Abstract
Background
Acacia catechu has been widely used in Ayurveda for treating many diseases. Its heartwood extract is used in asthma, cough, bronchitis, colic, diarrhea, dysentery, boils, skin afflictions, sores and for stomatitis. The decoction of heartwood is used for drinking purpose in southern part of India especially in Kerala.
Objective
The current study was carried out to evaluate immunomodulatory effects of heartwood extracts of A. catechu in Swiss albino mice.
Material and methods
In vivo immunomodulatory activity was analyzed by hemagglutinating antibody (HA) titer, plaque forming cell assay and delayed type hypersensitivity (DTH). In vitro immunomodulatory potential of the extracts was studied using peritoneal macrophages and splenocytes from mice. Effect of extracts on phagocytic activity of macrophages was analyzed by nitroblue tetrazolium (NBT) reduction assay and cellular lysosomal enzyme assay. Anti-inflammatory activity was studied by nitric oxide (NO) assay and production of TNF-α and IL-10.
Results
A dose dependent increase in antibody titer was observed with extracts treatment. Treatment with extracts produced an enhancement in the number of antibody producing cells in the spleen. DTH reaction was significantly decreased with extracts treatment. An increased phagocytic response was shown by peritoneal macrophages on treatment with the extracts as evidenced by its effect on NBT reduction and cellular lysosomal enzyme activity. The extracts inhibited the release of pro-inflammatory cytokine TNF-α and the production of NO. IL-10 production was significantly increased after extract treatment.
Conclusion
The results of the present study indicate the immunomodulatory effects of A. catechu extracts on humoral, cell mediated and non-specific immune functions.
Keywords: Acacia catechu, HA titer, DTH, Cytokine
1. Introduction
The vertebrate immune system is a versatile defense system that is involved in protecting the host from invading pathogenic microorganisms. It also maintains a surveillance system that continuously monitors the integrity of host tissues [1]. The immune system influences pathophysiology of many infectious diseases. Such diseases can be alleviated by the modulation of immune responses. The two objectives of immunomodulation which is used to control diseases are stimulation of the immune system to develop immunity and suppression of undesired immune responses. The traditional medicines play a pivotal role in stimulating and suppressing the host immune responses. Plant derived natural substances can serve as immunomodulators to control certain immune responses [2].
Acacia catechu, a deciduous tree of the Fabaceae family is indigenous in India, other Asian countries, and East Africa. It is commonly known as catechu, cachou and black cutch. This plant is widely used in Ayurveda for many diseases including skin diseases. Ayurveda uses bark and heartwood of this plant for various formulations. Khadirarishta is a famous Ayurvedic skin tonic prepared from A. catechu. Khadira Sara, the heartwood extract is used as ingredient in many medicines such as Lavangadi Vati [3]. “Ercha” is a traditional Chinese medicine prepared from the heartwood extract of catechu used in the treatment of cough, dysentery, skin ulcerations and lesions [4]. The gummy extract of the wood called black catechu, is used as an anodyne, astringent and bactericide. The heartwood extract is also used in asthma, bronchitis, colic, diarrhea, boils, skin afflictions, sores and stomatitis. The bark shows anti-helmintic, antipyretic and anti-inflammatory properties. It is used in the treatment of bronchitis, ulcers, psoriasis, anaemia and gum troubles. The immunomodulatory activity of aqueous extract of bark of A. catechu has been reported [5]. The sap of A. catechu is commonly used for the treatment of diarrhea and wounds in ruminants. In veterinary folk medicine, both the extracts of bark and heartwood are used for broken horn [6]. The decoction prepared from the heartwood of this plant is used for drinking purpose in southern part of India especially in Kerala.
The important chemical constituents isolated and characterized from A. catechu include catechin, rutin, isorhamnetin, epicatechin, kaempferol, 4-hydroxybenzoic acid, 3,4′,7-trihydroxyl-3′,5-dimethoxyflavone, quercetin, afzelechin, epiafzelechin, mesquitol, aromadendrin, ophioglonin, and phenol [7]. Catechins, rutin and isorhamnetin show antioxidant property by scavenging free radicals [8]. These compounds are largely present in the heartwood extract of A. catechu and may contribute to the biopotency of A. catechu [9]. In the present study, we report the immunomodulatory activity of heartwood extracts of A. catechu by analyzing their effect on phagocytosis, specific antibody and antibody producing cells in spleen, delayed type hypersensitivity (DTH), nitric oxide (NO) production, TNF-α and IL-10 production by LPS stimulated macrophages and splenocyte proliferation.
