Abstract
Background:
Bone marrow stimulation (BMS) is a common surgical intervention in the treatment of small osteochondral lesions of the talus (OLTs). Evidence has shown good clinical outcomes after BMS in the short term, but several studies have shown less favorable results at midterm and long-term follow-up because of fibrocartilaginous repair tissue degeneration.
Purpose:
To evaluate the clinical and radiological outcomes of BMS in the treatment of primary OLTs at midterm and long-term follow-up and to investigate reported data in these studies.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic search of the MEDLINE, Embase, and Cochrane Library databases was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Clinical and radiological outcomes as well as reported data were evaluated.
Results:
A total of 15 studies comprising 853 patients (858 ankles) were included at a weighted mean follow-up time of 71.9 months. There were 9 studies that used the American Orthopaedic Foot & Ankle Society (AOFAS) score, with a weighted mean postoperative score of 89.9. There were 3 studies that measured postoperative magnetic resonance imaging results in the midterm using the MOCART (magnetic resonance observation of cartilage repair tissue) scoring system and showed 48% of patients with complete filling, 74% with complete integration, and 76% with surface damage. There was a complication rate of 3.4% and a reoperation rate of 6.0% after BMS in the midterm.
Conclusion:
This systematic review found good clinical outcomes after BMS at midterm follow-up for primary OLTs. Radiological outcomes showed repair tissue surface damage in the majority of patients, which may be a harbinger for long-term problems. Data were variable, and numerous data were underreported. Further high-quality studies, a validated outcome scoring system, and further radiological reports at midterm follow-up are required to accurately assess the success of BMS in the midterm.
Keywords: bone marrow stimulation, talus, osteochondral, systematic review
Arthroscopic bone marrow stimulation (BMS), including microfracture, is the most common reparative surgical procedure performed for the treatment of primary osteochondral lesions of the talus (OLTs). This procedure is typically indicated for smaller sized lesions.8,9,34 BMS breaches the subchondral plate, which leads to the release of mesenchymal stem cells and growth factors that ultimately fill the defect with fibrocartilaginous repair tissue.6,27
BMS has been shown to provide favorable short-term clinical outcomes,9,36,44 but several clinical studies have shown that midterm to long-term outcomes have less satisfactory results, as fibrocartilage repair tissue may deteriorate over time.13,23,26,38,42 Recently, several studies have shown that subchondral bone is also not fully restored after BMS and may degrade over time.35,37,38 The deterioration in both cartilage and subchondral bone suggests that BMS may fail in the long term. Clinically, the progression to ankle osteoarthritis has been observed in up to one-third of patients after BMS in the mid- to long term.13,42 Despite the mounting evidence that BMS fails over time, there are conflicting results, with excellent mid- to long-term clinical outcomes of BMS having been reported in several studies.3,5,24 Therefore, there is still a lack of consensus regarding the success of BMS at midterm and longer term follow-up.
The purpose of this systematic review was to evaluate the clinical and radiological outcomes, analyzing the level of evidence (LOE) and quality of evidence (QOE), of BMS in the treatment of OLTs at greater than 4 years after surgery.39
Methods
Search Strategy
A systematic review of MEDLINE, Embase, and Cochrane Library databases was conducted by 2 authors (J.T., C.M.) in January 2018 based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.25 The search terms used were the following: (arthroscopy OR arthroscopic OR microfracture OR micro fracture OR drill OR drilling OR bone marrow stimulation) AND (osteochondral OR cartilage OR chondral OR osteochondritis dissecans OR transchondral) AND (ankle OR talus OR talar). The available articles were then screened for inclusion based on the inclusion and exclusion criteria in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
| Clinical studies reporting outcomes after primary bone marrow stimulation including microfracture, drilling, and abrasion |
| Treatment for osteochondral lesion of the talus |
| Follow-up ≥48 mo |
| Studies involving a minimum of 10 participants |
| Published in a peer-reviewed journal |
| Written in English |
| Full-text version available |
| Exclusion Criteria |
| Review articles |
| Nonprimary defects |
| Treatment involving debridement alone |
| Case reports |
| Cadaveric studies |
| Animal studies |
| Technique articles |
| Use of scaffolds and biological adjuncts |
Titles, abstracts, and full texts were compiled and reviewed by 2 independent reviewers using the criteria mentioned above. Any discrepancies were resolved by consensus, and if any disagreement persisted, final adjudication was made by the senior author (J.G.K.). A total of 15 studies met the inclusion criteria and were included in the study.
