Abstract
Background
Oroxylum indicum Vent., a Dasamula plant used in Ayurveda possesses antioxidant properties.
Objectives
To evaluate the cardioprotective effect of 70% methanolic extract of O. indicum Vent. root bark (OIM) against doxorubicin induced cardiomyopathy in female Sprague Dawley rats.
Materials and methods
Cardiotoxicity was induced by intra-peritoneal injection of doxorubicin 30 mg/kg body weight (b.w.) for 4 consecutive days after a ten-day pre-treatment of animals with OIM at 200 mg/kg b.w. and 400 mg/kg b.w (p.o.). Drug treatment continued up to day 14. Probucol, orally administered at a dose of 20 mg/kg b.w. served as standard. ECG was recorded. The animals were sacrificed on day 15 and comparative analysis of serum marker levels of creatine phosphokinase (CPK), lactate dehydrogenase (LDH), Serum Glutamate Oxaloacetate Transaminase (SGOT), Serum Glutamate Pyruvate Transaminase (SGPT), tissue antioxidant status based on Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), reduced Glutathione (GSH) and lipid peroxidation (LPO) was carried out. Histopathological examination was carried out using hematoxylin–eosin staining.
Results
ECG records of OIM treated animals showed normal pattern, in comparison to the control with ST depression and arrhythmia in cardiogram. Tissue antioxidant profile (SOD, GSH and GPx) was significantly (p < 0.01) elevated in the cardiac tissue of treated group in dose-dependent manner; lipid peroxidation level was found to decrease with treatment. Comparative analysis of serum markers – CPK, LDH, SGOT and SGPT – among untreated control, standard and extract treated groups revealed that OIM extract at 400 mg/kg b.w. dose significantly reduced the levels (p < 0.01). Histological analysis revealed normal myocardial architecture in OIM treated groups. HPTLC fingerprint of OIM revealed 8 bands and detected the presence of chrysin, apigenin and quercetin.
Conclusion
O. indicum root bark shows marked cardio-protective activity, possibly due to the presence of antioxidant compounds acting synergistically.
Keywords: Doxorubicin, Cardioprotection, Oroxylum indicum Vent., HPTLC
1. Introduction
Ayurveda is a holistic indigenous system of medicine which has widely flourished all over India for more than 5000 years. It focuses on maintenance of health and prevention of diseases by the use of countless herbs, recommendation of an orderly lifestyle pattern and practice of treatment modalities like massaging, nasal drug administration, induced purgation etc. The medicinal preparations in Ayurveda generally include Kwathas or Kashayams, Arishtas and Asavams, Lehyas, Ghritas, Churnas, Gulikas, Tailams etc. [1], with the drugs derived principally from plants belonging to different families. Dashamoola is an Ayurvedic formulation consisting of the roots of ten medicinal plants categorized as Mahatpanchamula, comprising five trees, and Laghupanchmula, comprising five smaller plants, and is widely used in Ayurvedic preparations. The plants are bestowed with incredible medicinal properties, individually as well as in combination and are also sources of various bioactive compounds [2], [3].
Oroxylum indicum Vent., known as Shyonaka in Sanskrit [4] is one among the ten plants whose roots form ingredients of the widely used Ayurvedic formulation, Dashamoola, which literally means ‘ten roots’. According to the Ayurvedic description, it tastes bitter, astringent (rasa – tikta, kasaya); attributed with light/dry physical properties (guna – laghu, rooksha) as well as hot potency (veerya – ushna), and pungent post-digestion effect (vipak – katu). This combination often prescribed as Dashamoolarishta, Dashamoola Churna, Dashamoola Ghrita, Dashamoola Kalpa, Dashamoola Kwatha and Dashamoola Oil, is used commonly in Ayurveda for many nerve, muscle, bone and joint-related disorders owing to its strong anti-inflammatory, antioxidant and analgesic actions [5], [6], [7], [8]. There is also mention of the usage of Shyonaka as a single ingredient in treating Ama Vata (∼rheumatoid arthritis), sotha (∼inflammation) and various other disorders as drug for internal administration or external application [9], [10].
The present study focuses on the cardioprotective activity of 70% methanolic extract of O. indicum Vent. root bark against doxorubicin induced cardiac damage. Doxorubicin, an anthracycline antibiotic is widely used in chemotherapy especially for hematological malignancies and some types of tissue sarcomas and carcinomas; yet it is reported to cause heart damage, being its most serious contra-indication. Cardiotoxicity-induced by doxorubicin is reported to be caused by oxidative stress, mitochondrial dysfunction, susceptibility of cardiac tissue to lipid peroxidation and the low levels of antioxidant defences associated with the heart tissue [11], [12]. Enhancement of antioxidant status of the heart muscle tissue can be achieved by the use of pharmacological agents attributed with oxidative stress-resistance. Hence, supplementation therapies with compounds of antioxidant nature are in demand, which neither interfere with the anti-cancer efficacy of doxorubicin nor impose serious myocardial damage [13], [14]. A previous study on O. indicum Vent. has disclosed that the different parts of the plant like leaves, fruits, stem, stem bark, roots and root bark possess in vitro free radical scavenging activities [15]. The ethyl acetate, methanolic, and aqueous extracts of the leaves are reported to be antioxidant in nature [16]. Petroleum ether, benzene, chloroform, ethanol and aqueous extracts of the stem bark powder have been analyzed to prove their in vitro antioxidant properties through β-carotene bleaching assay [17]. The n-butanol fraction of root bark proved to be immunostimulant in nature, derived from evaluation in rats based on sheep RBC hemagglutinating antibody titre and delayed hypersensitivity reactions [18]. In sodium fluoride induced oxidative stress induced models, treatment with 70% methanolic extract of O. indicum Vent. root bark at dosages of 200 and 400 mg/kg b.w. showed significant amelioration in stress as reported previously by the authors. The extract showed anti-inflammatory properties in acute and chronic paw edema models [19]. In the light of its reported in vitro and in vivo antioxidant properties, the present study aims at investigating the efficacy of the extract ameliorating doxorubicin-mediated cardiotoxicity.
