Abstract
Objective
The T-box gene Tbx15 is abundantly expressed in adipose tissues, especially subcutaneous and brown fat. Although its expression is correlated with obesity, its precise biological role in adipose tissue is poorly understood in vivo. Here we investigated the function of Tbx15 in brown adipose thermogenesis and white adipose browning in vivo.
Methods
In the present study, we generated adipose-specific Tbx15 knockout (AKO) mice by crossing Tbx15 floxed mice with adiponectin-Cre mice to delineate Tbx15 function in adipose tissues. We systematically investigated the influence of Tbx15 on brown adipose thermogenesis and white adipose browning in mice, as well as the possible underlying molecular mechanism.
Results
Upon cold exposure, adipocyte browning in inguinal adipose tissue was significantly impaired in Tbx15 AKO mice. Furthermore, ablation of Tbx15 blocked adipocyte browning induced by β3 adrenergic agonist CL 316243, which did not appear to alter the expression of Tbx15. Analysis of DNA binding sites using chromatin-immunoprecipitation (ChIP) revealed that TBX15 bound directly to a key region in the Prdm16 promoter, indicating it regulates transcription of Prdm16, the master gene for adipocyte thermogenesis and browning. Compared to control mice, Tbx15 AKO mice displayed increased body weight gain and decreased whole body energy expenditure in response to high fat diets.
Conclusion
Taken together, these findings suggest that Tbx15 regulates adipocyte browning and might be a potential target for the treatment of obesity.
Keywords: Tbx15, Adipocyte, Thermogenesis, Browning, Obesity
Highlights
-
•
Lack of Tbx15 significantly impairs adipocyte browning induced by cold or adrenergic agonist CL 316243.
-
•
Tbx15 was involved in adipocyte browning by regulating expression of Prdm16.
-
•
Tbx15 AKO mice are sensitive to body weight gain on high fat diets.
1. Introduction
Obesity is increasingly prevalent globally and associated with many metabolic disorders including type-2 diabetes and hypertension [1]. The cause of obesity is energy imbalance in which energy intake exceeds energy expenditure. Because brown and beige adipocytes can consume chemical energy and dissipate it in the form of heat, it may constitute a promising strategy to combat obesity by targeting their formation and activity [2], [3], [4], [5]. The mitochondrial uncoupling protein 1 (UCP1), specifically expressed in brown and beige adipocyte, is critical for adipocyte thermogenesis by uncoupling electron transport from ATP production, although creatine driven substrate cycling has also been shown to contribute in a UCP1 independent fashion [6], [7]. Brown and beige adipocytes are derived from distinct progenitors. Brown adipocytes share the same precursor cells as myocytes while beige adipocytes are induced within white adipose tissue by cold exposure and derived from either preadipocytes or mature white adipocytes through trans-differentiation [8], [9], [10].
Prdm16 (PRD1-BF1-RIZ1 homologous domain containing 16) is a master regulatory gene responsible for the brown and beige adipocyte phenotypes. Ectopic expression of Prdm16 in myoblasts induces their differentiation into brown adipocytes while loss of Prdm16 expression in brown preadipocytes promotes muscle differentiation [11], [12]. Similarly, ectopic expression of Prdm16 in inguinal adipose tissue promotes beige adipocyte formation while ablation of Prdm16 expression in inguinal adipose tissue facilitates formation of visceral adipose tissue in mice [13], [14].
Tbx15 (T-box 15) belongs to a phylogenetically conserved family, which has a similar characteristic sequence in the DNA binding domain and is involved in various developmental processes [15]. Either complete congenital inactivation of the Tbx15 gene in mice or loss of function TBX15 mutations in humans resulted in severe skeletal malformation [16], [17]. By comparison, heterozygous deletion of Tbx15 expression in mice resulted in glucose intolerance and obesity on high fat diets [18], [19].
A single nucleotide polymorphism near the TBX15 gene is correlated with the body fat distribution in patients of both European and African ancestries by genome-wide association studies [20], [21]. Evidence suggests that this link between aberrant TBX15 function and obesity is mediated through biological effects in adipose tissue. In mice, Tbx15 is expressed highly in brown adipose tissue (BAT) and inguinal white adipose tissue (IngWAT, a beige-competent depot), and minimally expressed in visceral white adipose tissues such as epididymal white adipose tissue (EpiWAT) [22], [23], [24]. In contrast, TBX15 expression is elevated in visceral versus subcutaneous adipose tissue in non-obese humans but strongly down-regulated in visceral adipose depots of overweight and obese individuals [25].
TBX15 also plays an important role during the adipogenesis of primary brown and beige adipocyte precursors but not primary white adipocytes. SiRNA knockdown of Tbx15 in mouse brown and inguinal adipose stromal cells impaired their differentiation into mature adipocytes but has no effect on adipogenesis of epididymal stromal cells [26]. By comparison, over-expression of Tbx15 in 3T3-L1 preadipocytes blunted their capacity to differentiate into adipocytes [27]. Other evidence shows that both preadipocytes and adipocytes within a single white adipose tissue depot in mice could be divided into populations characterized by low and high Tbx15 expression. These cellular populations were also metabolically distinct exhibiting increased oxidative and glycolytic behavior respectively [18], [19].
Taken together, these studies indicate that Tbx15 plays a critical biological role in preadipocytes and adipocytes in vitro. However, it is still unclear whether adipose tissue is the physiologically relevant target as animal studies and SNPs in humans affect function globally in all tissues where Tbx15 is expressed. The impetus for the present study was, therefore, to generate adipose-specific Tbx15 knockout mice to explore specifically the biological impact of Tbx15 on adipose tissue biology.
2. Materials and methods
2.1. Animal
Mice of both Tbx15 flanked by loxP sites (floxed) and adiponectin-Cre (C57BL/6J background, purchased from the Jackson Laboratory (Stock No: 010803), were maintained on a 12-h light/12-h dark cycle at 23 °C in a specific pathogen free environment. Mice were allowed access to different diets and water ad libitum and housed in groups of four in separate cages. 8-week-old control and AKO mice were fed a high fat diet (D12492, Research Diets) for 12 weeks for the obesity studies. This diet provided 21.9 kJ/g: 60% of energy from fat, 20% from protein, and 20% from carbohydrate. Food intake and body weights were measured weekly. After feeding, mice were sacrificed by cervical dislocation. Micro-computed tomography measurements of the control and AKO mice were performed on a Aloka LCT-200 (ALOKA) and analyzed by LaTheta V 3.61B (LaTheta LCT-200, Hitachi-Aloka Medical, Ltd., Tokyo, Japan). All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Guangzhou Institutes of Biomedicine and Health (GIBH), Chinese Academy of Sciences.
