Abstract
Gabapentin is one of the most used drugs to treat postoperative pain with antihyperalgesic properties and has a unique mechanism of action, which differentiates it from other commonly used drugs. Various studies have shown that the perioperative use of gabapentin reduces postoperative pain. In our study, fragments of gingival tissue of healthy volunteers were collected during operation. Gene expression of 29 genes was investigated in gingival fibroblasts cell culture treated with gabapentin, compared with untreated cells. Of the different chemokines and interleukins studied, only 10 were statistically significant (CCL1, CCR1, CCR4, CCR5, CCR6, ILI1A, ILI1B, IL5, IL6R, TNFSF10). The overexpression of these cytokines, obtained in many studies, leads us to think that gabapentin can interact and cause post-inflammatory gingival hyperplasia, but, probably, in our study the gabapentin has not the same effect, because we used gingival fibroblasts of healthy people.
Keywords: fibroblasts, gabapentin, gingival overgrowth, inflammation
Introduction
Gabapentin is one of the most used drugs to treat postoperative pain with antihyperalgesic properties and has a unique mechanism of action, which differentiates it from other commonly used drugs. Various studies have shown that the perioperative use of gabapentin reduces postoperative pain.1
Gabapentin acts by reducing the hyperexcitability induced by lesions of the posterior horn neurons responsible for central sensitization.1
The mechanism of the antihyperalgesic action may be a result of the postsynaptic binding of gabapentin to the alpha 2-delta subunit of the voltage-dependent calcium channels of the dorsal horn neurons, causing a decrease in calcium entry into the nerve endings and therefore decrease of neurotransmitter release. Other possible cellular mechanisms include the effects of gabapentin on NMDA receptors, sodium channels, monoaminergic pathways, and the opioid system.2–4
Gabapentin was initially introduced in 1994 as an antiepileptic drug (AED), mainly due to partial seizures. It is an anticonvulsant whose side effects are well tolerated end well absorbed after oral administration with the maximum plasma concentration observed after 2–3 h.2,5 Some of the most commonly reported side effects of gabapentin include dizziness, drowsiness, fatigue, ataxia, and peripheral edema.4,6 Gabapentin was also found beneficial for treatment of neuropathic pain related to post-herpetic neuralgia (PHN),7,8 postpoliomyelitis neuropathy,9 and reflexsympathetic dystrophy.10 Furthermore, it has gabapentin, which has been shown to play a role in the treatment of diabetes-related pain neuropathy11 in placebo-controlled clinical trials.
The mechanism of action of gabapentin for treating neuropathic pain depends on its binding to the alpha-2/delta subunit of neuronal voltage-gated calcium channels and possibly on its interference with neuronal Ca influx.
In this article, we wanted to study the effects of the gabapentin molecule on the “Inflammatory Cytokines and Receptors” pathway present in human fibroblasts of healthy volunteers, and in the future, to understand the possible effect of the drug on inflammation and gingival hyperplasia. The literature previously reported the side effect of gabapentin.
Materials and methods
Primary human fibroblast cells culture
Fragments of gingival tissue from healthy volunteers (11-year-old male, 68-year-old female and 20-year-old male) were collected during operation.
Human primary gingival fibroblasts (HFb) were purchased from ATCC.
The pieces were transferred in 75 cm2 culture flasks containing DMEM medium (Sigma Aldrich, Inc., St Louis, Mo, USA), supplemented with 20% fetal calf serum, antibiotics (Penicillin 100 U/mL, and Streptomycin 100 mg/mL-Sigma Aldrich, Inc., St Louis, Mo, USA).
Cells were incubated in a humidified atmosphere of 5% CO2 at 37°C. The medium was changed the next day and twice a week. After 15 days, the pieces of gingival tissue were removed from the culture flask. Cells were harvested after additional 24 h of incubation.
Cell viability test
A stock solution of gabapentin 1 mg/mL was prepared. Further dilutions were made with the culture medium to the desired concentrations just before use. Cell lines were seeded into 96-well plates at a density of 104 cells per well containing 100 µl of cell culture medium and incubated for 24 h to allow cell adherence.
Serial dilutions of gabapentin (5000 ng/mL, 2000 ng/mL, 1000 ng/mL, 500 ng/mL, 100 ng/mL) were added (three wells for each concentration). The cell culture medium alone was used as the negative control.
After 24 h of incubation, cell viability was measured using PrestoBlue™ Reagent Protocol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, PrestoBlue solution (10 µL) was added to each well containing 90 µL of treatment solution. The plates were then placed back into the incubator for 1 h, after which absorbance was measured at wavelengths of 570 nm excitation and 620 nm emission by an automated microplate reader (Sunrise™, Tecan Trading AG, Switzerland). The percentage of viable cells was determined by comparing the average absorbance in drug-treated wells with the average absorbance in control wells exposed to vehicle alone. The results were presented as the mean ± standard deviation of three measures.
Cell treatment
Cell lines were seeded at a density of 1.0 × 105 cells/mL into 9 cm2 (3 mL) wells and subjected to serum starvation for 16 h at 37°C. The cells were treated with 1000 ng/mL gabapentin solution for 24 h. This solution was obtained in DMEM supplemented with 2% FBS, antibiotics, and aminoacids.