Tree barks are rich sources of secondary metabolites attributed with medicinal properties. In studying the pharmacological properties of such bioactive compounds, steps such as extraction, isolation and characterization are pre-requisites. Quality assessment should follow international guidelines. Since plant extracts are mixtures of different types of compounds, the separation and analysis of compounds is to be attained precisely, which involves chromatographic procedures like TLC, HPLC, HPTLC, column chromatography etc., as well as non-chromatographic procedures such as phytochemical screening assays and immunoassays, following solvent extraction [20]. Among various analytical techniques, HPTLC has proved to be precise, accurate, fast and cost-effective in determination of the phytochemical profile of variety of plant extracts [21], [22], [23]. Using a small quantity of mobile phase, several samples can be analyzed at a time. So, besides its focus on the cardioprotective activity of 70% methanolic extract of O. indicum Vent. root bark, the study also involves a chromatographic fingerprinting of the extract using HPTLC.
2. Materials and methods
2.1. Collection and preparation of plant extract
O. indicum Vent. roots were collected in the month of August from the Ayurvedic Garden belonging to Amala Cancer Hospital campus. The plant was authenticated by Dr. P. Sujanapal, Scientist- B, Silviculture Department, KFRI, Peechi, Thrissur 680 653, Kerala (Voucher specimen No: KFRI/SILVA/GEN/06/11). The bark, after peeling was dried at 45 °C in hot air oven and the powdered material was extracted using 70% methanol as solvent. A dry residue (OIM) was obtained by filtering, concentration and evaporation of the extract. This was re-dissolved in distilled water and used for further studies. There was an average yield of 9.01% in this system of extraction.
2.2. Chemicals and reagents
Nitro blue tetrazolium (NBT), glutathione (GSH) and 5-5′ dithiobis (2-nitro benzoic acid) (DTNB) were purchased from Sisco Research Laboratories Pvt. Ltd, Mumbai, India; thiobarbituric acid from Hi Media Laboratories, Mumbai, India, doxorubicin from Naprod Life Sciences Pvt Ltd. and chrysin, apigenin, luteolin, quercetin, gallic acid and kaempferol (analytical grade standard compounds) from Sigma Aldrich Corporation (St. Luis, MO, USA). Biochemical kits manufactured by Euro Diagnostic Systems, Chennai, India, were used for estimation of Lactate Dehydrogenase (LDH), Creatine Phosphokinase (CPK), Serum Glutamate Oxaloacetate Transaminase (SGOT) and Serum Glutamate Pyruvate Transaminase (SGPT). All other chemicals and reagents used were of analytical grade.
2.3. Experimental animals
Female Sprague Dawley rats (200–220 g) were obtained from the Small Animal Breeding Station, Mannuthy, Kerala, and were maintained at standardized environmental conditions and brought up with standard rat feed and water ad libitum. All animal experiments conducted during the present study were with prior permission from Institutional Animal Ethics Committee, Amala Cancer Research Centre (Approval No. ACRC/IAEC/15/02-(01)) and strictly followed the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by the Animal Welfare Division, Government of India.
2.4. Experimental design
Thirty female Sprague Dawley rats (200–220 g body weight) were divided into five groups of six animals each and were kept under the following treatment schedule.
Group I – Normal reference group
Group II – Negative control
Group III – Standard (served as positive control)
Group IV – O. indicum Vent. extract low concentration (OIML)
Group V – O. indicum Vent. extract high concentration (OIMH)
Groups III, IV and V received 14 day long oral treatment of Probucol (20 mg/kg b.w.), OIML (200 mg/kg b.wt) and OIMH (400 mg/kg b.wt) respectively. Induction of cardiotoxicity started from day 11 of the oral treatment, up to day 14, when all the groups (except I), received intra-peritoneal injections of doxorubicin, making up a 4-day cumulative dose of 30 mg doxorubicin/kg of animal body weight. The dosage and administration were decided as per the procedure referred from Firdous and Kuttan [24] with slight modifications.
2.5. ECG analysis
On the 15th day, prior to sacrifice, ECG of the animals was recorded by anaesthetizing them with ketamine (80 mg/kg b.wt.: i.p.) [25], [26]. Needle electrodes were inserted under the skin for the limb lead at position II and ECG tracings were recorded using BPL Cardiart 6108 T instrument.
2.6. Evaluation of serum marker enzymes
After ECG recording, rats were sacrificed and blood was collected by heart puncture into non-heparinised vials to collect serum. Serum cardiac markers like CPK, LDH and SGOT were analyzed to evaluate the extent of heart tissue damage. Hepatic damage, if any, was also analyzed in terms of the liver marker enzyme SGPT.
2.7. Evaluation of myocardial lipid peroxidation level and tissue antioxidant status
10% homogenates of heart tissue were prepared in ice cold 0.1 M Tris–HCl. Lipid peroxidation measured as MDA level in the heart tissue homogenate (10%) was analyzed according to the method of Ohkawa et al. [27]. The cytosolic fraction of the heart tissue homogenate was used for the antioxidant studies such as SOD according to the method of McCord and Fridovich [28], GPx based on the oxidation of glutathione [29] and reduced GSH level according to Moron et al. [30].
2.8. Histopathological analysis
Small portion of the heart tissue was washed in phosphate buffered saline, fixed in 10% buffered formalin and embedded in paraffin wax. Sections of 5 μm thickness were made and stained with hematoxylin–eosin.
2.9. HPTLC fingerprinting
The 70% hydromethanolic extract of O. indicum Vent. root bark was re-dissolved in methanol (30 mg/ml) and subjected to qualitative HPTLC on pre-coated TLC plates of silica gel 60 F254 (Merck, India) using CAMAG automatic spotting device equipped with TLC Scanner 3 and WinCat software (CAMAG, Switzerland). The solvent system used was toluene:ethyl acetate:formic acid (7:3:0.2) and the plate was scanned at a wavelength of 254 nm. Standard compounds chrysin (≥98% – HPLC grade), apigenin (≥99% – HPLC grade), luteolin (≥97.0% – HPLC grade), quercetin (≥95% – HPLC grade), gallic acid (97%) and kaempferol (≥97.0% – HPLC grade) dissolved in methanol (0.1 mg/ml) were also subjected to HPTLC procedure simultaneously. HPTLC analysis was carried out according to the procedure referred from Sharma et al. [31], with slight modifications.