Assessment of LOE and Methodological Quality
The LOE of each study included was assessed using The Journal of Bone and Joint Surgery published criteria.29 The methodological quality of evidence was assessed using the modified Coleman Methodology Score (MCMS) based on the modification made to the scoring system, which ensures specificity to OLTs.34 A total of 2 independent reviewers determined the MCMS for each included study.10 If a disagreement existed, the score was reviewed by the senior author and consensus was reached. Studies were considered excellent if they scored between 85 and 100 points, good if they scored between 70 and 84 points, fair if they scored between 55 and 69 points, and poor if they scored less than 55 points.34
Data Extraction and Evaluation
A total of 2 reviewers independently extracted data from each study and assessed the variable reporting of outcome data using parameters of previously published criteria for the treatment of OLTs.17 We also collected clinical characteristics, including patient age, sex, body mass index (BMI), lesion location, lesion size, and follow-up time. Moreover, objective and subjective outcomes, postoperative imaging findings, complications, and revisions were extracted and evaluated. Data were extracted and collated using aggregated data from the studies and not based on individual patient outcomes or characteristics. We defined midterm follow-up as ≥48 months based on previous literature pertaining to OLTs.39
Statistical Analysis
All other statistical analyses were performed using SAS software version 9.3 (SAS Institute). Descriptive statistics were calculated for all continuous and categorical variables. Continuous variables were reported as the weighted mean and estimated standard deviation, whereas categorical variables were reported as frequencies with percentages. P < .05 was considered statistically significant.
Results
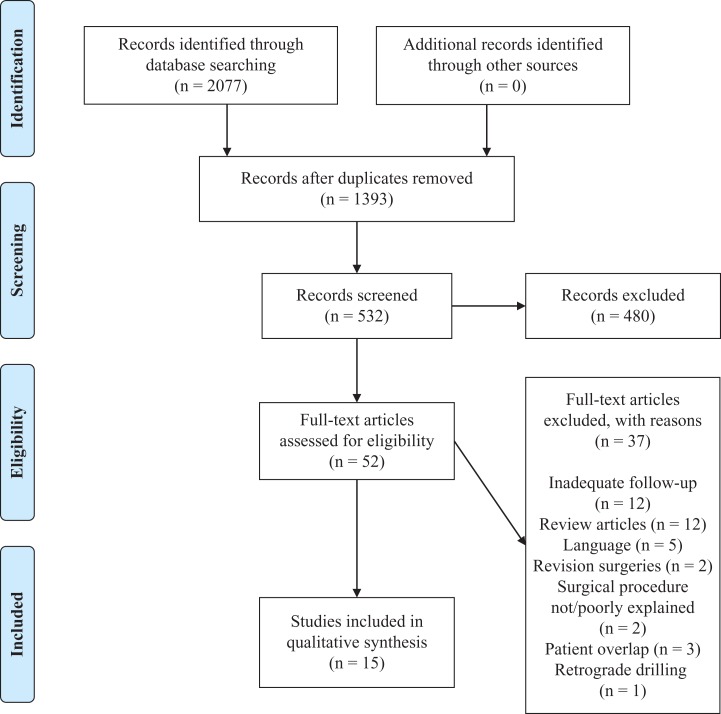
A total of 2077 results were obtained from the literature search. Of these studies, 15 studies met the inclusion criteria outlined and were included in the current review** (Figure 1). The 15 included studies were published between 1999 and 2016.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Patient Characteristics
A total of 853 patients were identified; in total, 858 ankles underwent BMS for primary OLTs. The weighted mean patient age was 35.3 years, and the weighted mean follow-up was 71.9 months (range, 48-141 months). The weighted mean lesion size was 110.5 mm2 (range, 87-140 mm2). Patient demographic and clinical characteristics are shown in Table 2.
Table 2.
