Abstract
Background
A recent study demonstrated that spine formation rates by ketamine in the prefrontal cortex (PFC) were not altered at 3–6 h following a single injection, but were markedly altered at 12–24 h. Here, we investigated the acute (3 h post-treatment) effects of (R)-ketamine in the decreased spine density in the medial PFC (mPFC) and hippocampus in susceptible mice after chronic social defeat stress (CSDS).
Methods
(R)-ketamine (10 mg/kg) or saline was administered intraperitoneally to CSDS-susceptible mice. Dendritic spine density in the mPFC and hippocampus was measured 3 h after a single injection.
Results
(R)-ketamine significantly ameliorated the decreased spine density in the prelimbic area of mPFC, Cornu Ammonis3, and dentate gyrus of the hippocampus of CSDS-susceptible mice
Conclusions
This study suggests that (R)-ketamine rapidly ameliorates the decreased spine density in the mPFC and hippocampus of CSDS-susceptible mice, resulting in its rapid-acting antidepressant effects.
Keywords: Rapid-acting antidepressant, dendritic spine density, (R)-ketamine, medial prefrontal cortex, hippocampus
Significance Statement.
The discovery of rapid-acting and sustained antidepressant effects of ketamine in patients with treatment-resistant depression is serendipitous. A recent study using single-cell 2-photon calcium imaging demonstrated that spine formation rates in the prefrontal cortex (PFC) by (R,S)-ketamine were slow. Here, we report that (R)-ketamine rapidly (<3 h) ameliorates the decreased spine density in the medial PFC of susceptible mice after chronic social defeat stress. Therefore, it is likely that the rapid rescue of spine formation by (R)-ketamine contributes to its rapid-acting antidepressant effects.
Introduction
The discovery of the rapid-acting and sustained antidepressant effects of the N-methyl-D-aspartate receptor (NMDAR) antagonist ketamine in treatment-resistant patients with depression was serendipitous (Krystal et al., 2019). However, the precise molecular mechanisms underlying the antidepressant effect of ketamine remain to be elucidated (Hashimoto, 2019; Zhang and Hashimoto, 2019a). Chronic stress paradigms, such as chronic social defeat stress (CSDS) and chronic unpredicted mild stress (CUMS), are known to profoundly reduce dendritic spine density and functions in the prefrontal cortex (PFC) and hippocampus; these effects could contribute to the morphological and functional alterations observed in patients with depression (Duman and Aghajanian, 2012; Duman and Duman, 2015). Ketamine (10 mg/kg, 24 h post-treatment) ameliorated the decreased spine synapse number in the medial PFC (mPFC) in mice after CUMS (Li et al., 2011), resulting in its sustained antidepressant effects. Notably, the rapid-acting antidepressant ketamine rapidly increases spine synapse numbers in the PFCs of rodents and reverses the effects of chronic stress. These findings support the neurotrophic and synaptogenic hypothesis of depression and rapid-acting antidepressant actions (Duman and Aghajanian, 2012; Duman and Duman, 2015).
(R,S)-ketamine (Ki = 0.53 μM for NMDAR) is an equal amount mixture of (R)-ketamine (or arketamine: Ki = 1.4 μM for NMDAR) and (S)-ketamine (or esketamine: Ki = 0.3 μM for NMDAR; Hashimoto, 2019). On 5 March 2019, the United State Food Drug Administration approved (S)-ketamine nasal spray for treatment-resistant depression. However, preclinical studies demonstrated that (R)-ketamine has greater potency and longer lasting antidepressant effects than (S)-ketamine in rodent models of depression (Zhang et al., 2014a; Yang et al., 2015; Fukumoto et al., 2017; Yang et al., 2017; Yang et al., 2018). Importantly, the side effects of (R)-ketamine in rodents were less than those of (R,S)-ketamine and (S)-ketamine (Yang et al., 2015; Hashimoto et al., 2017; Tian et al., 2018a; Chang et al., 2019). We reported that ketamine and its 2 enantiomers ([R]-ketamine and [S]-ketamine; 10 mg/kg at 8 days post-treatment] significantly ameliorated the decreased spine density in the mPFC and hippocampus in CSDS-susceptible mice (Yang et al., 2015; Dong et al., 2017). Interestingly, (R)-ketamine elicited a more potent beneficial effect on the decreased dendritic spine density in the mPFC and hippocampus of CSDS-susceptible mice than (S)-ketamine (Yang et al., 2015). Compared with (S)-ketamine, it is possible that the greater potency of (R)-ketamine for dendritic spine formation contributes to its longer-lasting antidepressant effects (Yang et al., 2015; Hashimoto, 2019).