2.2. Western blot analysis
Cells were lysed in a buffer containing 1% Nonidet-P 40, 150 mM NaCl, 10 mM Tris-Cl (pH7.5), and 1 mM EDTA. Lysates were resolved by 10% SDS-PAGE and transferred onto a PVDF membrane, which was then blotted with antibodies to TBX15 (Novas, NBP-49036), UCP1 (Abcam), GAPDH (Cell Signaling), HA (Cell Signaling) and FLAG epitopes (Cell Signaling).
2.3. RNA extraction and quantitative PCR
Total RNA was isolated with Trizol (Invitrogen), and first-strand cDNA synthesized with Superscript III Reverse Transcriptase (Invitrogen) with 0.5 μg of RNA as the template for each reaction. mRNA levels were quantified under optimized conditions with SYBR Premix Ex Taq (Takara Bio) following the manufacturer's instructions. The reference gene was 18S ribosomal RNA.
2.4. Histology and immunohistochemistry
Brown adipose tissue and inguinal and epididymal adipose tissues were fixed in 4% formaldehyde overnight at room temperature, embedded in paraffin, and cut into 5-μm section with a microtome. Slides were deparaffinized, rehydrated, and stained with hematoxylin and eosin (Sigma) using a standard protocol. Alternatively, sections were stained with anti-UCP1 (ab23841, Abcam; 1:200) and developed with SIGMAFAST DAB with Metal Enhancer (Sigma). Sections were examined by light microscopy (Motic BA600) and photographed with Moticam Pro 285A. Photomicrographs were scanned with an Abaton Scan 300/Color scanner.
2.5. Indirect calorimetry and calculated energy expenditure
Whole-body oxygen consumption was measured with an open-circuit indirect calorimetry system with automatic temperature and light controls (Comprehensive Lab Animal Monitoring System, Columbus Instruments). Mice had ad libitum access to chow and water in respiration chambers, and data were recorded for 48 h, including 24 h of acclimatization. Energy expenditure was calculated using both CLAX 2.2 as recommended by the manufacturer and CalR (https://calrapp.org/) [28].
2.6. Isolation and immortalization of adipose stromal cells from adipose tissues
Adipose tissues were dissected from wild type mice as described previously [14], rinsed in phosphate-buffered saline (PBS), minced, and digested for 40 min at 37 °C in 0.1% (w/v) type I collagenase solution (Sigma) with D-Hanks buffer. Digested tissue was filtered through a 250-μm nylon mesh and centrifuged at 800 ×g for 3 min. The sediment was resuspended in Dulbecco's modified Eagle's medium (DMEM, Gibco) with 10% fetal bovine serum (HyClone) and used for cell immortalization. Retrovirus medium was prepared by transfecting PMX-SV40 into PlatE cells, filtered through a 0.45 μm filter, and then added to the isolated adipose stromal cells. After passaging for 5 times, the immortalized cell line was further transfected with the PMX-HA-Tbx15 plasmid and used subsequently for ChIP analysis.
2.7. Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed using a Pierce ChIP Kit (Agarose 26156), following the manufacturer's protocol. Briefly, mature adipocytes derived from inguinal stromal cells were exposed to 1% formaldehyde at room temperature for 10 min to induce DNA cross-linking and then lysed and digested with MNase for 10 min on ice. The cell lysate supernatant was incubated with IgG, HA (Cell signal), or TBX15 antibody (Novas, NBP-49036) at 4 °C overnight and then with ChIP Grade Protein A/G Plus Agarose for 1h. Bound DNA was isolated and recovered as instructed by the manufacturer. The primer pairs used to detect the Prdm16 promoter were GAGGTGCAGAGGTGCAGGACGC and CTAACCCGGCTCCTCCGAAGC (proximal region, −1.0_0 kb), GTGCTACACCTCTAGGTGACC and GTTGTGGGAAGGCCTGGCTC (enhancer region, −2.0_-1.0 kb), GCATCTCTGGCCTGGAGCATAACAG and GCGGTCAGACATGAAGAATTCCTC (enhancer region, −3.0_-2.0 kb), CTGCTGGAATCCCTTAGGCGACTGTAAG and CTGAACAGAATGAAAGTGTTCAG (enhancer region, −4.0_-3.0 kb), GCAGAGGACCAGTGTTCTCTGAATAAG and CATATCCCTTTGCACTCCCGGCT (enhancer region, −5.0_-4.0 kb).
2.8. Immunoprecipitation
HEK 293 cells transfected with PMX-HA-Tbx15 and PMX-Flag-Prdm16 plasmids were washed with phosphate buffered saline (PBS) and then lysed in cell lysis buffer (25 mM HEPES, 5 mM EDTA, 1% Triton X-100, 50 mM NaF, 150 mM NaCl, 10 mM phenylmethylsulfonyl fluoride (PMSF), 1 M leupeptin, 1 M pepstatin, and 1 M aprotinin A (pH7.2). Cellular protein (100 μg) was mixed with 1 μg of IgG (cell signal), anti-HA (cell signal) or FLAG (cell signal) antibody and incubated overnight at 4 °C. Then, 10 μl of protein G Plus-agarose (Santa Cruz) was added and samples incubated for another 4 h at 4 °C. Samples were then washed three times with lysis buffer and resuspended in SDS sample buffer (125 mM Tris–HCl (pH 6.8), 20% (v/v) glycerol, 4% (w/v) SDS, 100 mM dithiothreitol, and 0.1% (w/v) bromophenol blue) and heated at 100 °C for 5 min prior to electrophoresis.
