Abstract
Background
There has been enormous curiosity in the development of alternative plant based medicines to control diabetes, oxidative stress and related disorders. One of the therapeutic approaches is to reduce postprandial release of glucose in the blood. Two key enzymes that are involved in reducing postprandial glucose are α-amylase and α-glucosidase. Mentha arvensis L. has been traditionally used by several tribes as a medicinal plant to treat various disorders.
Objective
The present study was undertaken to test M. arvenisis L. for inhibition of postprandial hyperglycemia.
Material and method
We performed various in vitro and in vivo tests to evaluate efficacy of M. arvenisis L. for antidiabetic activity (postprandial hyperglycemia).
Results
Methanolic extract of M. arvensis L. leaves showed DPPH free radical scavenging activity (more than 78% μg/μl) and high antiglycation potential (more than 90% inhibition of AGE formation). Methanolic extract also showed remarkable inhibitory effects on α-amylase (more than 50% μg/μl) and α-glucosidase (68% μg/μl) and significant inhibition of postprandial hyperglycemia in starch induced diabetic Wistar rats.
Conclusion
The non-insulin dependent antidiabetic or inhibition of postprandial hyperglycemic activity of methanolic extract of M. arvensis L. leaves was shown by using in vitro and in vivo approaches in the present study.
Keywords: Mentha arvensis L., DPPH, α-amylase, α-glucosidase, Postprandial hyperglycemia
Graphical abstract
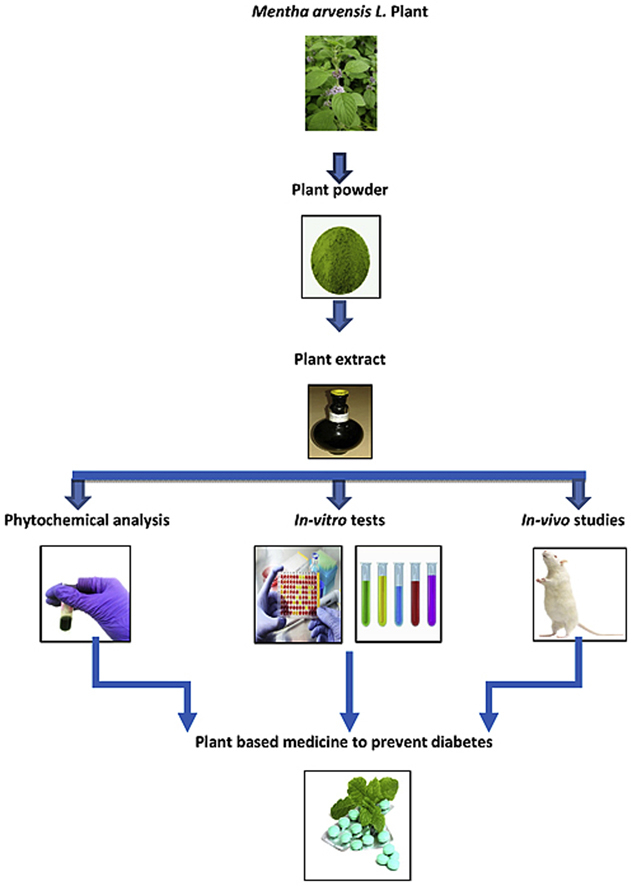
1. Introduction
Diabetes mellitus is a chronic metabolic disease, characterized by alteration in the carbohydrate, protein and lipid metabolism. The incidence of diabetes mellitus is continuously increasing in the world; it affects 340 million people out of which 70 million are in India alone [18]. India is the world's second most populous country, and having more people with type 2 diabetes mellitus, which is the major form of diabetes mellitus, accounting for 90% of cases worldwide [17]. Postprandial hyperglycemia plays a vital role in development of type 2 diabetes mellitus and other complications caused by diabetes mellitus, such as retinopathic, nephropathic, neuropathic and cardiovascular complications [24]. One therapeutic approach suggested to reduce postprandial hyperglycemia is by the inhibition of two key enzymes linked to type 2 diabetes mellitus, namely α-glucosidase and α-amylase, in the digestive organs [9]. Recently, it has been reported that compounds with combined antioxidant potential and antiglycation properties are effectively used to treat diabetes mellitus [8].