Patient Demographics and Clinical Characteristicsa
| Studies, n | 15 |
| Ankles/patients, n | 858/853 |
| Sex, male/female n | 512/341 |
| Age, y | 35.3 (24.7-41.9) |
| Body mass index, kg/m2 | 24.7 (23.4-25.4) |
| Duration of symptoms, mo | 24.1 (6.7-34.0) |
| Follow-up, mo | 71.9 (48.0-141.0) |
| Lesion size, mm2 | 110.5 (87.0-140.0) |
| Lesion location, % | |
| Medial | 73.4 |
| Central | 1.8 |
| Lateral | 24.4 |
aData are presented as weighted mean (range) unless otherwise indicated.
LOE and QOE
Of the 15 studies, there were 11 with LOE 4,†† there were 3 with LOE 3,5,7,18 there was 1 with LOE 2,24 and there were no studies with LOE 1. The mean MCMS was 60; there was 1 study of excellent quality, 1 study of good quality, 9 studies of fair quality, and 4 studies of poor quality using the MCMS criteria. The mean number of patients in each study was 57 (range, 12-298). There were 6 studies that had a study population of ≥50 patients that received BMS.
Methods of BMS Used
Within the 15 studies chosen, various methods of BMS were utilized. Microfracture and drilling were the most common methods of BMS implemented, while abrasion was less commonly used. Different methods of BMS were used within the same study on occasion.
Reported Data
The defined data reported in the included studies are shown in Table 3. While demographic information (sex and age) was well reported (100.0%), patient history was poorly reported (53.3%). Most aspects of the study design were well reported (84.2%); however, lesion classification systems utilized (53.3%) and methods of lesion size measurement (40.0%) were poorly reported. Clinical variables were also poorly reported (58.9%), including lesion size (60.0%), presence of cysts (53.3%), associated abnormality (33.3%), and concomitant procedures (20.0%). Soft tissue impingement was the most common associated abnormality, reported in 197 (50.4%) of 391 patients, and osteophytes were reported in 78 (20.0%) of 391 patients. Patient-reported outcomes (73.4%) were well reported; however, preoperative pain, function, and activity scale (46.7%) were inadequately described. Follow-up magnetic resonance imaging (MRI) was reported in 26.7% of studies, but only 3 studies (20.0%) reported MRI at midterm follow-up (weighted mean follow-up, 72.8 months). Follow-up radiographs (46.7%) and computed tomography (0.0%) were also poorly reported modalities.
Table 3.
Summary of Reported Dataa
| Percentage of Studies | |
|---|---|
| Characteristic information | 100.0 |
| Sex | 100.0 |
| Mean age | 100.0 |
| Patient history | 53.3 |
| Body mass index | 26.7 |
| Mean duration of symptoms | 53.3 |
| Previous traumatic experiences | 60.0 |
| Activities of daily living/athletic participation | 73.3 |
| Study design | 84.2 |
| Type of study | 100.0 |
| Number of patients | 100.0 |
| Percentage of patients in follow-up | 100.0 |
| Consecutive patients | 80.0 |
| Follow-up time | 100.0 |
| Method of lesion size measurement | 40.0 |
| Lesion classification system utilized | 53.3 |
| Surgical approach used to access lesion | 100.0 |
| Clinical variables | 58.9 |
| Lesion size | 60.0 |
| Lesion location | 93.3 |
| Presence of cyst | 53.3 |
| Associated abnormality | 33.3 |
| Concomitant procedures | 20.0 |
| Description of rehabilitation | 93.3 |
| Imaging data | 52.2 |
| Imaging used to identify lesion | 93.3 |
| Diagnostic radiograph | 66.7 |
| Diagnostic MRI | 60.0 |
| Diagnostic CT | 20.0 |
| Imaging used at follow-up | 75.0 |
| Follow-up radiograph | 46.7 |
| Follow-up MRI | 26.7 |
| Follow-up CT | 0.0 |
| Patient-reported outcomes | 73.4 |
| Pain, function, and activity scale preoperatively | 46.7 |
| Pain, function, and activity scale at follow-up | 100.0 |
aCT, computed tomography; MRI, magnetic resonance imaging.
