Abstract
Background:
Oculomotor impairments, dizziness, and imbalance are common after sports-related concussion (SRC) in adolescents and suggest a relationship between SRC and vestibular system dysfunction. However, it is not clear whether the source of these problems is attributable to the peripheral or central vestibular system.
Hypothesis:
The video Head Impulse Test (vHIT), which assesses peripheral vestibular function, will show differences in gain between adolescents with and without SRC. Furthermore, there will be an association between vHIT and clinical balance and vestibular/oculomotor testing.
Study Design:
Cross-sectional study.
Level of Evidence:
Level 2.
Methods:
Twenty-five symptomatic adolescents aged between 12 and 19 years with a recent (within 10 days) SRC and 22 healthy controls aged 13 to 20 years were assessed using the vHIT, Balance Error Scoring System (BESS), and Vestibular Ocular Motor Screening (VOMS) tools. The vestibulo-ocular reflex (VOR) gain was calculated independently for right and left head impulses. Independent-samples t tests or Mann-Whitney U tests for nonnormal distributions were used to compare concussed patients and controls on the measures. Spearman rank-order correlations were used to assess the association of vHIT with BESS and VOMS.
Results:
VOR gain in all adolescents with SRC was greater than 0.8, which is considered within normal limits. VOR gain and BESS scores were not significantly different between groups. Adolescents with SRC had significantly worse VOMS item scores than adolescents without SRC (P < 0.001). There were no significant correlations among vHIT gain and VOMS or BESS.
Conclusion:
There was no evidence for dysfunction in the peripheral horizontal semicircular canal function at high rotation speeds (ie, vHIT) after SRC, and vHIT was unrelated to balance and vestibular/oculomotor symptoms and dysfunction. However, adolescents with SRC scored worse on vestibular and oculomotor testing than those without SRC. Vestibular dysfunction and symptoms after SRC may be centrally derived.
Clinical Relevance:
We do not recommend the assessment of head impulse function in adolescents with SRC unless more definitive signs of peripheral vestibular injury are present. We recommend using the VOMS to assess symptoms of suspected SRC injury in adolescents.
Keywords: balance, vestibular, oculomotor, concussion, adolescents
Sports-related concussion (SRC) results in physical, cognitive, emotional, and/or sleep-related symptoms that usually resolve spontaneously within a few days.6,8,16 The high prevalence of dizziness (75%) and balance problems (79%) after SRC motivates the study of vestibular function in this population.13,19 Traumatic brain injury (TBI) may compromise different parts of the vestibular system, and may result in central and/or peripheral vestibular disorders.5 Furthermore, several previous reports that quantified the prevalence of peripheral vestibular disorders after mild traumatic brain injury (mTBI) found peripheral dysfunction in 25% to 74% of their sample.4,22,25
Recently, the high-velocity (>150 deg/s) Head Impulse Test (HIT) has been used to examine peripheral vestibular function after concussion. The bedside HIT7 was combined with video-oculography in 2009 (vHIT).11 The primary parameter of interest is the gain, which is the ratio of eye velocity to head velocity. A gain value of 1 indicates that the eyes are counter-rotating at the exact velocity as the head and thus an earth-fixed target will be stabilized on the retina. A gain value less than 1 means that the eyes are counter-rotating at a slower velocity than the head, resulting in movement of the target across the retina (ie, retinal slip), and a perception of blurred vision. Various values have been used to define a clinically reduced gain indicative of peripheral vestibular hypofunction, from 0.68 to 0.90.2,11,24 Using a threshold of 0.90 as a cutoff for normality for a computer-controlled HIT, Balaban et al2 reported that 62% of their adult acute mTBI cohort were impaired, with an average gain of 0.84. In adolescents with chronic mTBI, Alshehri et al1 observed an average gain of 1.05 in their sample.
The primary purpose of this study was to estimate the prevalence of reduced vestibulo-ocular reflex (VOR) function as assessed using the vHIT in adolescents with and without SRC. A secondary purpose was to examine the relationship between vHIT gain and commonly used clinical balance and vestibular/oculomotor tests to determine whether clinical balance and vestibular test performance depends on peripheral VOR function in people with concussion.
