Abstract
Background
Preclinical and some human data suggest allosteric modulation of the muscarinic M1 receptor (CHRM1) is a promising approach for the treatment of schizophrenia. However, it is suggested there is a subgroup of participants with schizophrenia who have profound loss of cortical CHRM1 (MRDS). This raises the possibility that some participants with schizophrenia may not respond optimally to CHRM1 allosteric modulation. Here we describe a novel methodology to measure positive allosteric modulation of CHRM1 in human CNS and the measurement of that response in the cortex, hippocampus, and striatum from participants with MRDS, non-MRDS and controls.
Methods
The cortex (Brodmann’s area 6), hippocampus, and striatum from 40 participants with schizophrenia (20 MRDS and 20 non-MRDS) and 20 controls were used to measure benzyl quinolone carboxylic acid-mediated shift in acetylcholine displacement of [3H]N-methylscopolamine using a novel in situ radioligand binding with autoradiography methodology.
Results
Compared with controls, participants with schizophrenia had lower levels of specific [3H]N-methylscopolamine binding in all CNS regions, whilst benzyl quinolone carboxylic acid-modulated binding was less in the striatum, Brodmann’s area 6, dentate gyrus, and subiculum. When divided by subgroup, only in MRDS was there lower specific [3H]N-methylscopolamine binding and less benzyl quinolone carboxylic acid-modulated binding in all cortical and subcortical regions studied.
Conclusions
In a subgroup of participants with schizophrenia, there is a widespread decreased responsiveness to a positive allosteric modulator at the CHRM1. This finding may have ramifications it positive allosteric modulators of the CHRM1 are used in clinical trials to treat schizophrenia as some participants may not have an optimal response.
Keywords: schizophrenia, musccarinic M1 receptor, positive allosteric modulation, hippocampus, striatum
Significance Statement.
Positive allosteric modulation of muscarinic receptors is strongly argued to be a novel way of treating a number of disorders of the human CNS, including schizophrenia. Here we report the development of a novel methodology that allows the positive allosteric modulation of the muscarinic M1 receptor to be quantified in human CNS, in particular in regions of the CNS that have complex structures such as the hippocampus. This methodology has been used to show, for the first time to our knowledge, that the response to the muscarinic M1 receptor positive allosteric modulator is less in a subgroup of participants with schizophrenia. This finding may explain why some individuals with schizophrenia may not respond to such treatments.
Introduction
There is a growing body of evidence that includes data from neuroimaging, postmortem CNS, and preclinical pharmacology suggesting that there is a role for the muscarinic M1 receptor (CHRM1) in the pathophysiology and treatment of schizophrenia (Raedler et al., 2007; Gibbons and Dean, 2016). Center to this hypothesis was evidence to suggest there were lower levels of CHRM1s in many CNS regions in participants with the disorder (Raedler et al., 2003) and that levels of CHRM1 in the dorsolateral prefrontal cortex were inversely related to cognitive ability and correlated with the severity of negative symptoms (Bakker et al., 2018). Notably, soon after the demonstration of lower levels of CHRM1 in the CNS from participants with schizophrenia, it was suggested that activating the CHRM1 could prove to have therapeutic benefits for those with the disorder (Felder et al., 2001; Dean et al., 2003). This hypothesis gained support from a study showing the drug xanomeline, a CHRM1 and CHRM4 agonist, improved cognitive deficits and the severity of positive and negative symptoms in participants with schizophrenia (Shekhar et al., 2008). The usefulness of xanomeline as a new treatment for schizophrenia is limited because of severe gastric side effects that are suggested to be due to the agonist activity of the drug at the peripheral CHRM1 (Alt et al., 2016). However, a new formulation including xanomeline and a peripheral CHRM antagonist, trospium, appears to have the therapeutic potential of xanomeline without the peripheral side effects (Miller et al., 2016). This advantageous outcome is likely due to trospium being unable to cross the blood-brain barrier (Chancellor et al., 2012) and therefore being able to selectively act to antagonize the CHRM1 in the periphery to block the unwanted agonists effects of xanomeline at that receptor (Hegde, 2006). Notably, there is currently a phase II trial determining the efficacy, safety, and tolerability of KarXT (xanomeline + trospium; Karuna Pharmaceuticals) and sustained efforts to show that other drugs that activate orthosteric and allosteric sites on the CHRM1 can be useful in treating schizophrenia (Conn et al., 2009).
One major limitation to currently available drugs used to treat schizophrenia is the high level of suboptimal responsiveness and even treatment resistance (Kane and McGlashan, 1995; Sharif, 1998). The therapeutic benefit from drugs currently used to treat schizophrenia was thought to come from their ability to either antagonize (Miyamoto et al., 2005) or act as partial agonists (Frampton, 2019) at the dopamine D2-like receptors. Therefore, individuals who do not respond to such treatments are thought to have forms of schizophrenia in which abnormal dopaminergic function is not central to the pathophysiology of the disorder (Jankowska et al., 2019). The notion that, in some individuals, the pathophysiology of schizophrenia can involve systems other than the dopaminergic system supports the argument that schizophrenia is a syndrome of disorders (Jablensky, 2006) and it therefore follows that drug responsiveness could be restricted to specific subgroups within this broad syndrome. In addition, schizophrenia has been suggested to have a number of symptom domains and that responsiveness to drugs may be a function of the specific symptom domain being targeted (Marder et al., 2019). Thus, given the importance of CHRM1 in cognition, it has been postulated that drugs that activate that receptor could be useful in alleviating the cognitive deficits associated with schizophrenia that have proven resistant to treatment with existing antipsychotic drugs (Hopper et al., 2016).
