Key Points
Question
Are there differences in cardiometabolic profiles and exercise hemodynamic parameters in men and women with heart failure with preserved ejection fraction (HFpEF)?
Findings
In this cross-sectional study including 295 participants, compared with men, women with HFpEF demonstrated similar reductions in the percent predicted peak maximum level of oxygen consumption and greater deficits in oxygen delivery and utilization during exercise despite a lower burden of comorbidities in a cohort of rigorously defined HFpEF.
Meaning
The study’s findings suggest that there are sex differences in the cardiac and skeletal muscle responses to exercise in HFpEF.
Abstract
Importance
Sex differences in heart failure with preserved ejection fraction (HFpEF) have been established, but insights into the mechanistic drivers of these differences are limited.
Objective
To examine sex differences in cardiometabolic profiles and exercise hemodynamic profiles among individuals with HFpEF.
Design, Setting, and Participants
This cross-sectional study was conducted at a single-center tertiary care referral hospital from December 2006 to June 2017 and included 295 participants who met hemodynamic criteria for HFpEF based on invasive cardiopulmonary exercise testing results. We examined sex differences in distinct components of oxygen transport and utilization during exercise using linear and logistic regression models. The data were analyzed from June 2018 to May 2019.
Main Outcomes and Measures
Resting and exercise gas exchange and hemodynamic parameters obtained during cardiopulmonary exercise testing.
Results
Of 295 participants, 121 (41.0%) were men (mean [SD] age, 64 [12] years) and 174 (59.0%) were women (mean [SD] age, 61 [13] years). Compared with men, women with HFpEF in this tertiary referral cohort had fewer comorbidities, including diabetes, insulin resistance, and hypertension, and a more favorable adipokine profile. Exercise capacity was similar in men and women (percent predicted peak oxygen [O2] consumption: 66% in women vs 68% in men; P = .38), but women had distinct deficits in components of the O2 pathway, including worse biventricular systolic reserve (multivariable-adjusted analyses: ΔLVEF β = −1.70; SE, 0.86; P < .05; ΔRVEF β = −2.39, SE=0.80; P = .003), diastolic reserve (PCWP/CO: β = 0.63; SE, 0.31; P = .04), and peripheral O2 extraction (C(a-v)O2 β=-0.90, SE=0.22; P < .001)).
Conclusions and Relevance
Despite a lower burden of cardiometabolic disease and a similar percent predicted exercise capacity, women with HFpEF demonstrated greater cardiac and extracardiac deficits, including systolic reserve, diastolic reserve, and peripheral O2 extraction. These sex differences in cardiac and skeletal muscle responses to exercise may illuminate the pathophysiology underlying the development of HFpEF and should be investigated further.
This cross-sectional study examines sex differences in cardiometabolic profiles and exercise hemodynamic profiles among individuals with heart failure with preserved ejection fraction.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is an important public health concern. It accounts for more than 1 million hospitalizations each year, and its prevalence is expected to exceed heart failure with reduced ejection fraction in the near term.1 Its clinical phenotype is highly heterogeneous, making its diagnosis and treatment challenging. To date, to our knowledge, there is no uniform definition and no existing targeted pharmacotherapies have been shown to improve outcomes in clinical trials.2 Sex differences in HFpEF have been well described and likely contribute to its clinical heterogeneity.3,4 The prevalence of HFpEF is markedly higher in women than men.5 Differences in cardiovascular structure, adaptations to physiologic stress, and comorbidity burden have all been proposed to explain this difference, but true mechanistic understanding is lacking, limited by the paucity of women enrolled in heart failure trials and the neutrality of these trials.6 Prior studies have shown that hypertension and aging preferentially predispose women to the development of left ventricular (LV) hypertrophy.7,8 Separately, a recent study demonstrated that cardiometabolic traits are more strongly associated with risk of future HFpEF compared with heart failure with reduced ejection fraction, particularly in women.9
Recognizing the importance of elucidating sex differences in HFpEF to advance our understanding of this condition, we sought to investigate differences in physiologic responses to exercise in men and women with HFpEF. The concept of a clinical phenotype–oriented approach to identify HFpEF subgroups has previously been introduced to help target diagnostics and therapies among this heterogenous population.10 We leveraged exercise hemodynamic measurements, including cardiac responses to exercise, to identify key derangements in oxygen transport and utilization in men and women with HFpEF. In this context, we further sought to investigate differences in cardiac vs extracardiac manifestations of HFpEF among men vs women with known HFpEF.
Methods
Study Sample
We included patients with preserved LV ejection fraction (LVEF ≥50%) and chronic New York Heart Association (NYHA) class II to IV symptoms who underwent clinically indicated cardiopulmonary exercise testing (CPET) with invasive hemodynamic monitoring and had physiologic evidence of HFpEF at Massachusetts General Hospital between December 2006 and June 2017. We defined HFpEF using the following physiologic criteria: evidence of elevated LV filling pressures at rest (resting pulmonary capillary wedge pressure [PCWP], ≥15 mm Hg) or during exercise, with a peak exPCWP of 15 mm Hg or more and abnormally steep increments in PCWP compared with cardiac output (CO; ΔPCWP/ΔCO slope of >2.0 mm Hg/L/min, calculated based on repeated measures of PCWP and CO during exercise within a given individual, as defined previously), and a percent predicted peak maximum oxygen consumption (VO2) of less than 80%.11,12 From this sample (n = 381), we excluded participants with pulmonary arterial hypertension (n = 8), a history of heart or lung transplant (n = 11), complex adult congenital heart disease (n = 11), mitochondrial disorder (n = 5), undergoing evaluation for lung transplant (n = 4), significant valvular disease (n = 38), and severe lung disease (n = 9). All participants provided written informed consent. The study was approved by the Massachusetts General Hospital institutional review board.