Cell medium alone was used as the control negative.
The cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. After the end of the exposure time the cells were trypsinized and processed for RNA extraction.
RNA isolation, reverse transcription, and quantitative real-time RT-PCR
Total RNA was isolated from cell lines using GenElute mammalian total RNA purification miniprep kit (Sigma-Aldrich) according to manufacturer’s instructions. Pure RNA was quantified at NanoDrop 2000 spectrophotometer (Thermo Scientific).
cDNA synthesis was performed starting from 500 ng of total RNA, using PrimeScript RT Master Mix (Takara Bio Inc.). The reaction was incubated at 37°C for 15 min and inactivated by heating at 70°C for 10 s. cDNA was amplified by real-time quantitative PCR, using the ViiA™ 7 System (Applied Biosystems).
All PCR reactions were performed in a 20 µL volume. Each reaction contained 10 µL of 2× qPCRBIO SYGreen Mix Lo-ROX (Pcrbiosystems), 400 nM concentration of each primer, and cDNA.
Custom primers belonging to the “Inflammatory Cytokines and Receptors” pathway were purchased from Sigma Aldrich. All experiments were performed including non-template controls to exclude reagents contamination. PCR was performed including two analytical replicates.
The amplification profile was initiated by 10 min incubation at 95°C, followed by two-step amplification of 15 s at 95°C and 60 s at 60°C for 40 cycles. As a final step, a melt curve dissociation analysis was performed.
Statistical analysis
The gene expression levels were normalized to the expression of the reference gene (RPL13) and were expressed as fold changes relative to the expression of the untreated cells. Quantification was done with the delta/delta Ct calculation method.12
Results
The optimal concentration of gabapentin to be used for cell treatment has been obtained using PrestoBlue cell viability test. Basing on this test the concentration used for the treatment was 1000 ng/mL.
The gene expression profile of 29 genes belonging to the “Inflammatory Cytokines and Receptors” pathway was analyzed (Table 1, Figure 1). Table 2 gives the significant deregulated genes and their fold change.
Table 1.
Selected genes used in real-time PCR belonging to “Inflammatory Cytokines and Receptors” pathway. In bold are the fold change of significant gene expression level.
| Gene | Fold change | Gene function |
|---|---|---|
| CCL1 | 2.20 | Chemokine |
| CCL2 | 1.02 | Chemokine |
| CCL2D | 0.74 | Chemokine |
| CCL5 | 1.63 | Chemokine |
| CCL8 | 1.09 | Chemokine |
| CXCL5 | 0.69 | Chemokine |
| CXCL10 | 1.41 | Chemokine |
| CCR1 | 0.29 | Chemokine receptor |
| CCR4 | 0.22 | Chemokine receptor |
| CCR5 | 0.47 | Chemokine receptor |
| CCR6 | 0.18 | Chemokine receptor |
| CCR10 | 0.78 | Chemokine receptor |
| CXCR5 | 0.59 | Chemokine receptor |
| IL1A | 0.34 | Interleukin |
| IL1B | 0.16 | Interleukin |
| IL5 | 0.14 | Interleukin |
| IL6 | 1.14 | Interleukin |
| IL7 | 1.18 | Interleukin |
| IL8 | 0.70 | Interleukin |
| ILR1 | 1.19 | Interleukin receptor |
| IL1RN | 1.01 | Interleukin receptor |
| IL6R | 0.23 | Interleukin receptor |
| IL10RB | 1.86 | Interleukin receptor |
| BMP2 | 1.99 | Cytokine |
| SPP1 | 1.44 | Cytokine |
| TNFRSF | 1.12 | Cytokine |
| TNFSF10 | 0.12 | Cytokine |
| VEGFA | 0.79 | Cytokine |
| RPL13 | 1.00 | Housekeeping gene |
Figure 1.
Amplification plot of fibroblast treated with gabapentin 1000 ng/mL.
Table 2.
Significant gene expression levels after 24 h treatment with gabapentin, compared with untreated cells.
| Gene | Fold change | SD (±) | Gene function |
|---|---|---|---|
| CCL1 | 2.20 | 0,59 | Chemokine |
| CCR1 | 0.29 | 0,07 | Chemokine receptor |
| CCR4 | 0.22 | 0,02 | Chemokine receptor |
| CCR5 | 0.47 | 0,06 | Chemokine receptor |
| CCR6 | 0.18 | 0,01 | Chemokine receptor |
| IL1A | 0.34 | 0,09 | Interleukin |
| IL1B | 0.16 | 0,01 | Interleukin |
| IL5 | 0.14 | 0,01 | Interleukin |
| IL6R | 0.23 | 0,01 | Interleukin receptor |
| TNFSF10 | 0.12 | 0,01 | Cytokine |
Figure 2 shows the effects of the gabapentin on the gene expression profiling of treated fibroblasts. In all, 10 genes (CCL1, CCR1, CCR4, CCR5, CCR6, IL1A, ILI1B, IL5, IL6R, TNFSF 10) were statistically significant. All but 1 gene resulted downregulated after 24 h of treatment with gabapentin. In fact, CCL1 was the only, although weakly, up-expressed genes.