The notion that schizophrenia is a syndrome is important when considering the use of drugs that activate the CHRM1 potential to treat the disorder because it has been reported that there is a subgroup (~25%) within the disorder, who have been termed muscarinic receptor deficit schizophrenia (MRDS) (Scarr et al., 2009), that can be separated because they have a marked loss of [3H]pirenzepine binding (approximately 75%) to CHRM1 in Brodmann’s cortical area (BA) 9. This initial finding has now been expanded to show participants with MRDS have a widespread loss of CHRM1 in other areas of the cortex (BA 6, 10, 24, 44, and 46) (Gibbons et al., 2013; Seo et al., 2014) as well as the striatum (Dean et al., 2016a). It appears the loss of CHRM1 in the cortex may be due to a marked decrease in pyramidal cells in laminae III and V expressing CHRM1 (Scarr et al., 2018b), which was detected as a decrease in levels of cortical CHRM1 gene expression (Scarr et al., 2018a). Most recently, it has been reported that compared with controls and participants with schizophrenia who have not lost cortical CHRM1 (non-MRDS), there are changes in gene expression in BA 9 that are unique to MRDS (Scarr et al., 2018a) consistent with some differences the molecular pathophysiology being present in the subgroup. Moreover, when considering drug responsiveness, it is significant that CHRM1 G-protein recruitment in response to an orthosteric agonist has been shown to be altered in tissue from participants with MRDS but not MRDS (Salah-Uddin et al., 2009), possibly indicating participants with MRDS would not respond optimally to CHRM1 orthosteric agonists. By contrast, CHRM1 G-protein recruitment in response to an allosteric agonist did not differ in participants with MRDS, suggesting they may respond optimally to drugs that target such sites on the receptor.
Currently it would appear that the amino acid sequence of the orthosteric binding site across all CHRMs has been subject to conservation with evolution, and hence it has not been possible to develop drugs targeting the orthosteric site on each of the 5 CHRMs (Gregory et al., 2007). By contrast, it would appear that the amino acid sequence of at least 1 of the allosteric sites on each CHRM is unique to its receptor, meaning it has been possible to develop drugs that specifically target each human CHRMs (Conn et al., 2009). One such drug, benzyl quinolone carboxylic acid (BQCA), is a positive allosteric modulator that specifically targets CHRM1 with the potency of BQCA-modulation being measurable by its ability to increase the ability of acetylcholine to displace [3H]N-methylscopolamine ([3H]NMS) from CHRM1 (Ma et al., 2009). Significantly, BQCA-mediated acetylcholine displacement of [3H]NMS has been reported to be lower in participants with MRDS compared with that in non-MRDS and controls (Dean et al., 2016b), suggesting that participants with MRDS may not respond optimally to treatments based on positive allosteric modulation.
The study of BQCA-mediated acetylcholine displacement of [3H]NMS in schizophrenia was based on the use of membrane homogenate (Dean et al., 2016b). This has limitations when studying complex regions in the CNS, such as the hippocampus, because it is difficult to dissect small regions and prepare membranes from those regions for study. This limitation can be overcome using in situ radioligand binding with autoradiography as this uses tissue slices to give quantitative data within discrete anatomical regions within tissue such as the hippocampus (Kerwin et al., 1990). Here we report a novel methodology that uses in situ radioligand binding with autoradiography to measure BQCA-mediated acetylcholine displacement of [3H]NMS binding in the human cortex (BA 6), hippocampus, and striatum and the outcome of using this methodology comparing this measurement in tissue from participants with MRDS, non-MRDS, and controls.
Methods
Ethical Considerations
For all tissue used in this study, the collection of human CNS tissue postmortem was approved by the Ethics Committee of the Victorian Institute of Forensic Medicine. CNS tissue was only collected after gaining written consent from the nearest next-of-kin.
Materials
[3H]NMS (specific activity 84.1 Ci/mmol; Lot# 1885805) and Ultima Gold scintillation cocktail were obtained from Perkin Elmer. BQCA was synthesized at Vanderbilt University as previously described (Shirey et al., 2009). 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, acetylcholine chloride, dimethyl sulfoxide, MgCl2, NaCl, and polyethylenimine were obtained from Sigma-Aldrich.
BQCA-Mediated Binding: General Methodology
To measure BQCA-mediated acetylcholine displacement of [3H]NMS binding (BQCA-mediated binding) using in situ radioligand binding and autoradiography, frozen sections (20 µm) were prepared from BA 6 using a cryostat (CryoCut 1800; Leica) and thaw mounted on gelatinized slides. For comparison with the membrane binding methodology (Dean et al., 2016b), tissue homogenates were prepared from the same blocks of cortical tissue used to prepare frozen tissue sections as described previously (Dean et al., 2016b). Tissue sections and membrane-enriched homogenate preparations were stored at −80°C until required.
For method optimization and studies on schizophrenia, all aspects of BQCA-mediated binding were measured in triplicate using the same assay buffer [20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 100 mM NaCl, 10 mM MgCl2, pH 7.4]. When using homogenates, the binding of the radioligand was terminated by filtering the reaction mixture through a Whatman GF/B filter soaked in polyethylenimine (0.1%) and washing the filters 3 times with 5 mL of ice-cold normal saline. The washed filter paper was then added to 5 mL Ultima Gold scintillation cocktail and the radioactivity retained on the filter paper measured using a TriCarb 2910 TR scintillation counter (PerkinElmer).
For in situ radioligand binding with autoradiography, reactions were stopped by submerging the frozen sections twice (3 minutes) in ice-cold assay buffer followed by a brief submerging (approximately 10 seconds) in ice-cold deionized water. Tissue sections were then partially fixed overnight in paraformaldehyde vapor and apposed to a BAS-TR2025 phospho-imaging plate (Fujifilm) with [3H]microscales (Amersham Biosciences) until an image of measurable intensity developed (7 days). Subsequently, BAS-TR2025 plates were scanned in a BAS 5000 high-resolution phosphoimager (Fujifilm), and binding intensity was determined as DPM/mg estimated wet weight tissue equivalents by comparing image intensity to a standard curve fitted to [3H]microscales using AIS imaging software (Imaging Research). Results from both autoradiography and membrane-enriched homogenate experiments were converted from DPM to femtomoles (fmol) based on the radioactive decay and specific activity of [3H]NMS (in this study: 84.1 Ci/mmol).
BQCA-Mediated Binding: Methodological Optimization
For in situ radioligand binding and autoradiography, it was necessary to use a single concentration of [3H]NMS, acetylcholine, and BQCA to measure BQCA-mediated binding (Dean et al., 1997). Routinely, it is proposed that the concentration of radioligand used in such studies should be approximately 3× dissociation constant (KD) for membrane binding (Rodbard, 1981) as this saturates available binding sites and allows a good estimate of the number of binding sites when using single-point saturation analyses. Thus, having previously shown the KD for [3H]NMS in human cortex to be 0.13 nM (Dean et al., 2016b), we used 0.4 nM [3H]NMS for all in situ radioligand binding experiments.
