Abstract
Mechanisms underlying pulmonary arterial hypertension (PAH) remain elusive. Pulmonary arterial hypertension and exercise PH share similar physiologic consequences; it is debated whether they share biologic mechanisms and if exercise PH represents an early phase of pulmonary arterial hypertension. We conducted an observational study to test if there is a graded metabolic disturbance along the severity of PH, which may indicate shared or disparate pathophysiology. Individuals referred to an academic medical dyspnea center with unexplained exertional intolerance underwent invasive cardiopulmonary exercise testing. We identified controls with no hemodynamic exercise limitation, individuals with exercise PH (mean pulmonary arterial pressure (mPAP) < 25 mmHg at rest but ≥ 30 mmHg during exercise without pulmonary venous hypertension) and pulmonary arterial hypertension (mPAP > 25 mmHg at rest without pulmonary venous hypertension) (n = 26 in each group). Unbiased metabolomics with chromatography mass spectrometry was performed on pulmonary arterial blood at rest and peak exercise. Random forest analysis and hierarchical clustering were used to quantify metabolite prediction of group membership and rank metabolites which were significantly different between groups. Compared to controls, pulmonary arterial hypertension subjects exhibited perturbations in pathways involving glycolysis, TCA cycle, fatty acid and complex lipid oxidation, collagen deposition and fibrosis, nucleotide metabolism, and others. The metabolic signature of exercise PH was uniquely between that of control and pulmonary arterial hypertension subjects. Accuracy predicting control, exercise PH, and pulmonary arterial hypertension group was 96%, 90%, and 88%, respectively, using paired rest-exercise metabolic changes. Our data suggest the metabolic profile of exercise PH is between that of controls and patients with pulmonary arterial hypertension.
Keywords: pulmonary hypertension, metabolomics, exercise
Introduction
Pulmonary hypertension (PH), a pulmonary vascular disorder with multiple causes, is defined by a mean pulmonary artery pressure (mPAP) > 20 mmHg at rest. PH manifests as decreased exercise tolerance, fatigue, or dyspnea. Regardless of the disease process associated with PH, when PH develops, it predisposes to increased morbidity and/or mortality. PH is often difficult to diagnose at its earliest stage when the disease can remain silent or symptomatically mimic other conditions, resulting in misdiagnosis and possibly missing an important window which could provide mechanistic insight and therapeutic opportunity.
Resting mPAP 19–24 mmHg is associated with increased risk of hospitalization and death,1 and resting mPAP 21–24 mmHg is associated with decreased exercise capacity and decreased survival.2 Our group and others have described the physiologic profile of subjects who at rest do not have PH but who develop PH with exercise (ePH)3,4 (mPAP ≥ 30 mmHg during exercise but < 25 mmHg at rest in the absence of pulmonary venous hypertension), as measured with invasive cardiopulmonary exercise testing (iCPET) or echocardiography.5 Twenty five percent to 86% of patients with resting mPAP 21-24 mmHg have ePH.6,7 ePH itself is associated with overt symptoms, reduced exercise capacity, and poorer clinical outcomes.3,8–13 Exercise PH may represent an early and clinically relevant stage of pulmonary arterial hypertension (PAH). Whether ePH inevitably progresses to PAH is unknown, and treatment is controversial, though ePH has been hypothesized as a possible target to prevent or delay development of overt PAH.3
In light of growing evidence that clinical outcomes worsen at lower mPAP values than previously used to define PAH, at the recent 6th World Symposium on Pulmonary Hypertension, the mPAP cutoff to help define PAH was lowered from 25 mmHg to 20 mmHg.14 The Task Force also reexamined if ePH should be included in the definition of PAH.14 The Task Force felt that ePH should not be included in the definition of PAH due, in part, to lack of certainty regarding the etiologic relationship of ePH to PAH.
We hypothesized that constructing metabolite signatures and comparing those profiles between controls with normal pulmonary vascular response to exercise versus patients with ePH and established PAH would provide insight as to overlap between these two disease states. Because treating ePH with pulmonary vasodilators is also debated, rather than comparing patient groups off and on treatment, for example, we compared metabolic profiles at rest and at peak exercise as a clinically relevant perturbation. We applied machine learning data reduction with random forest analysis to determine a tractable number of metabolites which may have highest predictive accuracy for delineating ePH and PH. To our knowledge, this is the first application of rigorous clinical phenotyping with iCPET and unbiased metabolomic testing to identify metabolic signatures along the progression of PH. Although observational and hypothesis generating, our data fill a critical gap in knowledge about the graded biologic pattern between ePH and PAH, and may help clarify whether ePH should be considered in the definition of PAH.
Methods
Study sample
The study sample was derived from patients obtaining routine care in the dyspnea clinic at Brigham and Women's Hospital (Boston, MA). Patients underwent a clinically indicated resting supine right heart catheterization followed by a symptom-limited upright iCPET as part of their diagnostic evaluation. The study was approved by the Partners Human Research Committee (2011P000272). Patients gave written informed consent for subsequent use of clinical data for research, including biological samples.