2.9. Glucose and insulin tolerance tests
For glucose tolerance tests, mice were fasted overnight and injected intraperitoneally (i.p.) with 20% glucose at a dose of 2 g/kg body weight. For insulin tolerance tests, mice were starved for 6 h and injected via the i.p. route with recombinant human insulin (Eli Lilly, 0.5 U/kg body weight). Blood glucose was monitored from tail vein blood using a glucometer (ACCU-CHEK Advantage; Roche Diagnostics China, Shanghai, China) at various time points.
2.10. RNA-seq analysis
RNA-seq analysis of inguinal adipose tissues from control and AKO mice was performed by Shanghai Majorbio Bio-pharm Technology Co. Ltd using the free online platform of Majorbio I-Sanger Cloud Platform (www.i-sanger.com). Briefly, total RNA was extracted from tissue using TRIzol® Reagent according the manufacturer's instructions (Invitrogen) and genomic DNA removed using DNase I (TaKara). A RNA-seq transcriptome library from 5 μg of total RNA was prepared using a TruSeqTM RNA sample preparation Kit (Illumina, San Diego, CA). Following quantification by TBS380, a paired-end RNA-seq sequencing library was sequenced with an Illumina Hiseq Xten (2 × 150 bp read length). To identify DEGs (differential expression genes) between two different samples, the expression level of each transcript was calculated according to the fragments per kilobase of exon per million mapped reads (FRKM) method. RSEM (http://deweylab.biostat.wisc.edu/rsem) was used to quantify gene abundances. R statistical package software EdgeR (Empirical analysis of Digital Gene Expression in R, http://www.bioconductor.org/packages/2.12/bioc/html/edgeR.html) was utilized for differential expression analysis.
2.11. Statistical analysis
Data are expressed as means ± SEM. ANOVA and unpaired, two-tailed t tests were used for most comparisons in GraphPad Prism 5 (GraphPad, San Diego, CA, USA). Post hoc tests were run only when F achieved P < 0.05 and there was no significant variance in homogeneity. P < 0.05 was considered significant.
3. Results
3.1. Generation of adipose specific Tbx15 knockout mice
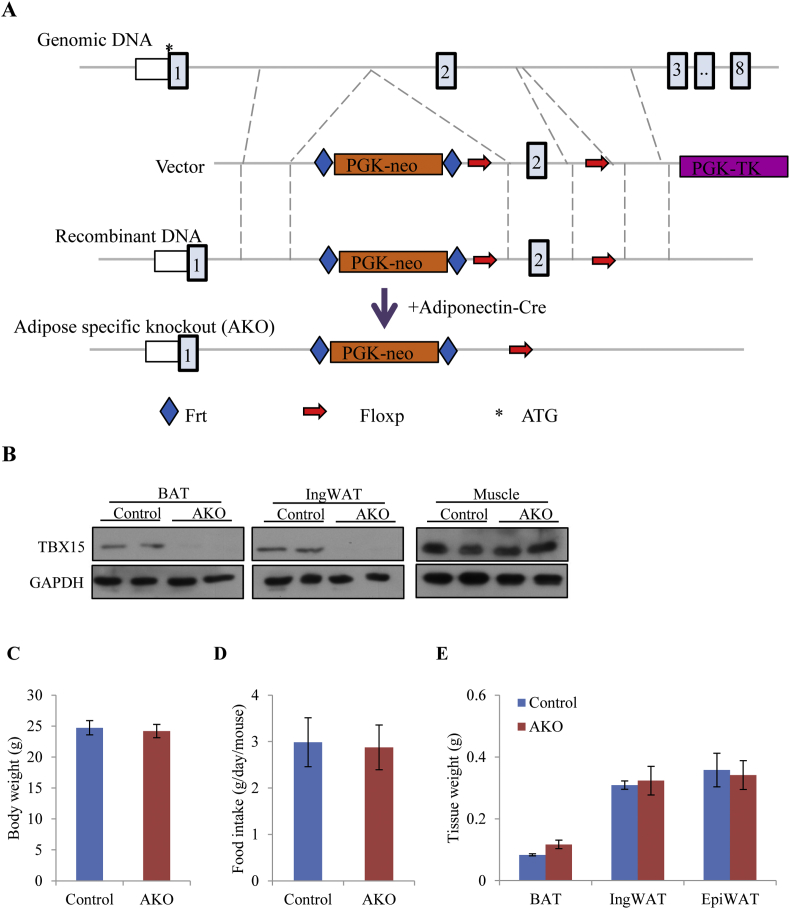
The transcriptional factor Tbx15 is highly expressed in brown and inguinal adipose tissues, suggesting it plays a biological function in adipose tissue [25]. To investigate its putative function, we generated adipose-specific Tbx15 knockout mice. As shown in Figure 1A, a method based on homologous recombination was used to construct a Tbx15 floxed mouse. A plasmid containing a PGK-Neo selection gene cassette flanked by two loxP sites in exon 2 was constructed and transfected into wild type embryonic stem cell (ES) cells. The correctly targeted embryonic stem cell (ES) clones were identified and used in the production of floxed mice.
Figure 1.
Generation of adipose specific Tbx15 knockout mice. A. Schematic showing the cloning strategy for the adipose specific Tbx15 knockout mouse model. B. Determination of TBX15 protein expression in AKO and control mice by western blot analysis. Body weight (C), food intake (D), and tissue weights (E) of 8-week-old AKO and control mice on standard chow diets.
Homozygous floxed mice developed normally with no phenotypic differences with wild type mice suggesting that the recombinant floxed cassette did not affect endogenous expression of Tbx15 since global knockout of Tbx15 leads to abnormal bone development and droopy ear syndrome [17], [29]. To generate adipose-specific Tbx15 knockout mice, adiponectin Cre mice, where Cre is expressed under control of the adiponectin promoter, were mated with Tbx15 floxed mice. Tbx15 floxed mice without Cre were used as the control group. Ablation of TBX15 protein expression in the adipose tissue of AKO mice was confirmed by western blot analysis (Figure 1B). There was no measurable detection of TBX15 in brown and white adipose tissues but retention of full expression in skeletal muscle of AKO mice. Taken together, these results show successful generation of Tbx15 adipose-specific knockout mice.
