Abstract
Hypoxia is associated with poor prognosis and therapeutic resistance in cancer patients. Accumulating evidence has shown that microRNA (miRNA) plays an important role in the acquired drug resistance in colorectal carcinoma (CRC). However, the role of miRNA in hypoxia-induced CRC drug resistance remains to be elucidated. Here, we identified a hypoxia-triggered feedback loop that involves hypoxia-inducible transcription factor 1α (HIF-1α)-mediated repression of miR-338-5p and confers drug resistance in CRC. In this study, the unbiased miRNA array screening revealed that miR-338-5p is downregulated in both hypoxic CRC cell lines tested. Repression of miR-338-5p was required for hypoxia-induced CRC drug resistance. Furthermore, we identified interleukin-6 (IL-6), which mediates STAT3/Bcl2 activation under hypoxic conditions, as a direct miR-338-5p target. The resulting HIF-1α/miR-338-5p/IL-6 feedback loop was necessary for drug resistance in colon cancer cell lines. Using CRC patient samples, we found miR-338-5p has a negative correlation with HIF-1α and IL-6. Finally, in a xenograft model, overexpressing miR-338-5p in CRC cells and HIF-1α inhibitor PX-478 were able to enhance the sensitivity of CRC to oxaliplatin (OXA) via suppressing the HIF-1α/miR-338-5p/IL-6 feedback loop in vivo. Taken together, our results uncovered an HIF-1α/miR-338-5p/IL-6 feedback circuit that is critical in hypoxia-mediated drug resistance in CRC; targeting each member of this feedback loop could potentially reverse hypoxia-induced drug resistance in CRC.
Keywords: miR-338-5p, HIF-1α, colorectal cancer, drug resistance, IL-6
Graphical Abstract

Hypoxia is associated with poor prognosis and therapeutic resistance in cancer patients. However, the role of microRNA in hypoxia-induced colorectal carcinoma drug resistance remains to be elucidated. Xu et al. identified a hypoxia-triggered feedback loop that involves HIF-1α-mediated repression of miR-338-5p and confers drug resistance in CRC.
Introduction
Acquired drug resistance is a foremost challenge in patients with advanced stage malignancies. The acquisition of chemotherapy resistance is a complex event involving the alteration of the tumor microenvironment, whose underlying mechanism has not been fully elucidated. Increasing evidence from experimental and clinical studies has revealed that the adaptability of cancer cells to hypoxia is closely related to cancer progression and contributes to the development of resistance to radiation and chemotherapy.1, 2, 3, 4, 5, 6
Hypoxia is a common feature of the tumor microenvironment that activates the HIF-signaling pathway in cancer cells. In the case of hypoxia, HIF is reported to be overexpressed in various cancer cells and associated with the progression and adverse clinical outcome of many different tumor entities, including colorectal cancer (CRC). HIF genes regulate the expression of many genes associated with angiogenesis, tumor growth, metastasis, metabolic reprogramming, and treatment resistance.3, 7, 8, 9, 10 In view of its significance as the main regulator of hypoxia-induced drug resistance, hypoxia-inducible transcription factor 1α (HIF-1α) has been regarded as an attractive therapeutic target for cancer treatment.
MicroRNAs (miRNAs) are small non-coding RNAs, 20–25 nt in length, acting through targeting the specific 3′ UTRs of mRNAs and regulating their expression.11 miRNAs are involved in a variety of physiological and pathological processes in cancer: they regulate cell differentiation, proliferation, infiltration, apoptosis, radioresistance, and chemoresistance.12, 13, 14, 15 miRNAs often form feedback loops, since they themselves are regulated by transcription factors, which they directly or indirectly target;16, 17 for example, miR-34a suppresses tumor progression by inhibiting an IL-6R/STAT3/miR-34a feedback loop.18 As a result, miRNA microarray is widely used as a powerful tool to analyze the response of cancer cells to treatment. miR-338-5p belongs to the miR-338 family and had been elucidated to participate in the development of different cancers.19, 20 Interestingly, by miRNA microarray we found that miR-338-5p was significantly downregulated in CRC under the hypoxia condition (Table 1), suggesting it may play a certain role in the drug resistance under the hypoxia condition.