Hemodynamic measurements
The technical aspects of our iCPET and exercise protocol have been described in detail previously.15,16 RHC was performed in the supine position with a 5-port pacing PA catheter (Edwards LifeSciences, Irvine, CA, USA) inserted percutaneously under ultrasound and fluoroscopic guidance into the internal jugular vein and a 5Fr radial artery catheter contemporaneously placed in the radial artery. Patients underwent a symptom-limited incremental CPET using an upright cycle ergometer with a breath-by-breath assessment of gas exchange (ULTIMA CPX; Medical Graphics Corporation, St Paul, MN, USA). Pulmonary and systemic hemodynamics was simultaneously monitored during exercise (Xper Cardio Physiomonitoring System; Phillips, Melbourne, FL, USA). Measurements were recorded at end expiration. Patients included in this analysis all reached or surpassed their predicted respiratory exchange ratio peak to demonstrate acceptable amount of exercise before defining their phenotype. Pulmonary pressures were recorded at the end of passive exhalation. Blood samples were obtained from the pulmonary artery (PA) and radial artery at rest, each minute of exercise, peak exercise, and 1 h after peak exercise – only blood samples from the PA were used in this analysis. Each specimen was centrifuged for at least 15 min at 2200–2500 RPM within 1 h of collection. Specimens were aliquoted and frozen at −80℃. Within an hour of the collection, the samples were processed and stored before shipment to the metabolomics laboratory.
PH group categorization
This study was performed during the period in which PAH was defined as resting mPAP > 25 mmHg, pulmonary arterial wedge pressure (PAWP) < 15 mmHg, and PVR ≥ 3 Woods units, acknowledging that in December 2018, the 6th World Symposium on Pulmonary Hypertension lowered the mPAP cutoff, defining PAH as > 20 mmHg.14 ePH was defined as, at peak exercise, mPAP > 30 mmHg and PAWP < 20 mmHg. Normal controls had resting mPAP < 20 mmHg, PAWP < 15 mmHg, PVR < 3 Woods units, and peak exercise mPAP < 30 mmHg and PAWP < 20 mmHg.
Metabolomic testing
From our database of over 1500 clinical iCPETs, 26 patients with ePH were selected randomly and age and gender matched to 26 patients with PAH and 26 patients with normal exercise hemodynamics. Only patients with frozen plasma stored in −80℃ for a period not greater than five years and with no previous freeze/thawed cycles were included. The following exclusion criteria were used: (1) submaximal exercise testing: peak respiratory exchange ratio (RER) < 1.05, peak heart rate (HRmax) < 85% and PvO2 > 27mmHg; (2) pulmonary mechanical limit to exercise: ventilatory reserve (VE/MVV) at the anaerobic threshold (AT) > 0.70; (3) moderate/severe valvular disease by the contemporary cardiac echocardiogram; and (4) severe anemia: hemoglobin concentration < 10. All blood samples were drawn before any patient began treatment for PH. We performed unbiased metabolomic testing using Metabolon on paired blood samples drawn at rest and peak exercise, measuring 1013 compounds of known identity. This allowed us to construct metabolic signatures at rest and during exercise both shared by and unique to ePH and PAH. Sample processing methods are available at www.metabolon.com, and described in the Supplement.
Statistical analysis
Baseline characteristic of control, ePH, and PAH patients were compared using one-way analysis of variance (ANOVA) with Tukey's post hoc analysis for continuous variables and using the Chi-square test for categorical variables. A two-sided p-value of ≤ 0.05 was used to determine significant differences among groups (GraphPad Prism 7). For analysis of metabolites, following log transformation and imputation of missing values, if any, with the minimum observed value for each compound, a two-way ANOVA with repeated measures was used to identify biochemicals that differed significantly between experimental groups from rest to peak exercise. Output displays relative spectral peaks and is therefore unitless. Random forest clustering was used to generate prediction trees for each group membership using the metabolic data and to determine the accuracy predicting group membership. To determine which metabolites make the largest contribution to the classification (ranking), we used the mean decrease accuracy, measured as a percent decrease, as the variable importance metric for ranking. For simplicity, we display the top 30 metabolites in biochemical importance plots as potentially worthy of further investigation.
Results
Baseline clinical characteristics of the patients at rest are shown in Table 1. As expected, patients with PAH had higher mPAP and PVR, and lower CO. Compared to controls, PAH patients were slightly older and had a greater burden of cardiovascular comorbidities. Patients with ePH and PAH had higher body mass index and a greater prevalence of hypertension than controls. No patients were using pulmonary vasodilator therapy at the time of iCPET. Hemodynamic differences between groups during upright exercise are displayed in Table 2. Of the 26 ePH patients included, 21 began PH specific treatment early after diagnosis: 11 began phosphodiesterase-5 inhibitor (5 tadalafil, 6 sildenafil); 11 began ambrisentan (8 of these were enrolled in an open-label trial of ambrisentan in ePH that has shown improvement in pulmonary hemodynamics in ePH); and 2 patients began prostacyclins. Two patients had connective tissue disease and progressed to resting PAH. One patient had a resting RHC five years before iCPET and had previously been diagnosed with PAH but had been lost to care for some time. No patient had a history of heart failure.
Table 1.