Clinical Variables and Biomarkers
History and physical examination results, including vital signs, body mass index (calculated as weight in kilograms divided by height in meters squared), and fasting blood testing, were obtained at the time of CPET. Medical history was obtained via review of medical records. Fasting blood samples were processed and stored at −80°C immediately. An immunoturbidimetric assay (Roche) was used to ascertain high-sensitivity C-reactive protein (hsCRP; intra-assay coefficient of variation [CV], 0.4%-8.4%), and an electrochemiluminescence immunoassay (Roche) was used to assay plasma N-terminal pro–B type natriuretic peptide (intra-assay CV, 2.4%-3.8%). Simple plex assays were used to ascertain human total adiponectin (ProteinSimple; intra-assay CV, 6.5%-8.9%), human interleukin 6 (IL-6) (ProteinSimple; intra-assay CV, 2.4%-3.9%), leptin (ProteinSimple; intra-assay CV, 3.3%-9.3%), and resistin (ProteinSimple; intra-assay CV, 6.4%-9.9%). Echocardiographic parameters obtained within 1 year of CPET were reviewed, including LVEF, LV hypertrophy, left atrial (LA) enlargement, and diastolic dysfunction.
Cardiopulmonary Exercise Testing
All patients underwent right heart catheterization via the internal jugular vein. Participants subsequently performed maximal upright cycle ergometry with a previously described ramp protocol (3-minute initial period of unloaded exercise followed by a 5-20 watts/min continuous ramp).13 Minute-by-minute gas exchange (MedGraphics) and hemodynamic measures were obtained at rest and during each minute of exercise. Key gas exchange and hemodynamic variables obtained included peak VO2 values, as determined by the highest 30-second average during the final 90 seconds of exercise, continuous respiratory exchange ratio, minute-by-minute right atrial pressure, pulmonary arterial pressures, PCWP, and Fick CO derived from a simultaneous assessment of arterial-venous O2 difference (C [a − v] O2) and VO2 values. Radionuclide first-pass ventriculography was also performed at rest and at peak exercise as described previously.14
Statistical Analysis
Results are reported as mean (SD) or medians (interquartile ranges [IQRs]) for continuous variables and percentages for dichotomous variables. Differences between men and women for baseline variables were examined using χ2, t tests, or the Wilcoxon rank sum test as appropriate. We examined sex differences in clinical characteristics and exercise parameters using multivariable linear and logistic regressions as appropriate. All comparisons were adjusted for age, body mass index, hypertension, diabetes, smoking status, and previous myocardial infarction (MI). In exploratory analyses, we further adjusted multivariable models examining exercise parameters for overall exercise capacity (peak VO2) and performed sensitivity analyses, excluding participants with MI and smoking history to account for differences in baseline comorbidities between men and women. Analyses for homeostatic model assessment of insulin resistance (HOMA-IR) excluded patients with prevalent diabetes. The HOMA-IR and biomarkers were natural log-transformed because of right-skewed distributions. All tests were 2-sided and a P value of <.05 was considered significant. Analyses were conducted using Stata, version 15.1 (StataCorp).
Results
A total of 295 patients with an LVEF of 50% or more and NYHA class II to IV symptoms met hemodynamic criteria for HFpEF and were included in our analysis. Of these, 174 (59%) were women. Of the 295 patients, 65 (22%) met HFpEF criteria on the basis of abnormal resting PCWP, the remainder based on abnormal exercise hemodynamics, with similar proportions among men and women. Table 1 displays the baseline clinical characteristics for men and women and Table 215,16 displays the gas exchange and hemodynamic parameters obtained during invasive cardiopulmonary testing. In brief, women were younger (women: mean [SD] age, 61 [13] years; men: mean [SD] age, 64 [12] years) and had fewer comorbidities, including diabetes (25 women [14%] vs 35 men [29%]), insulin resistance (median HOMA-IR, 1.9 mg·IU/dL·mL [IQR, 1.0-3.3] in women vs 2.7 [IQR, 1.5-4.1] mg·IU/dL·mL in men), hypertension (98 women [56%] vs 91 men [75%]), and previous MI (4 women [2%] vs 12 men [10%]). Concomitantly, fewer women were taking antihypertensive medications (111 women [64%] vs 92 men [76%]) with similar diuretic use (51 women [40%] vs 51 men [42%]). Among women, 15 (9%) reported hormone therapy use.