2. Materials and methods
2.1. Plant material and extraction
A. catechu was collected from Kannur District, Kerala, India in October, 2012. The specimen was authenticated by the taxonomist from Department of Botany, St. Thomas College, Pala, Kottayam, Kerala, India. Voucher specimens (Sunil MA 1504) have been deposited at herbarium of Department of Botany, St. Thomas College. Heartwood of the plant was shade dried, powdered and stored in sealed containers. The powder was defatted with hexane and then subjected to successive extraction using ethanol and water by Soxhlet extraction. The solvent was removed under reduced pressure and the extracts were stored at 4 °C, till used.
2.2. Animals
Swiss albino male mice (20–30 g) were obtained from Kerala Veterinary University, Thrissur, Kerala. They were maintained in animal house under standard conditions and fed with standard pellet diet and water ad libitum. The experiments were approved by Institutional Animal Ethics Committee (No: B21032014-08).
2.3. Sheep red blood cells (SRBCs) suspension
Fresh sheep blood from local slaughter house was collected in Alsever's solution (1:1 proportion) and was kept in the refrigerator. SRBC for immunization was prepared by centrifuging at 2000 rpm for 10 min and washing 4–5 times with physiological saline. The washed SRBCs were then suspended in buffered saline at desired concentration.
2.4. Treatment
The doses for the present study were fixed by Irwin test at 500, 1000 and 2000 mg/kg body weight concentrations [10]. The A. catechu water extract and ethanol extract were dissolved in phosphate-buffered saline (pH 7.2). For further assays, 1/10th–1/20th of the dose at which behavioral alterations were observed was considered as safe dose [11]. The extracts did not produce any toxic effect up to 2 g/kg b. w. concentration. 100 and 200 mg/kg b. w. were fixed as the dose range for further studies. Six groups of Swiss albino mice (6 Nos/group) were used in the present study. Group I was control which received PBS. Group II were treated with dexamethasone (DMS) at a concentration of 1.25 mg/kg b. w. Animals of group III, IV, V, and VI received 100 and 200 mg/kg b. w. of the two extracts orally in volumes of 0.2 ml/day for 30 days.
2.5. Preparation of peritoneal mouse macrophages
One milliliter of 3% Brewer thioglycollate medium (Himedia, India) was injected intraperitoneally into mice as a stimulant to elicit peritoneal macrophages. Four days later, the peritoneal exudate was collected by peritoneal lavage with 10 ml of RPMI-1640 medium (Himedia, India). The exudate was centrifuged at 400 × g, 4° C for 10 min. The supernatant was discarded and cell pellet was resuspended in complete RPMI-1640 medium. Contaminating erythrocytes were lysed using erythrocyte lysis buffer. The cell number was determined by counting in a hemocytometer and the cell viability was tested by the trypan-blue dye exclusion technique [12].
2.6. Preparation of mouse splenocytes
Splenocytes were prepared according to the method previously described [13] with a slight modification. Briefly, mice were sacrificed and their spleens were removed aseptically. The cell suspension was prepared by means of flushing. After centrifugation at 1000 rpm at 37 °C for 10 min, erythrocytes were lysed by lysis buffer and the cell pellets were washed twice with RPMI-1640 medium. The cells were re-suspended in complete RPMI-1640 medium and the cell number was adjusted to desired concentration. The viability of splenocytes was determined by the trypan-blue dye exclusion technique as described earlier.
2.7. Hemagglutinating antibody (HA) titer
Hemagglutinin titer assay was performed using the procedure of Bin-Hafeez et al [14]. On the day of termination of the treatment, animals were immunized with 0.2 ml of 10% SRBCs intraperitoneally. On the fifth day after immunization, blood was collected from orbital plexus of each mouse and serum was separated. Serum was then incubated at 56 °C for 15 min to inactivate complement. Serial two fold dilutions of serum was made in 50 μl of PBS (pH 7.2) in 96-well plates and mixed with 50 μl of SRBC (1%) suspension in PBS. After mixing, plates were kept at room temperature for 2 h. The highest serum dilution which showed visible hemagglutination was considered as the antibody titer.
2.8. Plaque forming cell (PFC) assay
The effect of A. catechu extracts on antibody producing cells was studied by the method of Davis and Kuttan [15]. On the day of termination of the extract treatment, animals were immunized with 2.5 × 108 SRBC, intraperitoneally. The animals were euthanized on the 5th day after immunization. Splenocytes were prepared as mentioned earlier. 50 μl each of spleen cell suspension (1 × 106 cells/ml) and antigen (SRBC 7%) were mixed with 0.5 ml of molten agarose kept at 45 °C and spread on slides. After solidifying the agarose, rabbit serum (complement) was applied over the gel and incubated at 37 °C for 1 h. The number of plaque forming cells (PFC) was determined by Jerne's plaque assay [16].