3.2. Adipose-specific ablation of Tbx15 attenuates cold-induced thermogenesis and browning
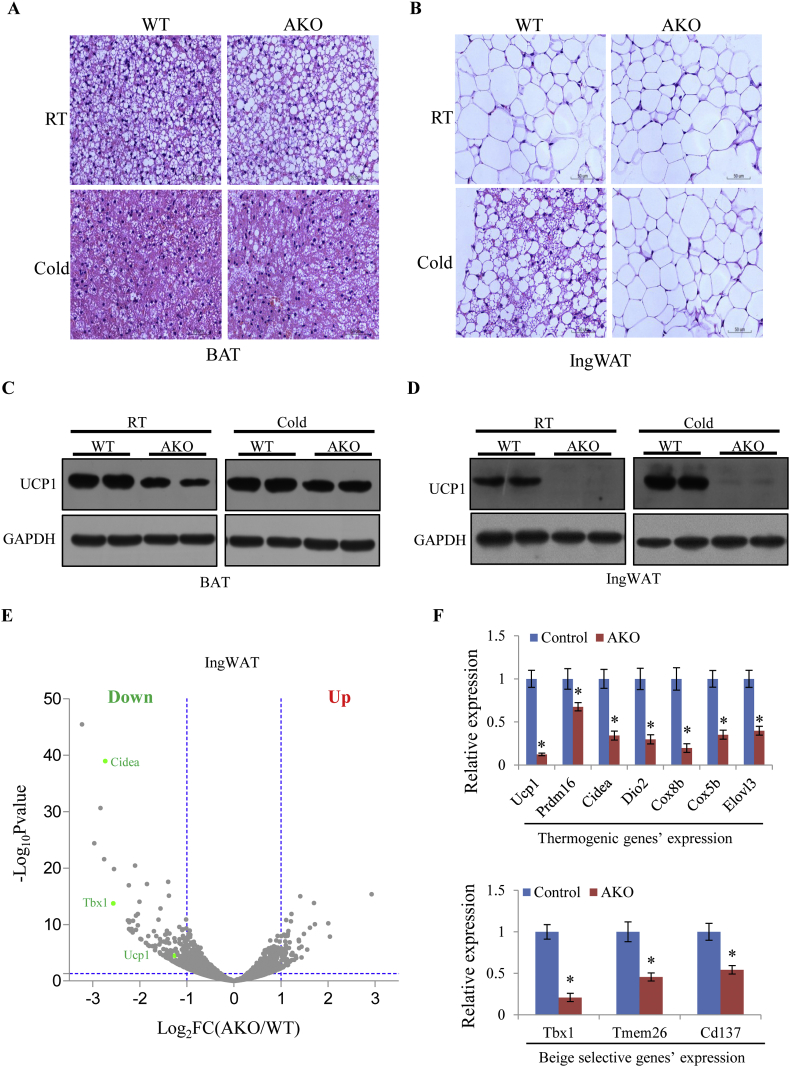
AKO mice showed no differences with control mice with regard to body weight, food intake and tissue weights of BAT, IngWAT and EpiWAT on standard chow diets at room temperature (Figure 1C–E). AKO and control Tbx15 floxed mice were then cold-challenged for 24 h and H&E staining analysis of isolated BAT, IngWAT and EpiWAT depots was performed. As shown in Figure 2A, B (upper panel) and Fig. S1A (upper panel), there were no obvious differences in adipocyte morphology in BAT, IngWAT and EpiWAT at room temperature. However, upon cold exposure, beige adipocytes within IngWAT, as indicated by H&E staining, were induced in the control mice but not in AKO mice (Figure 2B, lower panel). By comparison, a minor difference in adipocyte morphology was observed in BAT of control versus AKO mice following the 24 h cold challenge (Figure 2A, lower panel) with no obvious differences in EpiWAT (Fig. S1A, lower panel).
Figure 2.
Tbx15 adipose-specific knockout impairs cold-induced adipocyte thermogenesis and browning. A-B. Representative H&E staining of brown adipose tissue (BAT, A) and inguinal white adipose tissue (IngWAT, B) from mice at room temperature (RT, upper panel) or following cold exposure (4 °C, lower panel). C-D. Western blot analysis of UCP1 in BAT (C) and IngWAT (D) normalized to GAPDH. E. Volcano map analysis of IngWAT RNA-seq data in separate cohorts of AKO and control mice after 24 h cold exposure. F. qPCR analysis of thermogenic and beige selective gene expression in inguinal adipose tissue from control and AKO mice following cold exposure at 4 °C for 24 h (n = 5). Data represent mean ± SEM, *p < 0.05.
Western blot and UCP1 immunohistochemistry analyses further showed that UCP1 protein expression was slightly reduced in BAT (Figure 2C) but largely ablated in AKO IngWAT mice following 24 h cold exposure on separate mice cohorts (Figure 2D and Fig. S1B). As these results suggest Tbx15 is critical for white adipocyte browning, RNA-seq analysis of IngWAT isolated from separate cohorts AKO and control mice was performed. A volcano map and a heat map from RNA-seq analysis also clearly showed down-regulation of Cidea, Tbx1, and Ucp1 in AKO versus control mice respectively (Figure 2E and Fig. S1C). In inguinal adipose tissue, genes involved in the thermogenic program, including Ucp1, Prdm16, Cidea, Dio2, Cox8b, Cox5b, and Elvol3, were dramatically down-regulated in AKO mice as were the beige-specific genes, Tbx1, Cd137, and Tmem26. By comparison, the white-specific gene, Tcf21, was increased in the inguinal adipose tissue of AKO mice (Figure 2F and Fig. S1D). In brown adipose tissue, mRNA levels of both Ucp1 and Prdm16 were also decreased in AKO mice versus control mice (Fig. S1E). Taken together, this data shows that Tbx15 is involved in the regulation of adipocyte thermogenesis and adipocyte browning following cold exposure.