Table 1. Baseline Clinical Characteristics in Men and Women.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Men (n = 121) | Women (n = 174) | ||
| Clinical characteristic | |||
| Age, mean (SD), y | 64 (12) | 61 (13) | .05 |
| White | 114 (94) | 166 (95) | .36 |
| Current smoking | 2 (1.7) | 6 (3.5) | .48 |
| Total cholesterol, mg/dL | 156 (40) | 175 (36) | .01 |
| Prevalent MI | 12 (10) | 4 (2) | .01 |
| Prevalent AF | 35 (29) | 23 (13) | .001 |
| Previous HF admission | 11 (9) | 14 (8) | .83 |
| Paced rhythm | 4 (4) | 5 (4) | >.99 |
| Hemoglobin, mean (SD), g/dL | 14 (4) | 12 (1) | <.001 |
| eGFR, mean (SD), mL/min/1.73 m2 | 73 (23) | 79 (30) | .12 |
| Cardiometabolic traits | |||
| BMI, mean (SD)a | 30.9 (5.0) | 31.1 (7.8) | .89 |
| Diabetes | 35 (29) | 25 (14) | .003 |
| HOMA-IR, mg·IU/dL·mL | 2.7 (1.5-4.1) | 1.9 (1.0-3.3) | .01 |
| Hypertension | 91 (75) | 98 (56) | .001 |
| Pharmacotherapy | |||
| Diuretics | 51 (42) | 70 (40) | .81 |
| ACE inhibitor or ARB | 55 (46) | 40 (23) | <.001 |
| β-adrenergic blocker | 64 (53) | 70 (41) | .03 |
| Calcium channel blocker | 20 (17) | 33 (19) | .65 |
| Hormone therapy | NA | 15 (9) | NA |
| Biomarker profiles, median (IQR) | |||
| hsCRP, mg/L | 2.3 (1.1-5.1) | 3.0 (1.2-6.2) | .21 |
| IL-6, pg/mL | 5.7 (3.2-8.9) | 5.1 (3.2-8.4) | .24 |
| Adiponectin, ng/mL | 4973 (3566-8203) | 7466 (5238-10 843) | <.001 |
| Leptin, pg/mL | 12 133 (7048-22 221) | 31 241 (17 194-54 999) | <.001 |
| Resistin, pg/mL | 12 544 (9739-17 088) | 11 266 (8570-15 187) | .04 |
| NT-proBNP, pg/mL | 92 (46-429) | 118 (57-343) | .43 |
Abbreviations: ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; HF, heart failure; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; IQR, interquartile range; MI, myocardial infarction; NA, not applicable; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; hemoglobin to grams per liter, multiply by 10; hsCRP to nanomoles per liter, multiply by 9.524.
Calculated as weight in kilograms divided by height in meters squared.
Table 2. Baseline Gas Exchange and Hemodynamic Parameters in Men and Women.
| Measure | Mean (SD) | |||
|---|---|---|---|---|
| Men (n = 121) | Women (n = 174 | |||
| Rest | Exercise | Rest | Exercise | |
| Gas exchange parameters | ||||
| Peak VO2, mL/kg/min | NA | 15.5 (4.2) | NA | 14.0 (3.7)a |
| Predicted peak VO2, %15 | 68 (17) | 66 (14) | ||
| Work, W | 108 (39) | 79 (32)a | ||
| MVV, L/min | 97 (29) | 72 (24)a | ||
| VE/MVV | 0.70 (0.25) | 0.72 (0.21) | ||
| VE/VCO2 slope | 37 (10) | 39 (10) | ||
| Peak RER | 1.15 (0.11) | 1.16 (0.13) | ||
| C(a−v)O2, mL/100 mL | 6.2 (1.2) | 12.4 (2.2) | 5.8 (1.1)a | 10.7 (1.7)a |
| C(a−v)O2/Hb, mL/g | NA | 0.86 (0.13) | NA | 0.82 (0.11)a |
| Hemodynamic parameters | ||||
| SBP, mm Hg | 148 (22) | 186 (38) | 151 (24) | 189 (28) |
| DBP, mm Hg | 76 (13) | 87 (20) | 76 (11) | 90 (17) |
| HR, bpm | 73 (15) | 122 (25) | 77 (14)a | 132 (26)a |
| SV, mL | 77 (23) | 101 (27) | 64 (19)a | 81 (21)a |
| SVi, mL/m2 | 36 (10) | 48 (11) | 35 (10) | 44 (10)a |
| PAP, mm Hg | 25 (7) | 42 (10) | 23 (7)a | 38 (9)a |
| PCWP, mm Hg | 16 (7) | 26 (8) | 14 (6)a | 24 (6)a |
| Fick CO, L/min | 5.4 (1.7) | 11.4 (3.3) | 4.7 (1.4)a | 10.0 (3.0)a |
| CI, L/min/m2 | 2.5 (0.7) | 5.3 (1.3) | 2.6 (0.7) | 5.4 (1.4) |
| LVEF, % | 61.3 (9.2) | 65.4 (8.5) | 63.5 (7.6)a | 65.6 (7.8) |
| RVEF, % | 48.5 (7.7) | 52.0 (8.5) | 51.8 (8.1)a | 52.7 (9.1) |
| O2 pulse, mL/beat | 4.4 (1.1) | 12.9 (3.5) | 3.4 (0.9)a | 8.8 (2.6)a |
| Pulm distensibility, %/mm Hg16 | NA | 1.0 (0.5) | NA | 1.1 (0.6) |
| PAP/CO slope, mm Hg/L/min | 3.9 (2.1) | 4.3 (3.3) | ||
| PCWP/CO slope, mm Hg/L/min | 3.1 (2.2) | 3.6 (2.8) | ||
Abbreviations: bpm, beats per minute; C (a − v) O2, peripheral oxygen extraction; CI, cardiac index; CO, cardiac output; DBP, diastolic blood pressure; Hb, hemoglobin; HR, heart rate; LVEF, left ventricular ejection fraction; MVV, maximum voluntary ventilation; NA, not applicable; O2, oxygen; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RER, respiratory exchange ratio; RVEF, right ventricular ejection fraction; SBP, systolic blood pressure; SV, stroke volume; SVi, stroke volume index; VCO2, carbon dioxide production; VE, ventilatory efficiency; VO2, oxygen consumption.
