Abstract
Introduction
Beta-lactams (BL) are the most frequently reported drug allergy, but the vast majority of patients are found not to be genuinely allergic after evaluation. Few studies have investigated the clinical predictors of genuine BL allergy, and the prevalence in hospitalized Chinese patients is unknown.
Methods
Patients admitted to a tertiary hospital in Hong Kong (HK) were analyzed to identify the prevalence and factors associated with the presence of BL allergy labels among hospitalized Chinese patients. A combined cohort of patients having completed allergy investigation for suspected BL allergies in the United Kingdom (UK) and HK were analyzed to identify predictors of genuine allergy.
Results
The prevalence of BL allergy labels in hospitalized HK Chinese was 5%, which was associated with female gender and concomitant non-BL antibiotic allergy labels. The rate of genuine BL allergy patients referred for suspected allergies in the UK and HK cohort was only 14%. History of anaphylaxis and interval of less than a year since the index reaction were independent clinical predictors of genuine BL allergy. The negative predictive value of penicillin skin testing was 90%, confirming the need for drug provocation testing after negative skin testing. There was a high rate of confirmed piperacillin-tazobactam allergy.
Discussion
The estimated true prevalence of genuine BL allergy in hospitalized HK Chinese is around 0.5%. This high rate of BL mislabeling highlights the need for comprehensive allergy evaluation and screening. History of anaphylaxis and duration since the index reaction are important predictors of genuine allergy. Piperacillin-tazobactam allergy may pose a unique challenge in this population with a high prevalence of suspected allergies, surging antibiotic resistance, and lack of testing available.
Keywords: Allergy, Antibiotics, Beta-lactams, Chinese, Drug, Epidemiology, Hypersensitivity, Penicillins, Predictors, Prevalence
Introduction
Beta-lactams (BL), including penicillins, cephalosporins, carbapenems and monobactams, are the most widely used class of antibiotics and frequently reported culprits of drug allergy.1 In the United States (US), over 10% of all patients report a history of penicillin “allergy”, but up to 95% of these patients are not genuinely allergic after evaluation.2, 3, 4 Despite reassuring figures, BL are still among the leading causes of drug-induced anaphylaxis and a comprehensive allergy evaluation is necessary in every case of suspected BL allergy.5 Evaluation of suspected BL allergy includes history, skin tests (ST), including skin prick test (SPT) and intradermal tests (IDT) when indicated, and drug provocation tests (DPT) to confirm current tolerance.6, 7 Although a negative ST carries a negative predictive value (NPV) of above 90%, DPT still remains the “gold standard” and is necessary to confidently confirm tolerance of BL following a negative ST.8, 9
Mislabelling BL allergy leads to obligatory use of alternative antibiotics which is associated with a multitude of adverse clinical consequences including: increased healthcare costs, intensive care admissions, longer hospital stays, and death; as well as increased risks of multi-drug resistant organisms such as Clostridioides difficile, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus infections.10, 11, 12, 13, 14 This is likely an even greater problem in Hong Kong (HK) where an upsurge of MRSA, extended spectrum beta-lactamase (ESBL) producing Escherichia coli, carbapenem-resistant Acinetobacter baumannii (CRAB), multidrug-resistant A. baumannii (MRAB), and carbapenemase-producing Enterobacteriaceae (CPE) has been known for decades.15, 16 Despite the severe consequences of incorrect BL allergy labels, the limitations in testing capacity and testing costs remain a significant barrier to comprehensive testing.17, 18
Despite being an important and growing global public health issue, the epidemiology or impact of suspected and true BL allergy in Chinese people remains unknown, as reports outside the US and Europe are scarce. There also are marked variations in patterns of sensitization to BL among different populations. Furthermore, few studies have investigated the clinical predictors of genuine BL allergy, with previous reports mostly based on self-reported patient histories rather than confirmed cases after formal allergic evaluation.19, 20, 21, 22
To elucidate these areas of uncertainty, this study was performed to identify the prevalence and impact of BL allergy labels (i.e. suspected/unconfirmed allergies) in HK Chinese patients. Additionally, a combined cohort of 997 patients from the United Kingdom (UK) and HK with confirmed BL allergic status was analyzed to identify the clinical characteristics associated with genuine BL allergies in patients confirmed by ST or DPT.
Methods
Prevalence and impact of BL allergy labels in HK
All available medical records of patients admitted to the acute general medical wards of Queen Mary Hospital (Hong Kong), a tertiary hospital in HK, from July 1 to December 31, 2018, were analyzed by chart review. After admission, patients may be transferred to other convalescent hospitals for further management if not fit for direct discharge. Discharge summaries from the patients' admission and transferal to any subsequent convalescent hospitals (if any) were reviewed with clinical data extracted.