A recent study using single-cell 2-photon calcium imaging in awake mice showed that the effects of ketamine (10 mg/kg) on spine formation in the PFC were slower (Moda-Sava et al., 2019). Spine formation rates were not significantly altered at 3–6 h following a single injection of ketamine, but were markedly altered at 12–24 h (Moda-Sava et al., 2019). This study suggests that dendritic spine formation in the PFC was required for the sustained antidepressant effects of ketamine, but not for its acute antidepressant effects (Moda-Sava et al., 2019). Currently, there are no reports showing acute (3 h post-treatment) effects of ketamine on the decreased spine density in the brain after CSDS or CUMS.
The present study was performed to examine whether (R)-ketamine rapidly ameliorates the decreased spine density in the mPFC and hippocampus in CSDS-susceptible mice.
Methods and Materials
Animals
Male, adult, C57BL/6 mice aged 8 weeks (body weight 20-25 g; Japan SLC, Inc., Hamamatsu, Japan) and male, adult, CD1 (ICR) mice aged 13–15 weeks (body weight >40 g; Japan SLC, Inc., Hamamatsu, Japan) were used. Animals were housed under controlled temperatures and 12 hour light/dark cycles (lights on between 07:00 a.m.–7:00 p.m.), with ad libitum food (CE-2; CLEA Japan, Inc., Tokyo, Japan) and water. The protocol was approved by the Chiba University Institutional Animal Care and Use Committee (permission number 1–376). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in the United States. Animals were deeply anaesthetized with isoflurane before being killed by cervical dislocation. All efforts were made to minimize suffering.
Materials
(R)-ketamine hydrochloride was prepared by recrystallization of (R,S)-ketamine (Ketalar ketamine hydrochloride, Daiichi Sankyo Pharmaceutical Ltd., Tokyo, Japan) and D-(-)-tartaric acid, as described previously (Zhang et al., 2014a). The dose (10 mg/kg as hydrochloride) of (R)-ketamine dissolved in the physiological saline was used as previously reported (Zhang et al., 2014a; Yang et al., 2015; Yang et al., 2017; Yang et al., 2018).