Baseline characteristics of the study population at rest.
| Controls | ePH | PAH | p-value | |
|---|---|---|---|---|
| Subjects | 26 | 26 | 26 | |
| Age, years | 58 ± 15 | 64 ± 13 | 70 ± 13* | < 0.01 |
| Females | 20 (77) | 17 (65) | 19 (73) | 0.64 |
| Body mass index | 25 ± 4.1 | 31 ± 6.4** | 29 ± 5.4* | < 0.01 |
| Hb (g/dL) | 14 ± 1.5 | 13.5 ± 1.6 | 13.5 ± 1.6 | 0.18 |
| Comorbidities and risk factors | ||||
| Hypertension | 9 (35) | 18 (69)* | 18 (69)* | 0.01 |
| Diabetes mellitus | 0 (0) | 3 (12) | 7 (27)** | 0.01 |
| Dyslipidemia | 7 (27) | 10 (38) | 17 (65)* | 0.01 |
| Tobacco | 2 (8) | 0 (0) | 0 (0) | 0.13 |
| Resting supine hemodynamics | ||||
| RAP (mmHg) | 6 ± 2 | 6 ± 3 | 8 ± 4* | 0.03 |
| mPAP (mmHg) | 17 ± 3 | 22 ± 4** | 34 ± 6**,¶ | < 0.01 |
| PAWP (mmHg) | 10 ± 3 | 11 ± 3 | 12 ± 5* | 0.05 |
| CO (L/min) | 4.8 ± 0.6 | 5.5 ± 1.7 | 4.5 ± 1α | 0.01 |
| CI (L/min/m2) | 2.7 ± 0.4 | 2.9 ± 0.9 | 2.5 ± 0.4 | 0.08 |
| PVR (WU) | 1.3 ± 0.5 | 2.2 ± 1 | 5 ± 2.1**,¶ | < 0.01 |
Note: Data are presented as n, mean ± SD or n (%), unless otherwise stated.
*p < 0.05 compared with controls. **p < 0.01 compared with controls. ¶p < 0.01 comparing ePH versus PAH. αp < 0.05 comparing ePH versus PAH.
ePH: exercise pulmonary hypertension; PAH: pulmonary arterial hypertension; RAP: right atrial pressure; mPAP: mean pulmonary artery pressure; PAWP: pulmonary arterial wedge pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance.
Table 2.
Upright invasive cardiopulmonary exercise data.
| Controls | ePH | PAH | ANOVA p-value | |
|---|---|---|---|---|
| Subjects | 26 | 26 | 26 | |
| Peak Work, Watts | 132 ± 45 | 92 ± 35** | 61 ± 42**,α | <0.01 |
| Peak heart rate, bpm | 149 ± 25 | 134 ± 26 | 118 ± 24** | <0.01 |
| Peak heart rate, % predicted | 90 ± 13 | 85 ± 13 | 79 ± 13** | 0.01 |
| Peak RER | 1.15 ± 0.1 | 1.14 ± 0.2 | 1.06 ± 0.1 | 0.07 |
| VE/VCO2 slope | 31.9 ± 5.5 | 32.8 ± 7.6 | 44 ± 18.2**,¶ | <0.01 |
| Peak VO2, % predicted | 96 ± 15 | 75 ± 16** | 68 ± 21** | <0.01 |
| Peak VO2, mL/kg/min | 23.0 ± 8.7 | 14.7 ± 4** | 12.5 ± 4.4** | <0.01 |
| VO2 at AT (%VO2max predicted) | 51 ± 11 | 42 ± 11* | 41 ± 10** | <0.01 |
| Peak CO, L/min | 12.1 ± 3.2 | 10.5 ± 2.5 | 8.4 ± 3.6**,α | <0.01 |
| Peak CO, % predicted | 103 ± 15 | 92 ± 19 | 86 ± 25* | 0.01 |
| Peak Ca-vO2, mL/dL | 13.1 ± 1.6 | 11.5 ± 2.0* | 11.2 ± 2.1** | 0.01 |
| Peak CaO2, mL/dL | 19.3 ± 1.9 | 17.6 ± 2.3* | 16.6 ± 2.1** | 0.01 |
| Peak DO2, mL/min | 2323 ± 804 | 1870 ± 529* | 1421 ± 669** | <0.01 |
| Peak RAP, mmHg | 5 ± 3 | 8 ± 4* | 10 ± 5** | <0.01 |
| Peak mPAP, mmHg | 25 ± 7 | 41 ± 8** | 48 ± 13**,α | <0.01 |
| Peak PAWP, mmHg | 10 ± 4 | 13 ± 4 | 16 ± 6** | <0.01 |
| Peak TPG, mmHg | 15 ± 6 | 28 ± 7** | 32 ± 12** | <0.01 |
| Peak PVR, WU | 1.2 ± 0.6 | 2.7 ± 0.8** | 4.5 ± 2.2**,¶ | <0.01 |
| Peak PVC, mL/mmHg | 2.8 ± 0.7 | 2.3 ± 0.6* | 1.6 ± 0.8**,¶ | <0.01 |
Note: Data are presented as n, mean ± SD or n (%), unless otherwise stated.
*p < 0.05 compared with controls. **p < 0.01 compared with controls. ¶p < 0.01 comparing ePH versus PAH. αp < 0.05 comparing ePH versus PAH.
ePH: exercise pulmonary hypertension; PAH: pulmonary arterial hypertension; RER: respiratory exchange ratio; VE/VCO2 slope: ventilatory equivalent for carbon dioxide; VO2: maximum oxygen consumption; AT: anaerobic threshold; CO: cardiac output; Ca-vO2: arterial-venous oxygen content difference; CaO2: arterial oxygen content; DO2: oxygen delivery; RAP: right atrial pressure; mPAP: mean pulmonary artery pressure; PAWP: pulmonary arterial wedge pressure; TPG: transpulmonary gradient; PVR: pulmonary vascular resistance; PVC: pulmonary vascular compliance.