Clinical Outcomes
Clinical and functional outcomes are shown in Table 4. The American Orthopaedic Foot & Ankle Society (AOFAS) score was the most commonly reported outcome measure, followed by the visual analog scale (VAS). The AOFAS score was utilized in 9 (60.0%) included studies. The weighted mean postoperative AOFAS score was 89.9 (range, 78.4-91.8) at a weighted mean follow-up of 71.3 months (range, 48-141 months). Of the 9 studies, 6 studies provided both pre- and postoperative AOFAS scores, and the weighted mean improvement was 24.5 points (range, 16.0-38.5 points). Based on the available data, there appears to be no difference in AOFAS scores when comparing those with a longer term follow-up to those with shorter follow-up times. A total of 4 studies utilized the VAS. The weighted mean preoperative VAS score was 7.2 (range, 6.5-7.9) of a possible 10, and the weighted mean postoperative VAS score was 2.4 (range, 1.8-2.6). The weighted mean change from the pre- to postoperative VAS score was 4.9 (range, 4.1-6.1).
Table 4.
Summary of Clinical Outcomesa
| Author (Year) | n | Follow-up, mo | Clinical Outcome Measures Used | Preoperative/Postoperative Scoreb | Imaging | Complications, n | Reoperations, n |
|---|---|---|---|---|---|---|---|
| Baker and Morales1 (1999) | 12 | 121 | N/A | N/A | Radiograph | N/A | 1 |
| Becher et al2 (2010) | 45 | 70 | Modified HSS, VAS (pain) | HSS: 19 (49%) excellent, 12 (31%) good, 4 (10%) satisfactory VAS: 6.5/2.4 |
MRI (MOCART) | 0 | 4 |
| Becher et al3 (2015) | 15 | 94.8 | AOFAS, HSS | AOFAS: 90 | MRI (MOCART) | 0 | 0 |
| Bohnsack et al5 (2003) | 68 | 57 | AOFAS, HSS | AOFAS: 68/90 | N/A (none) | 1 | 14 |
| Choi et al7 (2013) (chondral) | 210 | 50 | AOFAS, VAS (pain) | AOFAS: 65.2/85.1 VAS: 7.2/2.6 |
N/A (none) | N/A | 4 |
| Choi et al7 (2013) (osteochondral) | 88 | 57 | AOFAS, VAS (pain) | AOFAS: 65.2/85.2 VAS: 6.9/2.6 |
N/A (none) | N/A | 2 |
| Domayer et al12 (2011) | 14 | 55 | AOFAS, Cincinnati | AOFAS: 39.9/78.4 | MRI (MOCART) | N/A | N/A |
| Ferkel et al13 (2008) | 50 | 71 | AOFAS, Alexander, modified Weber | AOFAS: 84 | Radiograph (van Dijk classification) | 7 | 7 |
| Gregush and Ferkel15 (2010) | 31 | 88 | AOFAS, Berndt and Harty, modified Weber, SANE, SF-36 | AOFAS: 75 (n = 13)/89 (all), 91 (n = 13)c | Radiograph (van Dijk classification) | 0 | 0 |
| Hannon et al18 (2016) (BMS only) | 12 | 77 | FAOS, SF-12 | FAOS: 54.8/68.3 | MRI (MOCART) at 2 y | 0 | N/A |
| Hunt and Sherman20 (2003) | 28 | 66 | Berndt and Harty, Martin, SANE | Berndt and Harty: 13 (46%) good, 13 (46%) fair, 2 (8%) poor | N/A (none) | 0 | 1 |
| Kumai et al22 (1999) | 17 | 55 | Berndt and Harty | Berndt and Harty: 12 (70.6%) good, 5 (29.4%) fair, 0 (0.0%) poor | Radiograph (Takakura classification) | N/A | N/A |
| Lee et al24 (2015) (cyst) | 45 | 48 | AOFAS, AAS, VAS | AOFAS: 64.8/91.8 VAS: 7.5/2.3 |
Radiograph (Berndt and Harty) | 0 | N/A |
| Lee et al24 (2015) (noncyst) | 57 | 48 | AOFAS, AAS, VAS | AOFAS: 66.2/91.3 VAS: 7.3/2.2 |
Radiograph (Berndt and Harty) | 0 | N/A |
| Ogilvie-Harris and Sarrosa32 (1999) | 33 | 106 | Ogilvie-Harris score | Ogilvie-Harris score: pain (25 excellent), swelling (32 excellent), stiffness (31 excellent), limping (31 excellent), activity (30 excellent) | Radiograph (Kellgren and Lawrence) | 0 | 0 |
| Polat et al33 (2016) | 82 | 121 | AOFAS, VAS (pain) | AOFAS: 58.7/85.5 VAS: 7.9/1.8 |