2.9. Delayed type hypersensitivity (DTH)
The DTH response was determined using the method of Bin-Hafeez et al [17]. On the day of termination of the treatment, animals were immunized with SRBC (1 × 109 cells), subcutaneously. On the fifth day of immunization, all the animals were again challenged with 1 × 108 cells in the left hind footpad. The right footpad injected with the same volume of normal saline served as a trauma control for non-specific swelling. Increase in footpad thickness was measured 24 h after the challenge using an engineering micrometer (Digimatic micrometer – Mitutoyo South Asia).
2.10. Nitroblue tetrazolium (NBT) reduction test
The NBT reduction assay was carried out as described by Rainard [18]. Macrophage suspension was seeded in each well of a 96-well plate and incubated for 2 h in 5% CO2 humidified incubator; then non-adherent cells were removed by washing in RPMI-1640 medium. The remaining adherent cells (1 × 106 cells/well) were cultured in 100 μl RPMI-1640 medium with plant extracts dissolved in 0.1% dimethyl sulfoxide (DMSO) in PBS so that their final concentrations were 10 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml and incubated for 24 h at 37 °C in 5% CO2 humidified atmosphere. The 0.1% DMSO in PBS (without plant extract) was used as control. After incubation, 20 μl of the heat inactivated yeast (Saccharomyces cerevisiae) suspension (5 × 107 particles/ml) and 20 μl of NBT (Sigma, USA) solution in PBS (1.5 mg/ml) were added and the mixture was further incubated under the same conditions. After incubation for 60 min, the adherent macrophages were rinsed vigorously with RPMI medium and washed four times with 200 μl methanol. After air-drying, 120 μl of 2M KOH and 140 μl of DMSO were added. The absorbance was measured by a well reader (ThermoScientific, USA) at 570 nm and the percentage of NBT reduction was calculated by following equation:
2.11. Cellular lysosomal enzyme assay
Cellular lysosomal enzyme activity was measured by determining acid phosphatase in macrophages as described by Suzuki et al [19]. Macrophage suspension was seeded in each well of a 96-well plate and incubated in 5% CO2 humidified incubator for 2 h; then non-adherent cells were removed by washing in RPMI-1640 medium. The remaining adherent cells (1 × 106 cells/well) were cultured in 100 μl RPMI-1640 medium with plant extracts dissolved in 0.1% dimethyl sulfoxide (DMSO) in PBS so that their final concentrations were 10 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml and incubated for 24 h at 37 °C in 5% CO2 humidified atmosphere. The 0.1% DMSO in PBS (without plant extract) was used as control. After incubation, the medium was removed by aspiration and 20 μl of 0.1% Triton X-100 (Himedia, India), 50 μl of 0.1 M citrate buffer (pH 5.0) and 100 μl of 10 mM p-nitrophenyl phosphate (p-NPP) (Himedia, India) solution were added into each well. The plate was further incubated for 30 min, 150 μl of 0.2M borate buffer was then added and the absorbance was measured at 405 nm. The lysosomal enzyme activity (%) was calculated by the following equation:
2.12. Nitric oxide (NO) assay
The peritoneal macrophages were seeded in 24-well plate and incubated for 2 h in 5% CO2 humidified incubator; then non-adherent cells were washed out using RPMI-1640 medium. The remaining adherent cells (2 × 106 cells/well) were cultured in 1 ml RPMI complete medium containing 10% fetal calf serum (FCS) and incubated for 24 h with different concentrations of the plant extract (dissolved in 0.1% DMSO in PBS) ranging from 10 to 100 μg/ml both in the absence and presence of LPS (1 μg/ml). NO2− concentration derived from NO in culture media was determined by the Griess method [20]. After incubation, 100 μl cell culture supernatant was added to 100 μl Griess reagent, and incubated at room temperature for 10 min in 96-well microplate. Absorbance was read at 540 nm in a microplate reader (ThermoScientific, USA). Sodium nitrite was diluted in the medium and used as a standard.