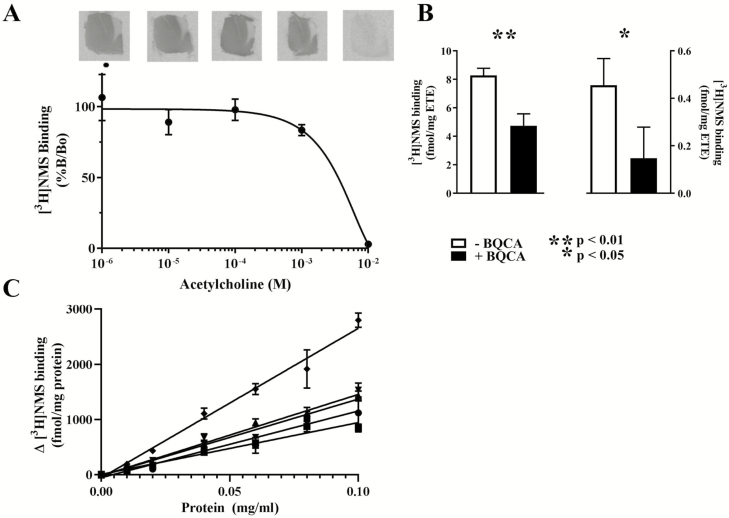
Having established the concentration of [3H]NMS to be used for in situ radioligand binding, we then measured the ability of acetylcholine (0 to 10–2 M) to displace [3H]NMS (0.4 nM) to BA 6 tissue sections from 3 participants with no history of psychiatric disorders. The lowest concentration of acetylcholine that consistently displaced [3H]NMS binding was 1 mM with nonspecific binding occurring in the presence of 10 nM acetylcholine (Figure 1A). Given BQCA increases the ability of acetylcholine to decrease [3H]NMS binding, we used the concentration of acetylcholine that first produced a reproducible decrease in [3H]NMS binding (1 mM) in ongoing experiments.
Figure 1.
Method optimization utilizing tissue from Brodmann’s area (BA) 6. (A) The binding of [3H]NMS (0.4 nM) (mean ± SEM) to frozen sections from 3 cases in the presence of increasing concentrations of acetylcholine. (B) The binding of [3H]NMS (0.4 nM) (mean ±SEM) to frozen sections from 3 cases in the presence of a standard concentration of acetylcholine and in the absence or presence of 3 µM benzyl quinolone carboxylic acid (BQCA). Example autoradiographs are included for each binding condition. (C) BQCA-mediated binding (mean ± SEM) measured as the difference between [3H]n-methyl scopolamine ([3H]NMS: 0.4 nM) binding to particulate membrane from 5 cases in the presence of a standard dose of acetylcholine (1 nM) and in the presence or absence of 3µM BQCA.
Using particulate membrane, we showed that BQCA (3 µM) most robustly and reproducibly increase in the ability of acetylcholine to displace [3H]NMS binding. Hence, using tissue sections from the same individuals, we measured BQCA (3 µM)-mediated acetylcholine displacement (1 and 10 mM) of [3H]NMS (0.4 nM) with the premise that BQCA could not further increase the displacement of [3H]NMS when acetylcholine had displaced all of the radioligand. As expected, in the presence of 1 mM acetylcholine, 3 µM causes a highly significant 43% reduction in [3H]NMS binding (Figure 1B). Surprisingly, in the presence of 10 mM acetylcholine, 3 µM reduced the binding of [3H]NMS by 68%, but because of very low radioligand binding there was more variability in results and therefore the reduction was less significant. Hence, to maximize the reproducibility of measuring BQCA-mediated binding, future experiments using frozen sections were carried out using 1 mM acetylcholine and 3 µM BQCA.
To determine if BQCA-mediated binding to frozen sections was a quantitative measure, the ability of BQCA (3 µM) to modulate the displacement of [3H]NMS (0.4 nM) at a standardized dose of acetylcholine (1 nM) from a tissue homogenate (in triplicated) prepared from BA 6 from 5 participants with no history of psychiatric disorders at a range of protein concentrations (0.01–0.10 mg/mL protein) was measured. There were strong correlations between BQCA-mediated binding and protein concertation (r2 from 0.95 to 0.99; P from <.0001 to .0002 for tissue from each of the 5 samples) (Figure 1C). Given the specificity of BQCA for CHRM1 (Ma et al., 2009), these strong relationships would suggest that under the standardized conditions used, BQCA-mediated binding gives some indication of the availability of CHRM1.
BQCA-Mediated Binding: Studies in Schizophrenia
Tissue Collection
For the study of BQCA-mediated binding in schizophrenia, CNS was collected from donors with a likely history of psychiatric illness and donors with no obvious history of such illnesses. Subsequently, a case-history review was completed using Diagnostic Instrument for Brain Studies (Hill et al., 1996) with information collected using this structured approach allowing a diagnosis to be made by consensus using DSMIV criteria (Roberts et al., 1998). Demographic and treatment information as well as CNS collection and processing related data were also collected. Subsequently, tissue was provided for this study from the hippocampal formation, striatum (caudate and putamen), and BA 6 (the anterior part of the paracentral lobule and the adjacent superior gyrus, the dorsal bank of the callosomarginal sulcus minus its posterior third, and the bases of superior and middle frontal gyrus and pre-central gyrus not included in BA 4).
[3H]NMS Binding and BQCA-Mediated Binding
[3H]NMS is a pan-muscarinic receptor antagonist (Moriya et al., 1999) and therefore the specific binding of that radioligand gives an indication as to the total density of CHRMs. Hence, based on our methodological optimization experiments, the binding of [3H]NMS (0.4 nM: 3 sections), minus the binding of the radioligand in the presence of acetylcholine (10 mM: 3 sections), to frozen tissue sections from BA 6, the hippocampus, and striatum was taken as a measure of specific binding of the radioligand to all available CHRMs.
BQCA-mediated binding was taken as the binding of [3H]NMS (0.4 nM) in the presence of acetylcholine (1 mM: 3 sections) and absence of BQCA minus the binding of the radioligand in the same condition in the presence of BQCA (3 µM: 3 sections).
Statistical Analysis
All statistical analysis was performed using GraphPad Prism 8 (GraphPad). Two group analyses were performed using unpaired, 2-tailed t test. Three group analyses were conducted using ordinary 1-way ANOVA and where statistically significant variance was observed, post-hoc comparisons were made between either MRDS or non-MRDS and controls with results adjusted using Dunnett’s correction for multiple comparisons. Frequency analysis was performed using χ 2 test (3 groups) or Fischer’s exact test (2 groups). Relationships between the demographic, CNS patient-related data and radioligand binding measures were identified using linear regression. A correlation was considered absent if r2 < 0.25, “weak” if 0.25 < r2 > 0.5, “moderate” if 0.5 < r2 > 0.75, and “strong” if r2 > 0.75 (Udovičić et al., 2007). Due to the sample size of this study, only strong correlations would be considered as indicating potential covariance requiring further analysis (Cook and Weisberg, 1999), in which case such covariates would be included in a secondary analysis using ANCOVA.
