Abstract
Background
We investigated the efficacy of electroconvulsive therapy in patients with major depressive disorder and concomitant anxiety symptoms and explored the relationships between depression symptoms and anxiety symptoms during acute electroconvulsive therapy.
Methods
Major depressive disorder inpatients (N = 130) requiring electroconvulsive therapy were recruited for a maximum of 12 treatments each. Depression symptoms, using the core factor subscale derived from the 17-item Hamilton Depression Rating Scale, and anxiety symptoms, using the anxiety/somatization subscale from the Hamilton Depression Rating Scale-17, were assessed before electroconvulsive therapy, after every 3 electroconvulsive therapy treatments, and after the final electroconvulsive therapy. Both core factor subscale and anxiety/somatization subscale scores were converted to T-score units to compare the degrees of changes between depression symptoms and anxiety symptoms after electroconvulsive therapy. The relationships between core factor subscale and anxiety/somatization subscale were analyzed using the cross-lagged longitudinal model during acute electroconvulsive therapy.
Results
A total 116 patients who completed at least the first 3 electroconvulsive therapy treatments were included in the analysis. Reduction of core factor scale T-scores was significantly greater than that of anxiety/somatization subscale T-scores. The model satisfied all indices of goodness-of-fit (chi-square = 30.204, df = 24, P = 0.178, Tucker-Lewis Index = 0.976, Comparative Fit Index = 0.989, Root Mean Square Error of Approximation = 0.047). Core factor subscale changes did not definitely predict subsequent anxiety/somatization subscale changes.
Conclusions
Electroconvulsive therapy is effective in the acute treatment of major depressive disorder patients associated with anxiety symptoms. Anxiety symptoms improved less than depression symptoms during acute electroconvulsive therapy. However, earlier reduction in depression symptoms does not definitely drive subsequent relief in anxiety symptoms.
Keywords: major depressive disorder, electroconvulsive therapy, core factor subscale, anxiety/somatization subscale, cross-lagged longitudinal model
Significance Statement.
This was a repeated measure study to explore the efficacy of electroconvulsive therapy (ECT) in patients with major depressive disorder (MDD) and concomitant anxiety symptoms and to investigate the relationships between depression symptoms and anxiety symptoms during acute ECT. We found that anxiety symptom improvement lagged behind rather than paralleled depression symptom improvement. Anxiety should therefore be regarded as a distinctive domain in MDD patients. Earlier reduction in depression symptoms did not definitely drive subsequent relief in anxiety symptoms. Our findings could help clinicians to understand the trajectories of depression and anxiety changes across the acute electroconvulsive therapy.
Introduction
Electroconvulsive therapy (ECT) is more effective than antidepressant drugs in decreasing depression symptoms for patients with severe depression and treatment-resistant depression (Group, 2003). Anxiety and depression are the most common coexisting psychological problems (Baldwin et al., 2002), and it is estimated that 40% to 60% of patients with major depressive disorder (MDD) also have anxiety symptoms (Gaspersz et al., 2017a). Therefore, anxiety has been regarded as one of the core features of MDD (Vaccarino et al., 2008). However, the DSM diagnostic criteria for MDD do not include current anxiety symptoms. Anxiety symptoms predict a more chronic course, more severe depression symptoms, greater risk of suicide, longer duration of illness, more psychosocial functional impairment, poorer quality of life, and greater likelihood of treatment nonresponse (Pfeiffer et al., 2009; Goldberg and Fawcett, 2012; Zimmerman et al., 2014; Gaspersz et al., 2017b). It is for this reason that anxiety symptoms should be routinely taken into consideration for treatment planning and monitoring of response to treatment (Goldberg and Fawcett, 2012).
Anxiety symptoms of MDD patients also respond to the antidepressant drugs that target depressive symptoms (Bandelow et al., 2007; Tourian et al., 2010; Thase et al., 2014). That is, treatment with antidepressant drugs can improve the symptoms of both anxiety and depression. Therefore, first-line psychopharmacological therapies may not be much different for an MDD patient with anxiety symptoms than for an MDD patient who has anxiety disorders with the full criteria of anxiety symptoms (Stahl, 2013). Although depression symptoms and anxiety symptoms are associated with one another, ECT textbooks only emphasize that ECT improves the depression symptoms but neglect the anxiolytic effect of ECT (APA, 2001; Mankad, 2010; Waite and Easton, 2013). To our knowledge, no studies have explored whether reduction of depression symptoms could predict subsequent reduction of anxiety symptoms, or vice versa throughout the course of acute ECT, and their associations with one another.
We hypothesized that ECT can reduce depression symptoms and anxiety symptoms simultaneously and that reduction of depression symptoms could predict subsequent reduction of anxiety symptoms, or vice versa. The first goal of the current study was to investigate the efficacy of ECT in patients with MDD and concomitant anxiety symptoms. The second was to longitudinally examine the reciprocal associations between anxiety symptoms and depression symptoms during acute ECT and to determine whether reduction of depression symptoms could predict subsequent reduction of anxiety symptoms, or vice versa. The current study examined the anxiety symptoms and depression symptoms across the course of acute ECT, and data were repeatedly measured during 5 assessments.