Extracted clinical data included age, gender, principle diagnoses for admission (infection-related or not), presence of any drug allergy labels (BL, non-BL antibiotics [aminoglycosides, glycopeptides, quinolones, macrolides, sulfonamides, tetracyclines or other]), length of stay (from day of admission to day of discharge [including stay at convalescent hospital] or death), and discharge outcomes (direct discharge/need for transfer to convalescence hospital, or death).
Factors associated with genuine BL allergies (UK and Chinese cohorts)
All available medical records of patients for BL allergy testing to Guy's and St. Thomas' National Health Service Foundation Trust (United Kingdom) (hereafter referred to as the “UK cohort”) between July 1 2010 and December 31, 2016, and to Queen Mary Hospital (Hong Kong) (hereafter referred to as the “Chinese cohort”) between April 1 2017 and December 31 2018, were retrieved by chart reviewer. Only patients who had completed BL allergy testing entirely were included (i.e. either confirmed allergic by positive ST or DPT, or confirmed non-allergic by a negative DPT).
Clinical parameters, including: age, gender, ethnicity (Chinese or non-Chinese), index drug (i.e. the suspected BL implicated in the index reaction), presenting manifestation (mucocutaneous [urticaria, pruritus, flushing, rash or angioedema] only, anaphylaxis [as per European Academy of Allergy and Clinical Immunology guidelines23], unknown/others), duration since index reaction (more or less than one year), and outcome of allergy testing (BL allergic or non-allergic) were extracted. Additional clinical parameters from the Chinese cohort included history of spontaneous urticaria, presence of concomitant drug allergy labels, more detailed breakdown of presenting manifestations (rash/urticaria, angioedema, anaphylaxis, gastrointestinal involvement, unknown/others) and details of allergy test results (immediate, delayed or negative ST/DPT results) were also extracted. These parameters were determined and confirmed by the attending allergist(s) at each site of study.
Beta-lactam ST (SPT/IDT) and DPT for UK and Chinese cohorts
Please refer to Supplementary Materials for ST and DPT protocols.
Statistical analysis
Categorical variables are expressed as number (percentage), and continuous variables are expressed as either mean (standard deviation) or median (range) when appropriate. Univariate and multivariate analyses were used to identify independent associations of BL allergy labels with clinical outcomes, and to identify clinical predictors of confirmed BL allergies in the UK and Chinese cohorts. The chi-squared statistic and independent samples t-test were used to compare categorical and continuous variables between groups in univariate analysis, respectively. Variables with a p value of 0.1 or less from univariate analysis were included in multivariate logistic to determine which variables were independently associated. A p value of less than 0.05 was considered statistically significant for the multivariate analysis. SPSS Statistics version 20 (IBM, Armonk, NY, USA) was used for all analyses.
Ethics
Data extraction for the UK cohort was conducted as part of an approved audit within the Allergy Department, Guy's and St. Thomas' Hospital National Health Service Foundation Trust. Data extraction for the Chinese cohort was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
Results
Prevalence of BL allergy labels was 5%, associated with female gender and concomitant non-BL antibiotic allergy labels
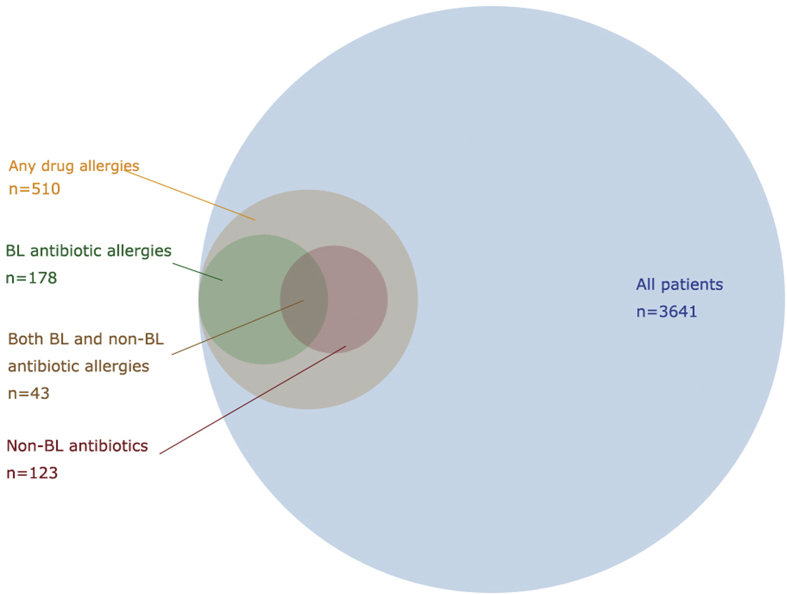
Regarding the prevalence and impact of BL allergy labels based on Chinese patients admitted to general medical wards in HK, 4361 records were available for analyses. These records were of 4361 admissions by 3641 individual patients, with a median of 1 (range: 1–8) admissions per patient. The clinical characteristics and results of univariate analysis are shown in Table 1. Five-hundred and ten patients (14.0%) had drug allergy labels, of which 178 (4.9%) had BL allergy labels and 123 (3.4%) had non-BL antibiotic allergy labels. Forty-three patients of the latter two groups (1.2%) had both concomitant BL and non-BL antibiotic allergy labels. The proportion of patients with any, BL or non-BL antibiotic allergy labels are depicted to scale in Fig. 1.