2.13. Assessment of cytokines secretion in macrophages
The macrophages were plated in 96-well plate and incubated for 2 h in 5% CO2 humidified incubator; then non-adherent cells were removed by washing in RPMI-1640 medium. The remaining adherent cells (1 × 106 cells/well) were cultured at 37 °C in 5% CO2 for 24 h in RPMI-1640 complete medium with or without LPS (1 μg/ml) in the presence of various concentrations (10–100 μg/ml) of the extracts. LPS treated cells were considered as positive control and cells without LPS treatment were considered as negative control. After 24 h, supernatants were collected by centrifugation at 2500 rpm for 20 min at 18 °C and assayed for IL-10 and TNF-α using ELISA kits (Biolegend, USA) according to the manufacturer's instructions.
2.14. Splenocyte proliferation assay
The proliferation assay of lymphocytes was carried out according to 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) method [21]. Briefly, 20 μl of various concentrations (10–100 μg/ml) of the plant extracts was added to 20 μl of spleen cell suspension (1 × 106 cells/ml) and 40 μl of RPMI-1640 medium in a 96-well plate. Proliferation of cells in the presence and absence of mitogens was investigated. The predetermined optimum dose of concanavalin-A (ConA), lipopolysaccharide (LPS) and phytohemagglutinin-M (PHA-M) at 5 μg/ml was used. After incubation at 37 °C in humidified 5% CO2 for 48 h, 20 μl of MTT (Himedia, India) at concentration of 5 mg/ml in PBS and 40 μl of RPMI-1640 medium were added to the wells and incubated for additional 4 h under the same conditions. The culture medium was removed and 100 μl of 0.04 M HCl in isopropyl alcohol were added to lyse cells. Then, the solution was diluted by adding 100 μl of distilled water and the absorbance was measured at 570 nm. The percentage of proliferation was calculated by the following equation:
2.15. Statistical analysis
All the values were expressed as mean ± SEM for six animals. Values for the in vitro assays were expressed as mean ± SEM for triplicate independent experiments. Statistical significance was analyzed using one-way ANOVA followed by Tukey's HSD using SPSS program (version 20). The differences were considered significant at p ≤ 0.05.
3. Results
3.1. Hemagglutinating antibody titer
The hemagglutinating antibody titer assay was used to assess the effect of extracts of A. catechu on humoral immune response. A dose dependent increase in the hemagglutinating antibody titer was observed with the extract treated groups compared to control groups. The animals treated with the ethanol extract of A. catechu (200 mg/kg b. w.) caused a significant rise in antibody titer (Table 1).
Table 1.
Effect of A. catechu on HA titer, plaque forming cells and delayed type hypersensitivity.
| Groups | HA titer | PFC/106 spleen cells | DTH (footpad thickness in mm) |
|---|---|---|---|
| Control (PBS) | 14.67 ± 1.33a | 330.83 ± 3.22b | 2.25 ± 0.07a |
| Water extract (100 mg/kg) | 29.33 ± 2.67a | 356.67 ± 1.71c | 1.05 ± 0.02b |
| Water extract (200 mg/kg) | 58.67 ± 5.33b | 370.50 ± 1.33d | 0.9783 ± 0.02b |
| Ethanol extract (100 mg/kg) | 58.67 ± 5.33b | 429.83 ± 1.51e | 0.2612 ± 0.01c |
| Ethanol extract (200 mg/kg) | 117.33 ± 10.67c | 535.67 ± 1.69f | 0.1842 ± 0.01c |
| Dexamethasone (1.25 mg/kg) | 7.33 ± 0.67a | 204 ± 2.62b | 0.1463 ± 0.00c |
Values represent mean ± SEM for six animals. Values carrying same alphabet did not vary significantly from each other (Tukey's HSD; p ≤ 0.05).
3.2. Effect of A. catechu extracts on antibody producing cells
The treatment with A. catechu increased the number of antibody producing cells in spleen (Table 1). The maximum number of plaque forming cells was observed in ethanol extract treated group (535.67 ± 1.69 PFC/106 spleen cells) at a dose of 200 mg/kg while the PBS treated animals had only 330.83 ± 3.22 PFC/106 spleen cells (p < 0.05).
3.3. Delayed type hypersensitivity (DTH) reaction
The effect of the extracts on T-cell mediated DTH reaction is shown in Table 1. The results showed that both the extracts at doses of 100 and 200 mg/kg significantly inhibited the foot paw edema compared to control groups.