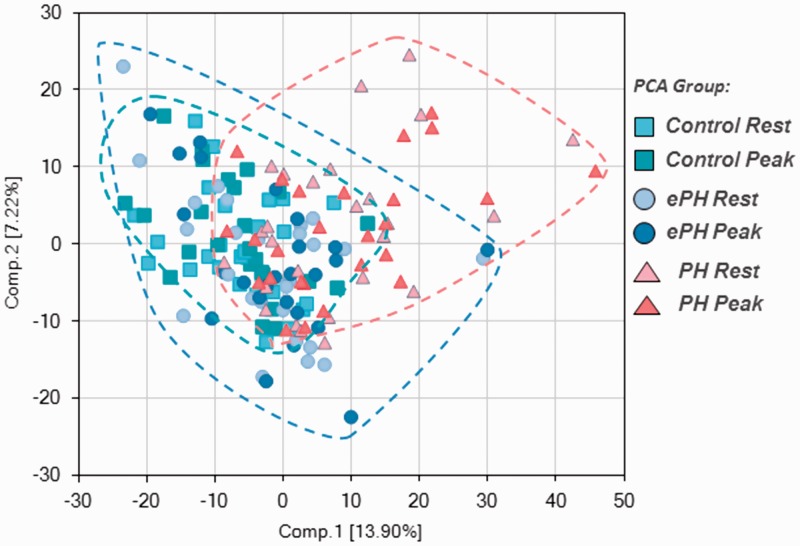
Overall, there were 319 metabolites associated with PH status (ePH vs. control, PAH vs. control, or ePH vs. PAH), 349 metabolites associated with exercise vs. rest (within each group), and 98 metabolites associated with both PH group and exercise (p ≤ 0.05). After principal component analysis, there was some separation between metabolite clusters of controls, ePH, and PH subjects, illustrating both shared and unique metabolites by disease status and severity (Fig. 1). Notably, samples from the same participant collected at rest and exercise clustered together, which is consistent with biochemical individuality often seen in human metabolic studies.
Fig. 1.
Principal component analysis depicting group clustering.
Energy generating pathways
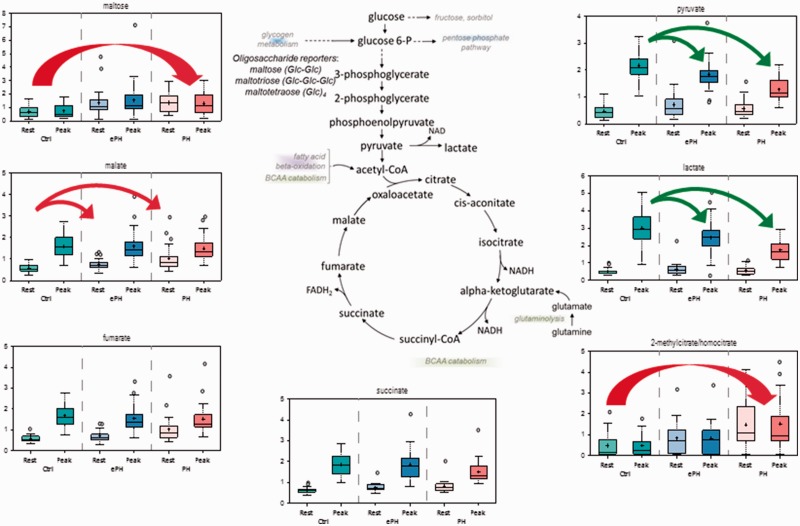
Random forest analyses illustrated that, in control participants, energy metabolism was altered at peak exercise compared to rest. We observed elevations in glycolytic intermediates pyruvate and lactate, and TCA cycle biochemicals succinate, fumarate, and malate (Fig. 2). This is expected given the need for increased energy utilization during exercise. Changes in energy utilization pathways with peak exercise vs. rest were also identified in controls vs. ePH and PAH, but the magnitude of the changes was blunted compared to that in controls. This was partially due to, in PAH subjects, higher rest fumarate and malate levels, and lower peak exercise levels of pyruvate, lactate, and succinate. Subjects with ePH had an intermediate metabolic signature. This indicates a graded poorer activation of glycolytic catabolism with exercise by PH severity, but also possibly higher glycolytic catabolism at rest with PH severity.
Fig. 2.
Changes in energy-generating pathways.