Table 1.
Univariate analysis between clinical characteristics and presence of BL allergy label compared to no BL allergy label.
| All patients (n = 3641) | No BL allergy label (n = 3463) | Has BL allergy label (n = 178) | p-value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 72 ± 17.9 | 72 ± 17.9 | 72 ± 17.9 | 0.84 |
| Female gender | 1965 (54.0%) | 1851 (53.5%) | 114 (64.0%) | 0.01 * |
| Presence of non-BL antibiotic allergy label | 123 (3.4%) | 80 (2.3%) | 43 (24.2%) | <0.01 * |
| Infection-related admission | 1311 (36.0%) | 1240 (35.8%) | 71 (39.9%) | 0.27 |
| Discharge outcomes | ||||
| Direct discharge | 2663 (73.1%) | 2532 (73.1%) | 131 (73.6%) | 0.89 |
| Death | 379 (10.4%) | 368 (10.6%) | 11 (6.2%) | 0.06 * |
| Length of hospital stay (days, mean ± SD) | 12 ± 19.1 | 12 ± 19.0 | 13 ± 20.6 | 0.80 |
* = p values < 0.10 and included in subsequent multivariate analysis (see Table 2).
Fig. 1.
Venn diagram of the presence of allergy labels in 3641 HK Chinese patients.
The prevalence of BL allergy labels was 208/4361 (4.8%) per admission or 178/3641 (4.9%) per individual patient. Gender, presence of a concomitant non-BL antibiotic allergy label and death were found to have a p < 0.10 in univariate analysis and were included in subsequent multivariate analysis (Table 2). Univariate analysis between rate of readmissions with presence of BL allergy labels did not reach statistical significance (data not shown). Multivariate analysis confirmed that female gender and presence of concomitant non-BL antibiotic allergy labels were both independently associated with presence of BL allergy labels. There was no significant difference between rate of infection-related admissions or measured outcomes (rate of direct discharge, death or length of stay) with patients with or without BL allergy labels. Associations with these measured outcomes remained statistically insignificant even after adjusting for infection-related admissions, concomitant non-BL antibiotic allergy or other drug allergy labels (data not shown).
Table 2.
Multivariate analysis between clinical characteristics and presence of BL allergy label compared to no BL allergy label (bold text indicates variables with p < 0.05).
| Variable | p-value | Odds ratio | 95% Confidence Interval |
|---|---|---|---|
| Female gender | 0.04 | 1.41 | 1.02–1.95 |
| Concomitant non-BL antibiotic allergy label | <0.01 | 13.0 | 8.59–19.55 |
| Death | 0.09 | 1.72 | 0.91–3.24 |
Only 14% of all suspected BL allergies in the Chinese cohort were genuine
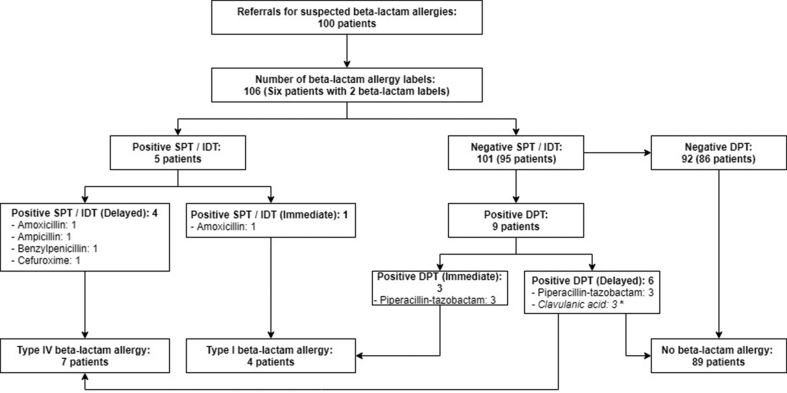
One hundred patient records were available from the Chinese cohort for analysis. Out of 100 patients, there were 106 BL allergy labels (6 patients had 2 BL allergy labels). After testing, only 14% of all suspected BL allergies in the Chinese cohort were confirmed to be genuine after complete investigation. The flow diagram of the patient diagnostic pathway and outcomes of the Chinese cohort is shown in Fig. 2. Univariate and multivariate analysis of the clinical characteristics between BL allergic and non-allergic patients are shown in Table 3 and Supplementary Table 1, respectively. A duration of more than one year since the index reaction was significantly inversely associated with genuine BL allergy.