3.4. In vitro phagocytic activity assay of plant extracts in mouse macrophages
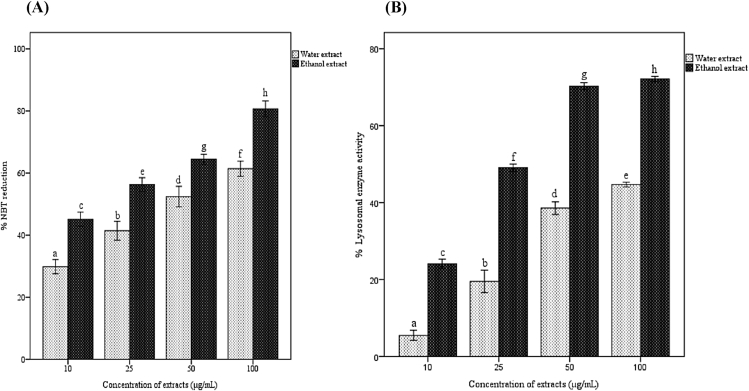
Effects of concentrations of the plant extracts on the reduction of NBT dye and lysosomal enzyme activity response in macrophages were demonstrated (Fig. 1A and B). Both water and ethanol extracts gave phagocytic modulation and a dose dependent response was observed. Phagocytic activity increased with increase in concentration of the extracts. Ethanol extract at a concentration of 100 μg/ml showed maximum activity in NBT dye reduction (80%) and lysosomal enzyme activity (72%).
Fig. 1.
Effect of A. catechu on in vitro phagocytosis assay. Effect of Acacia catechu on (A) NBT reduction and (B) cellular lysosomal enzyme activity. Values represent mean ± SEM of triplicate independent experiments; and values carrying same alphabet did not vary significantly from each other (Tukey's HSD; p ≤ 0.05).
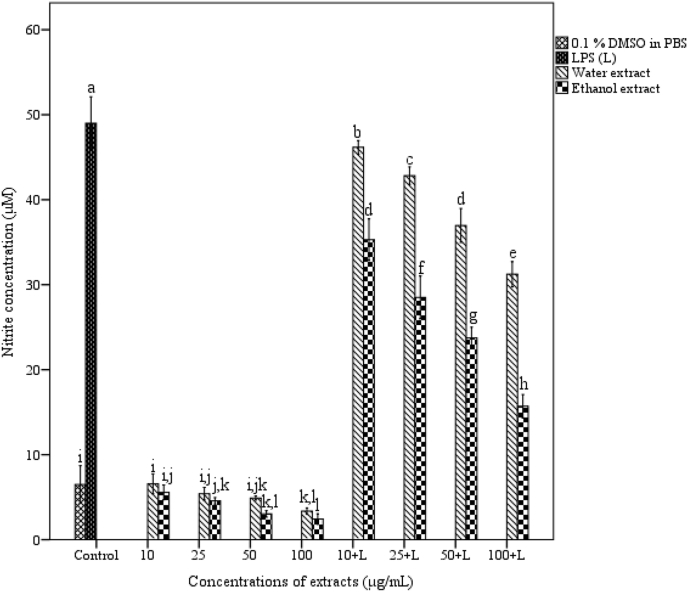
3.5. Effect of A. catechu on NO production
NO production by peritoneal macrophages was inhibited by extract treatment in a dose dependent manner (Fig. 2). LPS stimulated macrophages produced NO up to 49 ± 0.72 μM whereas untreated macrophages produced only 6.5 ± 0.51 μM.
Fig. 2.
Effect of A. catechu on NO production. Values represent mean ± SEM of triplicate independent experiments; and values carrying same alphabet did not vary significantly from each other (Tukey's HSD; p ≤ 0.05).