Fatty acid oxidation
Fatty acid oxidation is utilized to augment glucose metabolism. We noted alterations in fatty acid oxidation across groups at rest and peak exercise (Fig. 3). Consistent with increased energy demand during exercise, free fatty acids declined (e.g. stearate (18:0)), while acylcarnitines (e.g. laurylcarnitine (C12)) accumulated in all groups, indicating higher rates of beta-oxidation during exercise. Similarly, there was elevation in diacylglycerols (e.g. palmitoleoyl-oleoyl-glycerol (16:1/18:1)), suggesting enhanced hydrolysis of lipid stores during exercise. PAH and ePH groups had higher levels of acylcarnitines at rest and peak exercise compared to controls, suggesting diseased groups may either primarily utilize beta-oxidation more at rest and exercise to augment glycolytic catabolism, or may poorly utilize downstream metabolites, leading to secondary build-up of intermediate molecules in the acylcarnitine pathway. Ketone bodies acetoacetate and 3-hydroxybutarate (BHBA), which are produced by the liver and used by peripheral tissues, such as muscle, during exercise as a fuel source, significantly decreased during exercise in control and ePH groups. Ketone bodies declined in ePH and PAH as well, but the exercise-induced difference between rest and peak exercise was less pronounced than in controls. Subsequently, acetoacetate was higher in ePH and PAH participants vs. controls at peak exercise, which can be interpreted as failure to effectively oxidize ketone bodies in diseased states. Dicarboxylic acids (e.g. suberate (C8-DC), sebacate (C10-DC)) were elevated at rest and peak exercise in ePH and PAH vs. controls. Dicarboxylates are generated by omega-oxidations, which can serve as a rescue pathway when beta-oxidation is impaired and/or at times of high oxidative demand, and may indicate additional compensatory pathways in PH when beta-oxidation does not compensate for energy demand.
Fig. 3.
Changes in fatty acids oxidation.
Collagen deposition and fibrosis
N-terminal amino acids must be deacetylated so that the resulting free amino acids are available for protein synthesis. We observed marked elevations in acetylated amino acids in ePH and PAH vs. control at rest and peak exercise, which is consistent with altered protein degradation. C-glycosyltryptophan and 5-(galactosylhydroxy)-L-lysine were elevated in ePH and PAH vs. controls. 5-(galactosylhydroxy)-L-lysine is found almost exclusively within collagen. These changes suggest elevation in collagen deposition and/or turnover in PH. Collagen turnover is also associated with changes in aminosugars, which are involved in protein glycosylation reactions. Consistent with this reasoning, we found several aminosugar metabolites (e.g. N-acetylneuraminate, N-acetylglucosamine/N-acetylgalactosamine) were elevated in ePH and PAH, suggesting increased hexosamine synthesis and extracellular matrix remodeling. Dimethylarginine (SMDA + ADMA), which is derived from catabolism of proteins containing methylated arginine residues, was also elevated in ePH and PAH vs. controls at rest and peak exercise.
Nucleotide metabolism
In all groups, exercise was associated with higher levels of adenosine 3′,5′-cyclic monophosphate (cAMP), hypoxanthine, and inosine. The control, ePH, and PAH groups differed dramatically regarding nucleotide homeostasis. The PAH group had higher levels of several pyridine and purine nucleotides, including adenine, xanthosine, urate, and pseudouridine.
Prediction of group membership
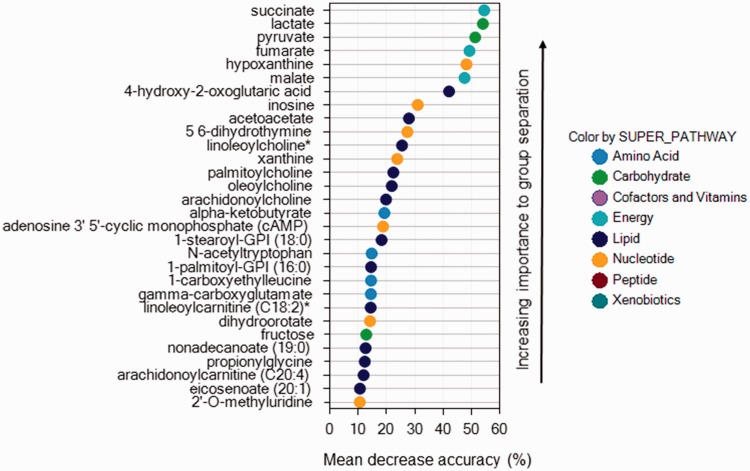
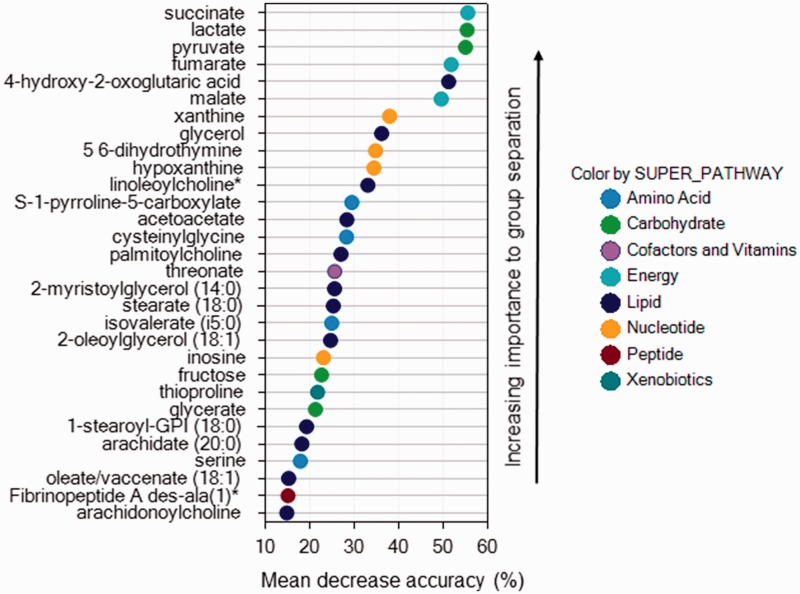
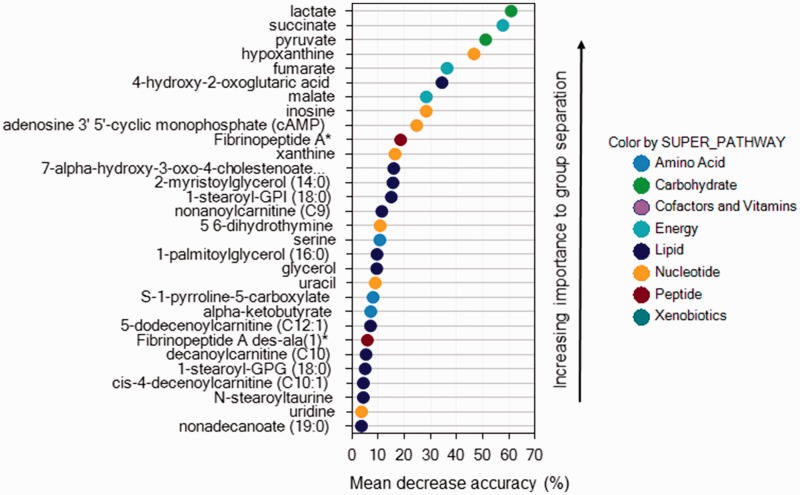