There are many mechanisms by which diabetes mellitus is developed and made chronic, which include oxidative stress and advance glycation end products (AGEs). Oxidative stress is a result of imbalance between oxidants and antioxidants in the body. One more important cause of diabetes mellitus is the interaction of glucose with a protein leading to AGEs [15]. Increase in glycation reaction, may increase tissue build-up of AGEs, leading to diabetic complications, because they can alter enzyme activity and ligand binding affinity and modify protein structure [14]. Therefore, glycation and oxidative stress are also important therapeutic targets for the treatment of diabetic complications.
There are many combinations of hereditary and environmental causes of diabetes mellitus, such as metabolic error in carbohydrate metabolism and changing lifestyle, dietary pattern, smoking, moderate coffee, alcohol consumption, highly processed food, calorie-dense, nutrient depleted diet, reduced physical activity, and due to the increase in obesity. For glucose transportation from the blood into the cells, the hormone – insulin is needed. Insulin is produced by the beta cells while glucagon is produced by the alpha cells of the pancreas. These hormones play an important role to sustain the blood glucose level.
In traditional and folk medicine many plants are used to control various diseases. This is typically because of varied combination of number and nature of secondary metabolites produced by different plants and their tissues. These are known to possess antioxidant, antibacterial, anti-inflammatory and many other medicinal properties [13]. There are many therapeutic treatments available to treat diabetes such as, acarbose and metformin; however, synthetic drugs are generally not preferred because of their high cost and many side effects, hence, it is necessary to develop traditional and alternative medicine which is specially based on plants. Herbal drugs play an important part of traditional medicine and literature shows that more than 400 plant species having antidiabetic activity. Plants which have antioxidant activity may show antidiabetic activity because oxidative stress may lead to diabetes mellitus. As Mentha arvenisis L. is already reported for antioxidant activity, hence, we selected this plant to check its antidiabetic property. The leaves of M. arvenisis L. are extensively used in traditional system for various medicinal purposes such as digestive expectorant, inflammation of liver, peptic ulcer, diarrhea, bronchitis, cardiotonic, diuretic, dentifrice, jaundice, hepatalgia, and skin diseases [7], [12], [13], [19]. It belongs to family Lamiaceae and is found throughout India. It is commonly known as 'pudina' in Hindi [11]. The present study was undertaken to extend the survey of M. arvensis L. for other medicinal properties than the ones reported so far. Hence, the methanolic extract of leaves of M. arvensis L. was used for its phytochemical analysis and evaluation of antioxidant and antiglycation potential through in vitro studies. Further inhibition of key enzymes linked to type 2 diabetes mellitus, i.e, α-amylase and α-glucosidase and inhibition of postprandial hyperglycemia in starch induced diabetic Wistar rats were also evaluated using methanolic extract of M. arvensis L. leaves.
2. Materials and methods
2.1. Collection of plant material, identification and authentication
Natural accessions of M. arvensis L. were collected from Maval area, district Pune, Maharashtra, India and authenticated by the Botanical Survey of India, Western Circle, Pune, Maharashtra, India. (No. BSI/WRC/Cert./2014/405 dated 1st December 2014).
2.2. Chemicals
All the chemicals and other solutions used for this study were of analytical grade. All the drugs and reagents were prepared freshly before use.
2.3. Animals
Healthy Wistar rats were obtained from National Institutional of Bioscience (CPCSEA Registration No. 1091/abc/07/CPCSEA) Pune, Maharashtra and were used for this study upon approval by the Institutional Animal Ethics Committee of Symbiosis School of Biomedical Sciences (CPCSEA Reg. No.1710/PO/a/13/CPCSEA). The study approval number is SSBS/IAEC/3/4.3.2015. Animals were maintained under standard laboratory conditions at Symbiosis School of Biomedical Sciences, Pune. Animal welfare guidelines were observed during the maintenance and experimentation period.
2.4. Preparation of methanolic extract
The plant material (leaves) was washed under running tap water. Further it was shade dried for 5–7 days and crushed to form coarse powder (500 g). The powdered material was taken in a separating funnel for extraction using methanol as solvent (2–3 lts) for 72 h. The methanolic extract was concentrated using Rotavapor [2]. Three biological replicates were prepared and extraction was performed for all the three replicates. The average yield of dried fraction of methanolic leaves extract of all three biological replicate of M. arvensis L. was 69.8 g.