Methods
Participants
The study was approved by the institutional review board at the University of Pittsburgh. Study investigators explained the study to interested adolescents and their parents (if younger than 18 years) in greater detail and obtained written informed consent from the participant (>18 years), or parental consent and participant assent (<18 years). A cross-sectional sample of 25 (16 male, 9 female) adolescent patients aged 12 to 19 years with a recent (within 10 days) SRC, and 22 (15 male, 7 female) age- and sex-matched controls aged 13 to 20 years were tested as part of a larger investigation assessing dual-task balance function in individuals with SRC. As part of this investigation, peripheral vestibular function was examined using the vHIT. Patients were recruited from multiple providers within a single sports concussion clinic after being diagnosed with SRC. Controls were recruited from middle and high schools in the greater Pittsburgh area and from the University of Pittsburgh. Participants in both groups were excluded if they had neck pain or injury, an injury with symptoms to the lower body, a history of a musculoskeletal disorder, a history of brain surgery, a history of substance abuse, a history of a major psychiatric or neurological disorder, a history of vestibular disorder, special education, or a history of TBI with a Glasgow Coma Score <13. Participants in the control group met the previous exclusion criteria and did not have an SRC. The following criteria were used to confirm a concussion diagnosis: (1) clear mechanism of injury, (2) presence of signs or symptoms of concussion at the time of injury (eg, posttraumatic amnesia, loss of consciousness, dizziness, headache), and (3) postconcussion impairment (balance, cognitive, vestibular, oculomotor) and/or increased symptoms from preconcussion levels.3,9
Measures
Video Head Impulse Test
The vHIT (EyeSeeCam Interacoustics; https://www.interacoustics.com/balance/software/eyeseecam) is a test of the VOR while maintaining gaze on an earth-fixed target during high-velocity head impulses. The vHIT was performed by rotating the head in the plane of the horizontal semicircular canals and measuring the horizontal eye movements. The vHIT system, which contains goggles secured on the head, incorporates a high-speed infrared camera to measure eye movements by tracking the pupil and an inertial measurement unit to record the velocity of the head impulse at a rate of 220 Hz.
Adolescents sat on a chair facing a wall (150 cm away) while viewing a small target. Participants were asked to keep their eyes on a fixed target on the wall in front of them while the examiner held the participants’ head with both hands and provided unpredictable, small amplitude, high-velocity head movements to the right and left. The amplitude of the head impulse was 10° to 20°, with a peak angular velocity of 150 to 300 deg/s and peak angular acceleration of 3000 to 6000 deg/s2. Ten head impulses in the horizontal plane in each direction were randomly provided.
Head movement was measured with the angular velocity sensor on the vHIT goggles. Eye position was measured using the calibrated video eye recordings and then differentiated with a phaseless 2-point difference equation to calculate eye velocity. VOR gain is equal to the ratio of the eye angular velocity to the head angular velocity. However, there is no gold standard for calculating the gain using video recordings. Therefore, we used 2 methods to calculate vHIT gain and compared the results from one method to the other. First, we calculated the instantaneous vHIT gain by dividing the eye velocity time series with the head velocity time series on a point-by-point basis, after aligning the peaks of the time series. The instantaneous gain was calculated as the average of 5 samples (approximately −10 to +10 ms) around the time point 20 ms before peak head velocity occurred. The second method of computing the vHIT gain was by estimating the regression slope between the eye velocity and the head velocity from the onset of movement to the peak velocity. Gain was calculated separately for the right and the left head impulses as the average of all head impulses to each side. To determine whether there were any abnormalities, the impulses from the side with the lower gain values were used in the statistical analysis.
Vestibular/Ocular Motor Screening
The Vestibular/Ocular Motor Screening (VOMS) is a brief clinical screening tool of the vestibulo-ocular system that assesses vestibular and ocular motor impairments not currently included in other concussion assessment tools.18 The VOMS consists of 7 tasks: smooth pursuits, horizontal saccades, vertical saccades, near point of convergence (NPC), horizontal VOR, vertical VOR, and a visual motion sensitivity (VMS) test. After performing each task, adolescents rated their symptoms using a 0 to 10 Likert-type scale, where 0 indicates no symptoms and 10 indicates severe symptoms. Symptoms included headache, dizziness, nausea, and fogginess.18
Balance Error Scoring System
The Balance Error Scoring System (BESS) assesses the vestibulospinal system using 6 balance tasks, each tested barefoot with eyes closed and hands on hips for 20 seconds while standing in double-leg stance, single-leg stance, and tandem stance. Each stance was performed on a firm surface and on an Airex foam pad.20
Postconcussion Symptom Scale
The Postconcussion Symptom Scale (PCSS) is a 22-item self-reported symptom questionnaire. The PCSS uses a 7-point Likert-type scale (range, 0-6) to assess concussion-related symptoms. Higher PCSS scores indicate worse symptoms.10
Procedures
Participants provided demographic information, medical history, and concussion history via questionnaire. Next, participants completed the following tests in this fixed order: PCSS, BESS, VOMS, and vHIT. All testing was conducted in a private examination area at the participants’ first clinical appointment. Controls were tested at convenient times outside of clinic hours. vHIT testing was performed on all participants by the same investigator. Because of the investigator’s availability, the vHIT was not performed on 4 patients with SRC. One patient with SRC withdrew from the study before completing the BESS, VOMS, and vHIT. One participant from the control group had missing VOMS data.