Applying random forest analysis allowed us to predict group membership from metabolites with high accuracy. Using blood samples paired between rest and peak exercise, the accuracy predicting control, ePH, and PAH group were 96%, 90%, and 88%, respectively. When comparing groups only at rest or at peak exercise, accuracy predicting group membership fell to 67% and 63%, respectively. Figures 4 to 6 display the biochemical importance plots for the top 30 metabolites from the paired rest–exercise blood samples predicting group membership. Larger mean decrease accuracy predicting group membership when the metabolite was permuted in the model equates to a more important metabolite.
Fig. 5.
Biochemical importance plot for exercise-induced pulmonary hypertension.
Fig. 4.
Biochemical importance plot for normal controls.
Fig. 6.
Biochemical importance plot for pulmonary arterial hypertension.
Discussion
To our knowledge, this is the first report of unbiased metabolomic profiling of PH in humans using detailed phenotyping with iCPET that allowed identification of ePH. We demonstrated global alterations in metabolism between control, ePH, and PAH patients, as well as unique and shared signatures between ePH and PAH patients. Our results suggest that the resting and exercise metabolic profile of patient with ePH sits between that of controls and patients with PAH.
Although our data are observational and we cannot draw direct causal relationships, our results suggest that PH patients have altered metabolism characterized by higher resting glycolytic activity and fatty acid oxidation, poorer glycolytic catabolism augmentation with exercise, and increased reliance on fatty acid oxidation, nucleotide metabolism, and catecholamine activation during exercise. PAH participants may have increases in some metabolic intermediates due to poor downstream utilization, leading to intermediate backup. Simultaneously, they have metabolite evidence of altered protein flux and extracellular matrix remodeling. These studies are in agreement with others, notably a metabolomic study utilizing tissue explant samples from PAH patients.17 Without global metabolic profiling, recognizing changes in up and downstream intermediates and across classes of metabolites would be more difficult, possibly leading to inappropriate conclusion about metabolic stoichiometry by disease severity or in response to exercise.
We found that participants with ePH and PAH had higher rest fumarate and malate levels compared to controls, suggesting that at rest PH patients may exist in a higher glycolytic catabolic state. With exercise, they also do not augment their glycolytic catabolism as effectively, illustrated by lower levels of pyruvate, lactate, and succinate at peak exercise. With poorer glycolytic catabolism at peak exercise, they must augment with increased fatty acid beta-oxidation, exemplified by lower levels of free fatty acids at peak exercise. Higher levels of acylcarnitines at rest and peak exercise may indicate that PH participants use fatty acid oxidation more than controls, or that PH participants have impaired ability to oxidize acyl-CoA species, leading to accumulations of acylcarnitine intermediates. In total, these results indicate that patients with ePH and PAH activate energy utilization systems differently at rest and with exercise compared to control subjects and compared to each other, suggesting a graded perturbation in metabolic phenotype that parallels PH progression. Targeted profiling of a range of metabolites, as identified by our random forest rankings, may allow earlier identification of PH or progression of PH before pathologic changes in resting central hemodynamics or symptoms. Prediction accuracy of our random forest models suggests that this may be possible with high confidence.