2.5. Qualitative analysis of M. arvensis L. extract
The extract was tested qualitatively to know the presence of phytochemicals such as tannins, flavonoids, terpenoids, alkaloids, saponins and phenols [1], [2], [3].
2.6. Estimation of total phenolic content
Total phenolic content of plant extracts was determined using Folin–Ciocalteu reagent method [23].
2.7. DPPH free radical scavenging activity
Free radical scavenging activity was performed as per method suggested by Ref. [4] by 2, 2- diphenyl-1-picrylhydrazyl (DPPH) assay.
2.8. α-amylase inhibitory assay
The assay was performed as described by Ref. [16], [20]. The % inhibition was calculated according to the following formula:
2.9. α-glucosidase inhibitory assay
The assay was performed as described by Ref. [20]. The α-glucosidase inhibitory activity was expressed by % inhibition according to the following formula:
2.10. BSA-AGE fluorescence assay/antiglycation assay
This assay was performed as described by Ref. [22]. Percentage inhibition was calculated using the given formula.
| % inhibition = (C−T)/C × 100, where C = Fluorescence intensity of glycated BSA and T = Test sample. |
2.11. Animal study
Postprandial non-insulin dependent anti-hyperglycemic activity of methanolic leaves extract of M. arvensis L. was determined by postprandial glycemic test. For this study, 18 male Wistar rats were divided into three groups each containing six male rats. All the animals were kept for overnight fasting. Next morning, blood was collected from the retro-orbital plexus and blood glucose level (‘0’ h) was estimated by auto blood analyzer (Bayer EXPRESS PLUS). All the groups of animals (A, B and C groups) were given soluble potato starch 2 g/kg b.w. to induce diabetes mellitus.
Group A (Diabetes mellitus Control): Diabetes mellitus rats received only distilled water followed with starch.
Group B (Standard compound): Diabetic rats received standard antidiabetic drug acarbose (50 mg/kg) 15 min before starch feeding.
Group C (Sample Treated): Diabetic rats treated with methanolic leaves extract of M. arvensis L. (50 mg/kg) 15 min before starch feeding.
Blood was collected at the intervals of 0, 30, 60, 90 and 120 minutes. Blood glucose levels were measured as described by Ref. [21].
All the data related to the animal study were analyzed by standard statistical methods. Determination of degree of significance p < 0.05 between the groups of animals was done by two-way ANOVA followed Tukey's multiple comparison test and was applied to compare difference between animal study groups.
3. Results
3.1. Primary phytochemical screening
The methanolic extract obtained from separatory funnel extraction was analyzed for the presence of various phyto-constituents. Phytochemical screening revealed the presence of tannins, flavonoids, terpenoids, alkaloids, saponins and phenols.
3.2. Biochemical analysis
All parameters were analyzed using three biological replicates and two technical replicates per biological replicate.
The average of total phenolic content in methanolic leaves extract of M. arvensis L. was observed to be 621.38 μg per milligram of plant extract.
3.3. DPPH frees radical scavenging activity
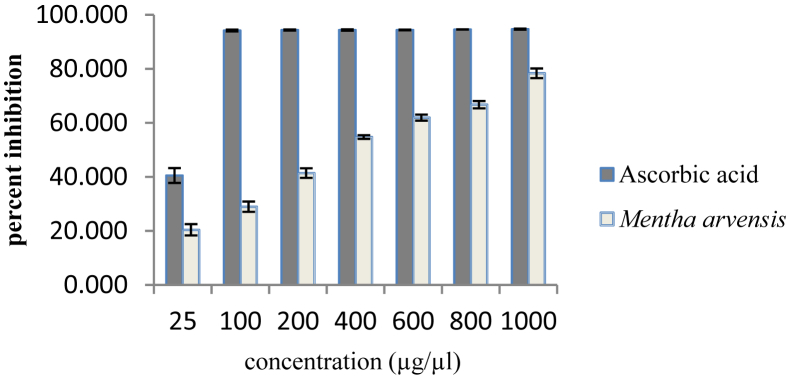
The ability of methanolic leaves extract of M. arvensis L. to scavenge DPPH free radical was calculated as percentage inhibition which was found to be 78% at concentration 1000 μg/μl, whereas percentage inhibition of ascorbic acid at the same concentration was 95% (Fig. 1).
Fig. 1.
DPPH scavenging activity of methanolic leaves extract of Mentha arvensis L.