P < .05 for comparison between men vs women in either rest or peak parameters.
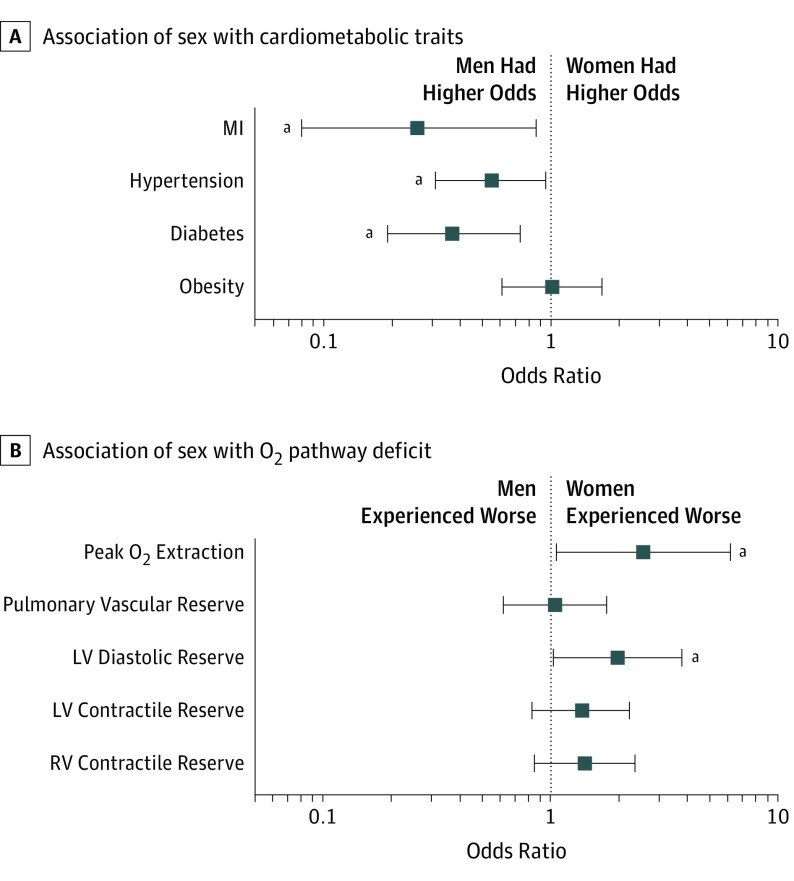
Sex differences in comorbid conditions persisted after accounting for clinical covariates in multivariable analyses: women with HFpEF had lower odds of diabetes (odds ratio [OR], 0.37; 95% CI, 0.19-0.73; P = .004), hypertension (OR, 0.55; 95% CI, 0.31-0.95; P = .03), and previous MI (OR, 0.26; 95% CI, 0.08-0.86; P = .03) but similar odds of obesity (OR, 1.01; 95% CI, 0.61-1.68; P = .97) (Figure 1A). In addition, adipokine profiles were more favorable among women compared with men with HFpEF (adiponectin: β = 0.52; SE, 0.11; P < .001; leptin β = 0.93; SE, 0.08; P < .001) (eTable 1 in the Supplement), although inflammatory biomarkers, including hsCRP and IL-6, were similar (hsCRP: ß = 0.19; SE, 0.12; P = .12; IL-6: ß = −0.09; SE, 0.13; P = .49).
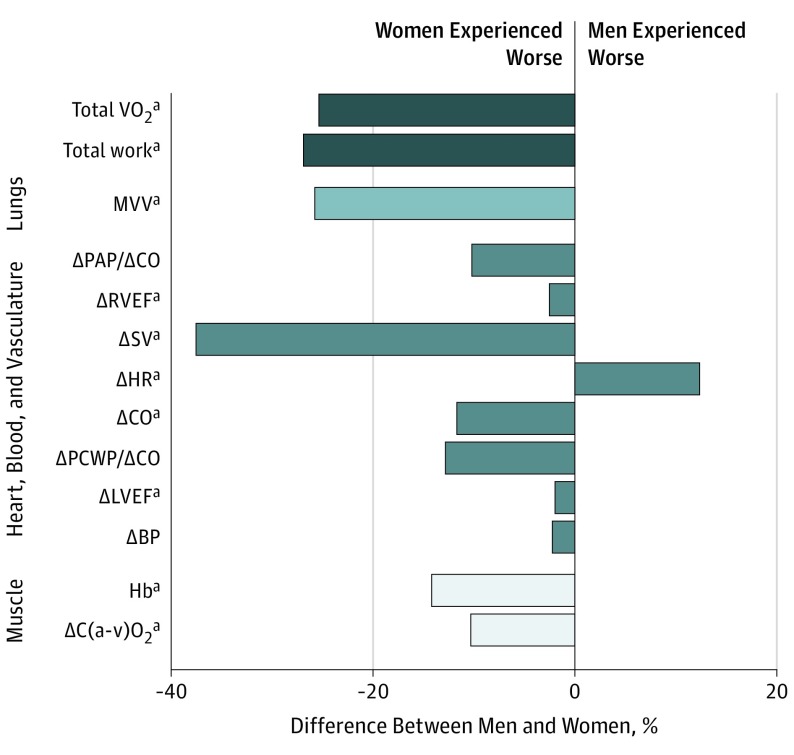
Figure 1. Association of Sex With Traits and Deficits.