Table 1.
miRNAs Differentially Expressed in Hypoxic or Normoxic CRC Cells
| miRNA | HCT116 Hypoxia or Normoxia | HCT8 Hypoxia or Normoxia |
|---|---|---|
| hsa-miR-150 | 2.14 | 0.93 |
| hsa-miR-203 | 0.41 | 1.26 |
| hsa-miR-221 | 0.30 | 0.49 |
| hsa-miR-222 | 0.34 | 0.39 |
| hsa-miR-301a | 0.39 | 1.07 |
| hsa-miR-324-3p | 0.41 | 0.56 |
| hsa-miR-338-5p | 0.30 | 0.29 |
| hsa-miR-431 | 0.39 | 0.67 |
| hsa-miR-483-5p | 2.80 | 1.02 |
| hsa-miR-513a-5p | 5.55 | 1.78 |
| hsa-miR-513b | 2.28 | 0.67 |
| hsa-miR-630 | 3.26 | 2.16 |
| hsa-miR-636 | 0.45 | 0.94 |
| hsa-miR-638 | 4.35 | 1.65 |
| hsa-miR-892b | 0.18 | 0.76 |
| hsa-miR-939 | 3.03 | 2.24 |
| hsa-miR-1202 | 5.03 | 2.15 |
| hsa-miR-1207-5p | 3.50 | 5.06 |
| hsa-miR-1268 | 4.16 | 2.36 |
| hsa-miR-1274a | 0.28 | 0.64 |
| hsa-miR-1274b | 0.34 | 0.93 |
| hsa-miR-1915 | 4.19 | 4.17 |
In this study, we investigated the role of miRNA in CRC drug resistance under hypoxia. We found an HIF-1α/miR-338-5p/IL-6 feedback circuit composed of HIF-1α-mediated inhibition of miR-338-5p and upregulation of IL-6/STAT3, which confers drug resistance in CRC. We furthermore provided strategies to reverse such resistance in CRC.
Results
Hypoxia Induces Drug Resistance in CRC through IL-6
Hypoxia is a common feature of the tumor microenvironment that activates the HIF-signaling pathway in cancer cells. It has been reported that hypoxia could regulate the expression of a number of genes involved in angiogenesis, tumor growth, metastasis, metabolic reprogramming, and treatment resistance.7, 8, 9, 10 To confirm whether hypoxia induces drug resistance in CRC cells, we treated the human CRC cell lines HCT116 and HCT8 with chemotherapeutic drugs commonly used in the clinical setting under either hypoxic or normoxic conditions.
Compared to normoxia, hypoxia impaired the cytotoxic effects of oxaliplatin (OXA), 5-fluorouracil (5-FU), doxorubicin (DOX), and cyclophosphamide (CTX) in both the HCT116 (Figure 1A) and HCT8 cells (Figure 1B). In addition, hypoxia significantly reduced the apoptosis of CRC cells induced by OXA (Figure 1C). Previous works showed hypoxia could induce IL-6 expression, and the IL-6/STAT3-signaling pathway plays a critical role in drug resistance. To determine whether IL-6 is the key mechanism of hypoxia-induced drug resistance in CRC cells, we compared the IL-6 level of the culture medium from hypoxic versus normoxic CRC cells and protein levels of IL-6, STAT3, and Bcl2 from cancer cells. Our result showed that hypoxia promoted IL-6 release compared to normoxia (Figure 1D), activated the IL-6/STAT3/Bcl2 pathway, and decreased the levels of cleaved caspase-3 (Figure 1E; Figure S1).
Figure 1.
Hypoxia Induces Colorectal Cancer Drug Resistance by IL-6
(A and B) The IC50 values of OXA, 5-Fu, DOX, and CTX in CRC (A) HCT116 and (B) HCT8 cells under hypoxic or normoxic conditions were determined using the CCK-8 assay. (C) Hypoxia decreased apoptosis level in CRC cells induced by oxaliplatin. A DNA fragmentation assay was used. (D) Culture supernatants from hypoxic or normoxic CRC cells with or without OXA were used to examine IL-6 level by ELISA. (E) Protein levels of the IL-6/STAT3/Bcl2 pathway were determined using western blot. (F) Anti-IL-6 slowed down the growth of hypoxic CRC cells under oxaliplatin treatment. A CCK-8 assay was used. (G) DNA fragmentation assays showed increased apoptosis level in hypoxic CRC cells induced by oxaliplatin (H) and decreased Bcl2 level or increased cleaved caspase-3 level by western blot analysis. *p < 0.05, **p < 0.01. Each bar represents the mean ± SD of three independent experiments.