3.4. In vitro α-amylase inhibitory assay
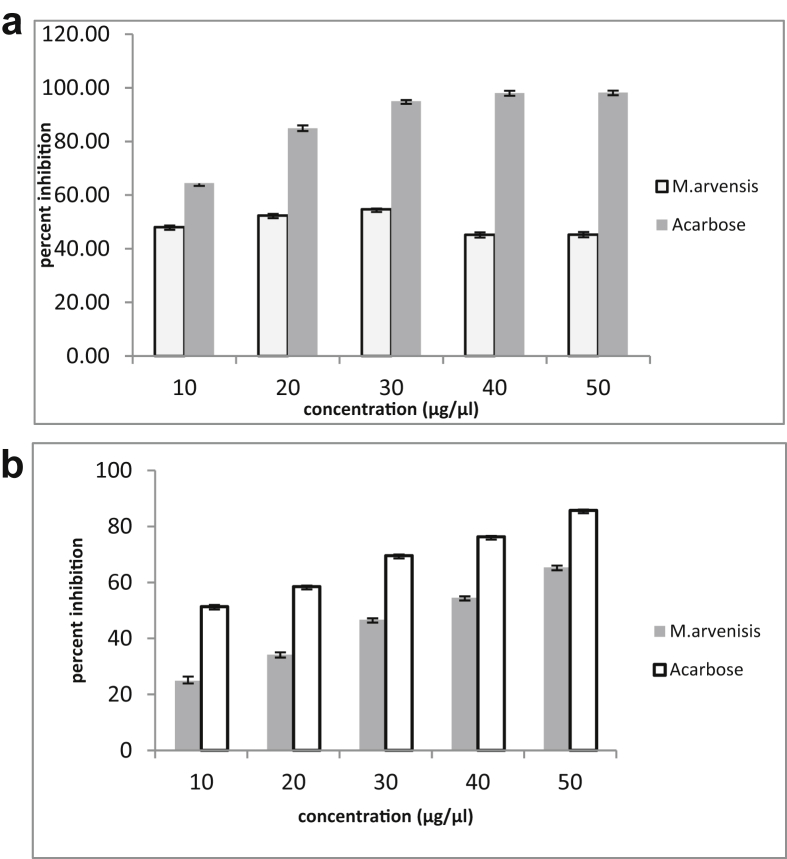
Inhibition of α-amylase by methanolic extract of M. arvensis L. was observed to be more than 50% inhibition at various concentrations (μg/μl) (Fig. 2a) as compared to the standard acarbose which showed more than 90% inhibition of α-amylase at the same concentrations.
Fig. 2.
a: α-amylase inhibitory activity of M. arvensis L. b: α-glucosidase inhibitory activity of M. arvensis L.
3.5. In vitro α-glucosidase inhibitory assay
The ability of methanolic leaves extract of M. arvensis L. to inhibit the α-glucosidase was calculated as percentage inhibition which was found to be 68% (Fig. 2b) at concentration 50 μg/μl, whereas percentage inhibition of acarbose at the same concentration was 85%.
3.6. BSA-AGE fluorescence assay
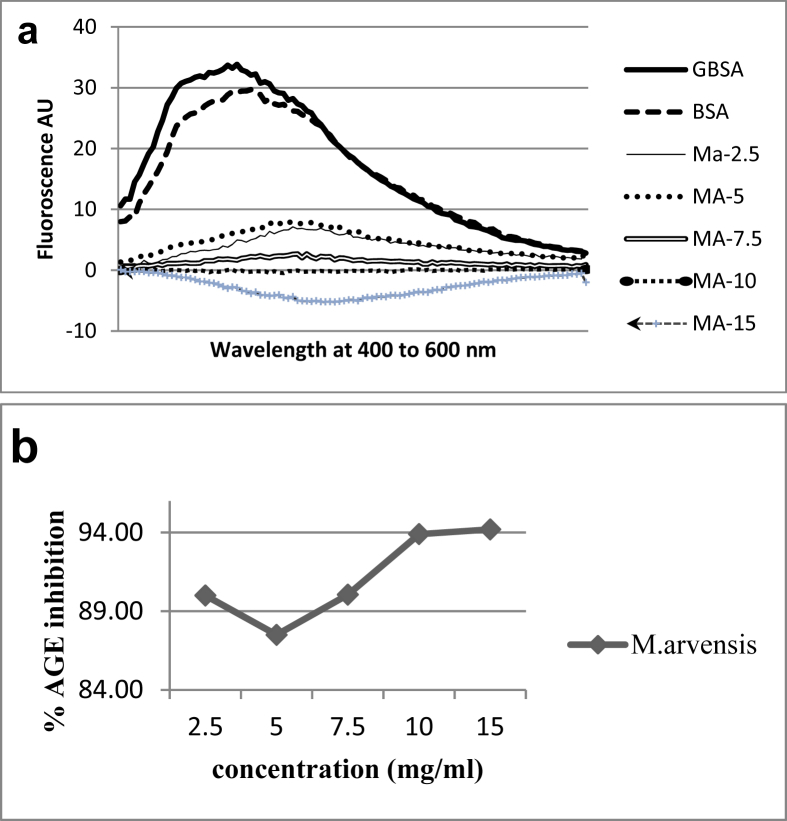
Effect of methanolic extract of M. arvensis L. on glucose mediated glycation revealed concentration dependent (2.5–15 mg/ml) increase in antiglycation activity (Fig. 3a).