RESULTS
Demographics
Tissue was obtained from 40 participants with schizophrenia consisting of 20 MRDS and 20 non-MRDS and 20 controls. There were no significant differences in age, PMI, CNS pH, or the proportionality of gender when comparing schizophrenia, MRDS, non-MRDS, and controls (Table 1). For MRDS and non-MRDS there was no significant differences in DOI, frequency of suicide completion, or treatment with antipsychotic or anticholinergic drugs. Neither levels of specific binding of [3H]NMS binding nor BQCA-mediated binding varied significantly in any region with gender or suicide completion (supplementary Table 1).
Table 1.
Demographic, Treatment, and CNS Collection Data Relating to the CNS Tissue Studied
| Sex | Age | PMI | Suicide | DOI | FRADD | ||
|---|---|---|---|---|---|---|---|
| Diagnoses | (M/F) | (y) | (hr) | CNS pH | Y / N | (y) | (Cpz) |
| Controls | 16/4 | 47 ± 3.6 | 43 ± 3.6 | 6.40 ± 0.04 | |||
| Schizophrenia | 31/9 | 48 ± 2.6 | 42 ± 2.2 | 6.30 ± 0.03 | 14/26 | 20 ± 2.4 | 426 ± 69 |
| P | >.9999 | .84 | .81 | .06 | |||
| MRDS | 15/5 | 46.2 ± 3.7 | 39.7 ± 2.8 | 6.28 ± 0.03 | 7/13 | 19 ± 3.4 | 513 ± 106 |
| Non-MRDS | 16/4 | 49.4 ± 3.8 | 44.9 ± 3.4 | 6.32 ± 0.05 | 7/13 | 20 ± 3.5 | 338 ± 86 |
| F2,57 = 0.21 | F2,57 = 0.66 | F2,57 = 2.07 | |||||
| P | .91 | .97 | .52 | .14 | >.9999 | .93 | .21 |
Abbreviations: Cpz, chlorpromazine equivalent dose; DOI, duration of illness; F, female; FRADD, final recorded antipsychotic drug dose; M, male; MRDS, muscarinic receptor deficit schizophrenia; non-MRDS, schizophrenia other than MRDS; PMI, postmortem interval; Sui, completed suicide.
Values are mean ± SEM or ratio.
Distribution of [3H]NMS binding and BQCA-mediated binding in BA 6, striatum, and hippocampus
[3H]NMS specific binding (supplementary Figure 1A) and BQCA-mediated binding (supplementary Figure 1B) were homogeneous across the cortical laminae in BA6; [3H]NMS binding was not significantly above nonspecific binding in white matter. No significant variation in the specific binding of [3H]NMS (supplementary Figure 1C) or BQCA-mediated binding (supplementary Figure 1D) could be detected across the caudate or putamen. Therefore, a single integrated measure of [3H]NMS binding and BQCA-mediated binding were taken across the cortical layers in BA 6 and the striatum.
Levels of specific [3H]NMS (supplementary Figure 1E) and BQCA-mediated binding (supplementary Figure 1F) varied across the hippocampus. In the dentate gyrus, there were 2 distinct layers with the layer with the higher binding density overlaying the molecular and granular layers and the layer with the lower binding density overlaying the polymorphic layer. [3H]NMS and BQCA-mediated binding was homogeneous throughout the cornu ammonis (CA) 3 field, but CA2 and CA1 showed higher intensity binding in a band overlaying the alveus layer through to the pyramidal layer and a lower intensity band overlaying the lacunosum moleculare and stratum radiatum. The subiculum also showed 2 layers of binding intensity with the layer of higher intensity overlaying the polymorphic and pyramidal layers and the lower intensity layer overlaying the molecular layer.
[3H]NMS binding in schizophrenia, MRDS, non-MRDS, and controls
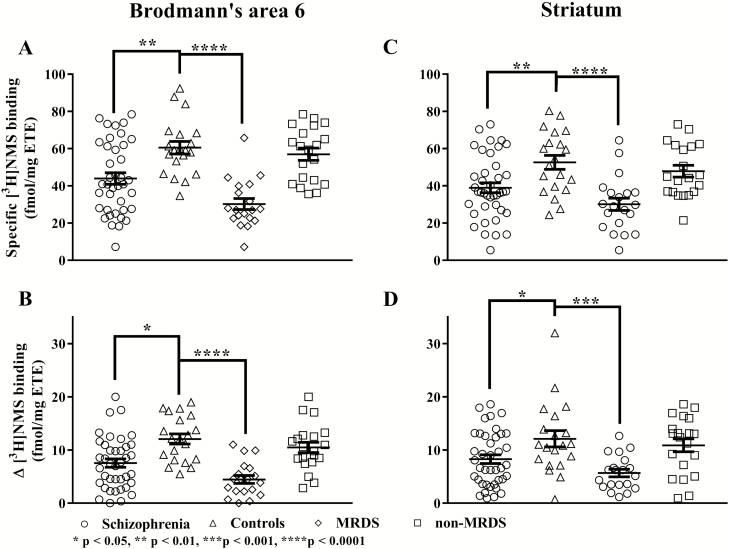
The specific binding of [3H]NMS was lower in BA 6 from participants with schizophrenia compared with controls (t57 = 3.35, P = .001; Figure 2A). After dividing participants with schizophrenia into MRDS and non-MRDS, there was significant variation in specific [3H]NMS binding with diagnoses (F2,56 = 26.1, P < .0001). Post-hoc analysis showed the variation in specific [3H]NMS binding was due to lower levels of binding in BA 6 from participants with MRDS (P = .0001), but not non-MRDS (P = .65), compared with controls.
Figure 2.
The specific binding of [3H]n-methyl scopolamine ([3H]NMS; A and B) and benzyl quinolone carboxylic acid (BQCA)-mediated [3H]NMS binding (C and D) in Brodmann’s area 6 (A and C) and the striatum (B and D) from participants with schizophrenia, sex-matched controls, muscarinic receptor deficit schizophrenia (MRDS) and participants with schizophrenia and no marked deficits in cortical muscarinic receptors (non-MRDS). Mean and SEM shown for each measure. ETE, estimated tissue equivalents.