Statistical Analysis
Demographic data were described and then compared between groups using independent-samples t tests for continuous variables and chi-square tests for dichotomous variables. Independent-samples t tests or Mann-Whitney U tests for nonnormal distributions were used to compare SRC and controls on the vHIT, VOMS, and BESS measures. Spearman rank-order correlation coefficients were used to assess the association of vHIT to BESS and VOMS. All statistical analyses were conducted using SPSS version 22.0 (SPSS), with a significance level of P < 0.05. The study was powered to detect balance differences between groups rather than differences in vHIT values. The power analysis indicated that 16 participants would be needed in each group, assuming an effect size of η2 = 0.17.
Results
Participants
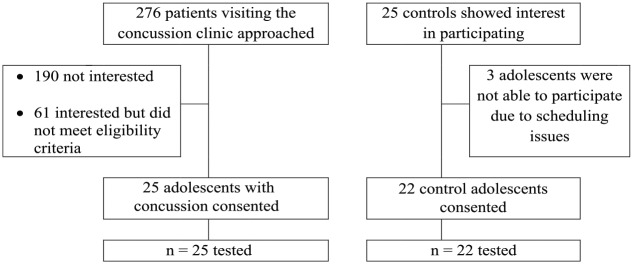
A total of 276 potentially eligible adolescents visiting the concussion clinic were screened for enrollment in the study. A flowchart indicating reasons for exclusion or inability to recruit participants is provided in Figure 1. A total of 25 of the 276 (9%) potentially eligible participants with SRC were enrolled into the study (mean days since injury, 5.8 days; SD, 2.7 days; range, 0-10 days). Demographic data are reported in Table 1. There were no significant differences in the age, sex, height, weight, or handedness for adolescents with and without SRC (Table 1).
Figure 1.
Participant enrollment flowchart.
Table 1.
Demographic and physical characteristics for sports-related concussion (SRC) and control groups
| Characteristics | SRC (n = 25) | Control (n = 22) | P |
|---|---|---|---|
| Age, y, mean (SD) | 15.1 (1.9) | 15.4 (2.1) | 0.59 a |
| Sex | 9 female, 16 male | 7 female, 15 male | 0.85 b |
| Weight, kg, mean (SD) | 64.1 (16.3) | 60.0 (8.9) | 0.28 a |
| Height, cm, mean (SD) | 171.0 (10.7) | 168.0 (10.5) | 0.15 a |
| Handedness | 20 right, 4 left | 19 right, 3 left | >0.99 b |
Independent-samples t test.
Pearson chi-square test.
SRCs occurred during participation in the following activities: recreational (n = 6); football (n = 5); basketball (n = 3); soccer (n = 3); hockey (n = 2); baseball (n = 1); cheerleading (n = 1); diving (n = 1); softball (n = 1); volleyball (n = 1); and wrestling (n = 1). At the time of their SRC, 24 (96%) adolescents reported dizziness, 13 (52%) reported confusion or disorientation, 4 (16%) reported anterograde amnesia, and 2 (8%) reported brief loss of consciousness. The PCSS total symptom severity score was significantly different between groups (P < 0.001): Patients with SRC had a mean symptom severity score of 30.4 (SD, 18.3), and controls had a mean symptom severity score of 6.4 (SD, 13.7).