2.10. Statistical analysis
The values were expressed as mean ± SD of 6 animals per group. Statistical evaluation of the data was done by one way ANOVA followed by Dunnett post hoc test using GraphPad InStat 3 software. Results were considered statistically significant when p < 0.05.
3. Results
3.1. ECG
While the normal group (devoid of doxorubicin induction) showed regular pattern of ECG (Fig. 1), the untreated doxorubicin-induced control animals presented clear ST segment depression, indicative of alterations in ventricular repolarisation phase. There were also changes such as reduction in QRS complex as well as prolongation of QT and PR intervals-changes, which were not visible in treated animals. The standard and OIMH groups revealed normal ST segment, QRS complex, QT and PR interval patterns when compared to the control group.
Fig. 1.
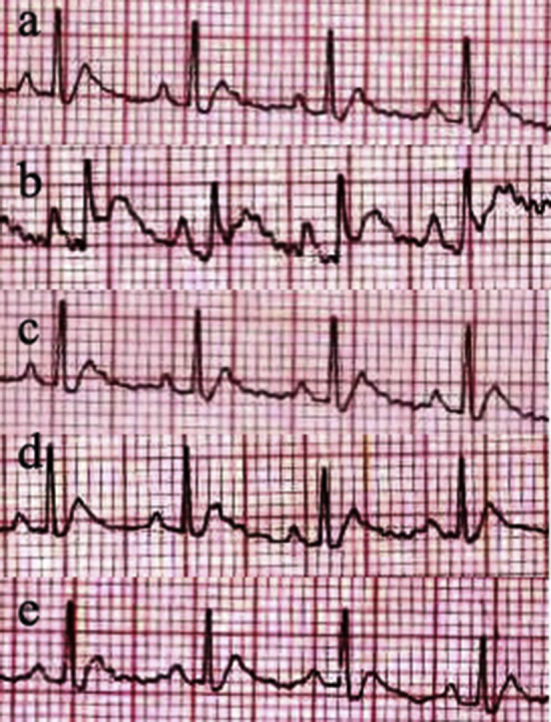
Effect of 70% hydromethanolic extract of O. indicum Vent. root bark on electrocardiogram of doxorubicin treated animals. a) ECG recording of normal group showed normal heart rate, ST deviation, PR, RR, ST and QT interval; b) Doxorubicin-induced untreated control group showed arrhythmia, ST segment depression and prolonged PR and QT intervals; c) Rats treated with Probucol (standard drug – 20 mg/kg bw) showed normal ECG pattern; Treatment with d) O. indicum extract low dose (200 mg/kg b.w.) and e) O. indicum extract high dose (400 mg/kg b.w.) showed normal electrocardiographic tracings.
3.2. Effect on serum marker enzyme levels
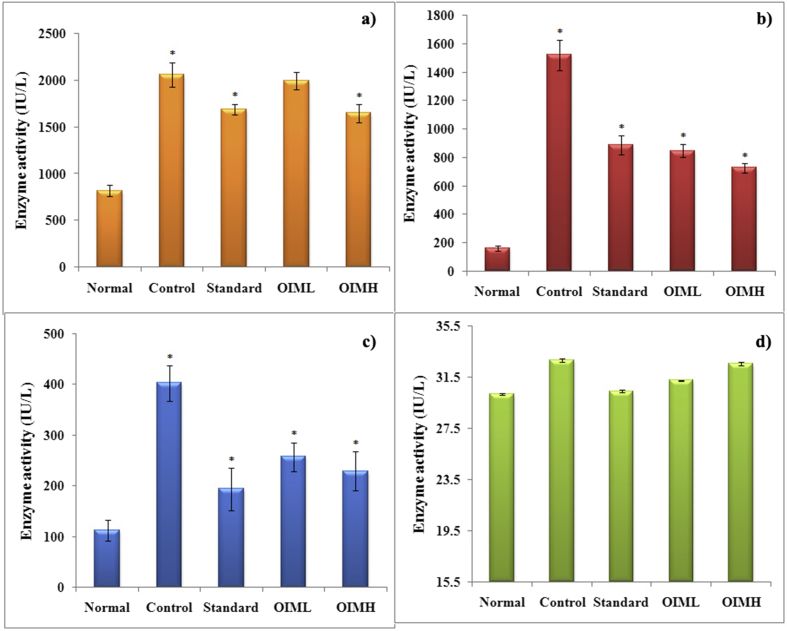
Significant (p < 0.01) increase in the level of cardiac marker enzymes – CPK, LDH and SGOT was discerned in the doxorubicin alone treated control group, compared to normal. In the standard and OIMH treated group, though the values were higher than normal, probably resulting from doxorubicin induction, they were inclined more towards normalcy, than a thorough devastating effect as seen in the control. But the levels of SGPT in all tested groups were kept within a narrow range, revealing that hepatic damage was not imposed by Dox (Fig. 2).
Fig. 2.
Effect of 70% hydromethanolic extract of O. indicum Vent. on serum cardiac markers in doxorubicin induced rats. Values are expressed as mean ± SD for 6 animals. a. LDH: Lactate Dehydrogenase; b. CPK: Creatine Phosphokinase; c. SGOT: Serum Glutamate Oxaloacetate Transaminase and d. SGPT: Serum Glutamate Pyruvate Transaminase: **p < 0.01, *p < 0.05 (control compared to normal, and groups – standard, OIML and OIMH compared to control).
3.3. Effect on myocardial lipid peroxidation level and tissue antioxidant status
The heart tissue isolated from control group, on biochemical evaluation, revealed enlarged level of lipid peroxidation, when compared to the normal group. But this was significantly low in the standard group and OIM treated group, operational in a dose-dependent manner. The antioxidant profile including SOD and GPx activities, as well as GSH levels were reduced in the control group, displaying deprived antioxidant defense status. The standard and the OIMH group, however disclosed a near normal antioxidant profile (Table 1).
Table 1.