The schematic depicts the sequence of inspired oxygen (O2) transport from inspired air to muscle. The bar graph depicts the percentage difference in absolute values or deltas (peak minus rest values) between men and women. BP indicates blood pressure; C (a − v) O2, peripheral oxygen extraction; CO, cardiac output; Hb, hemoglobin; HR, heart rate; LVEF, left ventricular ejection fraction; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RVEF, right ventricular ejection fraction; SV, stroke volume; VO2, oxygen consumption; VCO2, carbon dioxide production.
aP < .05.
Resting and exercise gas exchange and hemodynamic parameters were examined in women and men during invasive cardiopulmonary testing. During exercise, women and men achieved similar respiratory exchange ratios, indicative of maximal effort in both groups. In this cohort, women demonstrated lower peak VO2 values (14 mL/kg/min in women vs 16 mL/kg/min in men), mean (SD) peak workload (79 [32] W in women vs 108 [39] W in men), and mean (SD) O2 pulse (8.8 [2.6] mL/beat in women vs 12.9 [3.5] mL/beat in men), but the percent predicted VO2 were similar in men and women (66% in women vs 68% in men; P = .38) (Table 2).
We examined distinct steps of oxygen transport and utilization in the context of the oxygen pathway17 and found notable sex differences in exercise responses (Figure 1). Gas exchange parameters were consistently worse in women, including maximum voluntary ventilation, breathing reserve (1 − ventilatory efficiency [VE]/maximum voluntary ventilation), and VE (VE/ventilatory CO2 slope). When examining the right ventricular (RV)–pulmonary vascular circuit, we found lower RV reserve (ΔRVEF) in women but no differences in pulmonary distensibility or pulmonary vascular reserve (pulmonary artery pressure/CO slope) (Table 2). With respect to cardiac contributions to exercise capacity, women exhibited lower stroke volumes, heart rates, and Fick CO augmentation with exercise as well as worse diastolic reserve (PCWP/CO slope) and LV reserve (ΔLVEF) (Table 2). Finally, women exhibited lower peripheral oxygen extraction (C [a − v] O2) even after adjustment for hemoglobin levels.
Sex differences in peripheral oxygen extraction, diastolic reserve, and LV and RV reserve persisted after multivariable adjustment (Table 3). Specifically, peak CO was 1.9 L lower in women compared with men (β, −1.94; SE, 0.29; P < .001) and the PCWP/CO slope was 0.5 mm Hg/L/min steeper in women (β, 0.63; SE, 0.31; P = .04). Women had a 0.9 mL/100 mL–lower C [a − v] O2, indicating worse O2 extraction at peak exercise compared with men (β, −0.90; SE, 0.22; P < .001). Similarly, augmentation of RV and LV ejection fraction during exercise was 2.4% and 1.7%, respecitvely, lower in women compared with men, indicating worse RV reserve (β, −2.39; SE, 0.80; P = .003) and LV reserve (β, −1.70; SE, 0.86; P = .05) (Table 3). Differences in LV and RV reserve between men and women were attributable to baseline differences in resting LVEF and RVEF, with peak LVEF and RVEF being similar in men and women. Hemodynamic parameters were examined at 30 W of exercise and 50% peak workload and showed similar trends to peak values (eTable 2 in the Supplement). These sex differences in rest and exercise parameters persisted even after additional adjustment for baseline history of atrial fibrillation (eTables 3 and 4 in the Supplement).
Table 3. Association of Sex With Gas Exchange and Hemodynamic Measures.
| Measure | Rest | Exercise | ||
|---|---|---|---|---|
| βa (SE) | P Value | βa (SE) | P Value | |
| Gas exchange parameters | ||||
| Peak VO2 | NA | NA | −2.25 (0.37) | <.001 |
| % predicted peak VO2 | −2.91 (1.71) | .09 | ||
| Work, watts | −36.2 (3.5) | <.001 | ||
| MVV, L/min | −27.6 (2.7) | <.001 | ||
| VE/MVV | 0.005 (0.04) | .90 | ||
| VE/VCO2 slope | 2.58 (1.16) | .03 | ||
| Peak RER | −0.005 (0.02) | .72 | ||
| C(a-v)O2, mL/100 mL | −0.21 (0.14) | .13 | −0.90 (0.22) | <.001 |
| Hemodynamic parameters | ||||
| SBP, mmHg | 5.12 (2.72) | .06 | 1.67 (3.95) | .67 |
| HR, bpm | 3.06 (1.77) | .09 | 4.61 (2.78) | .10 |
| O2 pulse, mL/beat | −1.00 (0.11) | <.001 | −4.02 (0.32) | <.001 |
| PP, mmHg | 5.81 (2.20) | .01 | 0.61 (2.81) | .83 |
| PAP, mmHg | −2.20 (0.83) | .01 | −4.62 (1.12) | <.001 |
| PCWP, mmHg | −1.88 (0.71) | .01 | −2.51 (0.91) | .01 |
| Fick CO, L/min | −0.77 (0.16) | <.001 | −1.95 (0.29) | <.001 |
| PAP/CO slope, mmHg/L/min | NA | NA | 0.56 (0.35) | .11 |
| PCWP/CO slope, mmHg/L/min | 0.63 (0.31) | .04 | ||
| Pulm distensibility, %/mmHg | 0.11 (0.06) | .09 | ||
| ΔLVEF (peak − rest) | −1.70 (0.86) | .05 | ||
| ΔRVEF (peak − rest) | −2.39 (0.80) | .003 | ||
Abbreviations: C (a − v) O2, peripheral oxygen extraction; CO, cardiac output; HR, heart rate; LVEF, left ventricular ejection fraction; MVV, maximum voluntary ventilation; NA, not applicable; O2, oxygen; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PP, pulse pressure; RER, respiratory exchange ratio; RVEF, right ventricular ejection fraction; SBP, systolic blood pressure; VCO2, carbon dioxide production; VE, ventilatory efficiency; VO2, oxygen consumption.