3.3. TBX15 is involved in adipocyte browning by regulating expression of Prdm16
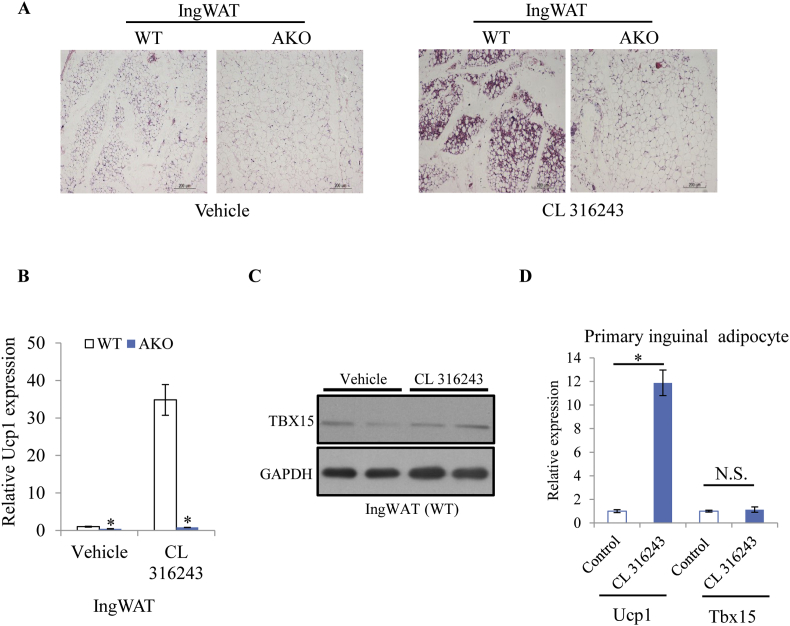
Cold exposure promotes adipocyte browning primarily through activation of the adrenergic signaling pathway. Our analysis of KEGG pathway revealed that genes regulated by Tbx15 were significantly enriched in the cAMP signaling pathway (Fig. S2). Therefore, the beta3 adrenergic agonist CL 316243 was used to treat the control and AKO mice for 1 day. As shown in Figure 3A, no apparent beige adipocytes were observed in AKO mice compared to the control mice after CL 316243 treatment. Consistent with this result, qPCR analysis suggested that the knockout of Tbx15 significantly reduced Ucp1 expression in inguinal adipose tissue treated with CL 316243 (Figure 3B). Next, western blot analysis of TBX15 in the inguinal adipose tissue of wild type mice suggested that TBX15 protein level was not altered by CL 316243 treatment (Figure 3C). To examine whether Tbx15 was upregulated by CL 316243, primary differentiated inguinal adipocytes derived from wild type mice were treated with CL 316243 for 24 h in vitro. The results showed that Ucp1 expression was significantly increased by CL 316243, but Tbx15 expression was unchanged (Figure 3D).
Figure 3.
Tbx15 is involved in adipocyte browning induced by beta3 adrenergic receptor agonism. A. Representative H&E staining of inguinal white adipose tissue (IngWAT) from control and AKO mice upon vehicle or CL 316243 treatment for 1 day. B. qPCR analysis of Ucp1 expression in inguinal adipose tissue from control and AKO mice upon vehicle or CL 316243 treatment for 1 day (n = 5). C. Western blot analysis of TBX15 in inguinal adipose tissue treated with CL 316243. D. qPCR analysis of Ucp1 and Tbx15 expression in primary inguinal differentiated adipocytes treated with DMSO or CL 316243 for 24 h (n = 3). Data represent mean ± SEM, *p < 0.05, unpaired student's t-test.
To investigate the molecular mechanism underlying Tbx15 regulation of adipocyte browning, a 4 kb Ucp1 promoter luciferase vector and PMX-Tbx15 or PMX-Pparγ were co-transfected into HEK293 cell respectively to examine whether TBX15 can directly regulate the expression of Ucp1. The results showed that PPARγ up-regulated the expression of Ucp1 as expected, but TBX15 did not (Fig. S3A). Next, ChIP analysis was carried out to investigate whether TBX15 could bind to the -2kb, -5 kb and −13 kb proximal and distal enhancer promoter regions of Ucp1, which were known key regions for binding transcription regulators reported as by Juro Sakai's group [30]. However, we found no evidence for binding interactions (Fig. S3B). Given that AKO mice displayed a phenotype similar to Prdm16 adipose-specific knockout mice. We therefore postulated that Tbx15 might interact with Prdm16 as a partner or regulate Prdm16 expression, which is a key factor for adipocyte browning. We therefore firstly investigated whether PRDM16 and TBX15 form a transcriptional complex through experiments using co-immunoprecipitation from HEK-293 cells transiently co-transfected with plasmids encoding HA-tagged TBX15 and FLAG-tagged PRDM16. However, under these experimental conditions we found no evidence for a stable protein interaction between PRDM16 and TBX15 (Fig. S3C). These results suggest that Tbx15 does not bind to known Ucp1 promoter regions nor does it interact with the PRDM16 protein to regulate Ucp1 expression, but TBX15 could still potentially interact with the other promoter region.
Next, we generated an immortalized inguinal adipose stromal cell line over-expressing HA-tagged Tbx15. The immortalized inguinal adipose stromal cell (ASC) line was first prepared by transfecting large antigen SV40 followed by transfection of HA-tagged Tbx15. Western blot analysis confirmed high expression of HA-Tbx15 in the immortalized cell line (Fig. S3D). ChIP analysis was then performed to examine whether Tbx15 regulates Prdm16 expression. As shown in Figure 4A, the promoter region of Prdm16 at approximately the -2kb position bound TBX15 as detected using either an HA or TBX15 antibody by qPCR analysis. This ChIP result was further confirmed by an agarose-gel assay (Figure 4B) showing that TBX15 bound directly at around the -2kb promoter position of Prdm16. Finally, qPCR analysis confirmed that increased ectopic expression of Tbx15 in adipose stromal cells up-regulated Prdm16 expression in accordance with the ChIP data (Figure 4C). Conversely, primary inguinal adipocytes lacking Tbx15 expression from AKO mice displayed decreased Prdm16 expression versus wild-type mice (Figure 4D). Over-expression of Tbx15 in the immortalized inguinal differentiated adipocytes promoted the expression of Ucp1, Dio2, and Cidea (Figure 4E). Thus, Tbx15 controls adipocyte browning through direct binding interactions with the Prdm16 promoter to regulate its expression.
Figure 4.