Effect of OIM on myocardial lipid peroxidation level and tissue antioxidant status.
| Animal groups | LPO (n moles of MDA/mg protein) | SOD (U/mg protein) | GPx (U/mg protein) | GSH (n moles/mg protein) |
|---|---|---|---|---|
| Normal | 0.409 ± 0.005 | 0.223 ± 0.009 | 35.15 ± 1.39 | 4.92 ± 0.133 |
| Control | 0.527 ± 0.006** | 0.073 ± 0.019** | 22.53 ± 1.29** | 3.11 ± 0.102** |
| Standard | 0.443 ± 0.013** | 0.181 ± 0.022** | 32.66 ± 1.73** | 4.69 ± 0.110** |
| OIML | 0.460 ± 0.011** | 0.165 ± 0.014** | 29.54 ± 1.09** | 4.36 ± 0.096** |
| OIMH | 0.426 ± 0.011** | 0.197 ± 0.008** | 31.31 ± 1.60c | 4.67 ± 0.167** |
Values are expressed as mean ± SD for 6 animals.
SD: standard deviation, OIM: O. indicum 70% methanolic extract, OIML: O. indicum 70% methanolic extract low dose, OIMH: O. indicum 70% methanolic extract high dose, LPO: lipid peroxidation, MDA: malondialdehyde, SOD: superoxide dismutase, GPx: glutathione peroxidase, GSH: glutathione.
**p < 0.01, *p < 0.05 normal compared to normal, and groups – standard, OIML and OIMH compared to control.
3.4. Histopathology
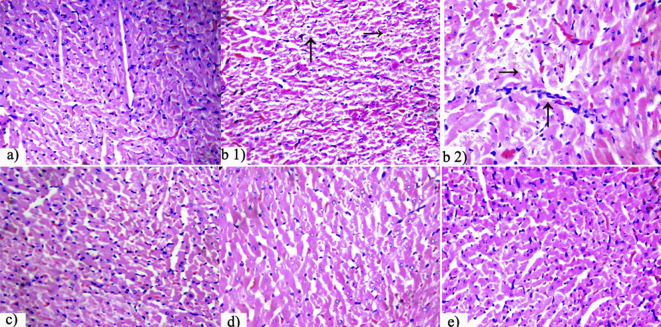
Light microscopic examination of normal heart stained with hematoxylin and eosin maintained a typical morphology, but doxorubicin-induced control rats, having received no treatment developed cardiotoxicity signs such as focal loss of tissue and fragmentation, myofibrillar disorganization and lack of well defined boundaries of cardiac fibers indicating necrosis of cardiac tissue (Fig. 3b.1), scattered cytoplasmic vacuolation in myocytes as well as lymphocyte infiltration (Fig. 3b.2). Amelioration of these effects has occurred in the Probucol (standard) treated rats as well as OIM high dose groups.
Fig. 3.
Effect of O. indicum Vent. extract on the histology of heart tissue (in light microscopic view – ×400) in doxorubicin induced rats. a) Cardiac histology of normal group with typical micro-architecture; (b) Doxorubicin induced untreated control group showed focal loss of tissue and fragmentation (small arrow to right), and lack of well defined boundaries of cardiac fibers indicating necrosis (big arrow pointed upwards) of cardiac tissue (b.1), cytoplasmic vacuolation (small arrow to right) and lymphocyte infiltration (big arrow pointed upwards) (b.2); c) Rats treated with Probucol (standard drug – 20 mg/kg bw) showed normal histology; Treatment with d) O. indicum extract low dose (200 mg/kg bw) revealed minimal damage and e) O. indicum extract high dose (400 mg/kg bw) showed normal cardiac tissue architecture (with hematoxylin–eosin staining).
3.5. HPTLC fingerprinting
In the HPTLC fingerprint of the 70% hydromethanolic extract of O. indicum Vent. (OIM) scanned at 254 nm, 8 dark bands were detected with Rf values at 0.30, 0.39, 0.46, 0.52, 0.62, 0.64, 0.70 and 0.83. The standard compounds chrysin, apigenin, luteolin, quercetin, gallic acid and kaempferol formed bands at Rf values 0.83, 0.46, 0.65, 0.70, 0.60 and 0.73 respectively (Fig. 4). This is suggestive of the presence of chrysin, apigenin, luteolin and quercetin in the plant extract.
Fig. 4.
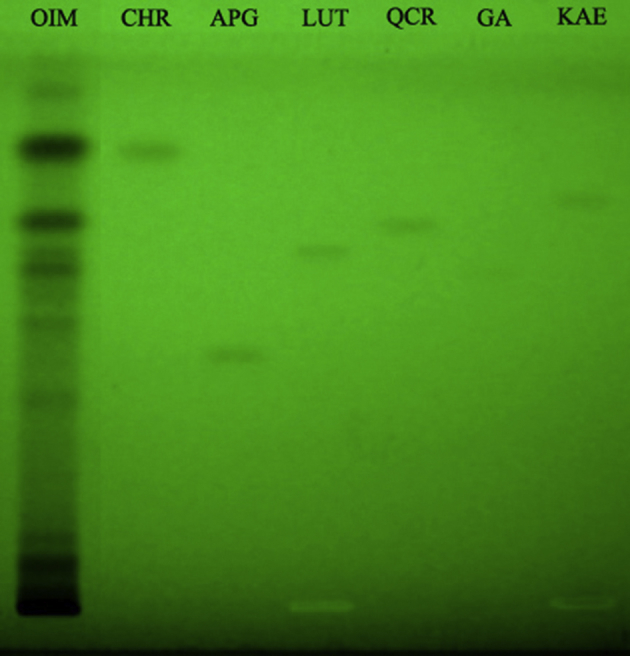
HPTLC plate developed on pre-coated TLC plates of silica gel 60 F254 using CAMAG automatic spotting device equipped with TLC Scanner 3 and WinCat software using toluene:ethyl acetate:formic acid (7:3:0.2) as solvent system. (Lanes:- OIM: O. indicum Vent. 70% hydromethanolic extract re-dissolved in methanol (30 mg/ml); Lanes CHR, APG, LUT, QCR, GA and KAE represent standard compounds chrysin, apigenin, luteolin, quercetin, gallic acid and kaempferol respectively dissolved in methanol (0.1 mg/ml).)