Methods
Ethics
This study was part of our earlier prospective, observational study, which has been documented elsewhere (Lin et al., 2016). The study was approved by Kai-Syuan Psychiatric Hospital’s institutional review board and conducted in accordance with Good Clinical Practice procedures and the current revision of the Declaration of Helsinki (project no. KSPH-2008–12). This study was registered on Clinicaltrials.gov (identifier: NCT02032576).
Participants
As previously described in detail (Lin et al., 2016), inpatients with MDD requiring ECT (i.e., need for a rapid and definitive response, at high suicide risk, with severe psychomotor retardation, and treatment-resistant depression) (Waite and Easton, 2013) were enrolled from the Inpatient Psychiatric Unit of Kai-Syuan Psychiatric Hospital, a major psychiatric center in south Taiwan. The study’s duration was from January 2008 to October 2013.
Participants were eligible if they were aged 18 years or older, satisfied the DSM-IV-TR criteria for MDD as confirmed using the Structured Clinical Interview for DSM-IV Axis I (APA, 1994), had a 17-item Hamilton Depression Rating Scale (HAMD-17) ≥18, and a Clinical Global Impression of Severity (Guy, 1976) ≥4 at baseline, had given written informed consent to participate in the study after the explanation of the efficacy of ECT, together with its side effects, and had not undergone ECT within the past 6 months. Exclusion criteria included histories of schizophrenia or other psychotic disorders, schizoaffective disorders, bipolar disorders, mental disorders due to organic factors, pregnancy, or severe cognitive impairment. Patients with serious medical conditions or neurological illnesses that restricted the use of ECT did not enter the study.
ECT Protocol
The implementation of ECT was in accordance with the American Psychiatric Association Task Force on ECT (APA, 2001). Psychotropic agents, including antidepressants, antipsychotics, and mood stabilizers, were discontinued for 3 days before initiating ECT, if emergency ECT was not required. Patients remained medication free during the ECT course, except for anxiolytic or sedative-hypnotic medications as needed for insomnia or severe anxiety. Standard bitemporal and modified ECT was performed. Anesthesia was induced by thiopental or thiamylal, both at doses of 1.5 to 2.0 mg/kg i.v. Neuromuscular blockade was induced by succinylcholine at a dosage of 0.5 to 1.0 mg/kg i.v. ECT was conducted using the Thymatron System IV machine with brief-pulse and constant current (pulse width, 0.5 ms; frequency, 60 Hz; current, 0.9A). Seizure duration was at least 20 seconds as measured by electromyogram and 25 seconds measured by electroencephalography. Treatment was given 3 times per week before August 2009 and 2 times per week thereafter, with a maximum of 12 treatments. The number of ECT treatments was determined by the treating psychiatrist, depending on whether remission (i.e., HAMD-17 ≤7) had been reached, if patients could not tolerate the side effects, or if patients decided to discontinue ECT (APA, 2001).
Measures
HAMD-17 anxiety/somatization subscale score was regarded as the severity of anxiety symptoms in MDD patients. The HAMD-17 anxiety/somatization subscale includes 6 items from the HAMD-17: the items for psychic anxiety (Item 10), somatic anxiety (Item 11), gastrointestinal somatic symptoms (Item 12), general somatic symptoms (Item 13), hypochondriasis (Item 15), and insight (Item 17) and was derived from a factor analysis of the HAMD-17 (P Cleary and Guy, 1977). The HAMD-17 anxiety/somatization subscale has been reported to have acceptable psychometric properties and can be used to identify anxiety symptoms in MDD patients for clinical or research purposes (McClintock et al., 2011). The HAMD-17 core factor subscale score was used to reflect the severity of depression symptoms in MDD patients. The core factor subscale, including 5 items from the HAMD-17—the items for depressed mood (Item 1), feelings of guilt (Item 2), suicide (Item 3), work and activities-loss of interest or pleasures (Item 7), and psychomotor retardation (Item 8)—was identified by an exploratory factor analysis (P. J. Cleary, 1981; Bech, 2006). Core factor subscale has been used to measure the efficacy of duloxetine in treating MDD patients (Detke et al., 2002). We used core factor subscale to rate the severity of depression symptoms (Kennedy, 2008) rather than McIntyre’s 7-item HAMD (items 1, 2, 3, 7, 10, 11, and 13) (McIntyre et al., 2005), Maier’s 6-item HAMD (items 1, 2, 7, 8, 9, and 10) (Maier and Philipp, 1985), or Beck’s 6-item HAMD (items 1, 2, 7, 8, 10, and 13) (Bech et al., 1981) because 3 short version scales include psychic anxiety item (Item 10).