TBX15 directly regulates Prdm16 expression through promoter interactions. A. ChIP analysis of TBX15 with the promoter of Prdm16 from −5 kb to 0 kb. B. Agarose gel analysis of PCR products from TBX15 ChIP within the −2∼-1 kb promoter of Prdm16. Input refers to bead flow through. C. mRNA expression of Prdm16 in HA-tagged Tbx15 ASC's versus control cells (transfected with PMX empty vector) (n = 5). D. mRNA expression of Prdm16 in Tbx15 AKO primary inguinal adipocytes and control adipocytes (n = 5). E. Over-expression of Tbx15 promotes thermogenic gene expression in inguinal differentiated adipocytes (n = 5). Data represent mean ± SEM, *p < 0.05, unpaired student's t-test.
3.4. AKO mice are sensitive to body weight gain on obesity-promoting diets
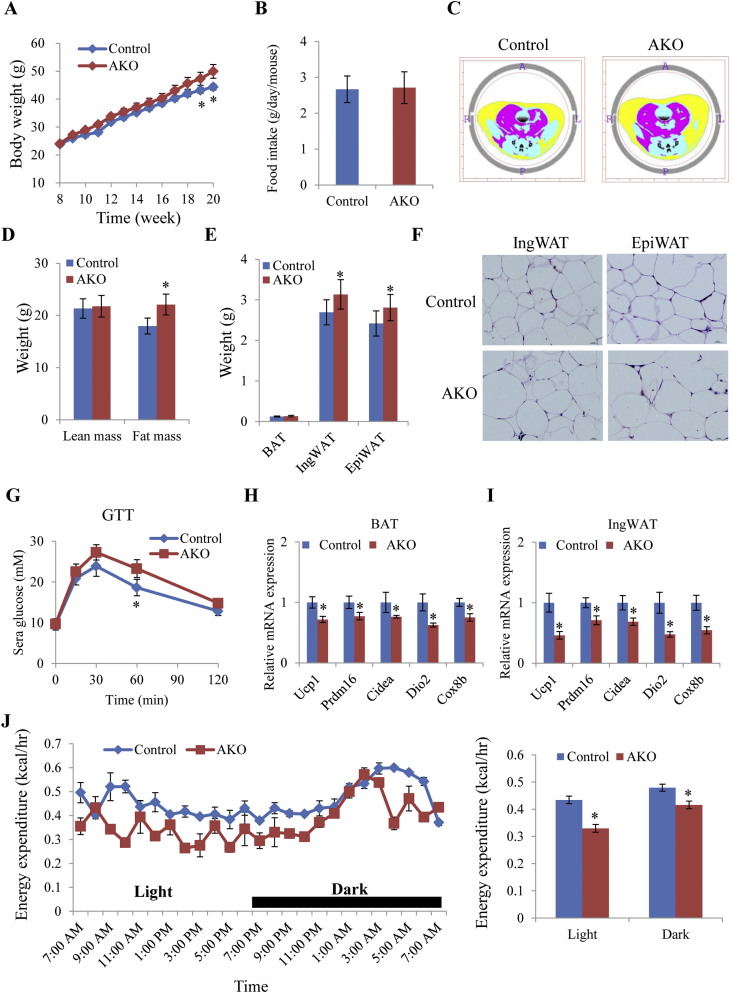
As adipocyte browning and thermogenesis regulate body weight, 8-week-old AKO and control mice were administered high fat diets for 10 weeks. AKO mice showed an increased propensity for weight gain compared to control mice (Figure 5A) without changes in food intake (Figure 5B). CT analysis further showed that the increased body weight in AKO mice was associated with increased total fat mass with no difference in lean body mass (Figure 5C, D). Tissue weights of IngWAT and EpiWAT in AKO mice were also higher than those in control mice (Figure 5E). Representative H&E staining also indicated larger adipocyte size in IngWAT and EpiWAT tissues isolated from AKO mice (Figure 5F and Fig. S4A).
Figure 5.
Adipose-specific Tbx15 knockout mice are predisposed toward obesity and display reduced energy expenditure on an obesity-promoting diet. Body weight (A) and food intake (B) of AKO and control mice on high fat diets (n = 10). C. Representative computed tomography (CT) images of AKO and control mice. D. Measurement of lean mass and fat mass in AKO and control mice. E. Tissue weights of adipose tissue in control and AKO mice. F. H&E staining of IngWAT and EpiWAT from control and AKO mouse. G. Glucose tolerant tests (n = 7). H–I Quantitative PCR analysis of thermogenic genes in BAT (H) and IngWAT (I) from control and AKO mice (n = 5). J. Energy expenditure (n = 5). Data represent mean ± SEM, *p < 0.05.
Glucose and insulin tolerance tests also showed that AKO mice were more insulin resistant than control mice (Figure 5G and Fig. S4B). Expression of thermogenic genes in brown (Figure 5H) and inguinal white (Figure 5I) adipose tissues in AKO mice were down-regulated compared to control mice as analyzed by qPCR (Figure 5H,I). Indirect calorimetry analyses also performed at 16th week where there was no significant body weight change. AKO mice showed reduced O2 consumption, CO2 production and energy expenditure (Figure 5J and Figs. S4C–D), consistent with impaired adipocyte thermogenesis/browning. Taken together, these results suggest Tbx15 regulates adipocyte thermogenesis/browning through Prdm16 expression. Specific ablation of Tbx15 expression in inguinal adipose tissue significantly blunts adipocyte browning induced by cold exposure and leads to an increased body weight gain with high fat feeding compared to the control mice. A diagram summarizing the role of TBX15 in regulation expression of Prdm16 and obesity is depicted in Figure 6.