Chronic Social Defeat Stress Model
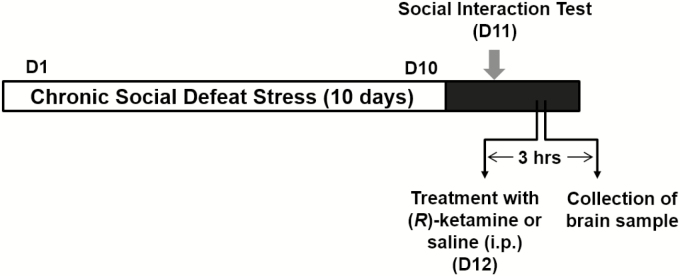
The procedure of CSDS was performed as previously reported (Figure 1; Yang et al., 2015; Dong et al., 2017; Yang et al., 2017; Qu et al., 2018; Yang et al., 2018). The C57BL/6 mice were exposed to a different CD1 aggressor mouse for 10 min per day for 10 consecutive days. When the social defeat session ended, the resident CD1 mouse and the intruder mouse were each housed in half of a cage, separated by a perforated Plexiglas divider to allow visual, olfactory, and auditory contact for the remainder of the 24-h period. At 24 h after the last session, all mice were housed individually. On Day 11, a social interaction test was performed to identify subgroups of mice that were susceptible and unsusceptible to social defeat stress. This was accomplished by placing mice in an interaction test box (42 × 42 cm) with an empty wire-mesh cage (10 × 4.5 cm) located at an end. The movement of the mice was tracked for 2.5 min, followed by 2.5 min in the presence of an unfamiliar aggressor who was confined in the wire-mesh cage. The duration of the subject's presence in the “interaction zone” (defined as the 8 cm–wide area surrounding the wire-mesh cage) was recorded by a stopwatch. The interaction ratio was calculated as the time spent in an interaction zone with an aggressor/time spent in an interaction zone without an aggressor. An interaction ratio of 1 was set as the cut-off: mice with scores <1 were defined as “susceptible” to social defeat stress and those with scores ≥1 were defined as “resilient.” Approximately 70–80% of mice were susceptible after CSDS. Susceptible mice were randomly divided in the subsequent experiments. Control C57BL/6 mice without CSDS were housed in the cage.
Figure 1.
Schedule of CSDS, treatment, and brain collection. CSDS was performed from Day 1 to Day 10. A social interaction test was performed on Day 11 to select susceptible mice. On Day 12, saline (10 ml/kg) or (R)-ketamine (10 mg/kg) was administered i.p. to susceptible mice. Saline (10 ml/kg) was also administered i.p. to control (no CSDS) mice. Golgi-Cox staining in the control mice and CSDS-susceptible mice was performed 3 hours after a single administration of saline or (R)-ketamine. Abbreviations: CSDS, chronic social defeat stress; D, day; i.p., intraperitoneal.
Golgi Staining
As previously reported (Zhang et al., 2014b; Yang et al., 2015; Dong et al., 2017; Qu et al., 2018), Golgi staining was performed using the FD Rapid Golgi Stain TM Kit (FD Neuro Technologies, Inc., Columbia, MD), following the manufacturer's instructions. At 3 h after a single intraperitoneal administration of saline (10 ml/kg) or (R)-ketamine (10 mg/kg), mice were deeply anesthetized with isoflurane and sodium pentobarbital; the brains were removed from the skull and rinsed in double-distilled water. Brains were immersed overnight in the impregnation solution, made by mixing equal volumes of Solution A and B, and were then stored in the dark in fresh solution for 2 weeks. Brains were transferred into Solution C overnight and then stored in the dark in fresh solution at 4°C for 1 week. Coronal brain sections (100 μm thickness) were cut on a cryostat (3050S, Leica Microsystems AG, Wetzlar, Germany), with the chamber temperature set at -20°C. Each section was mounted in Solution C, on saline-coated microscope slides. After absorption of excess solution, sections were dried naturally at room temperature. Dried sections were processed following the manufacturer's instructions. Briefly, images of dendrites within the prelimbic (PrL) area and infralimbic (IL) area of the mPFC, dentate gyrus (DG), Cornu Ammonis (CA1), and CA3 of the hippocampus were captured using a 100× objective with a Keyence BZ-9000 Generation microscope (Osaka, Japan). Spines were counted along mPFC, DG, CA1, and CA3 dendrites, starting from their point of origin from the primary dendrite, as previously reported (Yang et al., 2015; Dong et al., 2017; Qu et al., 2018). For spine density measurements, all clearly evaluable areas containing 50–100 μm of secondary dendrites from each imaged neuron were used. To determine the relative spine density, spines on multiple dendritic branches from a single neuron were counted to obtain an average spine number per 10 μm. For spine number measurements, only spines that emerged perpendicular to the dendritic shaft were counted. We analyzed 3 neurons per section and 3 sections per animal. The average value for each region was obtained in each individual. These individual averages were then combined to yield a grand average for each region.