4. Discussion
The aim of the current investigation was to evaluate the amelioration of cardiotoxicity induced by doxorubicin, by restoration of tissue redox balance. In overcoming the cardiac injury persuaded by doxorubicin, plant derived compounds and plant extracts as such, are effective as well. Pre-treatment of the animals with OIM extract regained near normalcy in the SOD, GSH and GPx levels, when compared to control animals treated with doxorubicin alone, pointing to the inference that the protective role of O. indicum Vent. root bark extract may be credited to its ability in enhancing the tissue antioxidant status. The results are in consistency with previous studies [32], [33], [34] of cardioprotection against the oxidative stress induced by doxorubicin. In an attempt to evaluate the cardioprotective role of aged garlic extract, grape seed proanthocyanidin and hazelnut, pre-supplementation of these compounds significantly enhanced the criteria such as cardiac superoxide dismutase and catalase activity in doxorubicin treated rats [35]. This has been attributed to the presence of water soluble allyl amino acid derivatives, lipid soluble allyl sulphides, flavonoids and saponins in aged garlic extract, the pro-anthocyanins of grape seed as well as the phenolic compounds in hazelnuts; all mediators in combating oxidative stress. Similar effects such as the enhancement of superoxide dismutase activity, reduction in malondialdehyde and LDH levels have been noticed in the presence of a peptide, ghrelin in cardiomyocytes exposed to doxorubicin induced oxidative stress [36]. Many notable similarities were found after extrapolating the conclusions of the above studies to the results of the current study.
In the current study, doxorubicin treated control animals exhibited elevated levels of these serum cardiac injury markers; meanwhile the experimental group pre-treated with O. indicum Vent. root bark extract showed lower levels. El-Demerdash et al. [37] demonstrated that pre-treatment with Probucol led to a significant increase in plasma doxorubicin clearance in rats. It has been proposed that cardioprotective effect of Probucol may be related to its antioxidant properties and enhancement of endogenous antioxidant enzyme activity [38], [39]. Swiss albino rats treated with simvastatin were found to withstand the noxious effects of doxorubicin, as indicated by a comparative increase in the reduced glutathione, GST and DT-diaphorase activity of heart tissue homogenate [40], again validating the role of oxidative stress in doxorubicin-induced cardiotoxicity. As reviewed by Nigam [41], serum AST (GOT) activity is an important biochemical marker of myocardial injury. Its level increases 3–8 h after the onset of the myocardial injury, elevating up to an average at 24 h and finally returning to normal levels in 3–6 days. Other known markers include lactate dehydrogenase and creatine kinase (its isoforms). An increase in serum LD activity is found following myocardial infarction beginning within 6–12 h and reaching a maximum at about 48 h and it remains elevated for 4–14 days before coming down to normal levels. Serum CK activity increases following myocardial injury beginning within 6 h and peaking on an average at 24 h and returning to normal within 2–3 days [42].
The efficacy of doxorubicin in combating solid tumors, leukemia, soft tissue sarcoma, breast cancer, small cell carcinoma of the lung etc. has been established through former studies [43], [44], [45]. Yet, restraints exist on the use of this drug due to the observed and postulated side effects, mostly owing to the cumulative dose of the drug. Transient electrocardiographic abnormalities such as non-specific ST-T changes and QT prolongation, pericarditis–myocarditis syndrome and ventricular dysfunction with congestive heart failure are the acute side effects [11]. Even if the minor contraindications such as tachycardia and hypotension may be omitted in the enumeration, it is the chronic and serious one that is to be accounted – cardiomyopathy. The postulated mechanisms of cardiotoxicity induction by anthracyclines include the oxidative stress hypothesis (superoxide and peroxynitrite generation initiated by a doxorubicin semiquinone radical) [46], metabolite theory (cardiac damage induced by parent doxorubicin or its metabolites) [47], [48] and changes imposed on calcium homeostasis. Therefore, adequate emphasis has been given on the innovation of negotiator compounds that can attenuate the toxic effects induced by doxorubicin treatment.
Electrocardiographic abnormalities are indicators of myocardial injury. While studying the protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity, intravenous injection of doxorubicin significantly increased ST deviation and T-wave amplitude in electrocardiographic analysis. Pre-treatment with the extract helped to reduce the doxorubicin induced increase in ST [49], though to a limited extent. ST segment depression with prolonged ST interval is constantly noticed in rat ECGs associated with studies of doxorubicin-induced cardiac damage [50], [51]. The ECG records of the current investigation also presented similar effects with the doxorubicin-induced control animals showing marked ST segment depression, which was not associated with the ECGs of standard or drug treated groups.
Doxorubicin is reported to cause inter-fibrillar hemorrhages, congestion, and focal areas of disrupted cardiac muscle fibers [52] as well as cardiac muscular dysfunction [53]. The heart tissue of OIM treated animals with a higher dose (400 mg/kg b.w.) maintained normal histological architecture as was observed in Probucol treated groups. In previously reported protective studies of Spirulina [54], Gmelina arborea [13], aliskiren (renin inhibitor) [55] and morphine [56] against doxorubicin induced cardiotoxicity, histopathological analysis of heart tissue revealed extensive myocardial degeneration manifested as the loss of myofibrils and focal cytoplasmic vacuolization, myocytic necrosis with moderate infiltration of lymphocytes and macrophages, granulated cytoplasm of myocytes etc. Comparable to the above, such effects were also noticed in the doxorubicin treated control animals.