In the striatum, there were lower levels of specific [3H]NMS binding in schizophrenia compared with controls (t58 = 2.97, P = .004; Figure 2C). On dividing participants with schizophrenia into MRDS and non-MRDS, there was significant variation in specific [3H]NMS binding (F2,57 = 12.2, P < .0001) with diagnosis. The variation in specific [3H]NMS binding was due to significantly lower levels of binding in striatum from participants with MRDS (P = .0001), but not non-MRDS (P = .51), compared with control participants.
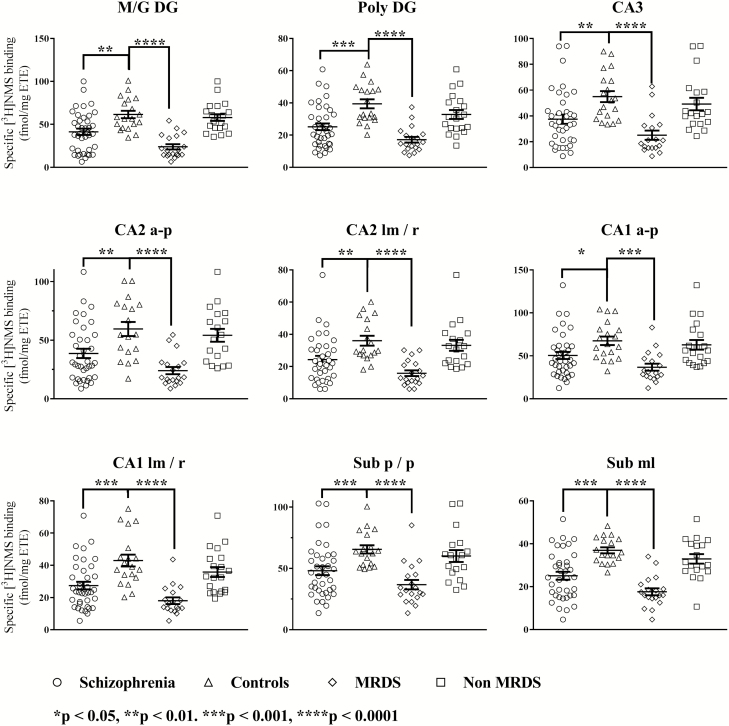
Compared with controls, specific [3H]NMS binding was lower in all subfields of the hippocampus from participants with schizophrenia (Figure 3). After dividing participants with schizophrenia into MRDS and non-MRDS, there was a significant variation in specific [3H]NMS binding with diagnoses in all hippocampal subfields. Post-hoc analysis showed this variation in specific [3H]NMS binding was due to lower levels of radioligand binding in all subfields of the hippocampus from participants with MRDS, but not non-MRDS (Figure 3).
Figure 3.
The specific binding of [3H]n-methyl scopolamine ([3H]NMS) to the hippocampus of participants with schizophrenia, controls, muscarinic receptor deficit schizophrenia (MRDS) and participants with schizophrenia and no marked deficits in cortical muscarinic receptors (non-MRDS). Mean and SEM shown for each measure. CA, cornu ammonis; a-p, alveus to pyramidal layers; lm/r, lacunosum moleculare and stratum radiatum; M/G DG, molecular and granular layers of the dentate gyrus; ML, molecular layer; Poly DG, polymorphic layer of the dentate gyrus; p/p, polymorphic and pyramidal layers.
BQCA-Modulated Binding in Schizophrenia, MRDS, non-MRDS, and Controls
BQCA-modulated binding was lower in BA 6 from participants with schizophrenia compared with controls (t57 = 3.50; P = .0009; Figure 2B). On dividing participants with schizophrenia into MRDS and non-MRDS, there was significant variation in BQCA-modulated binding with diagnosis (F2,56 = 19.4, P < .0001). Post-hoc analysis showed this variation was due to lower levels of specific [3H]NMS binding in BA 6 from participants with MRDS (P = .0001), but not non-MRDS (P = .36), compared with controls.
BQCA-modulated binding was lower in the striatum from participants with schizophrenia compared with controls (t58 = 2.44, P = .02; Figure 2D). After dividing participants with schizophrenia into MRDS and non-MRDS, BQCA-modulated binding varied significantly with diagnoses (F2,57 = 8.2, P = .0008). The variation in BQCA-modulated binding was due to significantly lower levels of binding in MRDS (P = .0007), but not non-MRDS (P = .69).
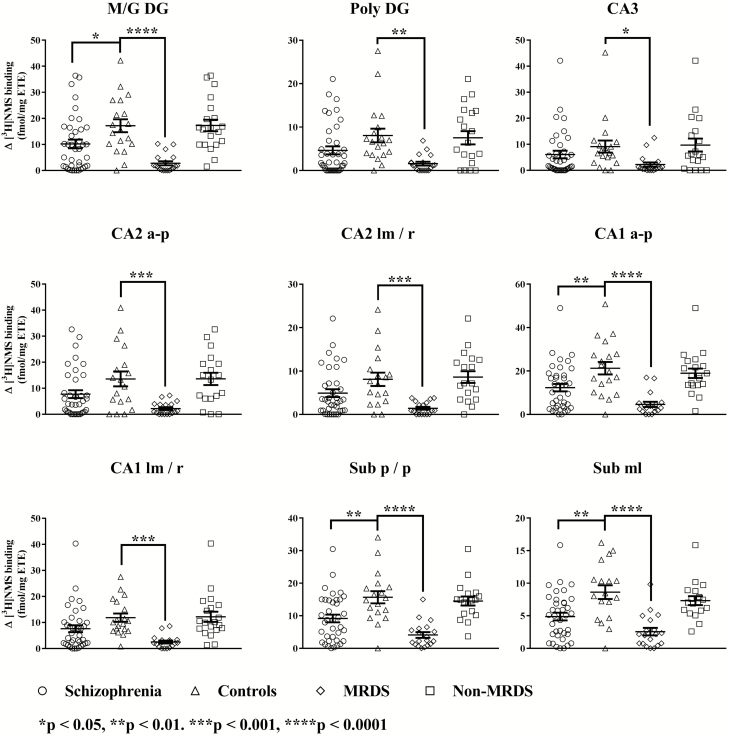
BQCA-modulated [3H]NMS binding was lower in the molecular and granular layer of the dentate gyrus, the alveus to pyramidal layers cornu ammonis 1 and the polymorphic and pyramidal layer, as well as the molecular layer of the subiculum from participants with schizophrenia (Figure 4). When comparing BQCA-modulated binding across MRDS, non-MRDS, and controls, there was significant variation in binding with diagnoses in all hippocampal subfields. Post-hoc analysis showed the variation in BQCA-modulated binding was due to lower levels of binding and modulation in all the subfields of the hippocampus from participants with MRDS, but not non-MRDS (Figure 4).