Statistical Analysis
The data are shown as the mean ± standard error of the mean. An analysis was performed using PASW Statistics 20 (formerly SPSS Statistics). Comparisons between groups were performed using a 1-way analysis of variance, followed by a post hoc Tukey test. A P value of less than 0.05 was considered statistically significant.
Results
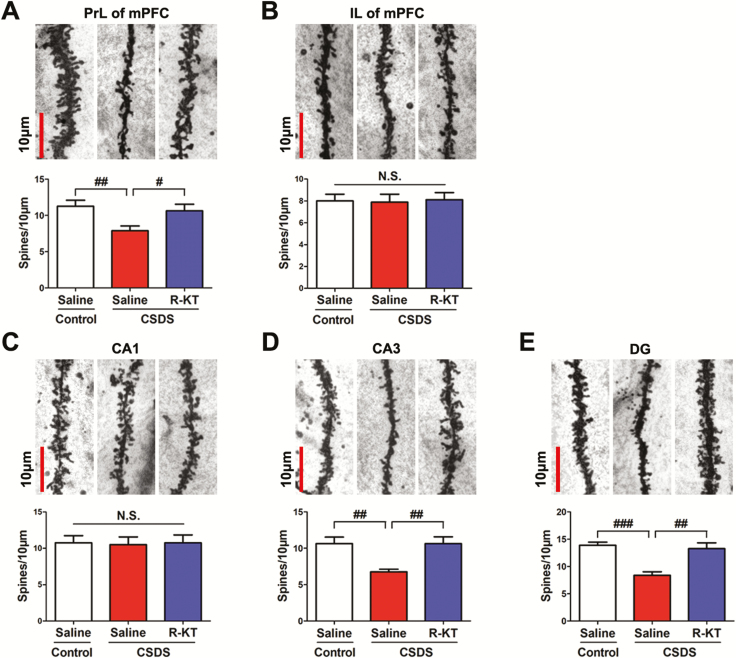
Dendritic spine densities in the PrL regions of the mPFC, CA3, and DG of the hippocampus from susceptible mice were significantly lower than those from control mice, consistent with previous reports (Figure 2A, D, andE; Yang et al., 2015; Dong et al., 2017; Qu et al., 2018). In contrast, spine densities in the IL regions of the mPFC and CA1 of the hippocampus from susceptible mice were not different from those in control mice, consistent with previous reports (Figure 2B and C; Yang et al., 2015; Dong et al., 2017; Qu et al., 2018). A single injection of (R)-ketamine (10 mg/kg at 3 h post-treatment) significantly ameliorated the decreased spine densities in the PrL areas of the mPFC, CA3, and DG of the hippocampus from susceptible mice (Figure 2A, D, andE).
Figure 2.
Rapid effects of (R)-ketamine on the decreased dendritic spine density in the prefrontal cortex and hippocampus in susceptible mice after CSDS. (A-E) Dendritic spine density in the PrL and IL regions of the mPFC, CA1, CA3, and DG of the hippocampus. The bar is 10 μm. (A) PrL region of mPFC (1-way ANOVA, F2,21 = 4.825; P = 0.019). (B) IL region of mPFC (1-way ANOVA, F2,21 = 0.027; P = 0.973). (C) CA1 of hippocampus (1-way ANOVA, F2,21 = 0.019; P = 0.982). (D) CA3 of hippocampus (1-way ANOVA, F2,21 = 8.144; P = 0.002). (E) DG of hippocampus (1-way ANOVA, F2,21 = 14.070; P < 0.001). The values represent the mean ± standard error of the mean (n = 8/group). #P < 0.05, ##P < 0.01, and ###P < 0.001, compared with saline-treated CSDS-susceptible mice. Abbreviations: ANOVA, analysis of variance; CSDS, chronic social defeat stress; DG, dentate gyrus; IL, infralimbic; mPFC, medial prefrontal cortex; N.S., not significant; PrL, prelimbic; R-KT, (R)-ketamine.