However, doxorubicin induced cardiotoxicity is generally attributed to free radical generation during redox cycling of doxorubicin and toxic action of doxorubicinol, a C 13-dihydrometabolite of doxorubicin in heart tissue. Therefore, pharmacological compounds which can inhibit the formation of both doxorubicinol and free radical species can ameliorate the cardiotoxic effects of doxorubicin. The authors have reported the in vivo antioxidant properties of OIM extract in sodium fluoride-induced oxidative stress model [19]. Similar findings supporting the ability of antioxidant rich plant extracts to ameliorate doxorubicin induced cardiotoxicity have been reported [32], [50], [57], signifying the direct relationship between the resistance of cardiac tissue to doxorubicin induced stress and its antioxidant defense status. In the study, the bands revealed from the HPTLC fingerprint of 70% hydromethanolic extract of O. indicum Vent. root bark revealed the presence of chrysin, apigenin and quercetin. The root bark of this plant has beforehand, been subjected to phytochemical analyses, which revealed the presence of phytoconstituents such as baicalein (flavonoids), biochanin-A and ellagic acid through TLC and RP-HPLC [58]. Chrysin [59], [60], apigenin [61], quercetin [62], biochanin-A [63] and ellagic acid [51] are compounds attributed with antioxidant properties. The anti lipo-peroxidative and cytoprotective effects of apigenin, baicalein, kaempferol, luteolin and quercetin against doxorubicin-induced oxidative stress were investigated in isolated rat heart cardiac myocytes, mitochondria and microsomes, and was found that all compounds, excepting apigenin were anti lipo-peroxidative to a significantly high level and all compounds including apigenin were cytoprotective than dexrazoxan, the standard used [64]. There are proven studies of cardioprotective effects of chrysin [65], quercetin [66] and ellagic acid [67] in doxorubicin-induced animal models. The biological properties of plant extracts are attributed to the synergistic action of the multifarious compounds present in them. It is hence, conclusive that the presence of antioxidant compounds in the OIM extract may possibly have mediated cardio-protection, through their combined action.
Moreover, O. indicum Vent. is one among Dashamoola – group of 10 roots, with potent anti- inflammatory activity, it is an ingredient of all Dashamoola formulations like Dasamoolarishtam, used for treating cold, fever and cough; Dhanwantaram tailam used in rheumatoid arthritis and osteo arthritis and Dhanwantararishtam, used in post natal care of mother. The plant is also attributed with vatahara properties. Free radicals are generated subsequent to the inflammatory changes in various parts of the body, and these cytotoxic reactive oxygen species cause oxidative damage to the cells. Medicinal preparations alleviating inflammation induced adverse changes are also identified as potent scavengers or inhibitors of free radicals. From the present study, undersigning the cardioprotective effect of O. indicum Vent., it was found that the extract restores the redox balance of the heart tissue, as evident from the blood and tissue marker levels. This also validates its use in the preparation of Dashamoola formulations which find use for treating inflammation related disorders owing to its strong anti-inflammatory and antioxidant actions.
5. Conclusion
The above results present the capability of O. indicum Vent. root bark extract in shielding the damaging and devastating effects of cumulative administration of doxorubicin (30 mg doxorubicin/kg of animal body weight) in murine models. Though it is not suggestive of an immediate clinical supplementation in humans undergoing doxorubicin therapy for cancer, the study extends the scope of exploring active fractions from this plant extract for using it as an adjuvant in doxorubicin mediated chemotherapy.
Sources of funding
Council of Scientific and Industrial Research, Government of India for the financial support in the form of Senior Research Fellowship (09/869 (0008)/2011-EMR-I).
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Somanathan A.R., Sadanandan K., Damodaran N.P. Standardisation of ayurvedic medicines – Dasamulam Kashayam. Ancient Sci Life. 1989;9(2):54–60. [PMC free article] [PubMed] [Google Scholar]
- 2.Rao M.L., Savithramma N. Phytochemical screening of Dasamoola – an ayurvedic drug. Int J Pharm Pharmaceut Sci. 2011;3(suppl 5):318–320. [Google Scholar]
- 3.A.P.I. 1 ed. vol. 1. Government of India; , New Delhi: 2007. The ayurvedic pharmacopoeia of India, part II – formulations. Y.N. Department of Ayurveda, Unani, Siddha and Homoeopathy, Ministry Of Health And Family Welfare. [Google Scholar]
- 4.Kapoor L.D. CRC Press; , New York: 1990. Handbook of ayurvedic medicinal plants: herbal reference library. [Google Scholar]
- 5.Singh R.S., Ahmad M., Wafai Z.A., Seth V., Moghe V.V., Upadhyaya P. Anti-inflammatory effects of Dashmula, an ayurvedic preparation, versus Diclofenac in animal models. J Chem Pharmaceut Res. 2011;3(6):882–888. [Google Scholar]
- 6.Bhalerao P.P., Pawade R.B., Joshi S. Evaluation of analgesic activity of Dashamoola formulation by using experimental models of pain. Indian J Basic Appl Med Res. 2015;4(3):245–255. [Google Scholar]
- 7.H.P.I. Regional Research Laboratory, Jammu and Indian Drug Manufacturer's Association; Mumbai, India: 1998. The herbal pharmacopoeia of India. [Google Scholar]
- 8.Sharma P.V. vol. 1. Chaukhamba Bharati Academy; Varanasi, India: 2006. Dravyaguna vijnana. [Google Scholar]
- 9.Chunekar K.C., Pandey G.S. 10th ed. Chaukhamba Bharati Academy; Varanasi, India: 1999. Bhavaprakasha nighantu; pp. 283–285. [Google Scholar]
- 10.Kirtikar K.R., Basu B.D. In: 2 ed. Blatter E., Caius J.F., Mhaskar K.S., editors. vol. 4. Periodical Experts; Delhi, India: 1975. pp. 1839–1841. (Indian medicinal plants). [Google Scholar]