Fig. 2.
Flow diagram of 100 patients from Chinese cohort having completed beta-lactam allergy testing.
Table 3.
Univariate analysis of clinical characteristics between confirmed BL allergic and non-allergic patients in Chinese cohort.
| All patients (n = 100) | BL non-allergic (n = 86) | BL allergic (n = 14) | p-value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 57 ± 15.1 | 56 ± 15.2 | 61 ± 12.6 | 0.30 |
| Female gender | 68 (68.0%) | 59 (68.6%) | 9 (64.3%) | 0.75 |
| History of spontaneous urticaria | 18 (18.0%) | 18 (20.9%) | 0 | 0.06 * |
| Presence of concomitant drug allergy labels | 63 (63.0%) | 53 (61.6%) | 10 (71.4%) | 0.48 |
| Presenting manifestation | ||||
| Rash/Urticaria | 87 (87.0%) | 73 (84.9%) | 14 (100.0%) | 0.12 |
| Angioedema | 9 (9.0%) | 8 (9.3%) | 1 (7.1%) | 0.79 |
| Anaphylaxis | 2 (2.0%) | 1 (1.2%) | 1 (7.1%) | 0.14 |
| Gastrointestinal involvement | 1 (1.0%) | 1 (1.2%) | 0 | 0.69 |
| Unknown/other | 20 (20.0%) | 19 (22.1%) | 1 (7.1%) | 0.20 |
| Duration since index reaction | ||||
| More than one year | 77 (77.0%) | 70 (81.4%) | 7 (50.0%) | 0.01 * |
* = p values < 0.10 and included in subsequent multivariate analysis (see Supplementary Table 1).
NPV of penicillin skin testing was 90%
In regards to suspected penicillin allergies only (excluding cephalosporins and carbapenems), the breakdown of allergy investigation results of confirmed allergic patients is shown in Supplementary Table 2. Out of 94 patients with suspected penicillin allergy, 4 had positive ST and the remaining 90 ST-negative patients proceeded to DPT. Out of 90 DPT, there were 9 positive tests, of which all reactions were mild and resolved with conservative management. Of note, 6/9 (67%) positive patients were with piperacillin-tazobactam. The overall NPV of ST for suspected penicillin allergy was therefore 81/90 (90%).
Clinical characteristics and comparison between allergy referrals in UK and Chinese cohorts
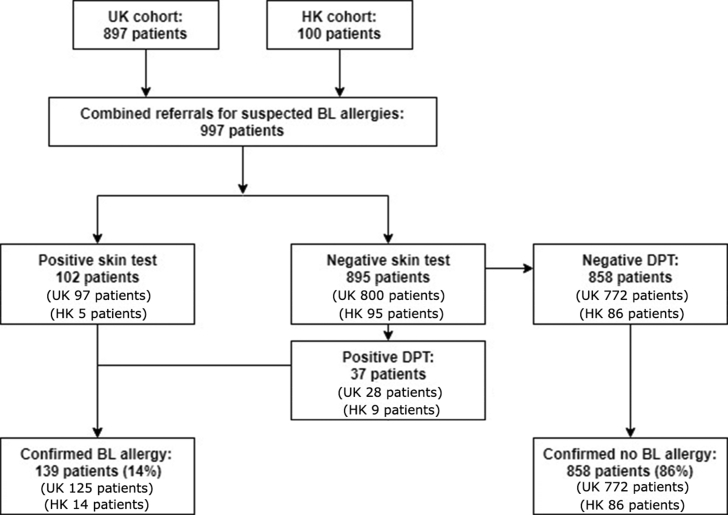
Regarding the factors associated with genuine BL allergy in the combined allergy cohorts (having confirmed allergy status after complete allergy investigation): 897 and 100 patient records were available from the UK and Chinese cohorts, respectively. In total, 997 patients with 1003 suspected BL allergy in total were analyzed (6 patients had two suspected BL allergies). The demographics and other clinical characteristics of the combined cohort is shown in Table 4. The flow diagram of the patient diagnostic pathway and outcomes for these 997 combined patients is shown in Supplementary Figure 1.