Figure 4.
Benzyl quinolone carboxylic acid (BQCA)-mediated binding to the hippocampus from participants with schizophrenia, controls, muscarinic receptor deficit schizophrenia (MRDS) and participants with schizophrenia and no marked deficits in cortical muscarinic receptors (non-MRDS). Mean and SEM shown for each measure. a-p, alveus to pyramidal layers; CA, cornu ammonis; lm/r, lacunosum moleculare and stratum radiatum; M/G DG, molecular and granular layers of the dentate gyrus; ML, molecular layer; Poly DG, polymorphic layer of the dentate gyrus; p/p, polymorphic and pyramidal layers.
Potential Confounds
The linear regression line describing the relationship between pH and PMI and the specific [3H]NMS binding in the molecular layer of the subiculum deviated significantly from the horizontal (supplementary Table 2A). However, the strength of these relationship were so low, neither pH nor PMI could be considered as a confounding factor when analyzing the specific [3H]NMS binding in the molecular layer of the subiculum in cohort sizes used in this study. There were no other significant relationships between radioligand binding measures and demographic, CNS collection, or schizophrenia-specific data (supplementary Table 2A–B).
Discussion
Here we report the outcomes from the development of a new methodology that allows the measurement of the ability of a positive allosteric modulator to affect the binding of acetylcholine to the CHRM1. Using this methodology, we have shown that a decreased response to the actions of BQCA, a CHRM1-positive allosteric modulator (Ma et al., 2009), is not only detectable in the cortex from participants with MRDS (Dean et al., 2016b) but is present in the striatum and a number of subfields in the hippocampus from participants with MRDS. This finding may be of clinical significance if drugs that modulate the allosteric site on the CHRM1 (Conn et al., 2009) enter clinical trials, as participants with MRDS may not be fully responsive to such treatments. However, such a conclusion must be tempered by the suggestion that there is a large reserve of CHRM1s (Porter et al., 2002; Scarr et al., 2016) and that occupancy of 15% of total available receptors by an orthosteric agonist can facilitate a full response (Porter et al., 2002). These data raise the possibility that even with decreased responsiveness in vitro, participants with MRDS may achieve significant clinical benefit from treatment with drugs targeting the allosteric site on the CHRM1.
This study also shows that the distribution of specific [3H]NMS binding in the striatum and hippocampus mirrors the distribution of [3H]pirenzepine binding (Scarr et al., 2007; Dean et al., 2016a) and that the lower levels of [3H]NMS binding in BA 6, striatum, and hippocampus from participants with schizophrenia are due to a marked loss of binding to tissue from participants with MRDS. Notably, our previous study using homogenate membrane preparations did not show lower levels of [3H]NMS binding in MRDS (Dean et al., 2016b). The difference in findings in our 2 studies may relate to the use of 2 different methodologies as our recent immunohistochemistry study reported that a significant number of CHRM1 appeared not to be localized to the cell membrane (Scarr et al., 2018b). Thus, our current data from the use of frozen sections measured [3H]NMS binding to membrane bound and non-membrane-bound CHRM1, whereas our previous study would only have measured membrane-bound receptors. If our new data is due to a loss of both membrane and non-membrane-bound CHRM1s, this is significant as it has been suggested that the 2 pools of receptors use different signaling systems (Anisuzzaman et al., 2013). Using membranes, we reported that the KD for [3H]NMS binding was higher in schizophrenia (Dean et al., 2016b), which raises the possibility that the 0.4 nM [3H]NMS used in this study was not saturating all available receptors. This could result in a low estimate of CHRM1 density (Dean et al., 1997). However, while the higher KD in our previous study was statistically significant in participants with schizophrenia, it was not of a magnitude that was likely to affect a single-point saturation analysis methodology. Notably, we previously observed a similar discrepancy between studies estimating the density of the serotonin 2A receptor (HTR2A) using autoradiography and particulate membrane (Dean and Hayes, 1996; Dean et al., 1996b). In studying this phenomena, we showed that that a low-molecular weight component of the cytosol, which would be washed away during membrane-enrichment, was reducing the availability of HTR2A binding sites in tissue homogenates and frozen sections (Dean et al., 2008). Therefore, a similar phenomenon may be causing the discrepancy between measures of specific [3H]NMS binding between our 2 studies. It has been proposed that there are endogenous ligands for the allosteric binding sites on CHRM1s (Jakubík and El-Fakahany, 2010), and it is therefore possible such a ligand could be the cause of differences in measuring [3H]NMS binding to membranes and tissue sections and/or tissue homogenates because they would be lost when preparing membranes.
Previously, based on gene expression studies, we argue the lower [3H]pirenzepine binding in the hippocampus from participants with schizophrenia was due to lower levels of muscarinic M4, not M1, receptors (Scarr et al., 2007). This hypothesis appeared to be supported by the finding that CHRM1+ neurons were not decreased in the hippocampus from participants with schizophrenia (Scarr et al., 2018b). However, as the effects of BQCA are specific to the CHRM1 (Ma et al., 2009), our new data would suggest there are lower levels of CHRM1s in a number of regions of the hippocampus from participants with MRDS. This is an important finding as hippocampal CHRM1s are known to affect N-methyl-d-aspartate receptor currents (Marino et al., 1998), are present in hippocampal memory circuits (Levey, 1996), and are important in hippocampal-dependent cognitive flexibility (Xiong et al., 2019). Therefore, a loss of CHRM1s could have a profound effect on hippocampal function in participants with MRDS.
As with the hippocampus, we found lower BQCA-modulated binding in the striatum from participants with MRDS, but not non-MRDS, mirroring our [3H]pirenzepine finding in the same participants (Dean et al., 2015). These data support our original proposition that there are fewer CHRM1 in the striatum from participants with CHRM1 (Dean et al., 1996a) but now suggest this decrease was due to participants with MRDS, some of whom were included in our earlier study. In the striatum, the CHRM1 modulates dopamine release (Zhang et al., 2002), enhances N-methyl-d-aspartate responsiveness (Calabresi et al., 1998), and facilitates motivation (Hailwood et al., 2019) and so the marked loss of striatal CHRM1s would affect striatal function in participants with MRDS.
