Key Points
Question
What is the association between cord plasma biomarkers of in utero acetaminophen exposure and risk of childhood attention-deficit/hyperactivity disorder and autism spectrum disorder?
Findings
In this cohort study of 996 mother-infant dyads from the Boston Birth Cohort, cord plasma biomarkers of fetal exposure to acetaminophen were associated with significantly increased risk of childhood attention-deficit/hyperactivity disorder and autism spectrum disorder.
Meaning
These findings suggest in utero exposure to acetaminophen is associated with increased risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in children and warrant additional investigations.
Abstract
Importance
Prior studies have raised concern about maternal acetaminophen use during pregnancy and increased risk of attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) in their children; however, most studies have relied on maternal self-report.
Objective
To examine the prospective associations between cord plasma acetaminophen metabolites and physician-diagnosed ADHD, ASD, both ADHD and ASD, and developmental disabilities (DDs) in childhood.
Design, Setting, and Participants
This prospective cohort study analyzed 996 mother-infant dyads, a subset of the Boston Birth Cohort, who were enrolled at birth and followed up prospectively at the Boston Medical Center from October 1, 1998, to June 30, 2018.
Exposures
Three cord acetaminophen metabolites (unchanged acetaminophen, acetaminophen glucuronide, and 3-[N-acetyl-l-cystein-S-yl]-acetaminophen) were measured in archived cord plasma samples collected at birth.
Main Outcomes and Measures
Physician-diagnosed ADHD, ASD, and other DDs as documented in the child’s medical records.
Results
Of 996 participants (mean [SD] age, 9.8 [3.9] years; 548 [55.0%] male), the final sample included 257 children (25.8%) with ADHD only, 66 (6.6%) with ASD only, 42 (4.2%) with both ADHD and ASD, 304 (30.5%) with other DDs, and 327 (32.8%) who were neurotypical. Unchanged acetaminophen levels were detectable in all cord plasma samples. Compared with being in the first tertile, being in the second and third tertiles of cord acetaminophen burden was associated with higher odds of ADHD diagnosis (odds ratio [OR] for second tertile, 2.26; 95% CI, 1.40-3.69; OR for third tertile, 2.86; 95% CI, 1.77-4.67) and ASD diagnosis (OR for second tertile, 2.14; 95% CI, 0.93-5.13; OR for third tertile, 3.62; 95% CI, 1.62-8.60). Sensitivity analyses and subgroup analyses found consistent associations between acetaminophen buden and ADHD and acetaminophen burden and ASD across strata of potential confounders, including maternal indication, substance use, preterm birth, and child age and sex, for which point estimates for the ORs vary from 2.3 to 3.5 for ADHD and 1.6 to 4.1 for ASD.
Conclusions and Relevance
Cord biomarkers of fetal exposure to acetaminophen were associated with significantly increased risk of childhood ADHD and ASD in a dose-response fashion. Our findings support previous studies regarding the association between prenatal and perinatal acetaminophen exposure and childhood neurodevelopmental risk and warrant additional investigations.
This cohort study of mother-infant dyads examines the associations between cord plasma acetaminophen metabolites and physician-diagnosed attention-deficit/hyperactivity disorder, autism spectrum disorder, and other developmental disabilities.
Introduction
Acetaminophen is the most commonly used medication for analgesic and antipyretic purposes among mothers during pregnancy and infants in early life.1,2,3,4,5,6,7,8 More than 65% of women in the United States and 50% in Europe ever used acetaminophen during pregnancy.7,9 Despite its widespread use, previous studies in animals and humans have found an association between prenatal acetaminophen exposure and increased risks of adverse childhood outcomes, including asthma,10,11 cryptorchidism,12 and neurodevelopmental disorders, including attention-deficit/hyperactivity disorder (ADHD)12,13,14,15,16,17,18,19,20 and autism spectrum disorder (ASD).17,21,22
Studies23,24 in rodents reported acetaminophen toxicity in cortical neurons and inhibition of fetal testosterone production, which would critically disrupt brain development. In addition, the therapeutic effect of acetaminophen can selectively inhibit cyclooxygenase 2, which may affect multiple brain functions, including long-term potentiation,25 spatial learning,26 and cerebellar development.27
Human studies12,13,14,15,16,17,18,19,20,21,22,28,29,30,31,32 have found that acetaminophen could cross the human placental barrier and remain in an infant’s blood circulation for a long duration.28,29 Ecologic and cohort studies have found an association between maternal acetaminophen use and risk of ADHD12,13,14,15,16,17,18,19,20 and ASD.17,21,22 In the past 5 years, an increasing number of large, prospective cohort studies (mostly from Europe) found significant associations between maternal self-reported acetaminophen use during pregnancy and increased risk of ADHD and related symptoms in offspring in later life.13,14,15,16,17,30 In addition, a longer duration of reported use was also associated with higher risk of ADHD.13 Two recent meta-analyses31,32 found significant associations between maternal-reported acetaminophen use during pregnancy and the risk of ADHD.
However, the Society for Maternal-Fetal Medicine (SMFM) and the US Food and Drug Administration (FDA) have refrained from making recommendations regarding use, citing limited evidence and methodologic concerns, including recall bias, lack of dose information, potential residual confounders, and multiple testing.33,34 Nonetheless, the FDA has called on pregnant women and health care professionals to carefully evaluate the benefits and risks of using acetaminophen during pregnancy.34 Similarly, the American Academy of Pediatrics (AAP) Grand Rounds concluded that there was no definitive causal link between acetaminophen exposure and ADHD.35
For the first time to our knowledge, we examined the prospective association between cord plasma acetaminophen metabolites (a direct evidence of fetal exposure) and childhood ADHD, ASD, and other developmental disabilities (DDs) using data from the Boston Birth Cohort (BBC). This study aimed to address the limitations highlighted by the SMFM, FDA, and AAP in relevant previous studies.33,34,35
Methods
Study Design and Setting
For this cohort study, we used data from the BBC, which consist of 3163 mother-infant dyads who enrolled at birth and remained in the follow-up study from October 1, 1998, to June 30, 2018 (eFigure 1 in the Supplement). A detailed description of the BBC can be found in previous studies.36,37 In brief, mothers who delivered singleton live births at Boston Medical Center (BMC) were invited into the BBC within 1 to 3 days after delivery. Mothers with the following conditions were not eligible for enrollment in the BBC: conception via in vitro fertilization, nonsingleton pregnancies (eg, twins or triplets), deliveries induced by maternal trauma, and/or newborns with major birth defects. Beginning at 6 months of age, enrolled infants who continued to receive pediatric primary or specialty care at the BMC were invited to participate in the postnatal follow-up study up to 21 years of age.36,38,39 The institutional review boards of BMC and the Johns Hopkins Bloomberg School of Public Health approved the study protocol for the baseline and follow-up studies. Written consent was obtained from all participating mothers. Depending on a child's age, verbal or written consent was also obtained from participating children. All the databases for research do not contain personal identifiers and are accessible only by authored investigators.