Table 4.
Baseline clinical characteristics and prevalence of genuine BL allergies in 997 combined patients (897 from UK and 100 from HK) having completed allergy testing.
| Combined cohort (n = 997) | UK cohort (n = 897) | Chinese cohort (n = 100) | p-value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 51 ± 17.3 | 50 ± 17.5 | 57 ± 15.1 | <0.01 |
| Female gender | 644 (64.6%) | 576 (64.2%) | 68 (68.0%) | 0.45 |
| Chinese ethnicity | 106 (10.6%) | 6 (0.7%) | 100 (100.0%) | <0.01 |
| Index drug | ||||
| Amoxicillin-clavulanate | 141 (14.1%) | 119 (13.3%) | 22 (22.0%) | 0.02 |
| Amoxicillin | 125 (12.5%) | 116 (12.9%) | 9 (9.0%) | 0.26 |
| Benzylpenicillin | 64 (6.4%) | 63 (7.0%) | 1 (1.0%) | 0.02 |
| Flucloxacillin | 31 (3.1%) | 31 (3.5%) | 0 | 0.06 |
| Piperacillin-tazobactam | 21 (0.2%) | 11 (1.2%) | 10 (10.0%) | <0.01 |
| Ampicillin | 17 (1.7%) | 5 (0.6%) | 12 (12.0%) | <0.01 |
| Cefuroxime | 15 (1.5%) | 10 (1.1%) | 5 (5.0%) | <0.01 |
| Cephalexin | 12 (1.2%) | 9 (1.0%) | 3 (3.0%) | 0.08 |
| Cloxacillin | 5 (0.5%) | 0 | 5 (5.0%) | <0.01 |
| Meropenem | 4 (0.4%) | 2 (0.2%) | 2 (2.0%) | 0.01 |
| Other BL antibiotics a | 6 (0.6%) | 2 (0.2%) | 4 (4.0%) | – |
| Unknown/forgotten | 562 (56.4%) | 529 (59.0%) | 33 (33.0%) | <0.01 |
| Presenting manifestation | ||||
| Mucocutaneous only | 617 (61.9%) | 528 (58.9%) | 89 (89.0%) | <0.01 |
| Anaphylaxis | 51 (5.1%) | 49 (5.5%) | 2 (2.0%) | 0.14 |
| Unknown/other | 34 (34.1%) | 320 (35.7%) | 20 (20.0%) | <0.01 |
| Duration since index reaction | ||||
| More than one year | 807 (80.7%) | 721 (81.2%) | 77 (77.0%) | 0.31 |
| Outcome of allergy testing | ||||
| Confirmed BL allergic | 139 (13.9%) | 125 (13.9%) | 14 (14.0%) | 0.99 |
Includes cefaclor (2), ampicillin-flucloxacillin (1), cefaclor (1), ceftazidime (1), ticarcillin-clavulanate (1).
There was no significant difference in the rate of genuine BL allergies between the UK and Chinese cohorts (13.9 vs. 14.0%, p = 0.99) despite significantly different ethnicity (only 0.7% Chinese patients in the UK cohort vs. 100% Chinese patients in the Chinese cohort) and age (median [range] of 49 [15–95] years vs. 58 [18–93] in the UK and Chinese cohorts, respectively). There were significantly more referrals for suspected amoxicillin-clavulanate, ampicillin, cefuroxime, cloxacillin, piperacillin-tazobactam and meropenem allergies; and fewer referrals for suspected benzylpenicillin in the Chinese cohort than the UK cohort. A significantly higher proportion of patients in the UK cohort presented with an unknown/forgotten index BL (59.0% vs. 33.0%, p < 0.01) as well as unknown/other (i.e. non-mucocutaneous only or non-anaphylactic) presenting manifestations (35.7% vs. 20.0%, p < 0.01).
History of anaphylaxis and <1 year since index reaction associated with genuine BL allergy
Univariate and multivariate analysis between clinical characteristics and BL allergic status are shown in Table 5 and Table 6, respectively. Similar to the Chinese cohort, a duration of more than one year since the index reaction was inversely associated with genuine BL allergy. In addition, an index history of anaphylaxis as the presenting manifestation was found to be significantly associated.
Table 5.