- 11.Schimmel K.J., Richel D.J., van den Brink R.B., Guchelaar H. J Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30(2):181–191. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Singal P.K., Iliskovic N., Li T., Kaur K. Heart failure due to doxorubicin. Kuwait Med J. 2001;33(2):111–115. [Google Scholar]
- 13.Vijay T., Dhana Rajan M.S., Sarumathy K., Palani S., Sakthivel K. Cardioprotective, antioxidant activities and Phytochemical analysis by GC-MS of Gmelina arborea (GA) in Doxorubicin-induced myocardial necrosis in Albino rats. J Appl Pharmaceut Sci. 2011;1(5):198–204. [Google Scholar]
- 14.Rajalakshmy I., Pydi R., Kavimani S. Cardioprotective medicinal plants – a review. Int J Pol Inf. 2011;1(1):24–41. [Google Scholar]
- 15.Mishra S.L., Sinhamahapatra P.K., Nayak A., Das R., Sannigrahi S. In vitro antioxidant potential of different parts of Oroxylum indicum: a comparative study. Indian J Pharmaceut Sci. 2010;72(2):267–269. doi: 10.4103/0250-474X.65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R.C., Sharma V., Sharma N., Kumar N., Singh B. In vitro antioxidant activity from leaves of Oroxylum indicum (L.) vent. – a north Indian highly threatened and vulnerable medicinal plant. J Pharm Res. 2008;1(1):65–72. [Google Scholar]
- 17.Kalaivani T., Mathew L. Phytochemistry and Free radical scavenging activities of Oroxylum indicum. Environ We: Int J Sci Technol. 2009;4:45–52. [Google Scholar]
- 18.Zaveri M., Gohil P., Jain S. Immunostimulant activity of n-butanol fraction of root bark of Oroxylum indicum Vent. J Immunot. 2006;3(2):83–99. doi: 10.1080/15476910600725942. [DOI] [PubMed] [Google Scholar]
- 19.Menon S., Lawrence L., Vipin P.S., Padikkala J. Phytochemistry and evaluation of in vivo antioxidant and anti-inflammatory activities of Oroxylum indicum Vent. Root bark. J Chem Pharmaceut Res. 2015;7(10):767–775. [Google Scholar]
- 20.Sasidharan S., Chen Y., Saravanan D., Sundram K.M., Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr J Tradit, Complementary Altern Med. 2011;8(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Yousefi K., Hamedeyazdan S., Torbati M., Fathiazad F. Chromatographic fingerprint analysis of marrubiin in marrubium vulgare L. via HPTLC technique. Adv Pharmaceut Bull. 2016;6(1):131–136. doi: 10.15171/apb.2016.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tambe R., Kulkarni M., Bhise K. Preliminary phytochemical screening and HPTLC fingerprinting of bark extracts of Symplocos racemosa. J Pharmacogn Phytochem. 2013;2(3):45–49. [Google Scholar]
- 23.Kulkarni M., Tambe R., Bhise K. Preliminary phytochemical screening and HPTLC Studies of extracts of Dried rhizomes of aspidium cicutarium. J Pharmacogn Phytochem. 2013;2(3):50–54. [Google Scholar]
- 24.Firdous A.P., Kuttan R. Chemo protective activity of carotenoid meso-zeaxanthin against doxorubicin-induced cardio toxicity. J Exp Therapeut Oncol. 2012;10(2):101–106. [PubMed] [Google Scholar]
- 25.Van Pelt L.F. Ketamine and xylazine for surgical anesthesia in rats. J Am Vet Med Assoc. 1977;171(9):842–844. [PubMed] [Google Scholar]
- 26.Kushawaha S., Malpani A., Aswar U.M., Bodhankar S.L., Malpani A., Shivakumar S.I. Effect of different anaesthetic agents on cardiovascular parameters in male Wistar rats. Research Journal of Pharmaceutical. Res J Pharm Biol Chem Sci. 2011;2(2):685–690. [Google Scholar]
- 27.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 29.Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104(5):580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 30.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582(1):67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 31.Sharma N., Prakash C., Gupta R.C., Rao C.V. Pharmacognostical, phytochemical investigations and HPTLC fingerprinting of Pentapetes phoenicea L. leaves. Indian J Nat Prod Resour. 2014;5(2):158–163. [Google Scholar]
- 32.Khan G., Haque S.E., Anwer T., Ahsan M.N., Safhi M.M., Alam M.F. Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Pol Pharm. 2014;71(5):861–868. [PubMed] [Google Scholar]
- 33.Rai S.S., Somashekar B., Shivalinge Gowda K.P. Cardioprotective effects of ethanolic leaf extract of Ipomoea batatas on doxorubicin induced cardiotoxicity in rats. Asian J Pharmaceut Clin Res. 2015;8(2):444–450. [Google Scholar]
- 34.Swamy A.V., Gulliaya S., Thippeswamy A., Koti B.C., Manjula D.V. Cardioprotective effect of curcumin against doxorubicin-induced myocardial toxicity in albino rats. Indian J Pharmacol. 2012;44(1):73–77. doi: 10.4103/0253-7613.91871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirkaya E., Avci A., Kesik V., Karslioglu Y., Oztas E., Kismet E. Cardioprotective roles of aged garlic extract, grape seed proanthocyanidin, and hazelnut on doxorubicin-induced cardiotoxicity. Can J Physiol Pharmacol. 2009;87(8):633–640. doi: 10.1139/y09-051. [DOI] [PubMed] [Google Scholar]
- 36.Xu A., Zhan J.C., Huang W.D. Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-kappaB pathways and mitochondrial protective mechanisms. Toxicology. 2008;247(2–3):133–138. doi: 10.1016/j.tox.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 37.El-Demerdash E., Ali A.A., Sayed-Ahmed M.M., Osman A.M. New aspects in probucol cardioprotection against doxorubicin-induced cardiotoxicity. Canc Chemother Pharmacol. 2003;52(5):411–416. doi: 10.1007/s00280-003-0676-y. [DOI] [PubMed] [Google Scholar]
- 38.Li T., Singal P.K. Adriamycin-induced early changes in myocardial antioxidant enzymes and their modulation by probucol. Circulation. 2000;102(17):2105–2110. doi: 10.1161/01.cir.102.17.2105. [DOI] [PubMed] [Google Scholar]
- 39.Siveski-Iliskovic N., Hill M., Chow D.A., Singal P.K. Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation. 1995;91(1):10–15. doi: 10.1161/01.cir.91.1.10. [DOI] [PubMed] [Google Scholar]
- 40.Naiyra A.A., Ali A.A., Ahmed R.A. Cardioprotective effect of simvastatin on doxorubicin induced oxidative cardiotoxicity in rats. J Basic Appl Sci. 2010;6(1):29–38. [Google Scholar]