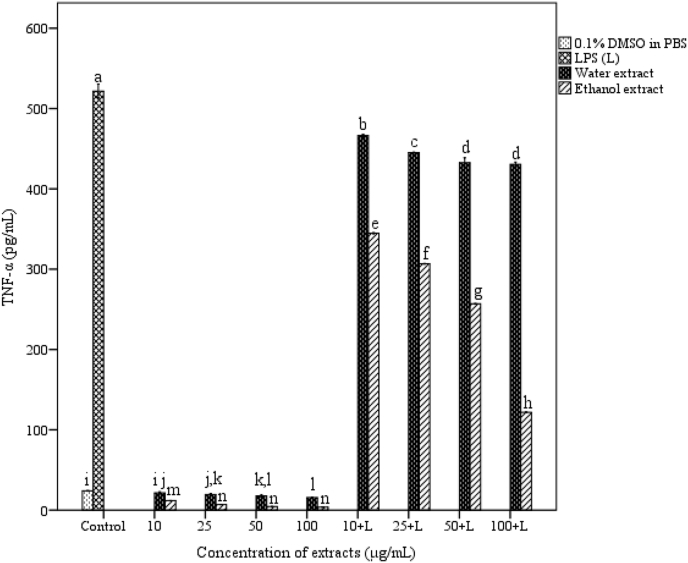
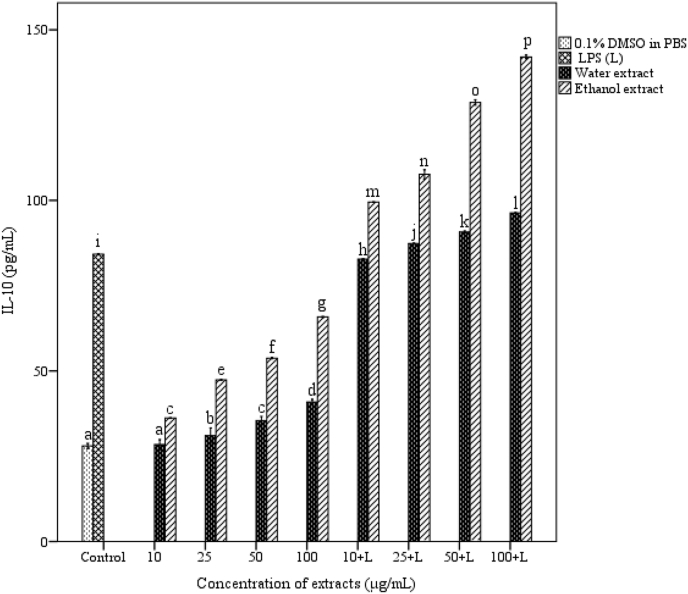
3.6. Effect of A. catechu on cytokine release
The effect of A. catechu on cytokine release in peritoneal macrophages is depicted in Fig. 3. TNF-α production by stimulated macrophages was significantly decreased in the presence of extracts. Ethanol extract showed more inhibition (121.78 ± 0.01 pg/mL) compared to water extract (430.53 ± 0.67 pg/mL) at a concentration of 100 μg/ml (p < 0.05) in the presence of LPS. Level of IL-10 was increased after extract treatment in a dose dependent manner (see Fig. 4).
Fig. 3.
Effect of A. catechu heartwood extracts on secretion of TNF-α in peritoneal macrophages. Values represent mean ± SEM of triplicate independent experiments; and values carrying same alphabet did not vary significantly from each other (Tukey's HSD; p ≤ 0.05).
Fig. 4.
Effect of A. catechu heartwood extracts on secretion of IL-10 in peritoneal macrophages. Values represent mean ± SEM of triplicate independent experiments; and values carrying same alphabet did not vary significantly from each other (Tukey's HSD; p ≤ 0.05).
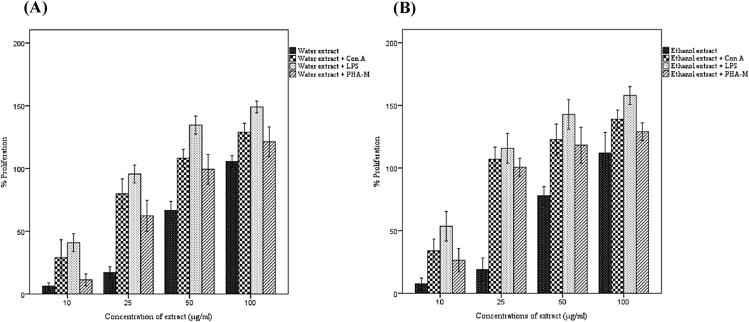
3.7. Effect of extracts on mouse splenocyte proliferation
Effect of A. catechu on splenocyte proliferation with or without mitogens is shown in Fig. 5. All extracts showed cell proliferation stimulation with a dose response relationship. The ethanol extract gave the maximum proliferation enhancement both in the presence and absence of mitogens. In the absence of mitogen, ethanol extract at 100 μg/ml enhanced proliferation by 111% (p < 0.05) compared to the control. In the presence of LPS, the ethanol extract stimulated splenocyte proliferation by 157% (p < 0.05) at 100 μg/ml.
Fig. 5.
Effect of A. catechu heartwood extracts on in vitro proliferation response of mouse splenocytes. (A) Effect of water extract on splenocyte proliferation. (B) Effect of ethanol extract on splenocyte proliferation; values represents mean ± SEM of triplicate independent experiments comparing to the control; and values carrying same alphabet did not vary significantly from each other (Tukey's HSD; p ≤ 0.05).
4. Discussion
Plants are good sources of biologically active natural products. Phytomedicines are used as health supplements or alternative therapies for treatment of diseases. Plant derived compounds have been used as the starting material for many semi-synthetic drugs. Herbal agents are often considered as safer and are of lower cost than conventional chemotherapeutics [22]. Immunomodulatory phytocompounds from herbal agents activate host defense mechanism and can provide an alternative therapy to conventional chemotherapy [23].