Univariate analysis of clinical characteristics between confirmed BL allergic and non-allergic patients.
| All patients (n = 997) | BL non-allergic (n = 858) | BL allergic (n = 139) | p-value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 51 ± 17.3 | 51 ± 17.3 | 51 ± 17.2 | 0.99 |
| Female gender | 644 (64.6%) | 542 (63.2%) | 102 (73.4%) | 0.02 * |
| Chinese ethnicity | 106 (10.6%) | 91 (10.6%) | 15 (10.8%) | 0.95 |
| Presenting manifestation | ||||
| Mucocutaneous only | 614 (61.9%) | 539 (62.8%) | 78 (56.1%) | 0.13 |
| Anaphylaxis | 51 (5.1%) | 13 (1.5%) | 38 (27.3%) | <0.01 * |
| Unknown/other | 340 (34.1%) | 317 (36.9%) | 23 (16.5%) | <0.01 * |
| Duration since index reaction | ||||
| More than one year | 798 (80.8%) | 728 (85.7%) | 70 (50.4%) | <0.01 * |
* = p values < 0.10 and included in subsequent multivariate analysis (see Table 6).
Table 6.
Multivariate analysis of clinical characteristics between confirmed BL allergic and non-allergic patients.
| p-value | Odds ratio | 95% Confidence Interval | |
|---|---|---|---|
| Anaphylaxis as presenting manifestation | <0.01 | 13.87 | 6.83–28.19 |
| More than one year since index reaction | <0.01 | 0.28 | 0.18–0.43 |
| Unknown/non-mucocutaneous/non-anaphylaxis as presenting manifestation | 0.20 | 0.71 | 0.43–1.91 |
| Female gender | 0.11 | 1.44 | 0.92–2.26 |
Discussion
This is one of the largest cohorts of patients with suspected BL allergy investigated by ST with or without DPT, and the first of such report in Chinese patients. This study identified the prevalence of suspected BL “allergy” or labels in HK Chinese to be 5%, which was associated with female gender and concomitant non-BL antibiotic allergy labels. However, the rate of BL allergy confirmed by skin testing or DPT (if skin test negative) in hospitalized HK Chinese referred for allergy evaluation was 14%, which was similar in the UK cohort, but much higher that noted in other populations.9 If these findings are extrapolated, then the prevalence of genuine BL allergy in HK would be around 0.5%. A history of anaphylaxis and an interval of less than one year since the index reaction were identified as independent clinical predictors of BL allergy confirmed by skin test or DPT (if skin test negative).
This prevalence of BL allergy labels (i.e. unconfirmed BL “allergy”) of 5% is lower than that of previous reports from the US and UK of around 10%.3, 24 This lower, and likely more accurate, point prevalence of BL allergy labelling may result from more accurate clinical histories based on the territory-wide electronic health record infrastructure in HK, which is not as widely available in many other countries. This may also partially explain why the Chinese cohort had significantly fewer patients with “unknown/forgotten” index drug or “unknown/other” manifestations compared to the UK cohort.
The presence of a BL allergy labels was also significantly associated with female gender, as well as other non-BL antibiotic allergy labels. The higher prevalence of allergy in females has been reported previously in different cohorts,19, 20, 21 although this association was not seen in the multivariate analysis for either the UK or Chinese cohorts. This suggests that gender may only be associated with BL allergy labelling, rather than genuine allergy per se. The significant greater number of non-BL antibiotic allergy labels in suspected BL allergic individuals (24% of all patients labelled with BL allergy, as depicted in Fig. 1) is alarming. This observation may be explained as patients labelled with BL allergies would be exposed to more non-BL antibiotics, leading to greater chance of a reported adverse reaction to these drugs. Alternatively, these patients with concomitant allergy labels may be intrinsically prone to developing multiple drug hypersensitivity.25
For the combined cohort, there were some significant differences in the baseline characteristics of the UK and Chinese allergy cohorts, including differences in age, ethnicity, index drugs as well as presenting manifestations. While the difference in ethnicity is expected, the significantly older Chinese cohort reflects the older age of HK's general population (44.4 years, vs 40.5 years).26 Significantly more patients from the Chinese cohort were also referred for suspected allergies to newer or broad-spectrum BL such as amoxicillin-clavulanate, piperacillin-tazobactam and meropenem. This likely reflects differences in prescribing practices rather than just mere differences of availability of drugs (such as flucloxacillin instead of cloxacillin in UK, and vice versa in HK).