Statistical Analyses
Analysis was on a modified intent-to-treat basis for participants reporting at least 1 post-baseline assessment on the HAMD-17. We first carried out descriptive statistics to summarize the data (i.e., percentages, means, and SDs). Paired t test was used to compare score changes in HAMD-17, core factor subscale, and anxiety/somatization subscale at each assessment.
Core factor subscale and anxiety/somatization subscale have different metrics. Effect size is a unitless measure and thus appropriate for comparisons involving scales with different metrics. Effect size (d) was used to demonstrate the level of improvement at endpoint. Effect size was defined as the mean of difference between baseline and posttreatment scores for each measure, divided by the SD of difference (Morris and DeShon, 2002). A d-value of 0.20 indicates a small effect size, 0.50 a medium effect size, and 0.80 a large effect size (Cohen, 1988). Large effect sizes indicate clinically relevant improvements at the end point. Effect size statistics has been recommended to determine clinically significant treatment effect (Hedges and Olkin, 2014).
To compare the degrees of changes between depression symptoms and anxiety symptoms after ECT, both the core factor subscale and anxiety/somatization subscale scores were converted to T-score units (mean = 50, SD = 10). This transformation has been used before (Vittengl et al., 2004; Dunn et al., 2012). The T score was calculated by the following formula (Minium et al., 1993):
The generalized estimating equations (GEE) method, with the first-order autoregressive working correlation structure (Zeger and Liang, 1986), was applied to compare degrees of depression symptom relief with those of anxiety symptom improvement at assessments 2, 3, 4, and 5. That is, the GEE method was used to compare the differences in T-score changes between core factor subscale T-scores and anxiety/somatization subscale T-scores at assessments 2, 3, 4, and 5.
Pearson’s correlation coefficient (r) was employed to quantify the association between core factor subscale and anxiety/somatization subscale. McHorney and colleagues (McHorney et al., 1993) define a strong association as a correlation >0.70, moderate to substantial as a correlation of 0.30 to 0.70, and weak as a correlation <0.30. The cross-lagged longitudinal model was analyzed by structural equation modeling (SEM) to test whether reduction of depression symptoms could predict subsequent reduction of anxiety symptoms, or vice versa. Path coefficients in the SEM model represented the strength of the path between 2 measured variables and were calculated using standardized regression coefficients (i.e., β values). The null hypothesis for SEM was that the model fits the data. The goodness-of-fit of the model was judged using the chi-square statistic (P > .05 indicates a good fit with the data) (Bollen, 1989; Tabachnick and Fidell, 2007), the Tucker-Lewis Index (TLI) (values >0.95 indicate a good fit), the Comparative Fit Index (values >0.90 indicate a good fit), and the Root Mean Square Error of Approximation value (values <0.08 indicate a good fit) (Bentler and Bonett, 1980; Bentler, 1990; Bollen and Long, 1993; Hu and Bentler, 1998; Kline, 2011). Missing data were estimated using the full information maximus likelihood methods. This method uses all available data to estimate the SEM model. It can provide robust parameter estimates and maximize statistical power due to the increased sample size (Schafer and Graham, 2002; Enders, 2011). Data were analyzed using the SPSS version 17.0 for Windows and the Analysis of Moment Structures version 17 (SPSS Inc., Chicago, IL). Statistical significance was defined as an alpha of < 0.05.
Results
Characteristics of Participants, Depression Symptoms, and Anxiety Symptoms at Each Assessment
A total 130 inpatients with MDD participated in the study. Fourteen of the 130 patients who did not complete the first 3 ECT treatments and thereby failed to have at least 1 posttreatment assessment were excluded. The remaining 116 (89.2%) patients entered the analysis. Twenty-seven (29.3%) were male, and 82 (70.7%) were female. The mean (SD) age was 46.9 (12.3) years and their mean (SD) age of MDD onset was 38.1 years (12.8). The mean (SD) number of ECT treatments was 8.9 (2.5). There were 23 patients treated with thrice-weekly ECT and 93 twice-weekly.