In the present study, treatment with A. catechu heartwood extracts enhanced the antibody titer. This indicates the stimulatory effect of the extracts on humoral response to SRBC [24]. Spleen plays an important role in developing immune responses to antigens in the bloodstream. Plaque forming cell assay was used to determine the number of antibody producing cells in the spleen. The significant enhancement of antibody producing cells in the spleen also indicates an activated humoral immune response.
DTH reaction directly correlates with cell-mediated immunity mediated by sensitized T-lymphocyte [25]. DTH response was significantly decreased after extracts treatment, indicating that immune response to SRBC is mainly due to the presence of high titer of antibodies and not due to generalized stimulation of immune responses involving T-cells. Reduction of NBT in NBT assay represents the activity of NADPH oxidase enzyme reflecting the stimulation of phagocytes [18]. NADPH oxidase enzyme plays an important role in the generation of reactive oxygen species such as superoxide radical which are involved in intracellular killing of pathogens by phagocytes. Yellow coloured, nitroblue tetrazolium undergo reduction by O2− and form blue NBT formazan. The cellular lysosomal enzyme assay is used to determine acid phosphatase secretion in macrophages. The membrane associated acid phosphatase of treated macrophages transforms p-nitrophenyl phosphate (p-NPP) to a coloured compound, indicating the activity of extract on degranulation of macrophages [19]. In the present study, lysosomal enzyme activity was significantly increased after extracts treatment. This also represents a stimulatory effect on phagocytes.
NO is an important inflammatory mediator. Small amount of NO is required for many physiological functions. Over production of NO may lead to inflammatory diseases. NO production was significantly decreased both in LPS stimulated and unstimulated macrophages treated with extracts. This indicates the anti-inflammatory effect of the extracts. The anti-inflammatory property of A. catechu was further demonstrated by analyzing the level of TNF-α and IL-10 secretion in macrophages. TNF-α, a strong mediator of inflammatory response, is secreted by monocytes and macrophages. It is involved in many of the clinical dilemma associated with autoimmune disorders such as rheumatoid arthritis, inflammatory bowel diseases and psoriasis. A. catechu inhibited the production of TNF-α. IL-10 plays an important role in immunoregulation and inflammation. IL-10 enhances proliferation of B cells, thymocytes and mast cells and can suppress the secretion of proinflammatory cytokines from macrophages. It can block the activity of NF-kB, a major transcription factor that regulates genes involved in inflammation. In this study, IL-10 level was increased with extract treatment. The extracts stimulated splenocyte proliferation both in the presence and absence of mitogens. Maximum proliferative effect was shown in the presence of LPS which is involved in T cell independent B cell proliferation.
The anti-inflammatory activity of A. catechu has been demonstrated by reducing the production of proinflammatory eicosanoids [26]. A. catechu heartwood is a rich source of catechin and epicatechin. Catechin and epicatechin are frequent components of traditional herbal remedies. Catechin is a plant flavonoid which possesses antioxidant properties. Presence of catechin may be the reason for its observed immunomodulatory activity.
5. Conclusion
The traditional knowledge on the medicinal uses of A. catechu has been scientifically validated in this study. The effect of the plant on enhancement of HA titers, PFC assay as well as its effect on DTH reaction and peritoneal macrophages indicate that it has appreciable immunomodulatory effect. The increased antibody titer indicates that daily consumption of A. catechu decoction may provide immunity against infections in general. A detailed study on the level of individual immunoglobulin classes is important to confirm the assumption. As it is not possible at this point to elucidate all the effective immunomodulatory constituents of A. catechu, further detailed studies in this regard will be helpful.
Sources of funding
University Grants Commission (UGC), New Delhi, India (F. No.41-78/2012).