Fig. 3.
a: AGE fluorescence spectra of BSA, GBSA and GBSA treated with M. arvensis L. b: Percentage inhibition of AGE by M. arvensis L.
The highest concentration (15 mg/ml) showed maximum glycation inhibition of 94% as shown in Fig. 3b.
3.7. Blood glucose levels in animal study
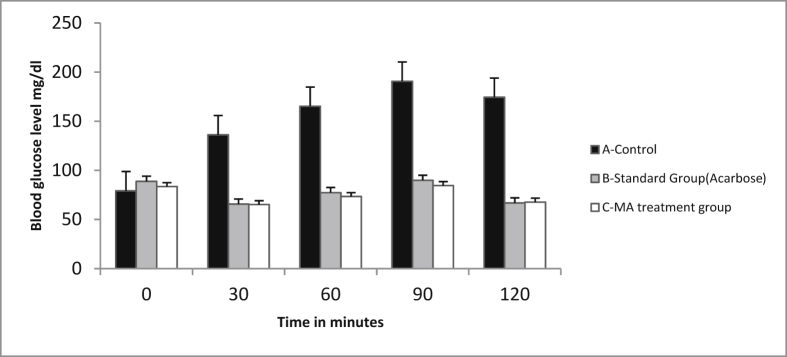
Anti-hyperglycemic activity of methanolic extract of M. arvensis L. was observed by blood glucose levels of rats after postprandial glycemic test at different time points 0, 30, 60, 90 and 120 min. The dose of leaves extract was selected as 50 mg/kg b.w. and for standard drug acarbose 50 mg/kg b.w. Two-way ANOVA analysis followed by Tukey's multiple comparison test was applied to find difference between the animal groups at *p < 0.05 when compared to the control (see Fig. 4).
Tukey's multiple comparison tests.
| 0 h | 30 min | 60 min | 90 min | 120 min | |
|---|---|---|---|---|---|
| D. Control vs. M. A | NS | **** | **** | **** | **** |
| D. Control vs. M. A | NS | **** | **** | **** | **** |
| Acarbose vs. M. A | NS | NS | NS | NS | NS |
Whereas NS – Non significant, **** – Highly significant.
Fig. 4.
Represents anti-hyperglycemic activity of methanolic extract of M. arvensis L. in rats.
4. Discussion
In the present study, preliminary phytochemical analysis of extract of M. arvensis L. leaves was performed and it was observed that the extract contained high amount of phenolic compounds along with other phytoconstituents, which are already studied for their medicinal activities. On this basis, we estimated total phenolic content of the extract and found that the extract contained higher amount of phenolic compounds. This reflected that the plant extract might have antioxidant and antiglycation activities and showed inhibition of two key enzymes linked to type 2 diabetes mellitus, such as α-amylase and α-glucosidase enzymes, because of its higher phenolic content along with other phytoconstituents. Phenols are very important plant constituents. They show high scavenging ability of free radicals due to their hydroxyl group. The high correlation between the content of phenolic concentration in plant extracts which leads to antioxidant activity is scientifically studied [5], [10]. Plant based antioxidants play an important role in neutralizing free radicals and protect important biological molecules from being damaged by free radicals. Antioxidants significantly prevent oxidation of cell content like proteins, lipids, carbohydrates and DNA. ROS (reactive oxygen species) is linked with diabetes mellitus, arteriosclerosis and age-related disorders. The antioxidant activity of extract of M. arvensis L. was determined using a DPPH reagent, which significantly scavenged DPPH free radical, and might prevent the complication of oxidative stress and related diseases.