The association between history of anaphylaxis and genuine BL allergy resonates with usual clinical observation. This reflects the objectivity and higher specificity of anaphylactic reactions for true IgE-mediated responses in comparison to other manifestations. Similarly, genuine BL allergic patients were also more likely to present for investigation within a year since their reaction. This finding corroborates previous reports of BL sensitivity waning over time.27
These results suggest that ascertaining the history of anaphylaxis and duration since the index reaction during history taking is important, and may aid the physician in pre-test evaluation or clinical decision making. Recommendations on risk stratification for penicillin allergy similarly based on duration since reaction and severity of index reactions were also recently published by authors from the US.8 Contrary to a previous report suggesting that Asian race may be protective in penicillin allergy compared with Caucasian, this association was not found in this study.19
The more detailed clinical data for the Chinese cohort also allowed further evaluation and breakdown of allergy testing results. Although the NPV of ST was comparable with other international cohorts, there was a disproportionately high number of patients allergic to piperacillin-tazobactam.8, 9 Piperacillin-tazobactam allergy contributed to over 40% of all confirmed BL allergies and, alarmingly, all piperacillin-tazobactam allergic patients had negative ST and were only diagnosed by a positive DPT. Fortunately, all DPT were performed using a graded approach and reactions were mild. Literature regarding piperacillin-tazobactam allergy is scarce, but a case of anaphylaxis despite negative ST results was previously reported.20 It has been postulated that the tazobactam component may be allergenic but poorly represented in SPT or IDT.
In contrast to previous reports, we were unable to identify any significant differences in rate of direct discharge, death, readmissions or length of hospital stay between patients with or without BL allergy labels. This was likely due to the limited retrospective nature of this study, as well as the low threshold of prescribing non-BL or broad spectrum (“big gun”) antibiotics in this locality. Furthermore, although the mean length stay was lengthened due to some chronic cases (range 1–139 days), the median length of stay with or without BL allergy labels was only 5 days, which would make it difficult to ascertain any significant differences between these two groups of patients. Dedicated and longer prospective studies, including analysis of overall healthcare costs, emergence of antibiotic resistance, and need for high dependency/intensive care admissions, are planned for the future.
Further limitations of this study also stem from the limitation of medical records review. Only a limited number of potential confounders could be assessed and variables such as demographics, duration since reaction and index reactions were not available for every patient. Retrospective data collection also includes potential referral or selection bias; for example, referring clinicians may tend to refer patients with more severe reactions or not refer patients with clear-cut histories of genuine BL allergies. The variable “duration since index reaction” could only be categorized into more or less than a year, and the variable “ethnicity” could only be categorized into Chinese or non-Chinese patients due to limitations of medical records. Furthermore, a combined cohort was required to empower the identification the clinical predictors of genuine BL allergy. Although no significant differences (other than age and ethnicity) were identified between the Chinese and UK cohorts, results from this cohort may be population-specific and likely pertain to the relevant regional prescribing practices and sensitization profiles. Further studies will be needed to corroborate the external validity of these findings.
In summary, the prevalence of BL allergy labels in HK Chinese patients was 5%, which was associated with female gender and concomitant non-BL antibiotic allergy labels. However, the rate of genuine BL allergy patients referred for suspected allergies was only 14%. This high rate of mislabeling highlights the need for comprehensive allergy evaluation and screening.
Analysis of a combined cohort also identified history of anaphylaxis and duration of less than a year since the index reaction as independent clinical predictors of genuine BL allergy. Physicians should be reminded to ascertain these crucial variables during history taking to aid clinical decision making. Subgroup analysis verified an NPV of penicillin ST of 90% but also observed a high rate of confirmed piperacillin-tazobactam allergy. In view of the lack of good predictive tests apart from DPT, piperacillin-tazobactam allergy may pose a unique challenge to this Chinese population with a high prevalence of suspected allergies and surging antibiotic resistance.
Author contributions
PHL, LQCS, IT, TJW and KLU collected the data. PHL and LQCS analyzed and interpreted the data. All authors contributed in the writing and revision of the article.
All authors consent for publication.
Source of funding
There was no source of funding for this study.
Potential competing interests
The authors report no competing interests. Approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100048.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. s1.
Flow diagram of 997 combined patients having completed BL allergy testing.