β coefficient: regression coefficients represent the difference between women (referent) and men for continuous variables. The multivariable model for gas exchange and hemodynamic parameters adjusts for age, body mass index (calculated as weight in kilograms divided by height in meters squared), hypertension (except for SBP), diabetes status, current smoking, previous myocardial infarction, and hemoglobin (only for C [a − v] O2).
When examining dichotomized traits to indicate specific deficits along the oxygen pathway, we found cardiac and extracardiac deficits were more prevalent in women compared with men with HFpEF (eFigure in the Supplement). Specifically, in multivariable-adjusted analyses, we found that women had a nearly 2-fold increased odds of having abnormal diastolic reserve (defined as an abnormal PCWP/CO slope >2; OR, 1.97; 95% CI, 1.03-3.78; P = .04) and a more than 2-fold increased odds of having abnormal peripheral O2 extraction (defined as C [a − v] O2 < hemoglobin; OR, 2.55; 95% CI, 1.06-6.16; P = .04) (Figure 2; eTable 5 in the Supplement). When dichotomizing systolic reserve using a change of more than 5% in ejection fraction with exercise, we found that 111 (64%) and 122 (70%) women and 67 (55%) and 77 (64%) men had abnormal RV and LV reserve, respectively, with no statistically significant sex differences. These sex differences in exercise parameters and hemodynamic abnormalities remained significant in sensitivity analyses, after exclusion of individuals with MI and active smoking (eTable 6 in the Supplement).
Figure 2. Relative Differences in Components of the Oxygen Pathway Between Men and Women.
A, Cross-sectional association of sex with binary cardiometabolic traits. B, Cross-sectional association of sex with deficits in oxygen transport and utilization. Error bars indicate 95% CIs. LV indicates left ventricle; MI, myocardial infarction; O2, inspired oxygen; RV, right ventricle.
aP < .05.
Discussion
In a HFpEF sample uniformly defined by rest and exercise hemodynamics, we highlight important sex differences in cardiac and skeletal muscle responses to exercise. Despite a similar percent predicted peak VO2, women exhibited worse peripheral O2 extraction with exercise, worse RV and LV systolic reserve as ascertained by measured CO and first-pass radionuclide ventriculography, and worse diastolic reserve as measured by the ΔPCWP/ΔCO response to exercise compared with men. These findings highlight important sex differences in oxygen delivery and utilization that contribute to HFpEF in men vs women.
Exercise intolerance is a central feature of HFpEF, and prior studies have described impairments in cardiac, vascular, and skeletal muscle reserve as contributors to impaired exercise tolerance.4,17,18,19,20,21 Exercise hemodynamics can help identify defects along the oxygen transport and utilization pathway that contribute to HFpEF and also carry clinical and prognostic information. For example, PCWP responses during exercise may indicate early HFpEF and predict poor exercise capacity and future heart failure outcomes.11,22 Little is known about sex differences in cardiac and extracardiac reserve with exercise, and available data are conflicting. In a study of 215 healthy men and women, cardiac power output and cardiac reserve decreased with age preferentially in men compared with women.23 By contrast, in the Baltimore Longitudinal Study of Aging of 200 healthy volunteers, women demonstrated increased resting and exercise vascular resistance and an increased reliance on chronotropic reserve with age compared with men.24 A recent investigation of differences in exercise hemodynamics between 47 men and 114 women with HFpEF found that women had greater impairments in diastolic reserve (evidenced by greater PCWP indexed to peak exercise workload) and more abnormal peripheral oxygen kinetics.25 Our findings are consistent, with further refinement of exercise physiology using multipoint measures of PCWP and CO, direct measurements of oxygen extraction throughout exercise, and an overlay of baseline cardiometabolic traits in a larger sample of uniformly physiologically defined HFpEF. Specifically, we show that women with HFpEF demonstrate lower exercise peripheral O2 extraction, lower LV and RV reserve, and a steeper PCWP/CO slope indicative of diastolic dysfunction as unmasked during exercise when compared with men. These sex differences persisted even after adjustment for hemoglobin values, which has been proposed to be a major driver of differences in oxygen transport and utilization in men and women. Ultimately, these differences in exercise response suggest that the underlying pathophysiologic drivers of HFpEF may be distinct among women and men.