Radiograph (Takakura classification) | 5 | 2 |
| van Bergen et al42 (2013) | 50 | 141 | AOFAS, Berndt and Harty, Ogilvie-Harris score, SF-12 | AOFAS: 88 (median) | Radiograph (van Dijk classification) | 4 | 6 |
aAAS, Ankle Activity Score; AOFAS, American Orthopaedic Foot & Ankle Society; FAOS, Foot and Ankle Outcome Score; HSS, Hannover Scoring System; MOCART, magnetic resonance observation of cartilage repair tissue; MRI, magnetic resonance imaging; N/A, not applicable; SANE, Single Assessment Numeric Evaluation; SF-12, 12-Item Short Form Health Survey; SF-36, 36-Item Short Form Health Survey; VAS, visual analog scale.
bPostoperative score if reported alone.
cn = 13 are patients who provided both pre- and postoperative AOFAS scores.
Radiological Outcomes
A total of 3 studies (20.0%) utilized MRI for follow-up assessments at midterm follow-up (48-month minimum follow-up). These 3 studies reported on a total of 54 patients using the MOCART (magnetic resonance observation of cartilage repair tissue) scoring system.28 At a weighted mean follow-up of 72.8 months (range, 55.0-94.8 months), 48% of patients showed complete cartilage filling of the defect, 28% showed hypertrophy, and 24% had incomplete defect filling after BMS. Moreover, 74% of patients showed complete integration of reparative tissue into adjacent cartilage, and 26% had incomplete integration with native cartilage. Surface damage (fibrillations, fissures, and ulcerations) was reported in 76% of patients, and 78% showed inhomogeneous reparative cartilage.
Follow-up radiographs were reported in 8 studies (53.3%) at a weighted mean follow-up time of 90.3 months (range, 48.0-141 months). A total of 4 separate radiographic systems were described, including the osteoarthritis classification described by van Dijk et al43 (3 studies), the Berndt and Harty4 classification (2 studies), the Kellgren and Lawrence21 classification (1 study), and the Takakura et al41 classification (1 study). According to the van Dijk et al43 classification, in a total of 119 patients, 9.5% of patients had stage 2 or 3 osteoarthritis, demonstrating joint space narrowing or total deformation of the joint space, at a weighted mean follow-up of 102 months (range, 71-141 months). Arthritic progression was observed in 64.2% of patients within this subgroup.
Complications and Revision Surgery
Complication rates ranged from 0% to 14% in the included studies. In total, 17 (3.4%) of 504 patients had postoperative complications. The most common complication was superficial peroneal nerve neuropathy (n = 8) and portal site pain (n = 3). Other complications included deep peroneal nerve paresthesia (n = 1), arterial bleeding (n = 1), plantar fasciitis (n = 1), saphenous neuropathy (n = 1), keloid formation at a portal site (n = 1), and sinus tract formation (n = 1). In total, of the 679 patients in whom reoperations were reported (10 studies), 41 (6.0%) underwent reoperations. A total of 14 patients underwent a reoperation of an unknown procedure, 6 patients underwent repeat arthroscopic BMS, 7 patients underwent repeat arthroscopic surgery and debridement, 8 patients underwent osteochondral autograft transfer, 2 patients underwent autologous chondrocyte implantation, 2 patients underwent arthroplasty, 1 patient underwent arthrodesis, and 1 patient underwent an open BMS procedure.