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaim.2017.10.010.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Delves P.J., Martin S.J., Burton D.R., Roitt I.M. 12th ed. 2011. Roitt's essential immunology. [Google Scholar]
- 2.Saravanan S., Prakash Babu N., Pandikumar P., Karunai Raj M., Gabriel Paulraj M., Ignacimuthu S. Immunomodulatory potential of Enicostema axillare (Lam.) A. Raynal, a traditional medicinal plant. J Ethnopharmacol. 2012;140:239–246. doi: 10.1016/j.jep.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Asolkar L.V., Kakkar K.K. Publications & Information Directorate, CSIR; New Delhi: 1992. Second supplement to glossary of Indian medicinal plants with active principles: A-K (1965–1981) [Google Scholar]
- 4.Shen D., Wu Q., Wang M., Yang Y., Lavoie E.J., Simon J.E. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization-mass spectrometry. J Agric Food Chem. 2006;54:3219–3224. doi: 10.1021/jf0531499. [DOI] [PubMed] [Google Scholar]
- 5.Ismail S., Asad M. Immunomodulatory activity of Acacia catechu. Indian J Physiol Pharmacol. 2009;53:25–33. [PubMed] [Google Scholar]
- 6.Williamson E.M. Churchill Livingstone; 2002. Major herbs of Ayurveda. [Google Scholar]
- 7.Nutan, Modi M., Dezzutti C.S., Kulshreshtha S., Rawat A.K.S., Srivastava S.K. Extracts from Acacia catechu suppress HIV-1 replication by inhibiting the activities of the viral protease and Tat. Virol J. 2013;10(309):1–16. doi: 10.1186/1743-422X-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X.-C., Liu C., Yang L.-X., Chen R.-Y. Phenolic compounds from the aqueous extract of Acacia catechu. J Asian Nat Prod Res. 2011;13:826–830. doi: 10.1080/10286020.2011.597384. [DOI] [PubMed] [Google Scholar]
- 9.Devi V.G., John A., Devi R.S., Prabhakaran V.A. Pharmacognostical studies on Acacia catechu Willd and identification of antioxidant principles. Int J Pharm Pharm Sci. 2011;3:108–111. [Google Scholar]
- 10.Roux S., Sablé E., Porsolt R.D. Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr Protoc Pharmacol. 2004;(Supplement 27) doi: 10.1002/0471141755.ph1010s27. 10.10.1-10.10.23. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira H.C., dos Santos M.P., Grigulo R., Lima L.L., Martins D.T.O., Lima J.C.S. Antidiabetic activity of Vatairea macrocarpa extract in rats. J Ethnopharmacol. 2008;115:515–519. doi: 10.1016/j.jep.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X., Goncalves R., Mosser D.M. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;(Supplement 83) doi: 10.1002/0471142735.im1401s83. 14.1.1-14.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manosroi A., Saraphanchotiwitthaya A., Manosroi J. Immunomodulatory activities of Clausena excavata Burm. f. wood extracts. J Ethnopharmacol. 2003;89:155–160. doi: 10.1016/s0378-8741(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 14.Bin-Hafeez B., Ahmad I., Haque R., Raisuddin S. Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13–18. doi: 10.1016/s0378-8741(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 15.Davis L., Kuttan G. Immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2000;71:193–200. doi: 10.1016/s0378-8741(99)00206-8. [DOI] [PubMed] [Google Scholar]
- 16.Jerne N.K., Nordin A.A. Plaque formation in agar by single antibody-producing cells. Science. 1963;140:405. [PubMed] [Google Scholar]
- 17.Bin-Hafeez B., Haque R., Parvez S., Pandey S., Sayeed I., Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 18.Rainard P. A colorimetric microassay for opsonins by reduction of NBT in phagocytosing bovine polymorphs. J Immunol Methods. 1986;90:197–201. doi: 10.1016/0022-1759(86)90076-1. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki I., Tanaka H., Yoshiyuki A., Yadomae T. Rapid measurement of phagocytosis by macrophages. Chem Pharm Bull. 1988;36:4871–4875. doi: 10.1248/cpb.36.4871. [DOI] [PubMed] [Google Scholar]
- 20.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival : application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad I., Aqil F., Owais M. WILEY-VCH Verlag GmbH & Co. KGaA; Weinheim: 2006. Modern phytomedicine. [Google Scholar]
- 23.Wagner H., Bladt S., Zgainski E.M. Springer-Verlag; Berlin Heidelberg GmbH: 1984. Plant drug analysis. [Google Scholar]
- 24.Shukla S., Mehta A., John J., Mehta P., Vyas S.P., Shukla S. Immunomodulatory activities of the ethanolic extract of Caesalpinia bonducella seeds. J Ethnopharmacol. 2009;125:252–256. doi: 10.1016/j.jep.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Owen J.A., Punt J., Stranford S.A. 7th ed. W. H. Freeman and Company; New York: 2013. Kuby immunology. [Google Scholar]
- 26.Burnett B.P., Silva S., Mesches M.H., Wilson S., Jia Q. Safety evaluation of a combination, defined extract of Scutellaria baicalensis and Acacia catechu. J Food Biochem. 2007;31:797–825. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.