There are limitations to our study. As with all studies using tissue from participants with schizophrenia who have been treated, drug treatment before death could be a confounding factor. However, case history reviews suggest that participants with MRDS and non-MRDS have had similar antipsychotic drug treatments. Thus, as lower levels of specific [3H]NMS binding and BQCA modulated binding only occurs in MRDS, this would argue such changes are not simply due to the MRDS group being treated with antipsychotic drugs. This argument is strengthened by the absence of any relationship between the final recorded antipsychotic medications and either specific [3H]NMS binding or BQCA-modulated binding and the observation that the frequency of treatment with relatively standardized doses of anticholinergic drugs does not differ between MRDS and non-MRDS. Finally, we have shown that treating rats with a number of antipsychotic drugs does not alter [3H]pirenzepine binding, suggesting such treatments to not affect levels of CHRM1.
In conclusion, this study strengthens the argument that there is a subgroup of participants with schizophrenia, the MRDS, that have a generalized loss of CHRM1 in many CNS regions (Gibbons et al., 2013). While we have shown a reduced response to BQCA in participants with MRDS, the fact the CHRM1 remains responsive to allosteric modulation and has a large receptor reserve would favor participants with MRDS showing a full or partial response to drugs targeting the CHRM1 allosteric site. This is important because it is argued such drugs will be a new treatment for schizophrenia (Conn et al., 2009). However, the use of either SPECT (Raedler et al., 2003; Bakker et al., 2018) or PET would be advisable to determine if participants with a marked loss of CHRM1s and schizophrenia had been recruited into drug trials of CHRM1 allosteric modulators to treat the disorder.
Supplementary Material
Acknowledgments
We thank the families of the tissue donors for making an invaluable contribution to this research.
This work was supported by the Australian Government: Department of Education, the Cooperative Research Centre for Mental Health, the National Health and Medical Research Council, the Rebecca L. Cooper Medical Research Foundation, One-in-Five and the Victorian Government’s Operational Infrastructure Support Programme.
Interest Statement
S.H., G.P., M.U., A.G., and B.D. have no conflicts of interest. J.C. is an inventor of patents that protect different classes of muscarinic receptor allosteric modulators but not the allosteric modulator used in our studies.
References
- Alt A, et al. (2016) Evidence for classical cholinergic toxicity associated with selective activation of M1 muscarinic receptors. J Pharmacol Exp Ther 356:293–304. [DOI] [PubMed] [Google Scholar]
- Anisuzzaman AS, Uwada J, Masuoka T, Yoshiki H, Nishio M, Ikegaya Y, Takahashi N, Matsuki N, Fujibayashi Y, Yonekura Y, Momiyama T, Muramatsu I (2013) Novel contribution of cell surface and intracellular M1-muscarinic acetylcholine receptors to synaptic plasticity in hippocampus. J Neurochem 126:360–371. [DOI] [PubMed] [Google Scholar]
- Bakker G, Vingerhoets C, Boucherie D, Caan M, Bloemen O, Eersels J, Booij J, van Amelsvoort T (2018) Relationship between muscarinic M1 receptor binding and cognition in medication-free subjects with psychosis. Neuroimage Clin 18:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G (1998) Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci 10:2887–2895. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Staskin DR, Kay GG, Sandage BW, Oefelein MG, Tsao JW (2012) Blood-brain barrier permeation and efflux exclusion of anticholinergics used in the treatment of overactive bladder. Drugs Aging 29:259–273. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW (2009) Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci 30:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RD, Weisberg S (1999) Applied regression including computing and graphics. Hoboken, NJ: Wiley. [Google Scholar]
- Dean B, Bymaster FP, Scarr E (2003) Muscarinic receptors in schizophrenia. Curr Mol Med 3:419–426. [DOI] [PubMed] [Google Scholar]
- Dean B, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL (1996a) The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol Psychiatry 1:54–58. [PubMed] [Google Scholar]
- Dean B, Crossland N, Boer S, Scarr E (2008) Evidence for altered post-receptor modulation of the serotonin 2a receptor in schizophrenia. Schizophr Res 104:185–197. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W (1996) Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophr Res 21:133–139. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W, Opeskin K, Naylor L, Pavey G, Hill C, Keks N, Copolov DL (1996b) Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Behav Brain Res 73:169–175. [DOI] [PubMed] [Google Scholar]
- Dean B, Hopper S, Conn PJ, Scarr E (2016b) Changes in BQCA allosteric modulation of [(3)H]NMS binding to human cortex within schizophrenia and by divalent cations. Neuropsychopharmacology 41:1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Pavey G, Opeskin K (1997) [3H]raclopride binding to brain tissue from subjects with schizophrenia: methodological aspects. Neuropharmacology 36:779–786. [DOI] [PubMed] [Google Scholar]
- Dean B, Thomas N, Lai CY, Chen WJ, Scarr E (2015) Changes in cholinergic and glutamatergic markers in the striatum from a sub-set of subjects with schizophrenia. Schizophr Res 169:83–88. [DOI] [PubMed] [Google Scholar]
- Dean B, Thomas N, Scarr E, Udawela M (2016a) Evidence for impaired glucose metabolism in the striatum, obtained postmortem, from some subjects with schizophrenia. Transl Psychiatry 6:e949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Porter AC, Skillman TL, Zhang L, Bymaster FP, Nathanson NM, Hamilton SE, Gomeza J, Wess J, McKinzie DL (2001) Elucidating the role of muscarinic receptors in psychosis. Life Sci 68:2605–2613. [DOI] [PubMed] [Google Scholar]
- Frampton JE. (2019) Brexpiprazole: a review in schizophrenia. Drugs 79:189–200. [DOI] [PubMed] [Google Scholar]
- Gibbons A, Dean B (2016) The cholinergic system: an emerging drug target for schizophrenia. Curr Pharm Des 22:2124–2133. [DOI] [PubMed] [Google Scholar]