Although our data are descriptive, the results are strengthened by consistent associations shared with mechanistic studies using tissue, animal models, or focusing on specific pathways in humans. Zhao et al.17 compared global metabolomic profiles in lung tissue from patients with severe PAH vs. controls; profiles were similar to our own, including those for glucose homeostasis and fatty acid utilization. These results are notably from patients with severe PAH only and do not depict changes with exercise, a stress which further differentiates the functional capability of PH patients from controls, as exemplified by higher predictive accuracy for PAH and ePH, compared to controls, from our random forest models using paired rest–exercise blood samples. Luo et al.18 also documented higher concentrations of long-chain plasma acylcarnitines in PAH cases versus controls, with different strengths of association based on pre vs. post-capillary PH status, highlighting the need for detailed PH phenotyping. Rhodes et al.19 demonstrated similar metabolic changes and patterns in patients with PAH in addition to metabolic profiles associating with increased risk of death. Barnes et al.20 noted that hexosamine biosynthetic pathway flux is increased in idiopathic PAH and drives O-linked β-N-acetylglucosamine transferase-mediated pulmonary artery smooth muscle cell proliferation20 Asymmetrical dimethylarginine (ADMA) has also been associated previously with unfavorable pulmonary hemodynamics and worse outcomes in patients with idiopathic PAH, and infusion with ADMA in healthy volunteers was associated with increased pulmonary vascular resistance and decreased stroke volume.21 Urate and xanthine oxidase have both been shown to be increased in PH.22–24 A rodent model of PH via chronic hypoxia has also shown enhanced xanthine oxidase activity, and blood pressure can be restored by treatment with specific xanthine oxidase inhibitors.25,26 These more targeted studies sit neatly within our global view of metabolites. Coupled with delineation of ePH, they collectively suggest a graded metabolic disarray paralleling the severity of PH.
Our study was strengthened by the use of iCPET, which allowed detailed clinical phenotyping and the ability to isolate a group of subjects with ePH but not PAH at rest, potentially finding a group of individuals earlier in their PH progression who may harbor unique early signatures of developing PAH. The importance of exercise stress in differentiating individuals who appear phenotypically similar at a single point in time is mirrored by the drop in accuracy predicting group membership using metabolites at either rest (67%) or peak exercise (63%), compared with the accuracy predicting group membership using change in metabolites between rest and peak exercise (accuracy > 88%). Subjects were able to serve as their own controls by drawing blood at rest and peak exercise, aiding statistical power and reducing confounding. We also deliberately used unbiased metabolomic testing in an attempt to survey as broad a swath of the metabolic space as currently possible to find novel pathways.
Our study has some limitations. We acknowledge the data are observational and hypothesis generating, though there are no animal models which are reflective of human ePH, and the specific goal of this study was to expand the depth of phenotyping to more clearly define a possible spectrum of disease or subcategory of disease. We felt that conducting an intervention study is not currently tenable. We tried to overcome this limitation by using exercise as a way to phenotype patients and as a safe and clinically relevant intervention which could be used to compare rest and peak metabolic profiles. The definition of PAH changed after our study was completed, lowering the mPAP cutoff from 25 mmHg to 20 mmHg. While this can be interpreted as introducing classification bias to our analysis (i.e. some control and ePH participants may be up-categorized to worse PH severity), our analysis is robust to the definition change because we included patients with ePH who had intermediate mPAP and PVR compared to controls and patients with PAH. This allowed us to examine the biologic gradation in metabolism across a range of mPAP and exercise-induced changes rather than relying solely on more disparate hemodynamic categories. It also suggests that it may be worthwhile to reexamine the PVR threshold currently used to define PAH, which was not changed in the updated consensus definition. We acknowledge our findings are inherently exploratory and require further validation, hopefully through experimental study, to inform biological relevance of identified metabolites. Our sample size is small and underpowered to allow adjustment for possible residual confounders. Using iCPET and exercise intervention allows patients to serve as their own controls for within individual comparisons, which greatly reduces the sample size needed for statistical significance and is robust to confounding but does not control for confounding due to differences in covariates between groups. We identified EDTA and its metabolite iminodiacetate (IDA) in both ePH and PH, which may suggest that sample collection technique and/or reagents could have contributed to batch separation, despite the fact that rigorous protocolized collection and reagent use was followed. Our study and most others measuring global metabolic signatures in PH have used the platform provided by Metabolon – this may engender publication bias through reliance on a single proprietary platform, but also enables comparison between studies to identify consistent associations. We only measured metabolites in blood from the pulmonary artery. It is unknown how metabolic flux across the lungs and systemic circulation may influence results. It would be useful to compare metabolites associations with PH status and outcomes across compartments (e.g., radial artery, peripheral venous, and central venous) to determine consistency and predictive accuracy, which may influence clinical applicability. There is variability in patient consumption of food (amount, composition, and timing) and adherence to medications (e.g. statins and antihyperglycemics), relative to when blood is drawn, and food consumption and medication adherence may differ by disease status in unknown ways. Although this may influence results in an unpredictable way, our results were nonetheless consistent in direction and magnitude across severity of PH, and did not change appreciably after adjustment for the measured content of medication metabolites. We acknowledge that despite matching, a gradation in age persisted across phenotypic categories. Although aging is known to increase systemic vascular resistance over time, the magnitude and pattern of effect (e.g., linear, exponential) on the pulmonary vasculature, at rest and exercise, are poorly defined, and warrant further investigation to inform how it may influence results. Our patients were not randomized as in a clinical trial, and so other unmeasured differences may confound our results, including the prevalence of other comorbidities. Nonetheless, we selected patients who presented for clinical evaluation for unexplained exertional intolerance and/or dyspnea and who had previously had extensive evaluations rather than participants who were included in a trial, and so our results may be more generalizable.