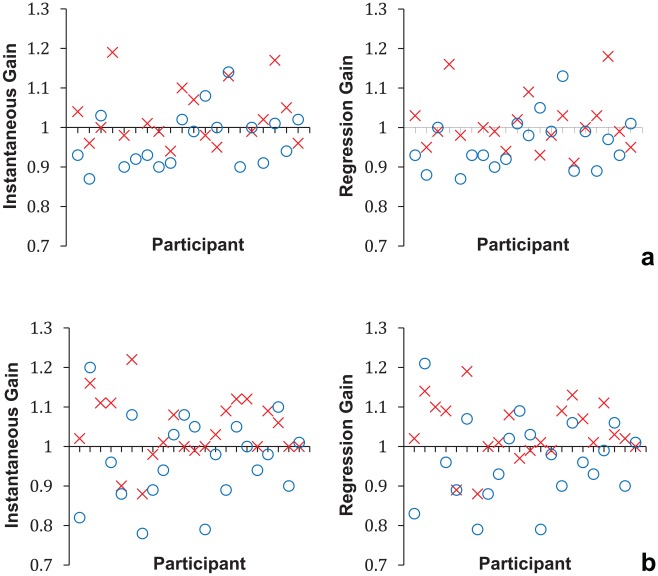
Figure 2 illustrates the interparticipant variability in individual gain responses for each of the gain measures for both participant groups, demonstrating a clustering of the values around a gain of 1.0, which represents normal VOR gain. Table 2 displays the mean values of the right and left impulses and asymmetry gain measures for both groups. Results of an independent-samples t test did not support group differences in the vHIT instantaneous and regression gains. In addition, the absolute asymmetry in gain between right and left sides was not different between groups.
Figure 2.
Individual video head impulse test (vHIT) gain values from right (x) and left (o) side head impulses for (a) adolescents with sports-related concussion (n = 20) and (b) controls (n = 22).
Table 2.
Video Head Impulse Test (vHIT) instantaneous and regression gain values for right and left impulses and asymmetry for the sports-related concussion (SRC) and control groups
| SRC (n = 20) | Control (n = 22) | P Valuea | ||||
|---|---|---|---|---|---|---|
| vHIT | Mean | SD | Mean | SD | ||
| Instantaneous gain 20 ms before peak | Right | 1.03 | 0.08 | 1.04 | 0.08 | 0.56 |
| Left | 0.97 | 0.07 | 0.97 | 0.11 | 0.98 | |
| Asymmetry | 0.08 | 0.04 | 0.09 | 0.06 | 0.27 | |
| Regression gain (no intercept) | Right | 1.01 | 0.07 | 1.04 | 0.08 | 0.21 |
| Left | 0.96 | 0.07 | 0.97 | 0.10 | 0.78 | |
| Asymmetry | 0.07 | 0.06 | 0.09 | 0.06 | 0.27 | |
Independent-samples t test.
Results of a Mann-Whitney U test indicated that all items of the VOMS were significantly different between groups (Table 3). Patients with SRC had worse symptoms and a longer NPC distance compared with participants without concussion. In the SRC group, there were no significant correlations between the vHIT instantaneous or regression gain and any of the VOMS subtests (Spearman ρ, <0.19; P > 0.05). BESS scores (Table 4) were not significantly different, with patients with SRC averaging 12 errors (SD, 5) and controls averaging 10 errors (SD, 4). There was also no difference between groups for the number of errors on either the firm or foam conditions. Finally, BESS performance did not correlate with vHIT performance (Spearman ρ, <0.3; P > 0.05).
Table 3.
Vestibular/Ocular Motor Screening (VOMS) scores in adolescents with sports-related concussion (SRC) and controls
| SRC (n = 24) | Control (n = 21) | ||||
|---|---|---|---|---|---|
| VOMS Items | Median | IQR | Median | IQR | P Valuea |
| Baseline symptoms | 7.5 | 5-13.75 | 0 | 0-2.75 | <0.001 |
| Smooth pursuits | 8 | 3.5-13 | 0 | 0-2.25 | <0.001 |
| Horizontal saccades | 8.5 | 5-14 | 0 | 0-2.25 | <0.001 |
| Vertical saccades | 9 | 4-13.25 | 0 | 0-2.25 | <0.001 |
| NPC–symptoms | 7 | 3-11 | 0 | 0-0.75 | <0.001 |
| Horizontal VOR | 9 | 3.5-13.75 | 0 | 0-0.75 | <0.001 |
| Vertical VOR | 9 | 4.25-14.75 | 0 | 0-1 | <0.001 |
| VMS | 10 | 5-14 | 0 | 0-0.75 | <0.001 |
| NPC–distance, cm | 3.75 | 1.8-13.9 | 0.5 | 0-1.3 | <0.001 |
IQR, interquartile range; NPC, near point of convergence; VMS, visual motion sensitivity; VOR, vestibulo-ocular reflex.aMann-Whitney U test.