Figure 2.
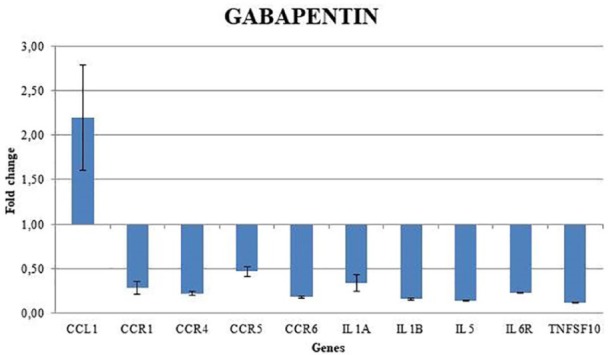
Gene expression profile of fibroblast treated with gabapentin 1000 ng/mL.
Significantly deregulated genes are those with a fold-change value superior to 2 (upregulated) and less than 0.5 (downregulated).
Discussion
The relationship between drugs and gingival tissue is influenced by several factors, such as age, genetic predisposition, presence of preexisting plaque, and gingival inflammation.12 There is a variable gingival response in patients taking drugs both in the variability in the extent and severity of the gingival changes.13,14
In this article, we wanted to study the effects of the gabapentin molecule on 29 genes belonging to “Inflammatory Cytokines and Receptors” pathway, present in human fibroblasts of healthy volunteers. Among the studied genes, only 10 genes (CCL1, CCR1, CCR4, CCR5, CCR6, IL1A, ILI1B, IL5, IL6R, TNFSF 10) were statistically significant. All but 1 gene resulted downregulated after 24 h of treatment with gabapentin. In fact, CCL1 was the only, although weakly, up-expressed gene. Probably, we have not highlighted overexpression of the other inflammatory molecules because the study was performed on healthy people.
In the future, it would be interesting to understand the possible effect of the drug on inflammation in patients with gingival hyperplasia.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dorina Lauritano  https://orcid.org/0000-0002-3550-1812
https://orcid.org/0000-0002-3550-1812
References
- 1. Maneuf YP, Gonzalez MI, Sutton KS, et al. (2003) Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cellular and Molecular Life Sciences 60(4): 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gidal BE. (2006) New and emerging treatment options for neuropathic pain. American Journal of Managed Care 12: S269–S278. [PubMed] [Google Scholar]
- 3. Taylor CP, Gee NS, Su TZ, et al. (1998) A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Research 29(3): 233–249. [DOI] [PubMed] [Google Scholar]
- 4. Dahl JB, Mathiesen O, Moiniche S. (2004) ‘Protective premedication’: An option with gabapentin and related drugs? A review of gabapentin and pregabalin in the treatment of post-operative pain. Acta Anaesthesiologica Scandinavica 48(9): 1130–1136. [DOI] [PubMed] [Google Scholar]
- 5. Elwes RD, Binnie CD. (1996) Lamotrigine, vigabatrin, gabapentin and oxcarbazepine. Clinical Pharmacokinetics 30(6): 403–415. [DOI] [PubMed] [Google Scholar]
- 6. Markman JD, Dworkin RH. (2006) Ion channel targets and treatment efficacy in neuropathic pain. The Journal of Pain 7(1 Suppl. 1): S38–S47. [DOI] [PubMed] [Google Scholar]
- 7. Segal AZ, Rordorf G. (1996) Gabapentin as a novel treatment for postherpetic neuralgia. Neurology 46(4): 1175–1176. [DOI] [PubMed] [Google Scholar]
- 8. Rosner H, Rubin L, Kestenbaum A. (1996) Gabapentin adjunctive therapy in neuropathic pain states. The Clinical Journal of Pain 12(1): 56–58. [DOI] [PubMed] [Google Scholar]
- 9. Zapp JJ. (1996) Postpoliomyelitis pain treated with gabapentin. American Family Physician 53(8): 2442, 2445. [PubMed] [Google Scholar]
- 10. Mellick GA, Mellick LB. (1997) Reflex sympathetic dystrophy treated with gabapentin. Archives of Physical Medicine and Rehabilitation 78(1): 98–105. [DOI] [PubMed] [Google Scholar]
- 11. Backonja M, Beydoun A, Edwards KR, et al. (1998) Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: A randomized controlled trial. JAMA 280(21): 1831–1836. [DOI] [PubMed] [Google Scholar]
- 12. Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- 13. Seymour RA, Thomason JM, Ellis JS. (1996) The pathogenesis of drug-induced gingival overgrowth. Journal of Clinical Periodontology 23(3 Pt. 1): 165–175. [DOI] [PubMed] [Google Scholar]
- 14. Seymour RA, Ellis JS, Thomason JM. (2000) Risk factors for drug-induced gingival overgrowth. Journal of Clinical Periodontology 27(4): 217–223. [DOI] [PubMed] [Google Scholar]