In conclusion, we show that ePH and PAH are metabolically distinct states compared to normal at rest and peak exercise. Compared to each other, ePH and PAH harbor unique and shared metabolic perturbations, but overall, ePH appears metabolically an early step along the PAH pathway, mirroring hemodynamic severity. Further investigation of ePH using unbiased testing to inform targeted mechanistic assessment may reveal novel pathways which can be leveraged to ameliorate the hemodynamic and/or metabolic consequences of evolving PH.
Supplemental Material
Supplemental material, PUL882623 Supplemetal Material for Metabolomics of exercise pulmonary hypertension are intermediate between controls and patients with pulmonary arterial hypertension by Jason L. Sanders, Yuchi Han, Mariana F. Urbina, David M. Systrom and Aaron B. Waxman in Pulmonary Circulation
Acknowledgements
None.
Authors' contributions
Study design and conception (DMS, ABW), Acquisition, analysis, and interpretation of data (JLS, YH, MFU, DMS, ABW), Drafting and critical revisions of the manuscript (JLS, ABW), Obtaining funding and administrative support (YH, DMS, ABW)
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Cardiovascular Medical Research and Education Fund (to YH and ABW).
Ethical approval
The study was approved by the Partners Human Research Committee (2011P000272).
Guarantor
Jason L. Sanders.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation 2016; 133: 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs G, Avian A, Tscherner M, et al. Characterization of patients with borderline pulmonary arterial pressure. Chest 2014; 146: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 3.Tolle JJ, Waxman AB, Van Horn TL, et al. Exercise-induced pulmonary arterial hypertension. Circulation 2008; 118: 2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naeije R, Vonk Noordegraaf A, Kovacs G. Exercise-induced pulmonary hypertension: at last!. Eur Respir J 2015; 46: 583–586. [DOI] [PubMed] [Google Scholar]
- 5.Naeije R, Vanderpool R, Dhakal BP, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 2013; 187: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira RKF, Faria-Urbina M, Maron BA, et al. Functional impact of exercise pulmonary hypertension in patients with borderline resting pulmonary arterial pressure. Pulm Circ 2017; 7: 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau EM, Godinas L, Sitbon O, et al. Resting pulmonary artery pressure of 21-24 mmHg predicts abnormal exercise haemodynamics. Eur Respir J 2016; 47: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 8.Steen V, Chou M, Shanmugam V, et al. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008; 134: 146–151. [DOI] [PubMed] [Google Scholar]
- 9.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. [DOI] [PubMed] [Google Scholar]
- 10.Fowler RM, Maiorana AJ, Jenkins SC, et al. Implications of exercise-induced pulmonary arterial hypertension. Med Sci Sports Exerc 2011; 43: 983–989. [DOI] [PubMed] [Google Scholar]
- 11.Saggar R, Khanna D, Furst DE, et al. Exercise-induced pulmonary hypertension associated with systemic sclerosis: four distinct entities. Arthritis Rheum 2010; 62: 3741–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira RK, Waxman AB, Agarwal M, et al. Pulmonary haemodynamics during recovery from maximum incremental cycling exercise. Eur Respir J 2016; 48: 158–167. [DOI] [PubMed] [Google Scholar]
- 13.Stamm A, Saxer S, Lichtblau M, et al. Exercise pulmonary haemodynamics predict outcome in patients with systemic sclerosis. Eur Respir J 2016; 48: 1658–1667. [DOI] [PubMed] [Google Scholar]
- 14.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53(1): 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maron BA, Cockrill BA, Waxman AB, et al. The invasive cardiopulmonary exercise test. Circulation 2013; 127: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 16.Berry NC, Manyoo A, Oldham WM, et al. Protocol for exercise hemodynamic assessment: performing an invasive cardiopulmonary exercise test in clinical practice. Pulm Circ 2015; 5: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Peng J, Lu C, et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One 2014; 9: e88727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo N, Craig D, Ilkayeva O, et al. Plasma acylcarnitines are associated with pulmonary hypertension. Pulm Circ 2017; 7: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodes CJ, Ghataorhe P, Wharton J, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017; 135: 460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes JW, Tian L, Heresi GA, et al. O-linked beta-N-acetylglucosamine transferase directs cell proliferation in idiopathic pulmonary arterial hypertension. Circulation 2015; 131: 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kielstein JT, Bode-Boger SM, Hesse G, et al. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2005; 25: 1414–1418. [DOI] [PubMed] [Google Scholar]
- 22.Bendayan D, Shitrit D, Ygla M, et al. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir Med 2003; 97: 130–133. [DOI] [PubMed] [Google Scholar]
- 23.Spiekermann S, Schenk K, Hoeper MM. Increased xanthine oxidase activity in idiopathic pulmonary arterial hypertension. Eur Respir J 2009; 34: 276. [DOI] [PubMed] [Google Scholar]
- 24.Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J 2008; 32: 503–512. [DOI] [PubMed] [Google Scholar]
- 25.Hoshikawa Y, Ono S, Suzuki S, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol 2001; 90: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 26.Jankov RP, Kantores C, Pan J, et al. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 2008; 294: L233–245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL882623 Supplemetal Material for Metabolomics of exercise pulmonary hypertension are intermediate between controls and patients with pulmonary arterial hypertension by Jason L. Sanders, Yuchi Han, Mariana F. Urbina, David M. Systrom and Aaron B. Waxman in Pulmonary Circulation