Degree of Depression Symptom Reduction vs Degree of Anxiety Symptom Improvement
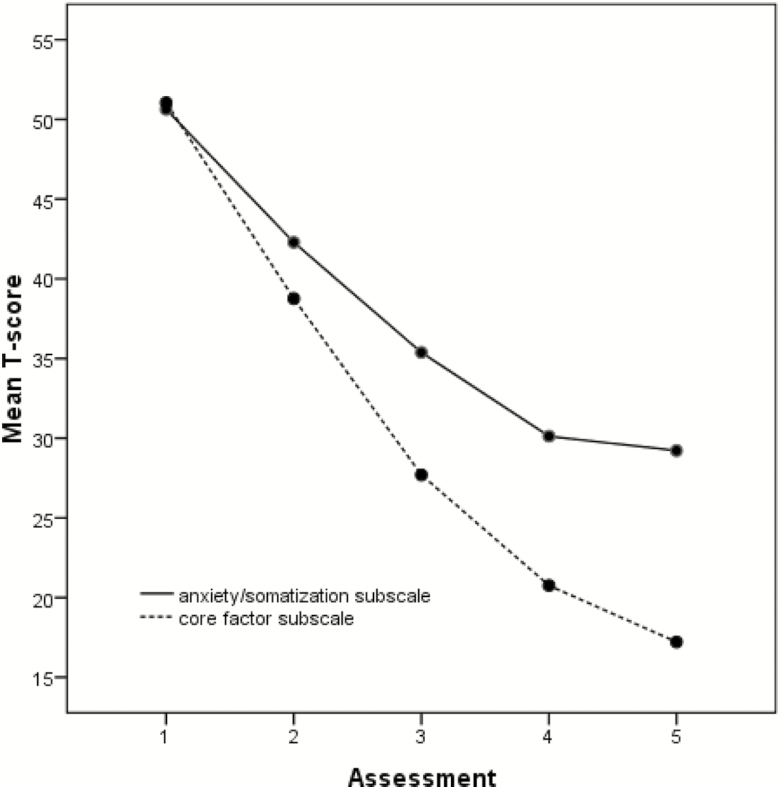
Table 1 contains raw scales, effect sizes, and T-scales of the core factor subscale and anxiety/somatization subscale at each assessment. Acute treatment with ECT resulted in large (d > 0.8) levels of changes in core factor subscale and anxiety/somatization subscale scores at assessment 2, 3, 4, and 5. Furthermore, the GEE was used to compare the reduction of T-scores for core factor subscale with that of T-scores for anxiety/somatization subscale at assessments 2, 3, 4, and 5. The reduction of core factor subscale T-scores was significantly greater than that of anxiety/somatization subscale T-scores, which began at assessment 2 (estimate = –5.8, P < .001) and persisted through assessment 3 (estimate = –10.2, P < .001), 4 (estimate = –11.5, P < .001), and 5 (estimate = –13.4, P < .001) (Table 2). Figure 1 illustrates the core factor subscale T-score and anxiety/somatization subscale T-score throughout acute ECT. The trajectory of depression reduction did not parallel that of anxiety reduction.
Table 1.
Raw Scores, Effect Sizes, and T Scores of HAMD-17a, Core Factor Subscaleb, and Anxiety/Somatization Subscalec at Each Assessment
| Assessment 1 (n = 116) | Assessment 2 (n = 116) | Assessment 3 (n = 107) | Assessment 4 (n = 73) | Assessment 5 (n = 61) | |
|---|---|---|---|---|---|
| HAMD-17, mean ± SDd | 30.9 ± 7.0 | 18.3 ± 7.6 | 11.1 ± 6.3 | 8.4 ± 5.9 | 7.1 ± 5.2 |
| Effect size | 1.59 | 2.34 | 2.76 | 2.77 | |
| Pe | <.001 | <.001 | <.001 | <.001 | |
| Core factor subscale, mean ± SD | 12.8 ± 3.1 | 7.6 ± 3.6 | 4.2 ± 3.2 | 2.9 ± 3.0 | 2.4 ± 2.7 |
| Effect size | 1.41 | 2.21 | 2.73 | 2.63 | |
| Pe | <.001 | <.001 | <.001 | <.001 | |
| Anxiety/somatization subscale, mean ± SD | 9.0 ± 3.0 | 5.7 ± 3.1 | 3.7 ± 2.3 | 2.8 ± 2.3 | 2.6 ± 2.0 |
| Effect size | 1.29 | 1.83 | 2.15 | 2.40 | |
| Pe | <.001 | <.001 | <.001 | <.001 | |
| HAMD-17 T-score, mean ± SD | 50.0 ± 10.0 | 32.0 ± 10.8 | 21.8 ± 9.0 | 17.9 ± 8.4 | 16.0 ± 7.5 |
| Core factor subscale T-score, mean ± SD | 50.0 ± 10.0 | 33.4 ± 11.4 | 22.5 ± 10.3 | 18.4 ± 9.6 | 16.8 ± 8.7 |
| Anxiety/somatization subscale T-score, mean ± SD | 50.0 ± 10.0 | 39.3 ± 10.1 | 32.7 ± 7.5 | 29.5 ± 7.7 | 28.9 ± 6.6 |
aHAMD-17 = 17-item Hamilton Depression Rating Scale.
bCore factor subscale = HAMD-17 Items 1(depressed mood), 2 (feelings of guilt), 3 (suicide), 7 (work and activities-loss of interest or pleasures), and 8 (motor retardation).
cAnxiety/somatization subscale = HAMD-17 Items 10 (psychic anxiety), 11 (somatic anxiety), 12 (gastrointestinal somatic symptoms), 13 (general somatic symptoms), 15 (hypochondriasis), and 17 (insight).
dSD = standard deviation.
ePaired t test = mean scores at the assessments 2, 3, 4, and 5 compared with mean scores at assessment 1.