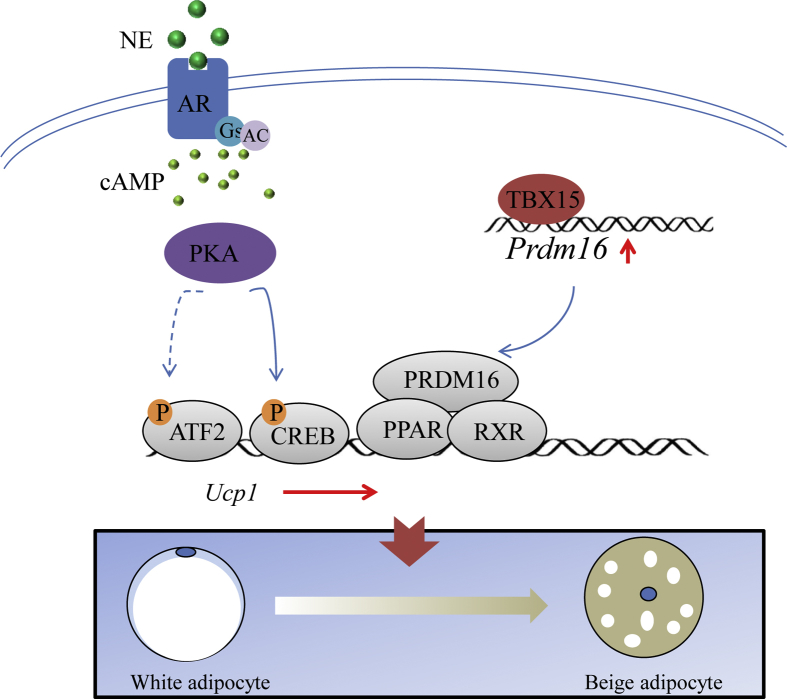
Figure 6.
Schematic diagram showing role of Tbx15 on adipocyte browning and obesity. The transcriptional factor TBX15 is crucial for adipocyte browning induced by adrenergic signaling pathway in vivo through direct regulation of Prdm16 expression.
4. Discussion
It is well established that adipocyte browning or thermogenesis in inguinal or brown adipose tissue plays a role in body weight regulation by increasing energy expenditure [4]. Recent studies suggest that expression of Tbx15 in brown and inguinal adipose tissues is tightly correlated with body weight in mammals. However, the underlying biological mechanisms involved have not been elucidated.
In our study, we first generated a Tbx15 adipose specific knockout mouse model to study the role of Tbx15 in adipose tissue. We found that adipocyte browning in inguinal adipose tissue was significantly impaired in the absence of TBX15 upon cold challenge. Beige marker genes were significantly reduced as shown by RNA-seq and qPCR analysis. Consistent with these findings, siRNA knockdown of TBX15 in human adipocytes has also been shown to decrease the expression of beige marker genes [31].
The phenotype of Tbx15 AKO mice is similar to that of Prdm16 AKO mice, with a comparatively minor effect on brown adipocyte thermogenesis but a pronounced effect on white adipocyte browning [14]. ChIP analysis of TBX15 showed a direct interaction with the Prdm16 promoter that regulates its expression. However, the tissue expression pattern of Tbx15 and Prdm16 is different in that Tbx15 is also highly expressed in myocytes but Prdm16 is not [11], [12]. This finding indicates that Tbx15 may not be the only determinant of Prdm16 expression. Also, Prdm16 global knockout mice die at birth whereas Tbx15 global knockout mice do not [32]. Thus, it is possible that other differentially-expressed genes between myocytes and adipocytes also participate in the regulation of Prdm16. Overall, our results suggest that Tbx15 is important for adipocyte browning and may therefore explain the widespread elevated expression of TBX15 in the Inuit people from Greenland who live in cold environments. Thus, elevated Tbx15 expression may promote adipocyte browning and facilitate adaptation to cold.
Consistent with the role of adipocyte browning and thermogenesis in body weight regulation, adipose-specific Tbx15 knockout mice displayed increased weight gain on high fat diets compared to control mice. Our findings suggest that downregulation of Tbx15 in adipose tissue contributes to this weight gain by inhibiting adipocyte browning and energy expenditure.
Our RNA-seq analyses in inguinal adipose tissue also independently supports a regulatory role for Tbx15 in fuel utilization in vivo as the expression of glycolytic genes was reduced in the inguinal adipose tissue of Tbx15 knockout mice (Figs. S5A–B). By comparison, overexpression of Tbx15 in primary inguinal adipocytes derived from control mice promoted glycolytic gene expression (Fig. S5C). Immunochemistry analysis supported this finding as protein expression of ENO1 and PKM2 (also called PKM) in IngWAT were also decreased in AKO mice (Fig. S5D). Consistent with these results, the respiratory exchange ratio was higher in AKO mice compared to control mice in the dark phase (Fig. S5E). These results suggest that knockout of Tbx15 within the fat depot shifts fuel utilization from fatty acid to glucose metabolism in vivo. ChIP experiments provided no evidence for regulation of Eno1 and Pkm2 gene expression by TBX15 through direct interactions with their respective promoter regions (Fig. S5F). Recently, Shingo Kajimura's group had identified a subset population of beige adipocytes with enhanced glucose oxidation as glycolytic beige fat [33]. Our data suggested that Tbx15 may be involved in glycolytic beige adipocyte development. Overall, our findings are consistent with reports that TBX15 levels are negatively correlated with obesity in humans [19], [25] and a genome-wide association study identifying an association between a single nucleotide polymorphism in the TBX15 locus and body mass index [20].
In conclusion, our results demonstrate that Tbx15 is important for adipocyte thermogenesis and browning in adipose tissue in vivo. Interventions that increase TBX15 expression may provide new therapeutic strategies to promote thermogenesis and combat obesity and related metabolic disorders.
Author contributions
W. S., X. Z., Z. W., and Y. C. carried out the research and analyzed the results; D. W. and T. N. supervised experiments, analyzed data, wrote and revised manuscript; Z. W. and L. M. conducted the experiments; S. L., X. G., X. H., S. J., S. T., Y. X., A. X., K. L., and C. W. reviewed and edited the manuscript; and D. W. and T. N. are the guarantors of this work and have full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgement
This study was supported in part by Natural Science Foundation of China (81700742), Frontier Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110105019), National Basic Research Program of China (2016YFC1305000), the Key International Collaborative funding from the Chinese Academy of Sciences (CAS09829841), the Strategic Priority Research Program on Development of New Drug of the Chinese Academy of Sciences (XDA12040325), Natural Science Foundation of Guangdong Province (2016A030310122), Science and Technology Planning Project of Guangdong Province (2017B030314056), Guangzhou International Collaborative Grant (2016201604030030), Guangzhou Science and Technology Program (201704020209, 201704030059) and Guangdong Provincial Public Interest Research and Capacity Building Projects (2014A010107024), X. H. wishes to acknowledge the grant support of National Science Foundation of China (81670800).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.07.004.