Of the 3163 mother-infant dyads enrolled in the BBC postnatal follow-up study, 996 had sufficient cord plasma samples for metabolite assays and met the definitions for ADHD, ASD, other DDs, or neurotypical development (ND) (eFigure 1 in the Supplement). Of the dyads with cord metabolite data, 805 also had maternal plasma metabolite data collected within 3 days after delivery. eTable 1 in the Supplement compares the mother-infant dyads included in these analyses with those who were excluded.
Definition of Diagnosis Groups
We defined 5 mutually exclusive groups based on physician diagnoses as documented in electronic medical records (EMRs) up to June 2018: ADHD only, ASD only, ADHD and ASD, other DDs, and ND. The group with ADHD consisted of children with diagnoses recorded with ADHD-related International Classification of Diseases, Ninth Revision (ICD-9) (codes 314.0-314.9) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) (codes F90.0-F90.9) codes but excluding ASD-related codes (ICD-9 codes 299.0-299.91 or ICD-10 codes F84.0-F84.9). The group with ASD consisted of children with diagnoses recorded with ASD-related ICD codes but excluding ADHD-related codes. The group with ADHD and ASD consisted of children with diagnoses recorded with ADHD-related and ASD-related ICD codes. The group with other DDs consisted of children diagnosed with mental, behavioral, and neurodevelopmental disorders (ICD-9 codes 290-319 or ICD-10 codes F01-F99 but excluding ADHD- and ASD-related ICD codes). The group with ND consisted of children without any of the aforementioned codes related to mental, behavioral, and neurodevelopmental disorders in their EMR. All the primary care and subspecialty visits were recorded in the EMRs at the BMC beginning in January 2004. The primary and secondary diagnoses for each visit were documented along with corresponding codes in the ICD-9 (before October 1, 2015) and ICD-10 (after October 1, 2015).
Cord Biomarkers of Acetaminophen Exposure
Cord plasma metabolites of acetaminophen were measured using umbilical cord plasma samples collected at birth. Maternal plasma metabolites of acetaminophen were measured using nonfasting plasma samples obtained within 3 days after delivery.40 The unchanged acetaminophen, acetaminophen glucuronide, and 3-(N-acetyl-l-cystein-S-yl)-acetaminophen levels in cord and maternal plasma samples were measured using liquid chromatography–tandem mass spectrometry techniques at the Broad Institute Metabolite Profiling Laboratory at Massachusetts Institute of Technology.41,42,43
Definition of Maternal Characteristics
Similar to a previous study40 on maternal plasma biomarkers of acetaminophen, we included the following maternal and child clinical and demographic variables as potential confounders: maternal age at delivery, maternal race/ethnicity, maternal educational level, marital status, stress during pregnancy, smoking before or during pregnancy, alcohol use before or during pregnancy, maternal body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), parity, breastfeeding, ever use of illicit drugs, stress during pregnancy, maternal fever during pregnancy, early childhood lead levels, child's sex, delivery type, preterm birth, and birth weight. Maternal demographic and nonclinical variables were obtained by BBC trained research staff using standard questionnaire interview. Clinically related variables were extracted from EMRs. The early childhood lead levels in the children (as part of pediatric routine lead screening) were extracted from their EMRs. The first lead levels measured were chosen for this analysis.
Statistical Analysis
The maternal and child characteristics of the study children by the 5 groups (ND, ADHD, ASD, ADHD and ASD, and other DDs) were compared using the Pearson χ2 test (or Fisher exact test for small cells) for categorical variables and the analysis of variance test for continuous variables. The distribution of peak intensities of maternal and cord acetaminophen metabolite exposure was compared across the 5 groups. Next, metabolites of acetaminophen were ranked using inverse normal transformation for all subsequent analyses. The transformed peak intensities of unchanged cord acetaminophen were categorized into tertiles. Because of a high rate of no detection, other acetaminophen metabolites were grouped into binary groups: no detection and any detection for further analysis. We evaluated the association between maternal and cord acetaminophen groups using the Pearson χ2 test. Because of difficulties in accurately assessing fetal metabolic conditions within the maternal system,44 we borrowed the metabolite proportions from the adult’s acetaminophen metabolic pathway to calculate a variable to reflect overall cord acetaminophen burden using the following formula: [cord acetaminophen burden = (unchanged acetaminophen × 5% + acetaminophen glucuronide × 50% + 3-(N-acetyl-l-cystein-S-yl)-acetaminophen × 5%)/60%].45 We also calculated alternative cord acetaminophen burden based on study of the neonate’s urine acetaminophen metabolites as follows: [cord acetaminophen burden = (unchanged acetaminophen/14% + acetaminophen glucuronide/14%)/2].46 Early childhood lead levels were converted into a binary variable (5 μg/dL as the cutoff) based on guidelines from the Centers for Disease Control and Prevention.47
Missing data for sociodemographic characteristics (<4%) were imputed using multiple imputation by chained equations (MICE) with the Predictive Mean Matching method via mice package in R (R Foundation).48 Adjusted logistic regression was used to examine the associations between cord acetaminophen metabolite categories and the risk of ADHD, ASD, ADHD and ASD, and other DDs using children with ND as the reference group. The final adjusted model included the following maternal and child variables: maternal age at delivery, maternal race/ethnicity, maternal educational level, marital status, stress during pregnancy, smoking before or during pregnancy, alcohol use before or during pregnancy, maternal BMI, parity, child's sex, delivery type, preterm birth, and low birth weight. We also performed stratified analyses by each stratum of covariates (including child’s sex, maternal race/ethnicity, preterm birth, breastfeeding, smoking, alcohol use, illicit drug use, early childhood lead exposure, stress, maternal fever, and child age at last visit group) for binary cord acetaminophen burden (second and third tertiles vs first tertile) using univariate logistic regression comparing children with a diagnosis of ADHD only and children with a diagnosis of ASD only with the children with ND. We further calculated the probability of being included in the study based on race/ethnicity, sex, preterm birth, and low birth weight. Then we performed a series of sensitivity analyses by further using inverse probability (probability of being included in the analysis) weighting; further adjusting for maternal diagnoses of ADHD, depression, and anxiety; further adjusting for intrauterine infection or inflammation; and using alternative acetaminophen burden calculation for neonates. We also repeated the analyses on the association between maternal metabolites and child ADHD using the current data set. R software, version 3.4.3 (R Foundation) was used to perform all analyses.49 The significance threshold is a 2-sided P < .05.