- 41.Nigam P.K. Biochemical markers of myocardial injury. Indian J Clin Biochem. 2007;22(1):10–17. doi: 10.1007/BF02912874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varley H., Gowenlock A.H., Bell M. William Heinemann Medical Books Ltd; 1984. Enzymes, in practical clinical biochemistry; pp. 685–770. [Google Scholar]
- 43.Blum R.H., Carter S.K. Adriamycin. A new anticancer drug with significant clinical activity. Ann Intern Med. 1974;80(2):249–259. doi: 10.7326/0003-4819-80-2-249. [DOI] [PubMed] [Google Scholar]
- 44.Denard B., Lee C., Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. eLife. 2012;1:e00090. doi: 10.7554/eLife.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trebunova M., Laputkova G., Slaba E., Lacjakova K., Verebova A. Effects of docetaxel, doxorubicin and cyclophosphamide on human breast cancer cell line MCF-7. Anticancer Res. 2012;32(7):2849–2854. [PubMed] [Google Scholar]
- 46.Vasquez-Vivar J., Martasek P., Hogg N., Masters B.S., Pritchard K.A., Jr., Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36(38):11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 47.Minotti G., Recalcati S., Mordente A., Liberi G., Calafiore A.M., Mancuso C. The secondary alcohol metabolite of doxorubicin irreversibly inactivates aconitase/iron regulatory protein-1 in cytosolic fractions from human myocardium. FASEB (Fed Am Soc Exp Biol) J. 1998;12(7):541–552. doi: 10.1096/fasebj.12.7.541. [DOI] [PubMed] [Google Scholar]
- 48.Zhou S., Starkov A., Froberg M.K., Leino R.L., Wallace K.B. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Canc Res. 2001;61(2):771–777. [PubMed] [Google Scholar]
- 49.Xin Y.F., Zhou G.L., Deng Z.Y., Chen Y.X., Wu Y.G., Xu P.S. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother Res. 2007;21(11):1020–1024. doi: 10.1002/ptr.2186. [DOI] [PubMed] [Google Scholar]
- 50.Koti B.C., Nagathan S., Vishwanathswamy A., Gadad P.C., Thippeswamy A. Cardioprotective effect of Vedic Guard against doxorubicin-induced cardiotoxicity in rats: a biochemical, electrocardiographic, and histopathological study. Phcog Mag. 2013;9(34):176–181. doi: 10.4103/0973-1296.111287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warpe V.S., Mali V.R., Arulmozhi S., Bodhankar S.L., Mahadik K.R. Cardioprotective effect of ellagic acid on doxorubicin induced cardiotoxicity in wistar rats. J Acute Med. 2015;5(1):1–8. [Google Scholar]
- 52.Shivakumar P., Rani M., UshaReddy A., GopalaAnjaneyulu Y. A study on the toxic effects of doxorubicin on the histology of certain organs. Toxicol Int. 2012;19(3):241–244. doi: 10.4103/0971-6580.103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayward R., Hydock D., Gibson N., Greufe S., Bredahl E., Parry T. Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. J Physiol Biochem. 2013;69(2):177–187. doi: 10.1007/s13105-012-0200-0. [DOI] [PubMed] [Google Scholar]
- 54.Khan M., Shobha J.C., Mohan I.K., Naidu M.U., Sundaram C., Singh S. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother Res. 2005;19(12):1030–1037. doi: 10.1002/ptr.1783. [DOI] [PubMed] [Google Scholar]
- 55.Rashikh A., Abul Kalam N., Akhtar M., Mahmood D., Pillai K.K., Ahmad S.J. Protective effects of aliskiren in doxorubicin-induced acute cardiomyopathy in rats. Hum Exp Toxicol. 2011;30(2):102–109. doi: 10.1177/0960327110369819. [DOI] [PubMed] [Google Scholar]
- 56.Kelishomi R.B., Ejtemaeemehr S., Tavangar S.M., Rahimian R., Mobarakeh J.I., Dehpour A.R. Morphine is protective against doxorubicin-induced cardiotoxicity in rat. Toxicology. 2008;243(1–2):96–104. doi: 10.1016/j.tox.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Hosseini A., Rajabian A. Protective effect of Rheum turkestanikum root against doxorubicin-induced toxicity in H9c2 cells. Rev Bras Farmacogn. 2016;26:347–351. [Google Scholar]
- 58.Zaveri M., Khandhar A., Jain S. Quantification of baicalein, chrysin, biochanin-a and ellagic acid in root bark of Oroxylum indicum by RP- HPLC with UV Detection. Eurasian J Anal Chem. 2008;3(2):245–257. [Google Scholar]
- 59.Pushpavalli G., Kalaiarasi P., Veeramani C., Pugalendi K. Effect of chrysin on hepatoprotective and antioxidant status in d-galactosamine-induced hepatitis in rats. Eur J Pharmacol. 2010;631(1–3):36–41. doi: 10.1016/j.ejphar.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 60.Veerappan R., Senthilkumar R. Chrysin enhances antioxidants and oxidative stress in L-NAME-induced hypertensive rats. Int J Nutr Pharmacol Neurol Dis. 2015;5(1):20–27. [Google Scholar]
- 61.Singh J.P., Selvendiran K., Banu S.M., Padmavathi R., Sakthisekaran D. Protective role of apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine. 2004;11(4):309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- 62.Romanova D., Vachalkova A., Cipak L., Ovesna Z., Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48(2):104–107. [PubMed] [Google Scholar]
- 63.Zhang D.Y., Zu Y.G., Fu Y.J., Luo M., Gu C.B., Wang W. Negative pressure cavitation extraction and antioxidant activity of biochanin A and genistein from the leaves of Dalbergia odorifera, in T. Chen. Separ Purif Technol. 2011;83:91–99. [Google Scholar]
- 64.Psotová J., Chlopčíková S., Miketová P., Hrbáč J., Šimánek V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part III. Apigenin, baicalelin, kaempherol, luteolin and quercetin. Phytother Res. 2004;18(7):516–521. doi: 10.1002/ptr.1462. [DOI] [PubMed] [Google Scholar]
- 65.Mantawy E.M., El-Bakly W.M., Esmat A., Badr A.M., El-Demerdash E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol. 2014;728:107–118. doi: 10.1016/j.ejphar.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 66.Pei T.X., Xu C.Q., Li B., Zhang Z.R., Gao X.X., Yu J. Protective effect of quercetin against adriamycin-induced cardiotoxicity and its mechanism in mice. Yao Xue Xue Bao. 2007;42(10):1029–1033. [PubMed] [Google Scholar]
- 67.Warpe V.S., Mali V.R., Arulmozhi S., Bodhankar S.L., Mahadik K.R. Cardioprotective effect of ellagic acid on doxorubicin induced cardiotoxicity in wistar rats. J Acute Med. 2015;5(1):1–8. [Google Scholar]