Table 2.
Differences (=Estimate) Between the Reduction of Core Factor Subscale T-score and Reduction of Anxiety/Somatization Subscale T-scores at Assessments 2, 3, 4, and 5 Using GEE Method
| Scalea × Time | Estimate | Standard error | P |
|---|---|---|---|
| Scale × Assessment 2 | –5.8 | 1.3 | <.001 |
| Scale × Assessment 3 | –10.2 | 1.5 | <.001 |
| Scale × Assessment 4 | –11.5 | 1.6 | <.001 |
| Scale × Assessment 5 | –13.4 | 1.6 | <.001 |
Abbreviations: GEE, generalized estimating equations.
a Scale = Core factor T-score vs anxiety/somatization T-score (as reference scale).
Figure 1.
Core factor subscale T-scores vs anxiety/somatization subscale T-scores at each assessment during acute electroconvulsive therapy (ECT). The symptom severity was assessed using the 17-item Hamilton Depression Rating Scale (HAMD-17) before ECT, after every 3 ECT treatments, and after the final ECT.
The Relationships Between Depression Symptoms and Anxiety Symptoms
The correlation coefficient between core factor subscale and anxiety/somatization subscale scores at assessments 1, 2, 3, 4, and 5 were 0.36, 0.52, 0.50, 0.47, and 0.26, respectively (Table 3). These results reveal that the correlations between depression symptoms and anxiety symptoms may be bidirectional, which represent relationships without an explicitly defined causal direction (van der Voort et al., 2015). All the correlation coefficients were only weak to moderate (McKnight and Kashdan, 2009).
Table 3.
Pearson Correlation Coefficient Matrix of the Measured Variables
| Core 1 | Core 2 | Core 3 | Core 4 | Core 5 | Anxiety 1 | Anxiety 2 | Anxiety 3 | Anxiety 4 | |
|---|---|---|---|---|---|---|---|---|---|
| Core 1 | 1.00 | ||||||||
| Core 2 | 0.41** | 1.00 | |||||||
| Core 3 | 0.26** | 0.73** | 1.00 | ||||||
| Core 4 | 0.27* | 0.54** | 0.85** | 1.00 | |||||
| Core 5 | 0.22 | 0.36** | 0.68** | 0.89** | 1.00 | ||||
| Anxiety 1 | 0.36** | 0.16 | –0.02 | –0.15 | –0.21 | 1.00 | |||
| Anxiety 2 | 0.18 | 0.52** | 0.27** | 0.04 | –0.16 | 0.66** | 1.00 | ||
| Anxiety 3 | 0.20* | 0.47** | 0.50** | 0.34** | 0.07 | 0.46** | 0.70** | 1.00 | |
| Anxiety 4 | 0.17 | 0.31** | 0.40** | 0.47** | 0.32* | 0.36** | 0.42** | 0.64** | 1.00 |
| Anxiety 5 | 0.09 | 0.06 | 0.09 | 0.19 | 0.26* | 0.54** | 0.47** | 0.51** | 0.77** |
Abbreviations: Core n = Core factor subscale scores at assessment n, n = 1, 2, 3, 4, or 5. Anxiety n = Anxiety/somatization subscale scores at assessment n, n = 1, 2, 3, 4, or 5.
* P < .05, ** P < .01.
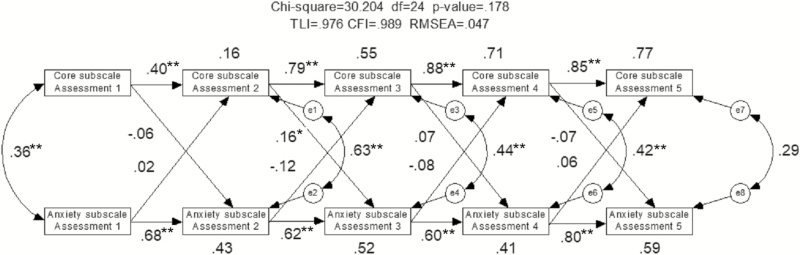
Figure 2 shows the results of the cross-lagged longitudinal data over 5 assessments analyzed using SEM. The model satisfied all indices of goodness-of-fit (chi-square = 30.204, df = 24, P = .178, TLI = 0.976, Comparative Fit Index = 0.989, Root Mean Square Error of Approximation = 0.047). The path between core factor subscale at assessment 2 and anxiety/somatization subscale at assessment 3 (i.e., core factor subscale at assessment 2→ anxiety/somatization subscale at assessment 3 in Figure 2) was significant (P = .037), indicating that core factor subscale score changes at assessment 2 were significantly associated with subsequent anxiety/somatization subscale score changes at assessment 3. However, anxiety/somatization subscale score changes did not predict subsequent core factor subscale score changes. The e1, e2, e3, e4, e5, e6, e7, and e8 indicate the error terms. The error term represents variance unexplained by independent variables.