Discussion
A recent study demonstrated that (R,S)-ketamine (10 mg/kg at 3–6 h post-treatment) did not affect the decreased spine density in the PFC of chronically corticosterone-treated mice, whereas (R,S)-ketamine markedly improved spine formation at 12–24 h (Moda-Sava et al., 2019). This observation suggests that spine formation is not involved in the rapid-acting antidepressant effects of (R,S)-ketamine, since (R,S)-ketamine (at 3–48 h post-treatment) rapidly attenuated the increased immobility time in the tail suspension test (TST).
In this study, we used (R)-ketamine, since (R)-ketamine elicited more potent antidepressant effects than (R,S)-ketamine and (S)-ketamine in a CSDS model (Chang et al., 2019). We found that (R)-ketamine (at 10 mg/kg) rapidly (<3 h) ameliorated the decreased spine densities in the PrL regions of the mPFC, CA3, and DG of the hippocampus in CSDS-susceptible mice. Furthermore, (R,S)-ketamine (or [R]-ketamine; at 10 mg/kg) rapidly (<3–4 h) attenuated the increased immobility time of TST in CSDS-susceptible mice (Tian et al., 2018b; Zhang and Hashimoto, 2019b). Although we did not investigate spines in the same regions of the same mice using the 2-photon imaging technique, it seems that (R)-ketamine could rapidly normalize the decreased spine densities of susceptible mice to control levels, resulting in its rapid-acting antidepressant effects.
It was reported that (R,S)-ketamine (5 mg/kg at 3 h post-treatment) rescued the decreased densities of thin spines, but not mushroom spines, in the mPFCs of male rats subjected to isolation stress (Sarkar and Kabbaj, 2016). Although the decline in total spine density was a cumulative effect of the declines in thin spines and mushroom spines, it has been suggested that the acute increase in spine density by ketamine was mediated mostly through an increase in thin spines (new, immature spines; Sarkar and Kabbaj, 2016). In addition, (S)-ketamine (15 mg/kg) reversed dendritic spine deficits in <1 h in CA1 pyramidal neurons of Flinders sensitive rats with a depression-like phenotype (Treccani et al., 2019). Cumulatively, it is likely that normalization of the decreased spine density in the brain by (R)-ketamine is more rapid.
Moda-Sava et al. (2019) used a chronic (21 days) corticosterone exposure model as a mouse model of depression. Chronic corticosterone treatment decreased dendritic spine density throughout the PFC, including in the anterior cingulate, PrL, and IL subregions, whereas ketamine had the opposite effect. Although ketamine showed a rapid (3 h post-treatment) antidepressant-like effect in TST, spine formation rates were not significantly altered at 3 to 6 hours post-treatment, but were markedly elevated at 12 to 24 hours. It has been suggested that the rescue of spine formation by ketamine is associated with its behavioral changes (Li et al., 2011; Duman and Aghajanian, 2012; Duman and Duman, 2015). However, this paper showed that ketamine-induced spine formation affects the immobility time of TST, but not anhedonia, in the sucrose preference test, suggesting a specific role of the PFC and other regions for motivated escape behavior and anhedonia (Moda-Sava et al., 2019). Lipopolysaccharide (LPS) is known to produce depression-like behaviors in rodents 24 hours after a single dose (Dantzer et al., 2008; Zhang et al., 2014b). In addition, in vivo imaging using 2-photon microscopy showed long-term changes in dendritic spines in the mouse brain after a single dose of LPS (Kondo et al., 2011). Therefore, it is of interest to investigate the acute (<3 h) and sustained (<24 h) effects of ketamine and its enantiomers for spine formation in LPS-treated rodents, using single-cell 2-photon calcium imaging.
