Sick leave and disability pension before and after treatment for obesity: a report from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 1999;23(6):619-624. doi: 10.1038/sj.ijo.0800890 [DOI] [PubMed] [Google Scholar]
- 46.Andersen JR, Hernaes UJ, Hufthammer KO, Vage V. Employment status and sick-leave following obesity surgery: a five-year prospective cohort study. PeerJ. 2015;3:e1285. doi: 10.7717/peerj.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathus-Vliegen EM, de Weerd S, de Wit LT. Health-related quality-of-life in patients with morbid obesity after gastric banding for surgically induced weight loss. Surgery. 2004;135(5):489-497. doi: 10.1016/j.surg.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 48.Turchiano M, Saunders JK, Fernandez G, Navie L, Labrador L, Parikh M. Bariatric surgery may improve employment status in unemployed, underserved, severely obese patients. Obes Surg. 2014;24(5):692-695. doi: 10.1007/s11695-013-1140-7 [DOI] [PubMed] [Google Scholar]
- 49.Sockalingam S, Wnuk S, Kantarovich K, et al. Employment outcomes one year after bariatric surgery: the role of patient and psychosocial factors. Obes Surg. 2015;25(3):514-522. doi: 10.1007/s11695-014-1443-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Number of Patients Remaining in 4 Cost Analyses
eFigure 1. Flow of Surgical Patients and Matched Patients from January 1, 2000, to September 30, 2011
eFigure 2. Unadjusted Median VA Inpatient Expenditures for Bariatric Surgery, By Procedure Type
eFigure 3. Model-Estimated Trends Without Covariate Adjustment in Estimated Total Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 4. Model-Estimated Trends Without Covariate Adjustment in Estimated Outpatient Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 5. Model-Estimated Trends Without Covariate Adjustment in Estimated Inpatient Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 6. Model-Estimated Trends Without Covariate Adjustment in Estimated Outpatient Pharmacy Expenditures for Surgical Patients and Nonsurgical Matches
eFigure 7. Model-Estimated Trends in Estimated Postsurgical Total Expenditures for Surgical Patients and Nonsurgical Matches From a Model That Adjusts for Outpatient Expenditures 13-24 Months Prior to Surgery
eFigure 8. Model-Estimated Trends in Estimated Total Expenditures for Surgical Patients and the Single Nonsurgical Match With the Closest Outpatient Expenditures 13-24 Months Prior to Surgery