Results
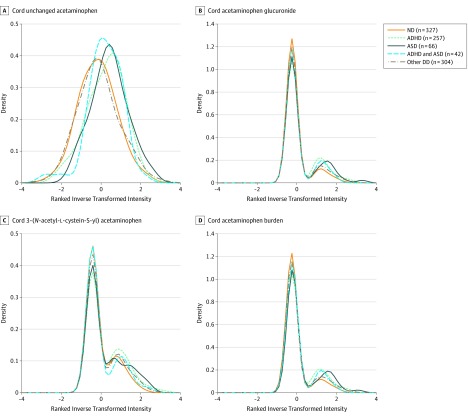
Of 996 participants (mean [SD] age, 9.8 [3.9] years; 548 [55.0%] male), the final sample included 257 children (25.8%) with ADHD only, 66 (6.6%) with ASD only, 42 (4.2%) with both ADHD and ASD, 304 (30.5%) with other DDs, and 327 (32.8%) who were neurotypical (eFigure 1 in the Supplement). This visual observation was supported by the data in Table 1. For instance, the third tertile of cord acetaminophen burden was 43.2% for ADHD, 43.9% for ASD, and 27.2% in ND (Figure 1 and eFigure 2 in the Supplement). Table 1 presents the univariate comparisons of maternal and child characteristics among the ND, ADHD, ASD, ADHD and ASD, and other DDs groups. The ADHD and ASD groups had higher exposures of cord unchanged acetaminophen and its metabolites compared with the other DDs and ND groups. Mothers of children in the ADHD only group were also more likely to have higher BMI (27.02 vs 25.99; P = .02), be non-Hispanic white (8.6% vs 3.4%; P = .049), be unmarried (72.4% vs 61.2%; P = .02), feel stressed during pregnancy (70.4% vs 54.4%; P = .001), ever smoked before or during pregnancy (quitter: 12.5% vs 5.8%; continuous: 8.9% vs 4.0%; P = .01), and ever used alcohol before or during pregnancy (7.4% vs 4.6%; P = .23) compared with mothers of children in the ND group. Children in the ADHD only group were more likely to be older (11.47 vs 8.56 years; P = .001), be male (76.3% vs 38.2%; P = 2.2 × 10−16), be born preterm (20.6% vs 8.9%; P = 1.1 × 10−06), and have low birth weight (18.3% vs 12.5%; P = .047) compared with their counterparts in the ND group. Mothers of children in the ASD group were also more likely to have higher BMI (28.66 vs 25.99; P = .02), be Hispanic (33.3% vs 19.9%; P = .04), be married (43.9% vs 38.8%; P = .02), have an educational level higher than college (48.5% vs 32.7%; P = .04), feel stressed during pregnancy (65.2% vs 54.4%; P = .001), ever smoked before or during pregnancy (quitter: 10.6% vs 5.8%; continuous: 10.6% vs 4.0%; P = .01), and ever used alcohol before or during pregnancy (7.6% vs 4.6%; P = .23) compared with mothers of children in the ND group. Children in the ASD group were more likely to be male (77.3% vs 38.2%; P = 2.2 × 10−16), born preterm (28.8% vs 8.9%; P = 1.1 × 10−06), and have low birth weight (25.8% vs 12.5%; P = .047) compared with their counterparts in the ND group. Dyads included in these analyses were more likely to have a Hispanic mother (23.8% vs 21.6%; P = .02), be older (9.52 vs 8.62 years; P < .001), and be male (55.0% vs 48.3; P < .001); and less likely to have had a preterm birth (17.9% vs 33.7%; P < .001) and low birth weight (17.2 vs 32.3; P < .001) (eTable 1 in the Supplement).
Table 1. Maternal and Child Characteristics According to Child Physician-Diagnosed Conditionsa.
| Variable | ND (n = 327) | ADHD (n = 257) | ASD (n = 66) | ADHD and ASD (n = 42) | Other DDs (n = 304) | P Valueb |
|---|---|---|---|---|---|---|
| Maternal age, y | ||||||
| <20 | 33 (10.1) | 26 (10.1) | 1 (1.5) | 0 (0.0) | 25 (8.2) | .09 |
| 20-34 | 241 (73.7) | 181 (70.4) | 50 (75.8) | 37 (88.1) | 219 (72.0) | |
| ≥35 | 53 (16.2) | 50 (19.5) | 15 (22.7) | 5 (11.9) | 60 (19.7) | |
| Maternal BMI, mean (SD) | 25.99 (6.05) | 27.02 (6.29) | 28.66 (7.52) | 26.43 (6.61) | 26.60 (6.13) | .02 |
| Parity | ||||||
| Nulliparous | 136 (41.6) | 107 (41.6) | 30 (45.5) | 15 (35.7) | 123 (40.5) | .89 |
| Multiparous | 191 (58.4) | 150 (58.4) | 36 (54.5) | 27 (64.3) | 181 (59.5) | |
| Income, $ | ||||||
| <30 000 | 133 (40.7) | 125 (48.6) | 30 (45.5) | 22 (52.4) | 165 (54.3) | .06 |
| ≥30 000 | 53 (16.2) | 40 (15.6) | 12 (18.2) | 6 (14.3) | 32 (10.5) | |
| Unknown | 141 (43.1) | 92 (35.8) | 24 (36.4) | 14 (33.3) | 107 (35.2) | |
| Race/ethnicity | ||||||
| Non-Hispanic black | 222 (67.9) | 161 (62.6) | 35 (53.0) | 23 (54.8) | 191 (62.8) | .049 |
| Non-Hispanic white | 11 (3.4) | 22 (8.6) | 2 (3.0) | 3 (7.1) | 15 (4.9) | |
| Hispanic | 65 (19.9) | 57 (22.2) | 22 (33.3) | 12 (28.6) | 81 (26.6) | |
| Others | 29 (8.9) | 17 (6.6) | 7 (10.6) | 4 (9.5) | 17 (5.6) | |
| Marital status | ||||||
| Married | 127 (38.8) | 71 (27.6) | 29 (43.9) | 12 (28.6) | 108 (35.5) | .02 |
| Not married | 200 (61.2) | 186 (72.4) | 37 (56.1) | 30 (71.4) | 196 (64.5) | |
| Maternal educational level | ||||||
| Below college | 220 (67.3) | 181 (70.4) | 34 (51.5) | 24 (57.1) | 200 (65.8) | .04 |
| Above college | 107 (32.7) | 76 (29.6) | 32 (48.5) | 18 (42.9) | 104 (34.2) | |
| Maternal stress during pregnancy | ||||||
| Not stressful | 149 (45.6) | 76 (29.6) | 23 (34.8) | 13 (31.0) | 125 (41.1) | <.001 |
| Stressful | 178 (54.4) | 181 (70.4) | 43 (65.2) | 29 (69.0) | 179 (58.9) | |
| Maternal smoking before or during pregnancy | ||||||