In this study, we did not examine the effects of (R)-ketamine in the spine formation of control mice, since (R)-ketamine did not show antidepressant-like effects in control (no CSDS) mice (Zhang et al., 2018). In addition, Nugent et al. (2019) reported that ketamine caused depressive symptoms (i.e., anhedonia) in healthy control subjects, indicating that ketamine does not have antidepressant effects in healthy control subjects. Nonetheless, it is also of interest to investigate whether (R)-ketamine can affect dendritic spine density in the control mice. In this study, we did not measure the synaptic proteins [i.e., PSD-95 (postsynaptic density protein 95) and GluA1 (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor 1)] in the mPFC and hippocampus in CSDS-susceptible mice. It is of interest to examine whether (R)-ketamine can ameliorate the decreased expression of these proteins in these regions, since ketamine and (R)-ketamine ameliorated these proteins (Duman and Aghajanian, 2012; Duman and Duman, 2015; Yang et al, 2015; Dong et al, 2017).
In conclusion, this study indicates that (R)-ketamine rapidly (<3 h) ameliorates the decreased spine densities in the PrL regions of the mPFC, CA3, and DG in susceptible mice after CSDS, suggesting this mechanism for its rapid-acting antidepressant effects. Further detailed study using single-cell 2-photon calcium imaging is needed to confirm the acute effects of ketamine and its enantiomers for spine formation in rodents with depression-like phenotypes.
Acknowledgments
This study was supported by Japan Agency for Medical Research and Development (grant number JP19dm0107119 to K. H.).
K. H. is an inventor on a filed patent application on “The use of R-ketamine in the treatment of psychiatric diseases” by Chiba University. The other authors have no conflicts of interest.
References
- Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, Ren Q, Dong C, Fujita Y, Hashimoto K (2019) Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav 181:53–59. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Zhang JC, Yao W, Ren Q, Ma M, Yang C, Chaki S, Hashimoto K (2017) Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol 20:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Duman RS (2015) Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett 601:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, Chaki S (2017) Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther 361:9–16. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. (2019) Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci doi: 10.1111/pcn.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H (2017) Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 267:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Kohsaka S, Okabe S (2011) Long-term changes of spine dynamics and microglia after transient peripheral immune response triggered by LPS in vivo. Mol Brain 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS (2019) Ketamine: a paradigm shift for depression research and treatment. Neuron 101:774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C (2019) Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364:eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA Jr (2019) Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry 24:1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K (2018) Regional differences in dendritic spine density confer resilience to chronic social defeat stress. Acta Neuropsychiatr 30:117–122. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M (2016) Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry 80:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Dong C, Fujita A, Fujita Y, Hashimoto K (2018a) Expression of heat shock protein HSP-70 in the retrosplenial cortex of rat brain after administration of (R,S)-ketamine and (S)-ketamine, but not (R)-ketamine. Pharmacol Biochem Behav 172:17–21. [DOI] [PubMed] [Google Scholar]
- Tian Z, Dong C, Zhang K, Chang L, Hashimoto K (2018b) Lack of antidepressant effects of low-voltage-sensitive T-type calcium channel blocker ethosuximide in a chronic social defeat stress model: comparison with (R)-ketamine. Int J Neuropsychopharmacol 21:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treccani G, Ardalan M, Chen F, Musazzi L, Popoli M, Wegener G, Nyengaard JR, Müller HK (2019) S-ketamine reverses hippocampal dendritic spine deficits in Flinders sensitive line rats within 1 h of administration. Mol Neurobiol. Advance online publication. Retrieved 29 April 2019. doi: 10.1007/s12035-019-1613-3. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017) Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry 7:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2018) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83:18–28. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K (2014a) R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K (2014b) Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol 18:pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K (2019a) An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Rev Neurother 19:83–92. [DOI] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K (2019b) Lack of opioid system in the antidepressant actions of ketamine. Biol Psychiatry 85:e25–e27. [DOI] [PubMed] [Google Scholar]
- Zhang K, Ma M, Dong C, Hashimoto K (2018) Role of inflammatory bone markers in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol 21:1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]