While sex differences in cardiac remodeling may provide the biologic basis for the female preponderance among patients with HFpEF,26,27 the mechanisms underlying these observed differences remain unclear. It is known that over the adult life course, women are more likely than men to develop concentric LV hypertrophy and diastolic dysfunction, particularly in the setting of hypertension.3,8,28 Moreover, cardiometabolic disease appears to have a greater association with future HFpEF risk among women when compared with men studied in longitudinal cohorts.9 It is postulated that cardiometabolic disease leads to systemic inflammation, activation of the inflammatory cascade, and ensuing coronary microvascular endothelial inflammation,29 impaired nitric oxide signaling, and increased production of profibrotic cytokines, which all converge to induce hypertrophy, interstitial fibrosis, and ultimately, increased LV stiffness and clinical HFpEF.30 Our findings suggest that impaired diastolic reserve appears particularly prominent among women with HFpEF. Whether distinct cardiovascular responses to cardiometabolic risk factors may explain sex differences in HFpEF remains unclear. In our tertiary referral sample, we found lower rates of hypertension, diabetes, and insulin resistance; more favorable cardioprotective adipokine profiles; and similar levels of the inflammatory markers hsCRP and IL-6 in women compared with men. In contrast to prior studies, we show that in this unique sample of patients referred to undergo invasive CPET, women meet the same rigorous physiologically defined HFpEF criteria with hemodynamic derangements and demonstrate limitations in exercise capacity commensurate to men despite a lower burden of cardiometabolic disease. Further studies in other HFpEF cohorts are needed to elucidate whether women with HFpEF are more susceptible to the deleterious effects of cardiometabolic disease on cardiovascular structure and reserve.
Limitations
Our study has several limitations. First, the study sample included consecutive patients with NYHA class II to IV symptoms with an LVEF of more than 50% who were referred for clinically indicated CPET. While men and women met the same hemodynamic criteria used to define HFpEF, women had a lower burden of comorbidities. Differences in baseline characteristics may reflect the possibility that men and women were differentially referred to clinical CPET, although this is less likely given the similar functional impairment between sexes. Direct comparisons between women and men may be affected by differences in disease severity. While men and women met the same hemodynamic criteria used to define HFpEF, women had a lower burden of comorbidities. To account for these differences, we performed sensitivity analyses excluding individuals with prior MI and current smokers, which demonstrated similar results. Second, the sample was younger with less comorbid disease burden compared with other HFpEF clinical trials or registries.31,32,33 As a result, the study sample may reflect a more proximal (albeit symptomatic with a reduced peak VO2) stage of HFpEF, and the generalizability to other samples, including prior HFpEF clinical trials or registries, may be limited (eTable 7 in the Supplement). Finally, this is a cross-sectional observational study and causal inferences cannot be made. Nevertheless, one of the strengths of this study was the use of invasive hemodynamic rest and exercise measures to rigorously define HFpEF using physiologic criteria. This allowed us to circumvent inherent sex differences in other commonly used HFpEF criteria, including echocardiographic measures and natriuretic peptides.34,35 Therefore, further study of sex differences in exercise responses in broader HFpEF samples is warranted.
Conclusions
These findings highlight important sex differences in exercise hemodynamics among women and men with physiologically defined HFpEF. We show that women with HFpEF are characterized by greater deficits in cardiac and skeletal muscle responses, including worse peripheral O2 extraction with exercise, RV and LV systolic reserve, and diastolic reserve compared with men. These findings may partially explain important sex differences in the epidemiology and clinical course of HFpEF. Whether sex differences can help further elucidate underlying mechanisms leading to HFpEF and ultimately lead to targeted therapies remains to be studied.
eFigure. Proportion of men and women with abnormal cardiac and extra-cardiac exercise hemodynamic profile
eTable 1. Sex as a predictor of cardiometabolic traits and biochemical profiles of inflammation
eTable 2. Gas Exchange and Hemodynamic Parameters at 30W and 50% peak workload
eTable 3. Sex as a predictor of cardiometabolic traits and biochemical profiles of inflammation further adjusted for history of AF
eTable 4. Sex as a predictor of gas exchange and hemodynamic measures further adjusted for history of AF
eTable 5. Sex as a predictor of abnormal cardiac and extra-cardiac exercise hemodynamic profiles
eTable 6. Sensitivity analyses of abnormal cardiac and extra-cardiac exercise hemodynamic profiles
eTable 7. Comparison of Baseline Characteristics by Sex of HFpEF Clinical Trials and Registries with the MGH CPET Cohort
References
- 1.Steinberg BA, Zhao X, Heidenreich PA, et al. ; Get With the Guidelines Scientific Advisory Committee and Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126(1):65-75. doi: 10.1161/CIRCULATIONAHA.111.080770 [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72(3):310-313. doi: 10.1016/0002-9149(93)90678-6 [DOI] [PubMed] [Google Scholar]
- 4.Gori M, Lam CS, Gupta DK, et al. ; PARAMOUNT Investigators . Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(5):535-542. doi: 10.1002/ejhf.67 [DOI] [PubMed] [Google Scholar]
- 5.Ho JE, Enserro D, Brouwers FP, et al. . Predicting heart failure with preserved and reduced ejection fraction: the International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9(6):e003116. doi: 10.1161/CIRCHEARTFAILURE.115.003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahhan AS, Vaduganathan M, Greene SJ, et al. . Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3(10):1011-1019. doi: 10.1001/jamacardio.2018.2559 [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557-1562. doi: 10.1001/jama.1996.03530440037034 [DOI] [PubMed] [Google Scholar]