The antiglycation activity of extract of M. arvensis L. was determined using AGE fluorescence assay wherein it significantly inhibited the protein glycation (Fig. 3a and b). This might be due to higher content of polyphenolics. It has been already studied that polyphenolics block the formation of AGEs and other glycated proteins [6]. Glycation reaction involves a series of non-enzymatic reactions between the carbonyl group on reducing sugars and the amino group on proteins to form advanced glycation end product (AGE's), which are involved in the pathogenesis of diabetes mellitus and aging-related complications [6]. In case of postprandial hyperglycemia there is an increase in blood glucose level which in turn contributes to increased glycation reaction; this can alter protein conformation and impair function by altering enzyme activity, altering immunogenicity, modifying protein half life and causing cross-linking of structural proteins.
There are many therapeutic approaches, which may prove to be beneficial for treatment of type 2 diabetes mellitus (postprandial hyperglycemia). This can be done by reducing the absorption of glucose through the inhibition of two key enzymes linked to type 2 diabetes mellitus (PPHG) in the digestive tract. It has been studied that the inhibition of carbohydrate hydrolyzing enzymes, like α-amylase and α-glucosidase are the therapeutic approaches for the treatment of type 2 diabetes mellitus [18]. Inhibitors of these enzymes delay carbohydrate digestion in the body and overall carbohydrate digestion time causing a significant decrease in the rate of glucose absorption by blunting the postprandial plasma glucose level. In the present study, we found that the methanolic extract of M. arvensis L. has significantly inhibited the α-glucosidase and in moderate α-amylase enzymes (Fig. 2a and b), may be due to higher content of polyphenolics.
5. Conclusion
We have done in vitro and in vivo evaluation of M. arvensis L. for antidiabetic activity. Any imbalance between the free radicles and antioxidants leads to production of a condition known as “oxidative stress” that results in the development of pathological condition among which one is diabetes mellitus. The leaves extracts of M. arvensis L. showed significant antioxidant potential and significantly inhibited protein glycation, which correlated well with its phenolics along with other phytoconstituents. It was further evaluated as a potent inhibitor of two key enzymes linked to type 2 diabetes mellitus such as, α-amylase and α-glucosidase enzymes. The methanolic extract of M. arvensis L. was also evaluated as potent inhibitor of type 2 diabetes mellitus (PPHG) during in vivo study. Overall, the methanolic extract of M. arvensis L. significantly reduced postprandial hyperglycemia and it might be helpful in prevention of onset as well as delaying the development of long term complications of diabetes mellitus. Thus, it has been rationalized that the tested extract has the potential to emerge as a new remedy for treatment of type 2 diabetes mellitus (postprandial hyperglycemia).
Sources of funding
Council of Scientific & Industrial Research, New Delhi and CSIR-National Chemical Laboratory, Pune: CSIR Network Project CSC-0133 (FUNHEALTH) and CSC-0111.
Conflict of interest
None.