Figure 2.
The cross-lagged longitudinal model illustrates the reciprocal causal relationship between depression and anxiety over time and whether the depression symptom relief predicts subsequent anxiety symptom improvement, and vice versa during acute electroconvulsive therapy (ECT). Rectangles represent the indices of depression symptom severity and anxiety symptom severity. Circles with an e are called error terms (i.e., e1–e8 = error term), which represent the effect of unexplained variation on the indices. Unidirectional arrows signify direct effects and bi-directional arrows represent correlations, *P < .05, **P < .01.
Discussion
The first finding of this study was that patients receiving ECT had continual improvement over baseline in both depression symptoms and anxiety symptoms, as seen at each assessment (Table 1). These results indicate that ECT can be effective not only in treating depression symptoms but also in treating anxiety symptoms. The second finding was that anxiety symptom improvement lagged behind rather than paralleled depression symptom improvement. Anxiety should therefore be regarded as a distinctive domain in MDD patients. The third was that there were reciprocal associations between anxiety symptoms and depression symptoms during acute ECT. This confirms previous reports that MDD patients with high levels of anxiety are associated with more severe depression (Joffe et al., 1993; Fava et al., 2004; Wiethoff et al., 2010; Chan et al., 2012). However, the correlations were weak to moderate.
The fourth was that higher levels of anxiety symptoms may not predict greater depression symptoms at sequential time points, and higher levels of depression symptoms are not associated with late severity of anxiety symptoms, except for severity of depression symptoms at assessment 2. This indicates that depression reduction may not definitely drive anxiety improvement. ECT may have a direct impact on anxiety symptoms.
We investigated the effects of ECT on depression symptoms and anxiety symptoms for MDD patients using the core factor subscale and anxiety/somatization subscale. Few studies have examined the efficacy of ECT in MDD patients with concomitant anxiety. The results of the current study indicate that the use of the paired outcome criteria (core factor subscale and anxiety/somatization subscale) provides a more relevant perspective of the treatment outcome for these patients. This approach may be useful for other ECT studies dealing with patients who have concomitant depression and anxiety. Our findings could help clinicians to understand the trajectories of depression and anxiety changes across the acute electroconvulsive therapy. This was a repeated measure study. The longitudinal follow-up data were obtained from the same participants. The cross-lagged longitudinal model can explore the underlying processes of reciprocal causality between anxiety symptoms and depression symptoms at various time points (e.g., to what extent earlier reduction in depression symptoms drives subsequent relief in anxiety symptoms, and vice versa) (Blalock, 2007). However, several limitations of this study should also be addressed. First, the patients were not blinded to treatment nor was there a control group. The effectiveness on anxiety may come from the anxiolytic or sedative-hypnotic use. However, such medications may not improve the MDD’s core symptoms (Bech, 2001). Second, all the participants were from a single psychiatric center. Independent studies should replicate our research in other units to generalize our results. Third, for SEM analysis, a minimum sample of 100 has been suggested (Hoyle, 1995; Loehlin, 2004). The current sample size (n = 116) was therefore too small to perform SEM analysis with many variables. There may be other models with larger case numbers that would fit the data better. Fourth, this trial used core factor subscale and anxiety/somatization subscale to assess depression symptoms and anxiety symptoms, respectively. Further study is required to explore whether using other anxiety scales that are designed specially to measure the severity anxiety in depressed patients (e.g., feeling keyed up or tense, feeling restless, difficulty concentrating because of worry, fear that something awful might happen, and feeling that one might lose control) (Zimmerman et al., 2017) will yield different results. Fifth, all participants were treated with bitemporal ECT. Therefore, whether the present findings can be extrapolated to those treated with bifrontal or right unilateral ECT requires additional study. Additionally, 6 to 12 treatments are necessary for most patients in usual clinical practice (APA, 2001; Kellner, 2012), but a patient with poor response after 12 treatments is not likely to have a favorable response even after receiving more ECT treatments (Waite and Easton, 2013). However, since the rate and quality of response to ECT are highly individualized, some patients may need as many as 20 treatments to obtain maximal improvement (Mankad, 2010).
In conclusion, ECT demonstrated significant improvements in depression symptoms and anxiety symptoms for MDD patients. Anxiety symptoms improved more slowly than did depression symptoms during acute ECT. There was a bi-directional association between anxiety symptoms and depression symptoms across the course of acute ECT. However, reduction in depression symptoms did not definitely predict subsequent reduction in anxiety symptoms.
Acknowledgments
We would like to thank all the participants in this study.
This work was supported by the Kaohsiung Municipal Kai-Syuan Psychiatric Hospital (KSPH-2008-12) and the Ministry of Science and Technology, Taiwan (MOST-103-2314-B-280-001-MY3).