- 8.Lieb W, Xanthakis V, Sullivan LM, et al. . Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the Framingham Offspring study. Circulation. 2009;119(24):3085-3092. doi: 10.1161/CIRCULATIONAHA.108.824243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savji N, Meijers WC, Bartz TM, et al. . The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6(8):701-709. doi: 10.1016/j.jchf.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samson R, Jaiswal A, Ennezat PV, Cassidy M, Le Jemtel TH. Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5(1):e002477. doi: 10.1161/JAHA.115.002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisman AS, Shah RV, Dhakal BP, et al. . Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11(5):e004750. doi: 10.1161/CIRCHEARTFAILURE.117.004750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher GF, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards: a statement for health professionals from the American Heart Association. Circulation. 1990;82(6):2286-2322. doi: 10.1161/01.CIR.82.6.2286 [DOI] [PubMed] [Google Scholar]
- 13.Malhotra R, Bakken K, D’Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4(8):607-616. doi: 10.1016/j.jchf.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 14.Lewis GD, Shah R, Shahzad K, et al. . Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555-1562. doi: 10.1161/CIRCULATIONAHA.107.716373 [DOI] [PubMed] [Google Scholar]
- 15.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131(5):700-708. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra R, Dhakal BP, Eisman AS, et al. . Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ Heart Fail. 2016;9(6):e003011. doi: 10.1161/CIRCHEARTFAILURE.115.003011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houstis NE, Eisman AS, Pappagianopoulos PP, et al. . Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation. 2018;137(2):148-161. doi: 10.1161/CIRCULATIONAHA.117.029058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borlaug BA, Olson TP, Lam CS, et al. . Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845-854. doi: 10.1016/j.jacc.2010.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzman DW, Haykowsky MJ, Tomczak CR. Making the case for skeletal muscle myopathy and its contribution to exercise intolerance in heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10(7):e004281. doi: 10.1161/CIRCHEARTFAILURE.117.004281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhakal BP, Malhotra R, Murphy RM, et al. . Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8(2):286-294. doi: 10.1161/CIRCHEARTFAILURE.114.001825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy YNV, Andersen MJ, Obokata M, et al. . Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70(2):136-148. doi: 10.1016/j.jacc.2017.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588-595. doi: 10.1161/CIRCHEARTFAILURE.109.930701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldspink DF, George KP, Chantler PD, et al. . A study of presbycardia, with gender differences favoring ageing women. Int J Cardiol. 2009;137(3):236-245. doi: 10.1016/j.ijcard.2008.06.086 [DOI] [PubMed] [Google Scholar]
- 24.Fleg JL, O’Connor F, Gerstenblith G, et al. . Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol (1985). 1995;78(3):890-900. doi: 10.1152/jappl.1995.78.3.890 [DOI] [PubMed] [Google Scholar]
- 25.Beale AL, Nanayakkara S, Segan L, et al. . Sex differences in heart failure with preserved ejection fraction pathophysiology. a detailed invasive hemodynamic and echocardiographic analysis. JACC Heart Fail. 2019;7(3):239-249. doi: 10.1016/j.jchf.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 26.Masoudi FA, Havranek EP, Smith G, et al. . Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41(2):217-223. doi: 10.1016/S0735-1097(02)02696-7 [DOI] [PubMed] [Google Scholar]
- 27.Ho JE, Gona P, Pencina MJ, et al. . Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J. 2012;33(14):1734-1741. doi: 10.1093/eurheartj/ehs070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112(15):2254-2262. doi: 10.1161/CIRCULATIONAHA.105.541078 [DOI] [PubMed] [Google Scholar]
- 29.Mohammed SF, Borlaug BA, Roger VL, et al. . Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5(6):710-719. doi: 10.1161/CIRCHEARTFAILURE.112.968594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263-271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 31.Lam CS, Carson PE, Anand IS, et al. . Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2012;5(5):571-578. doi: 10.1161/CIRCHEARTFAILURE.112.970061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail. 2019;7(3):228-238. doi: 10.1016/j.jchf.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsich EM, Grau-Sepulveda MV, Hernandez AF, et al. . Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J. 2012;163(3):430-437, 437.e1-437.e3. doi: 10.1016/j.ahj.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 34.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):e147-e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 35.McMurray JJ, Adamopoulos S, Anker SD, et al. ; ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Proportion of men and women with abnormal cardiac and extra-cardiac exercise hemodynamic profile
eTable 1. Sex as a predictor of cardiometabolic traits and biochemical profiles of inflammation
eTable 2. Gas Exchange and Hemodynamic Parameters at 30W and 50% peak workload
eTable 3. Sex as a predictor of cardiometabolic traits and biochemical profiles of inflammation further adjusted for history of AF
eTable 4. Sex as a predictor of gas exchange and hemodynamic measures further adjusted for history of AF
eTable 5. Sex as a predictor of abnormal cardiac and extra-cardiac exercise hemodynamic profiles
eTable 6. Sensitivity analyses of abnormal cardiac and extra-cardiac exercise hemodynamic profiles
eTable 7. Comparison of Baseline Characteristics by Sex of HFpEF Clinical Trials and Registries with the MGH CPET Cohort