- Gibbons AS, Scarr E, Boer S, Money T, Jeon WJ, Felder C, Dean B (2013) Widespread decreases in cortical muscarinic receptors in a subset of people with schizophrenia. Int J Neuropsychopharmacol 16:37–46. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Sexton PM, Christopoulos A (2007) Allosteric modulation of muscarinic acetylcholine receptors. Curr Neuropharmacol 5:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailwood JM, Heath CJ, Phillips BU, Robbins TW, Saksida LM, Bussey TJ (2019) Blockade of muscarinic acetylcholine receptors facilitates motivated behaviour and rescues a model of antipsychotic-induced amotivation. Neuropsychopharmacology 44:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS. (2006) Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol 147(Suppl 2):S80–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Keks N, Roberts S, Opeskin K, Dean B, MacKinnon A, Copolov D (1996) Problem of diagnosis in postmortem brain studies of schizophrenia. Am J Psychiatry 153:533–537. [DOI] [PubMed] [Google Scholar]
- Hopper S, Udawela M, Scarr E, Dean B (2016) Allosteric modulation of cholinergic system: potential approach to treating cognitive deficits of schizophrenia. World J Psychiatr 5:32–43. [Google Scholar]
- Jablensky A. (2006) Subtyping schizophrenia: implications for genetic research. Mol Psychiatry 11:815–836. [DOI] [PubMed] [Google Scholar]
- Jakubík J, El-Fakahany EE (2010) Allosteric modulation of muscarinic acetylcholine receptors. Pharmaceuticals (Basel) 3:2838–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska A, Satala G, Partyka A, Wesolowska A, Bojarski AJ, Pawlowski M, Chlon-Rzepa G (2019) Discovery and development of non-dopaminergic agents for the treatment of schizophrenia: overview of the preclinical and early clinical studies. Curr Med Chem doi: 10.2174/0929867326666190710172002. [DOI] [PubMed] [Google Scholar]
- Kane JM, McGlashan TH (1995) Treatment of schizophrenia. Lancet 346:820–825. [DOI] [PubMed] [Google Scholar]
- Kerwin R, Patel S, Meldrum B (1990) Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience 39:25–32. [DOI] [PubMed] [Google Scholar]
- Levey AI. (1996) Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc Natl Acad Sci U S A 93:13541–13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, et al. (2009) Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A 106:15950–15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder S, Fleischhacker WW, Earley W, Lu K, Zhong Y, Németh G, Laszlovszky I, Szalai E, Durgam S (2019) Efficacy of cariprazine across symptom domains in patients with acute exacerbation of schizophrenia: pooled analyses from 3 phase II/III studies. Eur Neuropsychopharmacol 29:127–136. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ (1998) Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A 95:11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Kavoussi R, Breier A (2016) Xanomeline plus tropsium: a novel strategy to enhance pro-muscarinic efficacy and mitigate peripheral side effects. Neuropsychopharmacol 41:S230. [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10:79–104. [DOI] [PubMed] [Google Scholar]
- Moriya H, Takagi Y, Nakanishi T, Hayashi M, Tani T, Hirotsu I (1999) Affinity profiles of various muscarinic antagonists for cloned human muscarinic acetylcholine receptor (mAChR) subtypes and mAChRs in rat heart and submandibular gland. Life Sci 64:2351–2358. [DOI] [PubMed] [Google Scholar]
- Porter AC, Bymaster FP, DeLapp NW, Yamada M, Wess J, Hamilton SE, Nathanson NM, Felder CC (2002) M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res 944:82–89. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, Egan MF, Coppola R, Weinberger DR (2003) In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry 160:118–127. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B (2007) Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry 12:232–246. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Hill CA, Dean B, Keks NA, Opeskin K, Copolov DL (1998) Confirmation of the diagnosis of schizophrenia after death using DSM-IV: a Victorian experience. Aust N Z J Psychiatry 32:73–76. [DOI] [PubMed] [Google Scholar]
- Rodbard D. (1981) Mathematics and statistics of ligand assays: an illustrated guide. In: Ligand assay: analysis of international developments on isotopic and nonisotopic immunoassay (Langan J, Clapp JJ, eds), pp 45–99. New York: Masson Publishing USA, Inc. [Google Scholar]
- Salah-Uddin H, Scarr E, Pavey G, Harris K, Hagan JJ, Dean B, Challiss RA, Watson JM (2009) Altered M(1) muscarinic acetylcholine receptor (CHRM1)-Galpha(q/11) coupling in a schizophrenia endophenotype. Neuropsychopharmacology 34:2156–2166. [DOI] [PubMed] [Google Scholar]
- Scarr E, Sundram S, Keriakous D, Dean B (2007) Altered hippocampal muscarinic M4, but not M1, receptor expression from subjects with schizophrenia. Biol Psychiatry 61:1161–1170. [DOI] [PubMed] [Google Scholar]
- Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B (2009) Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry 14:1017–1023. [DOI] [PubMed] [Google Scholar]
- Scarr E, Seo MS, Aumann TD, Chana G, Everall IP, Dean B (2016) The distribution of muscarinic M1 receptors in the human hippocampus. J Chem Neuroanat 77:187–192. [DOI] [PubMed] [Google Scholar]
- Scarr E, Udawela M, Thomas EA, Dean B (2018a) Changed gene expression in subjects with schizophrenia and low cortical muscarinic M1 receptors predicts disrupted upstream pathways interacting with that receptor. Mol Psychiatry 23:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr E, Hopper S, Vos V, Seo MS, Everall IP, Aumann TD, Chana G, Dean B (2018b) Low levels of muscarinic M1 receptor-positive neurons in cortical layers III and V in Brodmann areas 9 and 17 from individuals with schizophrenia. J Psychiatry Neurosci 43:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MS, Scarr E, Dean B (2014) An investigation of the factors that regulate muscarinic receptor expression in schizophrenia. Schizophr Res 158:247–254. [DOI] [PubMed] [Google Scholar]
- Sharif ZA. (1998) Treatment refractory schizophrenia: how should we proceed? Psychiatr Q 69:263–281. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC (2008) Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 165:1033–1039. [DOI] [PubMed] [Google Scholar]
- Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, Christian EP, Doherty JJ, Quirk MC, Snyder DH, Lah JJ, Levey AI, Nicolle MM, Lindsley CW, Conn PJ (2009) A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci 29:14271–14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udovičić M, Baždarić K, Bilić-Zulle L, Petrovečki M (2007) What we need to know when calculating the coefficient of correlation? Psychiatry Res 17:10–15. [Google Scholar]
- Xiong CH, Liu MG, Zhao LX, Chen MW, Tang L, Yan YH, Chen HZ, Qiu Y (2019) M1 muscarinic receptors facilitate hippocampus-dependent cognitive flexibility via modulating GluA2 subunit of AMPA receptors. Neuropharmacology 146:242–251. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J (2002) Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci 22:6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.