Discussion
The most important finding of our study was that BMS in the treatment of primary OLTs resulted in good clinical outcomes but concerning radiographic outcomes at midterm follow-up (≥48 months; mean, 71.9 months). Although the poor methodological quality and poor LOE make it difficult to draw clinical recommendations from the literature, this review gives insight into the natural history, clinical outcomes, and radiological outcomes after BMS in the treatment of primary OLTs. The current study found good clinical outcomes based on the AOFAS score; however, the radiological and MRI outcomes did not show similarly positive results. The complication rates after the procedure were relatively low, and the data showed a reoperation rate of 6.0% in the midterm. However, the current study shows that there is evident radiological deterioration after BMS at midterm follow-up, and this may be a harbinger for future clinical deterioration.
Both the AOFAS and VAS scores observed in the current study showed good midterm clinical outcomes after BMS. Several studies have shown recently that clinical outcomes deteriorate in the long term after BMS.15,38 This was not reflected in the current study, and therefore, it is reasonable to say that BMS may provide successful midterm functional outcomes for smaller OLTs. However, there is concern that a total of 13 different scoring systems were used to evaluate clinical outcomes after BMS for the treatment of OLTs. Despite the AOFAS score being the most utilized clinical outcome measure, this broad heterogeneity in outcome scoring systems highlights the absence of a validated outcome measure for OLTs. The AOFAS score has not been validated for the clinical outcomes of OLTs but nonetheless remains commonly utilized.
In comparison with excellent clinical outcomes at midterm follow-up, the radiological results after BMS were less promising. While postoperative MRI studies are crucial in the assessment of cartilage repair, only 3 studies reported MRI outcomes at midterm follow-up. A high rate of surface damage (fissures, fibrillations, and ulcerations), inhomogeneous repair tissue, and incomplete repair cartilage integration with native adjacent cartilage was found. This may suggest a potential concern for deterioration and failure of fibrous cartilage repair tissue in the longer term. However, fibrous tissue degradation has not shown to be clinically relevant in the midterm. Recent studies have also reported that the subchondral bone as well as fibrocartilage tissue deteriorates over time after microfracture.37,38 The durability of both the cartilage and underlying subchondral bone is of great concern in the long term. Two long-term studies have shown that on plain radiographs, a 1-grade increase in the arthritis level was observed in one-third of patients compared with their preoperative radiographs,33,42 but no MRI studies have been performed with long-term follow-up. This lack of midterm to long-term MRI data should be addressed by future studies, allowing for the assessment of the degree of repair and cartilage quality.
The current review demonstrated significant variability and underreporting of clinical data in the included studies. Although basic information, including the age and number of patients, was well reported, other critical information was poorly reported. The mean lesion size was reported by only 60.0% of studies, despite this being the most reliable prognostic factor of clinical outcomes after BMS for the treatment of OLTs.34 BMI has also shown to be a prognostic factor of clinical outcomes,2,12 but BMI was reported in only 26.7% of studies in the current analysis. Although the presence of a cyst, associated abnormalities, and concomitant procedures can have a significant effect on patient outcomes, these data were poorly reported. This variability and underreporting, along with other factors, make it difficult to draw significantly robust conclusions regarding predictors of the effectiveness of BMS at midterm follow-up from the included studies.
The current review reported a 3.2% complication rate and a 6.0% reoperation rate at midterm follow-up after microfracture. Based on the predicted degeneration of fibrocartilaginous repair tissue and reported MRI deterioration, there is concern that reoperation rates may rise in the long term. To improve the longevity of cartilage repair tissue, biological augmentation including platelet-rich plasma and concentrated bone marrow aspirate have become increasingly popular for the treatment of OLTs by enhancing the biological environment during healing.19 There is evidence demonstrating the beneficial effects of platelet-rich plasma and concentrated bone marrow aspirate on cartilage repair.14,16,18,40 In the current investigation, no study reported on the use of any biological augmentation. Therefore, further studies are necessary to investigate the effects of these biologics on the longevity of repair cartilage and longer term outcomes in the treatment of OLTs.