| Never smoke | 295 (90.2) | 202 (78.6) | 52 (78.8) | 32 (76.2) | 243 (79.9) | .01 |
| Quitter | 19 (5.8) | 32 (12.5) | 7 (10.6) | 6 (14.3) | 34 (11.2) | |
| Continuous | 13 (4.0) | 23 (8.9) | 7 (10.6) | 4 (9.5) | 27 (8.9) | |
| Maternal alcohol use before or during pregnancy | ||||||
| No | 312 (95.4) | 238 (92.6) | 61 (92.4) | 37 (88.1) | 278 (91.4) | .23 |
| Yes | 15 (4.6) | 19 (7.4) | 5 (7.6) | 5 (11.9) | 26 (8.6) | |
| Child age by last visit, mean (SD), y | 8.56 (3.82) | 11.47 (3.42) | 7.85 (3.25) | 10.93 (3.72) | 9.08 (3.63) | <.001 |
| Child's sex | ||||||
| Male | 125 (38.2) | 196 (76.3) | 51 (77.3) | 34 (81.0) | 142 (46.7) | 2.2 × 10−16 |
| Female | 202 (61.8) | 61 (23.7) | 15 (22.7) | 8 (19.0) | 162 (53.3) | |
| Delivery type | ||||||
| Cesarean | 97 (29.7) | 92 (35.8) | 29 (43.9) | 15 (35.7) | 101 (33.2) | .19 |
| Vaginal | 230 (70.3) | 165 (64.2) | 37 (56.1) | 27 (64.3) | 203 (66.8) | |
| Preterm birth | ||||||
| No | 298 (91.1) | 204 (79.4) | 47 (71.2) | 28 (66.7) | 241 (79.3) | 1.1 × 10−06 |
| Yes | 29 (8.9) | 53 (20.6) | 19 (28.8) | 14 (33.3) | 63 (20.7) | |
| Low birth weight | ||||||
| No | 286 (87.5) | 210 (81.7) | 49 (74.2) | 33 (78.6) | 247 (81.2) | .047 |
| Yes | 41 (12.5) | 47 (18.3) | 17 (25.8) | 9 (21.4) | 57 (18.8) | |
| Cord unchanged acetaminophen | ||||||
| First tertile | 132 (40.4) | 62 (24.1) | 12 (18.2) | 8 (19.0) | 118 (38.8) | 5.1 × 10−09 |
| Second tertile | 112 (34.3) | 75 (29.2) | 21 (31.8) | 17 (40.5) | 107 (35.2) | |
| Third tertile | 83 (25.4) | 120 (46.7) | 33 (50.0) | 17 (40.5) | 79 (26.0) | |
| Cord acetaminophen glucuronide | ||||||
| No detection | 283 (86.5) | 190 (73.9) | 50 (75.8) | 34 (81.0) | 247 (81.2) | .01 |
| Below median | 24 (7.3) | 35 (13.6) | 5 (7.6) | 5 (11.9) | 27 (8.9) | |
| Above median | 20 (6.1) | 32 (12.5) | 11 (16.7) | 3 (7.1) | 30 (9.9) | |
| Cord 3-(N-acetyl-l-cystein-S-yl)-acetaminophen | ||||||
| No detection | 234 (71.6) | 151 (58.8) | 41 (62.1) | 30 (71.4) | 205 (67.4) | .04 |
| Below median | 54 (16.5) | 47 (18.3) | 11 (16.7) | 5 (11.9) | 51 (16.8) | |
| Above median | 39 (11.9) | 59 (23.0) | 14 (21.2) | 7 (16.7) | 48 (15.8) | |
| Cord acetaminophen burdenc | ||||||
| First tertile | 133 (40.7) | 57 (22.2) | 11 (16.7) | 9 (21.4) | 122 (40.1) | 5.4 × 10−07 |
| Second tertile | 105 (32.1) | 89 (34.6) | 26 (39.4) | 17 (40.5) | 95 (31.2) | |
| Third tertile | 89 (27.2) | 111 (43.2) | 29 (43.9) | 16 (38.1) | 87 (28.6) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DDs, developmental disabilities; ND, neurotypical development.
Data are presented as number (percentage) of patients unless otherwise indicated.
The P values were obtained using Pearson χ2 test (or Fisher exact test for small cells) for categorical variables and analysis of variance test for continuous variables.
Cord acetaminophen burden was estimated using the sum of all the acetaminophen metabolites.
Figure 1. Distribution of Cord Plasma Acetaminophen Metabolites.
Distribution of cord plasma acetaminophen metabolites by the following child physician-diagnosed conditions: neurotypical development (ND), attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), ADHD and ASD, and other developmental disabilities (DDs).
All cord acetaminophen metabolites showed similar significant positive associations with the risk of ADHD diagnosis. The point estimates of the odds ratios (ORs) vary from 1.69 to 2.88 for ADHD and 1.38 to 3.72 for ASD (Table 2). Moreover, we identified dose-response patterns for cord unchanged acetaminophen and cord acetaminophen burden with the risk of ADHD. For instance, compared with being in the first tertile, being in the second tertile of cord acetaminophen burden was associated with 126% higher odds of ADHD diagnosis (OR, 2.26; 95% CI, 1.40-3.69), and being in the third tertile was associated with 186% higher odds of ADHD diagnosis (OR, 2.86; 95% CI, 1.77-4.67). Cord unchanged acetaminophen, cord acetaminophen glucuronide, and cord acetaminophen burden were also significantly associated with increased risk of ASD diagnosis. For instance, the third tertile cord acetaminophen burden was associated with 262% higher odds of ASD diagnosis compared with the first tertile (OR, 3.62; 95% CI, 1.62-8.60). In contrast, only the third tertile cord unchanged acetaminophen was significantly associated with the risk of ADHD and ASD (OR, 3.38; 95% CI, 1.25-9.85). The results of sensitivity analyses by further using inverse probability weighting (eTable 2 in the Supplement); further adjusting for maternal diagnoses of ADHD, depression, and anxiety (eTable 3 in the Supplement); further adjusting for intrauterine infection/inflammation (eTable 4 in the Supplement); and using alternative acetaminophen burden (eTable 5 in the Supplement) are comparable to the results presented in Table 2.