Table 4.
Balance Error Scoring System (BESS) mean number of errors for sports-related concussion (SRC) and control groups
| SRC (n = 24) | Control (n = 22) | P Valuea | |||
|---|---|---|---|---|---|
| BESS | Median | IQR | Median | IQR | |
| Firm | 3.5 | 2-5 | 3.0 | 1-4 | 0.357 |
| Foam pad | 9.0 | 6-10 | 7.0 | 6-9 | 0.079 |
| Total | 11.5 | 9-15.75 | 11.0 | 7-13 | 0.213 |
IQR, interquartile range.aMann-Whitney U test.
Discussion
The key finding was that the vHIT gain value was not significantly different for patients with SRC and controls. Furthermore, none of the adolescents with SRC had a clinically reduced vHIT gain value, which was defined as having a vHIT gain ratio or slope lower than 0.80.12,14,21,23 Finally, the results indicated no correlation between the vHIT performance and VOMS symptom report or BESS performance.
The current findings support previous research of adolescents and adults with concussion,1 where no abnormalities were found on vHIT gain values. Participants in the study by Alshehri et al1 were more chronic (median number of days since concussion, 51; range, 11-803 days) than our sample (mean days since concussion, 5.8 days; SD, 2.7 days). Therefore, the vHIT gain may not be a useful tool to differentiate adolescents with and without concussion. Balaban et al2 assessed the computer-controlled rotational head impulse test (crHIT) gain, which provides whole-body impulses using a rotational chair while recording eye movement, and found that the crHIT gain was decreased in 62 of 100 patients with concussion compared with controls, using a threshold of 0.90 (both groups age range, 18-45 years). Possible reasons for discrepancies in the findings of the current study compared with the study by Balaban et al2 is that our participants were younger, tested approximately 3 days later, and received clinician-delivered head-on-body impulses rather than the computer-controlled whole-body impulses. It is not clear how these factors contribute to the outcomes.
Another goal of the study was to compare VOMS symptom provocation in adolescents with and without SRC. The baseline and provoked symptoms were higher (worse) in patients with SRC. The current findings support a previous study by Mucha et al,18 which demonstrated that adolescents with an SRC had a greater increase of symptoms on the VMS and had longer (worse) NPC distance than healthy controls.18 Our results suggest that the VOMS is more beneficial than the vHIT and the BESS in distinguishing adolescents with and without concussion approximately 1 week after the SRC.
The vHIT gain was unrelated to VOMS scores in adolescents after SRC. This is not surprising given that all adolescents with SRC had vHIT gains within normal limits. Although vHIT and VOMS were proposed to assess vestibular function, the vHIT tests the horizontal semicircular canals at a higher frequency than the VOMS VOR provocation test.
Limitations
The main limitation of this study is its lack of a comparison test of vestibular dysfunction, such as caloric testing. Inclusion of such a test could help in confirming vestibular system involvement. Another significant limitation is that included patients were up to 10 days post-SRC, which is a longer duration from injury onset compared with other studies that found resolution of reported symptoms and balance impairment (assessed using BESS) within 3 to 7 days after the SRC.15,17 Other limitations include that the examiner was blinded neither to those who had SRC nor to controls, and that the study was not powered to detect group differences with a small effect size.
Conclusion
The current study’s findings do not support the utility of the vHIT to assess head impulse function in adolescents after SRC, unless more definitive signs of peripheral vestibular injury (eg, spontaneous nystagmus, vertigo, hearing changes) are present.
Acknowledgments
The authors thank the Deanship of Scientific Research at Majmaah University for supporting this work under Project Number No. 1440-128.