Limitations
A high level of clinical evidence and good methodological quality are fundamental to making decisions about interventions for treating patients. A low LOE and methodological quality of evidence are more likely to show bias in comparison with a higher level LOE and methodological quality of evidence.30,31 In the current systematic review, the LOE was poor in the reported literature. There were no level 1 studies included, and 73.3% of studies were level 4. The MCMS showed that only 1 study was regarded as excellent. This is consistent with the literature on cartilage repair as a whole. Level 1 evidence is absent for midterm to long-term outcomes after BMS, so caution should be taken when assessing reported outcomes after BMS. Furthermore, retrospective case series were pooled. Methodological bias may therefore be found because of evidence being retrieved from a simplified pooling method based on a lower LOE. As a result, pooled calculated success rates should not be used when making decisions about a specific treatment technique but should be used to inform patients of the potential success percentage rates of a particular surgical strategy.11
Conclusion
The current systematic review found good clinical outcomes after primary BMS at midterm follow-up. Radiological outcomes were less promising, exposing repair tissue surface damage in the majority of patients. This may lead to recurrence and reoperations at long-term follow-up based on data reporting repair tissue degradation. The systematic review also exposed that data were variable and numerous predictive aspects were largely underreported. This makes a meaningful analysis challenging and may confound any recommendation based on this systematic review. Further high-quality studies, a validated outcome scoring system, and further radiological reports at longer term follow-up are required to accurately assess the success of BMS in the midterm and beyond.
Footnotes
One or more of the authors declared the following potential conflict of interest or source of funding: J.G.K. has received consulting fees from Arteriocyte Medical Systems and Isto Biologics. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Baker CL, Morales RW. Arthroscopic treatment of transchondral talar dome fractures: a long-term follow-up study. Arthroscopy. 1999;15(2):197–202. [DOI] [PubMed] [Google Scholar]
- 2. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656–663. [DOI] [PubMed] [Google Scholar]
- 3. Becher C, Zühlke D, Plaas C, et al. T2-mapping at 3 T after microfracture in the treatment of osteochondral defects of the talus at an average follow-up of 8 years. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2406–2412. [DOI] [PubMed] [Google Scholar]
- 4. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 2004;86-A(6):1336. [DOI] [PubMed] [Google Scholar]
- 5. Bohnsack M, Fischer J, Lipka W, et al. The influence of limited postoperative weight-bearing on the outcome of drilling in osteochondritis dissecans tali. Arch Orthop Trauma Surg. 2003;123(9):447–450. [DOI] [PubMed] [Google Scholar]
- 6. Buckwalter JA, Mow VC, Ratcliffe A. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg. 1994;2(4):192–201. [DOI] [PubMed] [Google Scholar]
- 7. Choi GW, Choi WJ, Youn HK, Park YJ, Lee JW. Osteochondral lesions of the talus: are there any differences between osteochondral and chondral types? Am J Sports Med. 2013;41(3):504–510. [DOI] [PubMed] [Google Scholar]
- 8. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974–1980. [DOI] [PubMed] [Google Scholar]
- 9. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24(1):106–112. [DOI] [PubMed] [Google Scholar]
- 10. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Scand J Med Sci Sports. 2000;10(1):2–11. [DOI] [PubMed] [Google Scholar]
- 11. Dahmen J, Lambers KTA, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs G. No superior treatment for primary osteochondral defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2018;26(7):2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domayer SE, Welsch GH, Stelzeneder D, et al. Microfracture in the ankle: clinical results and MRI with T2-mapping at 3.0 T after 1 to 8 years. Cartilage. 2011;2(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750–1762. [DOI] [PubMed] [Google Scholar]
- 14. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927–1937. [DOI] [PubMed] [Google Scholar]
- 15. Gregush RV, Ferkel RD. Treatment of the unstable ankle with an osteochondral lesion: results and long-term follow-up. Am J Sports Med. 2010;38(4):782–790. [DOI] [PubMed] [Google Scholar]