References
- APA (1994) American Psychiatric Association: structured clinical interview for DSM-IV. Washington, DC: American Psychiatric Press. [Google Scholar]
- APA (2001) The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- Baldwin DS, Evans DL, Hirschfeld RM, Kasper S (2002) Can we distinguish anxiety from depression? Psychopharmacol Bull 36(Suppl 2):158–165. [PubMed] [Google Scholar]
- Bandelow B, Andersen HF, Dolberg OT (2007) Escitalopram in the treatment of anxiety symptoms associated with depression. Depress Anxiety 24:53–61. [DOI] [PubMed] [Google Scholar]
- Bech P. (2001) Meta-analysis of placebo-controlled trials with mirtazapine using the core items of the Hamilton Depression Scale as evidence of a pure antidepressive effect in the short-term treatment of major depression. Int J Neuropsychopharmacol 4:337–345. [DOI] [PubMed] [Google Scholar]
- Bech P. (2006) Rating scales in depression: limitations and pitfalls. Dialogues Clin Neurosci 8:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech P, Allerup P, Gram LF, Reisby N, Rosenberg R, Jacobsen O, Nagy A (1981) The Hamilton depression scale. Evaluation of objectivity using logistic models. Acta Psychiatr Scand 63:290–299. [DOI] [PubMed] [Google Scholar]
- Bentler PM. (1990) Comparative fit indexes in structural models. Psychol Bull 107:238–246. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Bonett DG (1980) Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull 88:588–606. [Google Scholar]
- Blalock HM. (2007) Causal models in experimental designs. New Brunswick, NJ: AldineTransaction. [Google Scholar]
- Bollen KA. (1989) Structural equations with latent variables. New York; Chichester: Wiley. [Google Scholar]
- Bollen KA, Long JS (1993) Testing structural equation models. Newbury Park, CA: : Sage Publications. [Google Scholar]
- Chan HN, Rush AJ, Nierenberg AA, Trivedi M, Wisniewski SR, Balasubramani GK, Friedman ES, Gaynes BN, Davis L, Morris D, Fava M (2012) Correlates and outcomes of depressed out-patients with greater and fewer anxious symptoms: a CO-MED report. Int J Neuropsychopharmacol 15:1387–1399. [DOI] [PubMed] [Google Scholar]
- Cleary P, Guy W (1977) Factor analysis of the Hamilton depression scale. Drugs Exp Clin Res 1:115–120. [Google Scholar]
- Cleary PJ. (1981) Problems of internal consistency and scaling in life event schedules. J Psychosom Res 25:309–320. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, NJ: L. Erlbaum Associates. [Google Scholar]
- Detke MJ, Lu Y, Goldstein DJ, McNamara RK, Demitrack MA (2002) Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psychiatr Res 36:383–390. [DOI] [PubMed] [Google Scholar]
- Dunn TW, Vittengl JR, Clark LA, Carmody T, Thase ME, Jarrett RB (2012) Change in psychosocial functioning and depressive symptoms during acute-phase cognitive therapy for depression. Psychol Med 42:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. (2011) Analyzing longitudinal data with missing values. Rehabil Psychol 56:267–288. [DOI] [PubMed] [Google Scholar]
- Fava M, Alpert JE, Carmin CN, Wisniewski SR, Trivedi MH, Biggs MM, Shores-Wilson K, Morgan D, Schwartz T, Balasubramani GK, Rush AJ (2004) Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med 34:1299–1308. [DOI] [PubMed] [Google Scholar]
- Gaspersz R, Lames F, Kent JM, Beekman AT, Smit JH, van Hemert AM, Schoevers RA, Penninx BW (2017a) Longitudinal predictive validity of the DSM-5 anxious distress specifier for clinical outcomes in a large cohort of patients with major depressive disorder. J Clin Psychiatry 78:207–213. [DOI] [PubMed] [Google Scholar]
- Gaspersz R, Lamers F, Kent JM, Beekman ATF, Smit JH, van Hemert AM, Schoevers RA, Penninx BWJH (2017b) Anxious distress predicts subsequent treatment outcome and side effects in depressed patients starting antidepressant treatment. J Psychiatr Res 84:41–48. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Fawcett J (2012) The importance of anxiety in both major depression and bipolar disorder. Depress Anxiety 29:471–478. [DOI] [PubMed] [Google Scholar]
- Group UER. (2003) Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 361:799–808. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976) ECDEU Assessment manual for psychopharmacology. Washington DC: DHEW Publication 76338. [Google Scholar]
- Hedges LV, Olkin I (2014) Statistical methods for meta-analysis. Orlando: Academic Press. [Google Scholar]
- Hoyle RH. (1995) Structural equation modeling: concepts, issues, and applications. Thousand Oaks, Calif.; London: Sage Publications. [Google Scholar]
- Hu L-T, Bentler PM (1998) Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods 3:424. [Google Scholar]
- Joffe RT, Bagby RM, Levitt A (1993) Anxious and nonanxious depression. Am J Psychiatry 150:1257–1258. [DOI] [PubMed] [Google Scholar]