Acknowledgement
Authors are thankful to Director of SSBS, Pune for their help. We are thankful to Mr. Suraj Chavan and Miss. Vishakha Tiwari for their technical help.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Wadood A., Ghufran M., Jamal S.B., Naeem M., Khan A., Ghaffar R. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem. & Anal. Biochem. 2013;2:4. [Google Scholar]
- 2.Akbar M.D., Mubashir H.M., Adil F.W., Mudasir A.M., Nida S.S. Antioxidant potential of methanol root extract of Mentha arvensis L. from Kashmir region. J Appl Pharm Sci. 2014;4(03):50–57. [Google Scholar]
- 3.Anjali S., Sheetal S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacogn Phytochem. 2013;2(4):22–29. [Google Scholar]
- 4.Braca A., Tommasi N.D., Bari L.D., Pizza C., Politi M., Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 5.Borneo R., Leon E.A., Aguirre A., Ribotta P., Cantero J.J. Antioxidant capacity of medicinal plants from the Province of Cordoba (Argentina) and their in vitro testing in model food system. Food Chem. 2008;112:664–670. [Google Scholar]
- 6.Chilukuri H., Kolekar Y.M., Bhosle G.S., Godbole R.K., Kazi R.S., Kulkarni M.J. N-(3-Aminoalkyl) proline derivatives with potent antigycation activity. RSC Adv. 2015;5(94):77332–77340. [Google Scholar]
- 7.Chopra R.N., Chopra I.C. 2nd ed. Academic Publishers; Calcutta, India: 1994. Indigenous drugs of India; p. 196. [Google Scholar]
- 8.Duraisamy Y., Gaffney J., Slevin M., Smith C.A., Williamson K., Ahmed N. Aminosalicylic acid reduces the antiproliferative effect of hyperglycaemia, advanced glycation endproducts and glycated basic fibroblast growth factor in cultured bovine aortic endothelial cells: comparison with aminoguanidine. Mol Cell Biochem. 2003;246:143–153. [PubMed] [Google Scholar]
- 9.Holman R.R., Cull C.A., Turner R.C. A randomized double-blind trial of acarbose in type 2 diabetes mellitus shows improved glycemic control over 3 years. Diabetes mellit Care. 1999;22:960–964. doi: 10.2337/diacare.22.6.960. [DOI] [PubMed] [Google Scholar]
- 10.Katalinic V., Milos M., Kulisic T., Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2004;94:550–557. [Google Scholar]
- 11.Khan S.W., Khatoon S. Ethan botanical studies on some useful herbs of Haramosh and Bugrote Valleys in Gilgit, Northern areas of Pakistan. Pak J Bot. 2008;40(1):43–58. [Google Scholar]
- 12.Khare C.P. Springerverlag Berlin Heidol; 2004. Encyclopedia of Indian medicinal plants; pp. 309–310. [Google Scholar]
- 13.Kiritikar K.R., Basu B.D. vol. III. International Book Distributors; Dehradun, India: 1999. (Indian medicinal plants). [Google Scholar]
- 14.Kostolanska J., Jakus V., Barak L. Monitoring of early and advanced glycation in relation to the occurrence of micro vascular complications in children and adolescents with type1 diabetes mellitus. Physiol Res. 2009;58:553–561. doi: 10.33549/physiolres.931612. [DOI] [PubMed] [Google Scholar]
- 15.Maritim A.C., Sanders R.A., Watkins J.B. Diabetes mellitus, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 16.Ponnusamy S., Ravindran R., Zinjarde S., Bhargava S., Kumar A. Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid-Based Complement Alternat Med. 2011;2011:515647. doi: 10.1155/2011/515647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevugan A., Subramanian K., Balamuthu K., Abdul B.A.A., Mohammed A.A., Mandali V.R. Antidiabetic activity of leaf and callus extracts of Aegle marmelos in rabbit. Sci Asia. 2006;34:317–321. [Google Scholar]
- 18.Shobana S., Sreerama Y.N., Malleshi N.G. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) Seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. [Google Scholar]
- 19.Sola A.V. Orient Longman Private Ltd; 1995. Indian medicinal plants. [Google Scholar]
- 20.Suthindhiran K., Jayasri M.A., Kannabiran K. α-glucosidase and α-amylase inhibitory activity of micromonospora sp. VITSDK3 (EU551238) Int J Integr Biol. 2009;6(3):115. [Google Scholar]
- 21.Tiwari A.K., Reddy K.S., Radhakrishnan J., Kumar D.A., Zehra A., Agawane S.B. Influence of antioxidant rich fresh vegetable juices on starch induced postprandial hyperglycemia in rats. Food Funct. 2011;2:521–528. doi: 10.1039/c1fo10093a. [DOI] [PubMed] [Google Scholar]
- 22.Waheed A.G., Miana A., Ahmad S.I. Clinical investigation of hypoglycemic effects of seeds Azadirachta-indica in type-2 (NIDDM) diabetes mellitus. Pak J Res Pharm Sci. 2006;19:322–325. [PubMed] [Google Scholar]
- 23.Yao Y., Sang W., Zhou M., Ren G. Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J Agric Food Chem. 2010;58:770–774. doi: 10.1021/jf903234c. [DOI] [PubMed] [Google Scholar]
- 24.Zaman R. High prevalence of diabetes mellitus and promoting factors among human urban population of Bahawalpur district, Pakistan: cross-sectional study. Res J Med Sci. 2006;3(2):62–69. [Google Scholar]