- 16. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384–2389. [DOI] [PubMed] [Google Scholar]
- 17. Hannon CP, Murawski CD, Fansa AM, Smyth NA, Do H, Kennedy JG. Microfracture for osteochondral lesions of the talus: a systematic review of reporting of outcome data. Am J Sports Med. 2013;41(3):689–695. [DOI] [PubMed] [Google Scholar]
- 18. Hannon CP, Ross KA, Murawski CD, et al. Arthroscopic bone marrow stimulation and concentrated bone marrow aspirate for osteochondral lesions of the talus: a case-control study of functional and magnetic resonance observation of cartilage repair tissue outcomes. Arthroscopy. 2016;32(2):339–347. [DOI] [PubMed] [Google Scholar]
- 19. Hannon CP, Smyth NA, Murawski CD, et al. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96-B(2):164–171. [DOI] [PubMed] [Google Scholar]
- 20. Hunt SA, Sherman O. Arthroscopic treatment of osteochondral lesions of the talus with correlation of outcome scoring systems. Arthroscopy. 2003;19(4):360–367. [DOI] [PubMed] [Google Scholar]
- 21. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81(9):1229–1235. [DOI] [PubMed] [Google Scholar]
- 23. Lee KB, Bai LB, Yoon TR, Jung ST, Seon JK. Second-look arthroscopic findings and clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2009;37(suppl 1):63S–70S. [DOI] [PubMed] [Google Scholar]
- 24. Lee KB, Park HW, Cho HJ, Seon JK. Comparison of arthroscopic microfracture for osteochondral lesions of the talus with and without subchondral cyst. Am J Sports Med. 2015;43(8):1951–1956. [DOI] [PubMed] [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. [DOI] [PubMed] [Google Scholar]
- 26. Lynn AK, Brooks RA, Bonfield W, Rushton N. Repair of defects in articular joints: prospects for material-based solutions in tissue engineering. J Bone Joint Surg Br. 2004;86(8):1093–1099. [DOI] [PubMed] [Google Scholar]
- 27. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–466. [PubMed] [Google Scholar]
- 28. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. [DOI] [PubMed] [Google Scholar]
- 29. Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am. 2015;97(1):1–2. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Cook DJ, Jadad AR, et al. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess. 1999;3(12):i–iv, 1–98. [PubMed] [Google Scholar]
- 31. Moher D, Jones A, Cook DJ, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. [DOI] [PubMed] [Google Scholar]
- 32. Ogilvie-Harris DJ, Sarrosa EA. Arthroscopic treatment of osteochondritis dissecans of the talus. Arthroscopy. 1999;15(8):805–808. [DOI] [PubMed] [Google Scholar]
- 33. Polat G, Erşen A, Erdil ME, Kızılkurt T, Kılıçoğlu Ö, Aşık M. Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1299–1303. [DOI] [PubMed] [Google Scholar]
- 34. Ramponi L, Yasui Y, Murawski CD, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45(7):1698–1705. [DOI] [PubMed] [Google Scholar]
- 35. Reilingh ML, van Bergen CJ, Blankevoort L, et al. Computed tomography analysis of osteochondral defects of the talus after arthroscopic debridement and microfracture. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680–1687. [DOI] [PubMed] [Google Scholar]
- 37. Seow D, Yasui Y, Hutchinson ID, Hurley ET, Shimozono Y, Kennedy JG. The subchondral bone is affected by bone marrow stimulation: a systematic review of preclinical animal studies. Cartilage. 2019;10(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimozono Y, Coale M, Yasui Y, O’Halloran A, Deyer TW, Kennedy JG. Subchondral bone degradation after microfracture for osteochondral lesions of the talus: an MRI analysis. Am J Sports Med. 2018;46(3):642–648. [DOI] [PubMed] [Google Scholar]
- 39. Shimozono Y, Hurley ET, Myerson CL, Kennedy JG. Good clinical and functional outcomes at mid-term following autologous osteochondral transplantation for osteochondral lesions of the talus. Knee Sports Traumatol Arthrosc. 2018;26(10):3055–3062. [DOI] [PubMed] [Google Scholar]
- 40. Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29(8):1399–1409. [DOI] [PubMed] [Google Scholar]
- 41. Takakura Y, Tanaka Y, Kumai T, Tamai S. Low tibial osteotomy for osteoarthritis of the ankle: results of a new operation in 18 patients. J Bone Joint Surg Br. 1995;77(1):50–54. [PubMed] [Google Scholar]
- 42. van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GM, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95(6):519–525. [DOI] [PubMed] [Google Scholar]
- 43. van Dijk CN, Verhagen RA, Tol JL. Arthroscopy for problems after ankle fracture. J Bone Joint Surg Br. 1997;79(2):280–284. [DOI] [PubMed] [Google Scholar]
- 44. Zengerink M, Struijs PA, Tol JL, Van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]