Footnotes
The following authors declared potential conflicts of interest: A.P.K. performed work that resulted in a grant awarded to the University of Pittsburgh by Abbott and El Minda Ltd. S.L.W. received payment for lectures and, as a board member from the American Physical Therapy Association, received consulting fees from the Interacoustics Speakers Bureau and receives royalties from Oxford University Press. E.R.A. received a grant from the National Institutes of Health. P.J.S. has grants pending from the Department of Defense and the National Institutes of Health. This research was supported in part by a grant to the University of Pittsburgh from the National Institute on Deafness and Other Communication Disorders (1K01DC012332-01A1) to A.P.K.
References
- 1. Alshehri MM, Sparto PJ, Furman JM, et al. The usefulness of the video head impulse test in children and adults post-concussion. J Vestib Res. 2017;26:439-446. [DOI] [PubMed] [Google Scholar]
- 2. Balaban C, Hoffer ME, Szczupak M, et al. Oculomotor, vestibular, and reaction time tests in mild traumatic brain injury. PLoS One. 2016;11:e0162168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40:1303-1312. [DOI] [PubMed] [Google Scholar]
- 4. Davies RA, Luxon LM. Dizziness following head injury: a neuro-otological study. J Neurol. 1995;242:222-230. [DOI] [PubMed] [Google Scholar]
- 5. Ernst A, Basta D, Seidl RO, Todt I, Scherer H, Clarke A. Management of posttraumatic vertigo. Otolaryngol Head Neck Surg. 2005;132:554-558. [DOI] [PubMed] [Google Scholar]
- 6. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association position statement: management of sport-related concussion. J Athl Train. 2004;39:280-297. [PMC free article] [PubMed] [Google Scholar]
- 7. Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737-739. [DOI] [PubMed] [Google Scholar]
- 8. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15-26. [DOI] [PubMed] [Google Scholar]
- 9. Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain Inj. 2006;20:245-252. [DOI] [PubMed] [Google Scholar]
- 10. Iverson GL, Lovell MR, Collins MW. Interpreting change on ImPACT following sport concussion. Clin Neuropsychol. 2003;17:460-467. [DOI] [PubMed] [Google Scholar]
- 11. MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahringer A, Rambold HA. Caloric test and video-head-impulse: a study of vertigo/dizziness patients in a community hospital. Eur Arch Otorhinolaryngol. 2014;271:463-472. [DOI] [PubMed] [Google Scholar]
- 13. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40:747-755. [DOI] [PubMed] [Google Scholar]
- 14. McCaslin DL, Jacobson GP, Bennett ML, Gruenwald JM, Green AP. Predictive properties of the video head impulse test. Ear Hear. 2014;35:e185-e191. [DOI] [PubMed] [Google Scholar]
- 15. McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2556-2563. [DOI] [PubMed] [Google Scholar]
- 16. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. J Am Coll Surg 2013;216:e55-e71. [DOI] [PubMed] [Google Scholar]
- 17. Meehan WP, d’Hemecourt P, Comstock RD. High school concussions in the 2008-2009 academic year: mechanism, symptoms, and management. Am J Sports Med. 2010;38:2405-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mucha A, Collins MW, Elbin RJ, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42:2479-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson CL, Ferrara MS, Mrazik M, Piland S, Elliott R. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13:230-237. [DOI] [PubMed] [Google Scholar]
- 20. Riemann BL, Guskiewicz KM. Effects of mild head injury on postural balance testing. J Athl Train. 2000;35:19-25. [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor RL, Kong J, Flanagan S, et al. Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. J Neurol. 2015;262:1228-1237. [DOI] [PubMed] [Google Scholar]
- 22. Toglia JU, Rosenberg PE, Ronis ML. Posttraumatic dizziness; vestibular, audiologic, and medicolegal aspects. Arch Otolaryngol. 1970;92:485-492. [DOI] [PubMed] [Google Scholar]
- 23. van Esch BF, Nobel-Hoff GE, van Benthem PP, van der Zaag-Loonen HJ, Bruintjes TD. Determining vestibular hypofunction: start with the video-head impulse test. Eur Arch Otorhinolaryngol. 2016;273:1-7. [DOI] [PubMed] [Google Scholar]
- 24. Zellhuber S, Mahringer A, Rambold HA. Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. Eur Arch Otorhinolaryngol. 2014;271:2375-2383. [DOI] [PubMed] [Google Scholar]
- 25. Zhou G, Brodsky JR. Objective vestibular testing of children with dizziness and balance complaints following sports-related concussions. Otolaryngol Head Neck Surg. 2015;152:1133-1139. [DOI] [PubMed] [Google Scholar]