Table 2. Adjusted Associations Between Cord Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditionsa .
| Model | No. of Dyads | ADHD (n = 257) | ASD (n = 66) | ADHD and ASD (n = 42) | Other DDs (n = 304) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ND Group | No (%) | aOR (95% CI) | P Value | No. (%) | aOR (95% CI) | P Value | No. (%) | aOR (95% CI) | P Value | No. (%) | aOR (95% CI) | P Value | |
| Cord Unchanged Acetaminophenb | ||||||||||||||
| First tertile | 332 | 132 | 62 (18.7) | 1 [Reference] | NA | 12 (3.6) | 1 [Reference] | NA | 8 (2.4) | 1 [Reference] | NA | 118 (35.5) | 1 [Reference] | NA |
| Second tertile | 332 | 83 | 75 (22.6) | 1.48 (0.92-2.39) | .10 | 21 (6.3) | 1.33 (0.57-3.18) | .51 | 17 (5.1) | 2.01 (0.75-5.72) | .17 | 107 (32.2) | 0.93 (0.64-1.37) | .73 |
| Third tertile | 332 | NA | 120 (36.1) | 2.88 (1.80-4.66) | <.001 | 33 (9.9) | 3.72 (1.70-8.55) | <.001 | 17 (5.1) | 3.38 (1.25-9.85) | .02 | 79 (23.8) | 0.86 (0.56-1.31) | .48 |
| Cord Acetaminophen Glucuronideb | ||||||||||||||
| No detection | 804 | 283 | 190 (23.6) | 1 [Reference] | NA | 50 (6.2) | 1 [Reference] | NA | 34 (4.2) | 1 [Reference] | NA | 247 (30.7) | 1 [Reference] | NA |
| Any detection | 192 | 44 | 67 (34.9) | 2.25 (1.39-3.68) | <.001 | 16 (8.3) | 2.29 (1.06-4.85) | .03 | 8 (4.2) | 1.55 (0.53-4.15) | .40 | 57 (29.7) | 1.27 (0.81-2.01) | .30 |
| Cord 3-(N-Acetyl-l-Cystein-S-yl)-Acetaminophenb | ||||||||||||||
| No detection | 661 | 234 | 151 (22.8) | 1 [Reference] | NA | 41 (6.2) | 1 [Reference] | NA | 30 (4.5) | 1 [Reference] | NA | 205 (31.0) | 1 [Reference] | NA |
| Any detection | 335 | 93 | 106 (31.6) | 1.69 (1.13-2.53) | .01 | 25 (7.5) | 1.38 (0.72-2.63) | .33 | 12 (3.6) | 0.72 (0.29-1.66) | .45 | 99 (29.6) | 1.02 (0.71-1.46) | .93 |
| Cord Acetaminophen Burdenc | ||||||||||||||
| First tertile | 332 | 133 | 57 (17.2) | 1 [Reference] | NA | 11 (3.3) | 1 [Reference] | NA | 9 (2.7) | 1 [Reference] | NA | 122 (36.7) | 1 [Reference] | NA |
| Second tertile | 332 | 105 | 89 (26.8) | 2.26 (1.40-3.69) | <.001 | 26 (7.8) | 2.14 (0.93-5.13) | .08 | 17 (5.1) | 2.1 (0.81-5.72) | .13 | 95 (28.6) | 0.92 (0.62-1.35) | .66 |
| Third tertile | 332 | 89 | 111 (33.4) | 2.86 (1.77-4.67) | <.001 | 29 (8.7) | 3.62 (1.62-8.60) | .002 | 16 (4.8) | 2.44 (0.92-6.82) | .08 | 87 (26.2) | 0.83 (0.55-1.26) | .39 |
Abbreviations: ADHD, attention deficit/hyperactivity disorder; aOR, adjusted odds ratio; ASD, autism spectrum disorder; DDs, developmental disabilities; NA, not applicable; ND, neurotypical development.
All adjusted models were compared with the ND group with adjustment for maternal age at delivery, maternal race/ethnicity, maternal educational level, marital status, stress during pregnancy, smoking before or during pregnancy, alcohol use before or during pregnancy, maternal body mass index, parity, child's sex, delivery type, preterm birth, and low birth weight.
Inverse normal transformed intensity.
Sum of all the acetaminophen metabolites.
The point estimates of the associations between cord acetaminophen burden and ADHD and cord acetaminophen burden and ASD were in the positive direction across strata of covariates (Figure 2). Sensitivity analyses and subgroup analyses found consistent associations between acetaminophen and ADHD and acetaminophen and ASD across strata of potential confounders, including maternal indication, substance use, preterm birth, and child age and sex, for which point estimates for the ORs vary from 2.3 to 3.5 for ADHD and 1.6 to 4.1 for ASD. A larger difference in the point estimate of the ORs for ADHD only was observed across strata of child’s sex (OR within females, 3.3; 95% CI, 1.6-7.2; OR within males, 2.4; 95% CI, 1.5-3.9), breastfeeding (OR within bottle-only group, 4.3; 95% CI, 2.1-9.1; OR within both or breastfed groups, 2.0; 95% CI, 1.3-3.1), and maternal smoking before and during pregnancy (OR within never smokers, 2.2; 95% CI, 1.5-3.3; OR within quitters, 6.9; 95% CI, 1.4-51.8; OR within continuous smokers, 1.1; 95% CI, 0.2-5.4). A larger difference in the point estimate of the ORs for ASD only was observed across strata of child’s sex (OR within females, 2.6; 95% CI, 0.8-11.5; OR within males, 4.1; 95% CI, 1.9-10.0), maternal race/ethnicity (OR within black individuals, 2.1; 95% CI, 1.0-4.9; OR within nonblack individuals, 8.9; 95% CI, 2.5-57.1), maternal alcohol use before and during pregnancy (OR within no alcohol use group, 3.6; 95% CI, 1.8-7.7; OR within alcohol use group, 2.0; 95% CI, 0.2-44.8), maternal ever drug use (OR within no drug use group, 4.1; 95% CI, 2.0-9.7; OR within drug use group, 1.6; 95% CI, 0.4-7.8), stress during pregnancy (OR within those not stressful, 4.6; 95% CI, 1.5-20.2; OR within those stressful, 3.0; 95% CI, 1.4-7.3), and last diagnosis age (OR within those <9 years old, 3.4; 95% CI, 1.8-7.1; OR within those ≥9 years old, 2.1; 95% CI, 0.9-5.7). However, tests of interactions between each covariate and cord acetaminophen burden did not produce significant findings.