- Kellner CH. (2012) Brain stimulation in psychiatry: ECT, DBS, TMS, and other modalities. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Kennedy SH. (2008) Core symptoms of major depressive disorder: relevance to diagnosis and treatment. Dialogues Clin Neurosci 10:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. (2011) Principles and practice of structural equation modeling, 3rd ed. New York; London: Guilford. [Google Scholar]
- Lin CH, Chen MC, Yang WC, Lane HY (2016) Early improvement predicts outcome of major depressive patients treated with electroconvulsive therapy. Eur Neuropsychopharmacol 26:225–233. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. (2004) Latent variable models: an introduction to factor, path, and structural equation analysis, 4th ed. Mahwah, NJ; London: Lawrence Erlbaum Associates. [Google Scholar]
- Maier W, Philipp M (1985) Improving the assessment of severity of depressive states: a reduction of the Hamilton depression scale. Pharmacopsychiatry 18:114–115. [Google Scholar]
- Mankad MV. (2010) Clinical manual of electroconvulsive therapy, 1st ed. Washington, DC: American Psychiatric Pub. [Google Scholar]
- McClintock SM, Husain MM, Bernstein IH, Wisniewski SR, Trivedi MH, Morris D, Alpert J, Warden D, Luther JF, Kornstein SG, Biggs MM, Fava M, Rush AJ (2011) Assessing anxious features in depressed outpatients. Int J Methods Psychiatr Res 20:e69–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Ware JE Jr, Raczek AE (1993) The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31:247–263. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Konarski JZ, Mancini DA, Fulton KA, Parikh SV, Grigoriadis S, Grupp LA, Bakish D, Filteau MJ, Gorman C, Nemeroff CB, Kennedy SH (2005) Measuring the severity of depression and remission in primary care: validation of the HAMD-7 scale. CMAJ 173:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight PE, Kashdan TB (2009) The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clin Psychol Rev 29:243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minium EW, King BM, Bear G, eds. (1993) Statistical reasoning in psychology and education, 3rd ed. New York; Chichester: Wiley. [Google Scholar]
- Morris SB, DeShon RP (2002) Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods 7:105–125. [DOI] [PubMed] [Google Scholar]
- Pfeiffer PN, Ganoczy D, Ilgen M, Zivin K, Valenstein M (2009) Comorbid anxiety as a suicide risk factor among depressed veterans. Depress Anxiety 26:752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW (2002) Missing data: our view of the state of the art. Psychol Methods 7:147–177. [PubMed] [Google Scholar]
- Stahl SM. (2013) Stahl’s essential psychopharmacology: neuroscientific basis and practical application, 4th ed. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Tabachnick BG, Fidell LS (2007) Using multivariate statistics, 5th ed. Boston; London: Pearson/Allyn & Bacon. [Google Scholar]
- Thase ME, Chen D, Edwards J, Ruth A (2014) Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol 29:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourian KA, Jiang Q, Ninan PT (2010) Analysis of the effect of desvenlafaxine on anxiety symptoms associated with major depressive disorder: pooled data from 9 short-term, double-blind, placebo-controlled trials. CNS Spectr 15:187–193. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Evans KR, Sills TL, Kalali AH (2008) Symptoms of anxiety in depression: assessment of item performance of the Hamilton anxiety rating scale in patients with depression. Depress Anxiety 25:1006–1013. [DOI] [PubMed] [Google Scholar]
- van der Voort TY, Seldenrijk A, van Meijel B, Goossens PJ, Beekman AT, Penninx BW, Kupka RW (2015) Functional versus syndromal recovery in patients with major depressive disorder and bipolar disorder. J Clin Psychiatry 76:e809–e814. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB (2004) Improvement in social-interpersonal functioning after cognitive therapy for recurrent depression. Psychol Med 34:643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite J, Easton A (2013) The ECT handbook, 3rd ed. London: Royal College of Psychiatrists. [Google Scholar]
- Wiethoff K, Bauer M, Baghai TC, Möller HJ, Fisher R, Hollinde D, Kiermeir J, Hauth I, Laux G, Cordes J, Brieger P, Kronmüller KT, Zeiler J, Adli M (2010) Prevalence and treatment outcome in anxious versus nonanxious depression: results from the German algorithm project. J Clin Psychiatry 71:1047–1054. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130. [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Young D, Dalrymple K, Walsh E, Rosenstein L (2014) A clinically useful self-report measure of the DSM-5 anxious distress specifier for major depressive disorder. J Clin Psychiatry 75:601–607. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Clark H, McGonigal P, Harris L, Holst CG, Martin J (2017) Reliability and validity of the DSM-5 anxious distress specifier interview. Compr Psychiatry 76:11–17. [DOI] [PubMed] [Google Scholar]