Contributor Information
Donghai Wu, Email: wu_donghai@gibh.ac.cn.
Tao Nie, Email: nietaoly@126.com.
Conflict of interest
The authors declare no competing financial interests.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Collaborators GBDO. Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K. Health effects of overweight and obesity in 195 countries over 25 years. New England Journal of Medicine. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kajimura S., Spiegelman B.M., Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metabolism. 2015;22(4):546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S.H., Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metabolism Journal. 2016;40(1):12–21. doi: 10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 5.Bartelt A., Heeren J. Adipose tissue browning and metabolic health. Nature Reviews Endocrinology. 2014;10(1):24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 6.Kazak L., Chouchani E.T., Jedrychowski M.P., Erickson B.K., Shinoda K., Cohen P. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163(3):643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazak L., Chouchani E.T., Lu G.Z., Jedrychowski M.P., Bare C.J., Mina A.I. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metabolism. 2017;26(4):660–671. doi: 10.1016/j.cmet.2017.08.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Himms-Hagen J., Melnyk A., Zingaretti M.C., Ceresi E., Barbatelli G., Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. American Journal of Physiology Cell Physiology. 2000;279(3):C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 9.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moisan A., Lee Y.K., Zhang J.D., Hudak C.S., Meyer C.A., Prummer M. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nature Cell Biology. 2015;17(1):57–67. doi: 10.1038/ncb3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seale P., Kajimura S., Yang W., Chin S., Rohas L.M., Uldry M. Transcriptional control of brown fat determination by PRDM16. Cell Metabolism. 2007;6(1):38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harms M.J., Ishibashi J., Wang W., Lim H.W., Goyama S., Sato T. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metabolism. 2014;19(4):593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seale P., Conroe H.M., Estall J., Kajimura S., Frontini A., Ishibashi J. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. Journal of Clinical Investigation. 2011;121(1):96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen P., Levy J.D., Zhang Y., Frontini A., Kolodin D.P., Svensson K.J. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1–2):304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheeba C.J., Logan M.P. The roles of T-box genes in vertebrate limb development. Current Topics in Developmental Biology. 2017;122:355–381. doi: 10.1016/bs.ctdb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Singh M.K., Petry M., Haenig B., Lescher B., Leitges M., Kispert A. The T-box transcription factor Tbx15 is required for skeletal development. Mechanisms of Development. 2005;122(2):131–144. doi: 10.1016/j.mod.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Lausch E., Hermanns P., Farin H.F., Alanay Y., Unger S., Nikkel S. TBX15 mutations cause craniofacial dysmorphism, hypoplasia of scapula and pelvis, and short stature in Cousin syndrome. The American Journal of Human Genetics. 2008;83(5):649–655. doi: 10.1016/j.ajhg.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K.Y., Singh M.K., Ussar S., Wetzel P., Hirshman M.F., Goodyear L.J. Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism. Nature Communications. 2015;6:8054. doi: 10.1038/ncomms9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K.Y., Sharma R., Gase G., Ussar S., Li Y., Welch L. Tbx15 defines a glycolytic subpopulation and white adipocyte heterogeneity. Diabetes. 2017;66(11):2822–2829. doi: 10.2337/db17-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heid I.M., Jackson A.U., Randall J.C., Winkler T.W., Qi L., Steinthorsdottir V. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nature Genetics. 2010;42(11):949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C.T., Monda K.L., Taylor K.C., Lange L., Demerath E.W., Palmas W. Genome-wide association of body fat distribution in African ancestry populations suggests new loci. PLoS Genetics. 2013;9(8) doi: 10.1371/journal.pgen.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto Y., Gesta S., Lee K.Y., Tran T.T., Saadatirad P., Kahn C.R. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring, Md) 2010;18(5):872–878. doi: 10.1038/oby.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walden T.B., Hansen I.R., Timmons J.A., Cannon B., Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, "brite," and white adipose tissues. American Journal of Physiology Endocrinology and Metabolism. 2012;302(1):E19–E31. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 24.Timmons J.A., Wennmalm K., Larsson O., Walden T.B., Lassmann T., Petrovic N. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gesta S., Bluher M., Yamamoto Y., Norris A.W., Berndt J., Kralisch S. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(17):6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gburcik V., Cawthorn W.P., Nedergaard J., Timmons J.A., Cannon B. An essential role for Tbx15 in the differentiation of brown and "brite" but not white adipocytes. American Journal of Physiology Endocrinology and Metabolism. 2012;303(8):E1053–E1060. doi: 10.1152/ajpendo.00104.2012. [DOI] [PubMed] [Google Scholar]
- 27.Gesta S., Bezy O., Mori M.A., Macotela Y., Lee K.Y., Kahn C.R. Mesodermal developmental gene Tbx15 impairs adipocyte differentiation and mitochondrial respiration. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2771–2776. doi: 10.1073/pnas.1019704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mina A.I., LeClair R.A., LeClair K.B., Cohen D.E., Lantier L., Banks A.S. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metabolism. 2018;28(4):656–666. doi: 10.1016/j.cmet.2018.06.019. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candille S.I., Van Raamsdonk C.D., Chen C., Kuijper S., Chen-Tsai Y., Russ A. Dorsoventral patterning of the mouse coat by Tbx15. PLoS Biology. 2004;2(1):E3. doi: 10.1371/journal.pbio.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe Y., Rozqie R., Matsumura Y., Kawamura T., Nakaki R., Tsurutani Y. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nature Communications. 2015;6:7052. doi: 10.1038/ncomms8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ejarque M., Ceperuelo-Mallafre V., Serena C., Maymo-Masip E., Duran X., Diaz-Ramos A. Adipose tissue mitochondrial dysfunction in human obesity is linked to a specific DNA methylation signature in adipose-derived stem cells. International Journal of Obesity (London) 2018 doi: 10.1038/s41366-018-0219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Ikeda K., Yoneshiro T., Scaramozza A., Tajima K., Wang Q. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature. 2019;565(7738):180–185. doi: 10.1038/s41586-018-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.