Figure 2. Forest Plots Summarizing the Subgroup Analyses.
Subgroup analyses of the association between cord acetaminophen burden (second and third tertiles vs first tertile) and the risk of attention-deficit/hyperactivity disorder (ADHD) only (A) and the risk of autism spectrum disorder (ASD) only (B) in childhood. Squares represent mean values, with whiskers indicating 95% CIs. NA indicates not applicable.
eTable 6 in the Supplement gives the association between maternal and cord plasma acetaminophen metabolites. The Pearson χ2 tests found a significant association for all forms of acetaminophen metabolites between cord and maternal categories (unchanged acetaminophen: P < .001; acetaminophen glucuronide: P < 2.2 × 10−16; 3-[N-acetyl-l-cystein-S-yl]-acetaminophen: P = 2.2 × 10−16; acetaminophen burden: P = 3.3 × 10−10). Among children with nondetectable maternal acetaminophen glucuronide and 3-(N-acetyl-l-cystein-S-yl)-acetaminophen, 315 children (48.6%) with nondetectable maternal acetaminophen glucuronide and 279 (52.1%) with nondetectable 3-(N-acetyl-l-cystein-S-yl)-acetaminophen had detectable corresponding cord acetaminophen metabolites. The results of subsequent analyses on maternal metabolites and child ADHD using the current data set (eTable 7 in the Supplement) are also compatible with a previously published study40 (OR for second tertile acetaminophen burden, 1.75; 95% CI, 1.12-2.76; OR for third tertile acetaminophen burden, 2.45; 95% CI, 1.50-4.03).
Discussion
In this prospective birth cohort study, we identified a significant positive association between cord plasma acetaminophen metabolites and the risk of ADHD diagnosis and the risk of ASD in childhood. The positive associations between cord acetaminophen and ADHD and the cord acetaminophen and ASD were observed across strata of pertinent covariates, including maternal fever during pregnancy, which is an indicator for acetaminophen use. The associations also persisted after a series of further adjustment of potential confounders and differential inclusions. Furthermore, there were dose-response patterns for cord uncharged acetaminophen and cord acetaminophen burden with the risk of ADHD and ASD. The findings from this study contribute knowledge to ongoing research regarding the potential adverse neurodevelopmental consequences of perinatal acetaminophen exposure.
To our knowledge, this was the first prospective birth cohort study to examine the associations between cord plasma metabolites of acetaminophen and several types of neurodevelopmental disabilities with adjustments for many potential covariates. A previous study40 using maternal acetaminophen metabolites only partially overcame the methodologic limitations in previous studies13,14,15,16,17,30 that used self-reported acetaminophen use as an exposure indicator and lacked quantification of acetaminophen intake. However, the previous study40 only measured maternal acetaminophen metabolites at one time within 3 days after delivery, which limited the strength of the evidence. The current study using cord acetaminophen metabolites addressed the major limitations of the previous studies.13,14,15,16,17,30 The cord plasma metabolites provide a direct measurement of fetal acetaminophen exposure before delivery. In addition, the dose-response associations found in the current study also addressed the methodologic issues identified by the SMFM, FDA, and AAP regarding the reliance on maternal self-reported acetaminophen exposures in previous cohort studies.33,34
In this study, all cord samples had detectable unchanged acetaminophen. Among children whose maternal acetaminophen exposures were in the first tertile, more than 60% had second and third tertile cord unchanged acetaminophen exposure. This phenomenon may reflect the differences in metabolic capacity for acetaminophen between adults and neonates. This finding is also supported by a case study29 that reported that acetaminophen and its metabolites were detectable until measurement of the infant’s 47-hour urine sample after maternal intake of phenacetin-containing tablets 5.5 hours before delivery.
The liver is the primary location for metabolism of acetaminophen.50 In adults with healthy liver function, 5% to 10% of acetaminophen is processed into the highly toxic metabolite N-acetyl-p-benzoquinone imine, which is responsible for the major hepatotoxicity of acetaminophen45 and ultimately detoxified as 3-(N-acetyl-l-cystein-S-yl)-acetaminophen.50,51 In neonates, because of limited metabolism capacity, the acetaminophen and its toxic metabolites remain for longer after in utero exposure. Furthermore, acetaminophen could rapidly enter cerebrospinal fluid52,53,54,55 and inhibit prostaglandin synthesis.25,26,27 Although findings related to a low toxic burden on the liver, kidney, and intestines support the safety of acetaminophen use in the short term,56,57 the long-term potential association of neurodisruption with acetaminophen exposure remains to be clarified.58
Whether there is a specific time window when the developing brain is most sensitive to acetaminophen exposure remains unclear. Animal experiments suggest that the perinatal period is the critical exposure window for acetaminophen to induce behavioral abnormalities in mice.59 Our study consistently found that cord biomarkers of acetaminophen (reflecting perinatal exposure) were significantly associated with increased risk of ADHD and ASD in children.
Limitations
The present study has some limitations. First, it only included a 1-time measurement of cord acetaminophen metabolites at birth. Given that the half-life of acetaminophen in adults is less than 3 hours,44 the cord plasma measurement may at most reflect maternal use of acetaminophen during the peripartum period. Second, the metabolome panel did not capture acetaminophen sulfate, which is one of the major metabolites for acetaminophen.45 This missing biomarker limits our ability to evaluate the overall cord acetaminophen burden for newborns. Third, we did not have a true nonexposed group as reference because of the 100% detection of unchanged acetaminophen, which may have biased our results toward the null. Fourth, because of our observational study design, we were unable to exclude the potential residual confounders because of unmeasured genetic and environmental factors. Fifth, caution is needed to apply our findings to other populations with different characteristics.