References
- 1.Versporten A., Coenen S., Adriaenssens N. European surveillance of antimicrobial consumption (ESAC): outpatient penicillin use in Europe (1997-2009) J Antimicrob Chemother. 2011;66(suppl 6) doi: 10.1093/jac/dkr454. vi13-23. [DOI] [PubMed] [Google Scholar]
- 2.Joint Task Force on Practice Parameters Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhou L., Dhopeshwarkar N., Blumenthal K.G. Drug allergies documented in electronic health records of a large healthcare system. Allergy. 2016;71(9):1305–1313. doi: 10.1111/all.12881. [DOI] [PubMed] [Google Scholar]
- 4.Sacco K.A., Bates A., Brigham T.J., Imam J.S., Burton M.C. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72(9):1288–1296. doi: 10.1111/all.13168. [DOI] [PubMed] [Google Scholar]
- 5.Jerschow E., Lin R.Y., Scaperotti M.M., McGinn A.P. Fatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134(6):1318–1328. doi: 10.1016/j.jaci.2014.08.018. e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanca M., Romano A., Torres M.J. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64(2):183–193. doi: 10.1111/j.1398-9995.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 7.Mirakian R., Leech S.C., Krishna M.T. Management of allergy to penicillins and other beta-lactams. Clin Exp Allergy. 2015;45(2):300–327. doi: 10.1111/cea.12468. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy E.S., Macy E., Rowe T., Blumenthal K.G. Evaluation and management of penicillin allergy: a review. J Am Med Assoc. 2019;321(2):188–199. doi: 10.1001/jama.2018.19283. [DOI] [PubMed] [Google Scholar]
- 9.Macy E., Ngor E.W. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258–263. doi: 10.1016/j.jaip.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Mattingly T.J., 2nd, Fulton A., Lumish R.A. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6(5):1649–1654. doi: 10.1016/j.jaip.2017.12.033. e1644. [DOI] [PubMed] [Google Scholar]
- 11.MacFadden D.R., LaDelfa A., Leen J. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis. 2016;63(7):904–910. doi: 10.1093/cid/ciw462. [DOI] [PubMed] [Google Scholar]
- 12.Macy E., Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790–796. doi: 10.1016/j.jaci.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Blumenthal K.G., Lu N., Zhang Y., Li Y., Walensky R.P., Choi H.K. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ. 2018;361 doi: 10.1136/bmj.k2400. k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres M.J., Adkinson N.F., Jr., Caubet J.C. Controversies in drug allergy: beta-lactam hypersensitivity testing. J Allergy Clin Immunol Pract. 2019;7(1):40–45. doi: 10.1016/j.jaip.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Cheng V.C., Wong S.C., Ho P.L., Yuen K.Y. Strategic measures for the control of surging antimicrobial resistance in Hong Kong and mainland of China. Emerg Microb Infect. 2015;4(2) doi: 10.1038/emi.2015.8. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P.H., Cheng V.C., Yip T., Yap D.Y., Lui S.L., Lo W.K. Epidemiology and clinical characteristics of acinetobacter peritoneal dialysis-related peritonitis in Hong Kong-with a perspective on multi-drug and carbapenem resistance. Perit Dial Int. 2017;37(2):177–182. doi: 10.3747/pdi.2016.00123. [DOI] [PubMed] [Google Scholar]
- 17.Chan Y.T., Ho H.K., Lai C.K. Allergy in Hong Kong: an unmet need in service provision and training. Hong Kong Med J. 2015;21(1):52–60. doi: 10.12809/hkmj144410. [DOI] [PubMed] [Google Scholar]
- 18.Lee T.H., Leung T.F., Wong G. The unmet provision of allergy services in Hong Kong impairs capability for allergy prevention-implications for the Asia Pacific region. Asian Pac J Allergy Immunol. 2019 Mar;37(1):1–8. doi: 10.12932/AP-250817-0150. [DOI] [PubMed] [Google Scholar]
- 19.Albin S., Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35(6):489–494. doi: 10.2500/aap.2014.35.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rank M.A., Park M.A. Anaphylaxis to piperacillin-tazobactam despite a negative penicillin skin test. Allergy. 2007;62(8):964–965. doi: 10.1111/j.1398-9995.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- 21.Macy E., Poon K.Y.T. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8) doi: 10.1016/j.amjmed.2009.01.034. 778 e771-777. [DOI] [PubMed] [Google Scholar]
- 22.Apter A.J., Schelleman H., Walker A., Addya K., Rebbeck T. Clinical and genetic risk factors of self-reported penicillin allergy. J Allergy Clin Immunol. 2008;122(1):152–158. doi: 10.1016/j.jaci.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Muraro A., Roberts G., Worm M. Anaphylaxis: guidelines from the European Academy of allergy and clinical Immunology. Allergy. 2014;69(8):1026–1045. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 24.Kerr J.R. Penicillin allergy: a study of incidence as reported by patients. Br J Clin Pract. 1994;48(1):5–7. [PubMed] [Google Scholar]
- 25.Pichler W.J., Srinoulprasert Y., Yun J., Hausmann O. Multiple drug hypersensitivity. Int Arch Allergy Immunol. 2017;172(3):129–138. doi: 10.1159/000458725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United Nations DoESA, Population Division . 2017. World Population Prospects: The 2017 Revision. [Google Scholar]
- 27.Blanca M., Torres M.J., Garcia J.J. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5 Pt 1):918–924. doi: 10.1016/s0091-6749(99)70439-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.