Conclusions
In this study, cord biomarkers of fetal exposure to acetaminophen were associated with significantly increased risk of childhood ADHD and ASD in a dose-response fashion. Our findings support previous studies regarding the association between prenatal and perinatal acetaminophen exposure and childhood neurodevelopmental risk and warrant additional investigations.
eFigure 1. Flowchart of the Sample Inclusion and Exclusion
eFigure 2. Distribution of Maternal and Cord Plasma Acetaminophen Metabolites
eTable 1. Maternal and Child Characteristics Between Mother-Infant Dyads Included and Excluded in the Analyses
eTable 2. Adjusted Associations Between Cord Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood, Using Inverse Probability Weighting
eTable 3. Adjusted Associations Between Cord Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood, With Further Adjustment for Maternal Diagnoses of ADHD, Depression, and Anxiety
eTable 4. Adjusted Associations Between Cord Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood, With Further Adjustment for Maternal Intrauterine Infection/Inflammation
eTable 5. Adjusted Associations Between Cord Plasma Acetaminophen Burden (Alternative Calculation) and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood
eTable 6. Correlation Between Maternal and Cord Plasma Acetaminophen Metabolites
eTable 7. Adjusted Associations Between Maternal Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood
References
- 1.Babb M, Koren G, Einarson A. Treating pain during pregnancy. Can Fam Physician. 2010;56(1):25-27, 27. [PMC free article] [PubMed] [Google Scholar]
- 2.Headley J, Northstone K, Simmons H, Golding J; ALSPAC Study Team . Medication use during pregnancy: data from the Avon Longitudinal Study of Parents and Children. Eur J Clin Pharmacol. 2004;60(5):355-361. doi: 10.1007/s00228-004-0775-7 [DOI] [PubMed] [Google Scholar]
- 3.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263-268. [PubMed] [Google Scholar]
- 4.Rubin JD, Ferencz C, Loffredo C; Baltimore-Washington Infant Study Group . Use of prescription and non-prescription drugs in pregnancy. J Clin Epidemiol. 1993;46(6):581-589. doi: 10.1016/0895-4356(93)90132-K [DOI] [PubMed] [Google Scholar]
- 5.Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188(4):1039-1045. doi: 10.1067/mob.2003.223 [DOI] [PubMed] [Google Scholar]
- 6.Daw JR, Hanley GE, Greyson DL, Morgan SG. Prescription drug use during pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf. 2011;20(9):895-902. doi: 10.1002/pds.2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193(3 Pt 1):771-777. doi: 10.1016/j.ajog.2005.02.100 [DOI] [PubMed] [Google Scholar]
- 8.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr, Smith PB; Best Pharmaceuticals for Children Act—Pediatric Trials Network . Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31(9):811-821. doi: 10.1055/s-0033-1361933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupattelli A, Spigset O, Twigg MJ, et al. . Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4(2):e004365. doi: 10.1136/bmjopen-2013-004365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amberbir A, Medhin G, Alem A, Britton J, Davey G, Venn A. The role of acetaminophen and geohelminth infection on the incidence of wheeze and eczema: a longitudinal birth-cohort study. Am J Respir Crit Care Med. 2011;183(2):165-170. doi: 10.1164/rccm.201006-0989OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beasley R, Clayton T, Crane J, et al. ; ISAAC Phase Three Study Group . Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6-7 years: analysis from Phase Three of the ISAAC programme. Lancet. 2008;372(9643):1039-1048. doi: 10.1016/S0140-6736(08)61445-2 [DOI] [PubMed] [Google Scholar]
- 12.Gurney J, Richiardi L, McGlynn KA, Signal V, Sarfati D. Analgesia use during pregnancy and risk of cryptorchidism: a systematic review and meta-analysis. Hum Reprod. 2017;32(5):1118-1129. doi: 10.1093/humrep/dex047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ystrom E, Gustavson K, Brandlistuen RE, et al. . Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics. 2017;140(5):pii: e20163840. doi: 10.1542/peds.2016-3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liew Z, Ritz B, Rebordosa C, Lee P-CC, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014;168(4):313-320. doi: 10.1001/jamapediatrics.2013.4914 [DOI] [PubMed] [Google Scholar]
- 15.Thompson JM, Waldie KE, Wall CR, Murphy R, Mitchell EA; ABC Study Group . Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS One. 2014;9(9):e108210. doi: 10.1371/journal.pone.0108210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stergiakouli E, Thapar A, Davey Smith G. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. JAMA Pediatr. 2016;170(10):964-970. doi: 10.1001/jamapediatrics.2016.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avella-Garcia CB, Julvez J, Fortuny J, et al. . Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int J Epidemiol. 2016;45(6):1987-1996. doi: 10.1093/ije/dyw115 [DOI] [PubMed] [Google Scholar]
- 18.Ghanizadeh A. Acetaminophen may mediate oxidative stress and neurotoxicity in autism. Med Hypotheses. 2012;78(2):351. doi: 10.1016/j.mehy.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 19.Liew Z, Ritz B, Olsen J. Characteristics of acetaminophen users compared with nonusers during pregnancy, behavioral problems, and hyperkinetic disorders–reply. JAMA Pediatr. 2014;168(9):865-866. doi: 10.1001/jamapediatrics.2014.983 [DOI] [PubMed] [Google Scholar]
- 20.Liew Z, Ritz B, Virk J, Olsen J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: a Danish national birth cohort study. Autism Res. 2016;9(9):951-958. doi: 10.1002/aur.1591 [DOI] [PubMed] [Google Scholar]
- 21.Bauer AZ, Kriebel D. Prenatal and perinatal analgesic exposure and autism: an ecological link. Environ Health. 2013;12:41. doi: 10.1186/1476-069X-12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liew Z, Ritz B, Virk J, Olsen J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: a Danish national birth cohort study. Autism Res. 2016;9(9):951-958. doi: 10.1002/aur.1591 [DOI] [PubMed] [Google Scholar]
- 23.Kristensen DM, Lesné L, Le Fol V, et al. . Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377-384. doi: 10.1111/j.1365-2605.2012.01282.x [DOI] [PubMed] [Google Scholar]
- 24.Posadas I, Santos P, Blanco A, Muñoz-Fernández M, Ceña V. Acetaminophen induces apoptosis in rat cortical neurons. PLoS One. 2010;5(12):e15360. doi: 10.1371/journal.pone.0015360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11(2):371-386. doi: 10.1016/0896-6273(93)90192-T [DOI] [PubMed] [Google Scholar]
- 26.Shaw KN, Commins S, O’Mara SM. Deficits in spatial learning and synaptic plasticity induced by the rapid and competitive broad-spectrum cyclooxygenase inhibitor ibuprofen are reversed by increasing endogenous brain-derived neurotrophic factor. Eur J Neurosci. 2003;17(11):2438-2446. doi: 10.1046/j.1460-9568.2003.02643.x [DOI] [PubMed] [Google Scholar]
- 27.Dean SL, Knutson JF, Krebs-Kraft DL, McCarthy MM. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci. 2012;35(8):1218-1229. doi: 10.1111/j.1460-9568.2012.08032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weigand UW, Chou RC, Maulik D, Levy G. Assessment of biotransformation during transfer of propoxyphene and acetaminophen across the isolated perfused human placenta. Pediatr Pharmacol (New York). 1984;4(3):145-153. [PubMed] [Google Scholar]
- 29.Levy G, Garrettson LK, Soda DM. Letter: evidence of placental transfer of acetaminophen. Pediatrics. 1975;55(6):895. [PubMed] [Google Scholar]
- 30.Tovo-Rodrigues L, Schneider BC, Martins-Silva T, et al. . Is intrauterine exposure to acetaminophen associated with emotional and hyperactivity problems during childhood? findings from the 2004 Pelotas birth cohort. BMC Psychiatry. 2018;18(1):368. doi: 10.1186/s12888-018-1942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gou X, Wang Y, Tang Y, et al. . Association of maternal prenatal acetaminophen use with the risk of attention deficit/hyperactivity disorder in offspring: a meta-analysis. Aust N Z J Psychiatry. 2019;53(3):195-206. doi: 10.1177/0004867418823276 [DOI] [PubMed] [Google Scholar]
- 32.Masarwa R, Levine H, Gorelik E, Reif S, Perlman A, Matok I. Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: a systematic review, meta-analysis, and meta-regression analysis of cohort studies. Am J Epidemiol. 2018;187(8):1817-1827. doi: 10.1093/aje/kwy086 [DOI] [PubMed] [Google Scholar]
- 33.Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Prenatal acetaminophen use and outcomes in children. Am J Obstet Gynecol. 2017;216(3):B14-B15. doi: 10.1016/j.ajog.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 34.Food and Drug Administration FDA has reviewed possible risks of pain medication during pregnancy. https://www.fda.gov/Drugs/DrugSafety/ucm429117.htm. Accessed January 7, 2018.
- 35.Ystrom E, Gustavson K, Brandlistuen RE, et al. . Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics. 2017;140(5):e20163840. doi: 10.1542/peds.2016-3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, Divall S, Radovick S, et al. . Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311(6):587-596. doi: 10.1001/jama.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Zuckerman B, Pearson C, et al. . Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195-202. doi: 10.1001/jama.287.2.195 [DOI] [PubMed] [Google Scholar]
- 38.Li M, Fallin MD, Riley A, et al. . The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics. 2016;137(2):e20152206. doi: 10.1542/peds.2015-2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar R, Tsai HJ, Hong X, et al. . Race, ancestry, and development of food-allergen sensitization in early childhood. Pediatrics. 2011;128(4):e821-e829. doi: 10.1542/peds.2011-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Y, Riley AW, Lee LC, et al. . Maternal biomarkers of acetaminophen use and offspring attention deficit hyperactivity disorder. Brain Sci. 2018;8(7):pii: E127. doi: 10.3390/brainsci8070127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Sullivan JF, Morningstar JE, Yang Q, et al. . Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127(12):4394-4402. doi: 10.1172/JCI95995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowan S, Jiang S, Korem T, et al. . Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci U S A. 2017;114(22):E4472-E4481. doi: 10.1073/pnas.1702302114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascanfroni ID, Takenaka MC, Yeste A, et al. . Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21(6):638-646. doi: 10.1038/nm.3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(suppl 2):291S-298S. doi: 10.1111/j.1365-2125.1980.tb01812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazaleuskaya LL, Sangkuhl K, Thorn CF, FitzGerald GA, Altman RB, Klein TE. PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics. 2015;25(8):416-426. doi: 10.1097/FPC.0000000000000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allegaert K, de Hoon J, Verbesselt R, Vanhole C, Devlieger H, Tibboel D. Intra- and interindividual variability of glucuronidation of paracetamol during repeated administration of propacetamol in neonates. Acta Paediatr. 2005;94(9):1273-1279. doi: 10.1111/j.1651-2227.2005.tb02088.x [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention.” https://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf. Accessed August 25, 2012.
- 48.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2010;45(3):1-68. [Google Scholar]
- 49.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 50.Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)–induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31(1):55-138. doi: 10.1080/20014091111677 [DOI] [PubMed] [Google Scholar]
- 51.Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499-516. doi: 10.1016/j.ccc.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 52.Randles D, Heine SJ, Santos N. The common pain of surrealism and death: acetaminophen reduces compensatory affirmation following meaning threats. Psychol Sci. 2013;24(6):966-973. doi: 10.1177/0956797612464786 [DOI] [PubMed] [Google Scholar]
- 53.Durso GR, Luttrell A, Way BM. Over-the-counter relief from pains and pleasures alike: acetaminophen blunts evaluation sensitivity to both negative and positive stimuli. Psychol Sci. 2015;26(6):750-758. doi: 10.1177/0956797615570366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dewall CN, Macdonald G, Webster GD, et al. . Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-937. doi: 10.1177/0956797610374741 [DOI] [PubMed] [Google Scholar]
- 55.Randles D, Kam JW, Heine SJ, Inzlicht M, Handy TC. Acetaminophen attenuates error evaluation in cortex. Soc Cogn Affect Neurosci. 2016;11(6):899-906. doi: 10.1093/scan/nsw023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Penna A, Buchanan N. Paracetamol poisoning in children and hepatotoxicity. Br J Clin Pharmacol. 1991;32(2):143-149. doi: 10.1111/j.1365-2125.1991.tb03873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics. 1999;104(4):e39. doi: 10.1542/peds.104.4.e39 [DOI] [PubMed] [Google Scholar]
- 58.Parker W, Hornik CD, Bilbo S, et al. . The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J Int Med Res. 2017;45(2):407-438. doi: 10.1177/0300060517693423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Philippot G, Gordh T, Fredriksson A, Viberg H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): characterization of a critical period. J Appl Toxicol. 2017;37(10):1174-1181. doi: 10.1002/jat.3473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of the Sample Inclusion and Exclusion
eFigure 2. Distribution of Maternal and Cord Plasma Acetaminophen Metabolites
eTable 1. Maternal and Child Characteristics Between Mother-Infant Dyads Included and Excluded in the Analyses
eTable 2. Adjusted Associations Between Cord Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood, Using Inverse Probability Weighting
eTable 3. Adjusted Associations Between Cord Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood, With Further Adjustment for Maternal Diagnoses of ADHD, Depression, and Anxiety
eTable 4. Adjusted Associations Between Cord Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood, With Further Adjustment for Maternal Intrauterine Infection/Inflammation
eTable 5. Adjusted Associations Between Cord Plasma Acetaminophen Burden (Alternative Calculation) and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood
eTable 6. Correlation Between Maternal and Cord Plasma Acetaminophen Metabolites
eTable 7. Adjusted Associations Between Maternal Plasma Acetaminophen Biomarkers and the Risk of Physician-Diagnosed Conditions (Mutually Exclusive): Attention-Deficit/Hyperactivity Disorder (ADHD), Autism Spectrum Disorder (ASD), Both ADHD and ASD, and Developmental Disabilities (Other DD) in Childhood