To confirm the role of IL-6 in hypoxia-induced drug resistance, we used IL-6 antibody to block the function of IL-6. We found that blocking IL-6 could reverse the drug resistance to 5-FU, DOX, and CTX, which was induced by hypoxia (Figure S2). Furthermore, we observed that blocking IL-6 re-sensitized hypoxic CRC cells to OXA treatment, as proven by a left shift of the growth inhibition curve (Figure 1F); significantly increased apoptosis (Figure 1G); and decreased levels of Bcl2 and increased cleaved caspase-3 (Figure 1H). Conversely, when we treated normoxic CRC cells with IL-6 recombination proteins, we found that IL-6 protected OXA-induced apoptosis in HCT116 and HCT8 cells under normoxic conditions, which was confirmed by the right shift of the growth inhibition curve (Figure S3) and the significant reduction in apoptosis (Figure S3). Taken together, the above experiments illustrated that IL-6 is indispensable in hypoxia-induced drug resistance in CRC cells.
Hypoxia Decreases miR-338-5p Expression to Confer the Resistance of CRC Cells to OXA
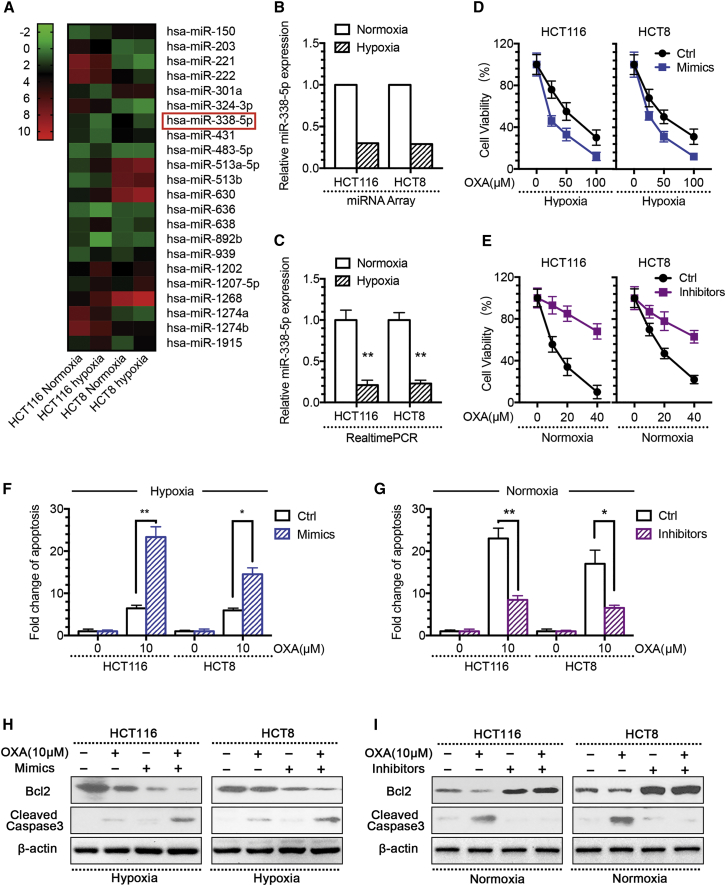
Ample evidence indicates that miRNA plays critical roles in cancer drug resistance.11, 12, 13, 14, 15 To determine whether miRNAs are involved in the development of hypoxia-induced drug resistance in CRC cells, we compared the miRNA expression profiles of the two CRC cell lines, HCT116 and HCT8, under either hypoxic or normoxic conditions, by using the miRNA microarray containing 2,578 human mature miRNA probes. The heatmap shows and Table 1 lists the 22 miRNAs expressed in hypoxic cells at levels different from their expression levels in normoxic cells, with the same trend in both the CRC cell lines (Figure 2A). Of note, miR-338-5p was reduced at the most significant level in both hypoxic cell lines, making us hypothesize that miR-338-5p plays certain roles in the development of acquired resistance to OXA in CRC cells.
Figure 2.
Hypoxia-Decreased miR-338-5p Expression Confers the Resistance of CRC Cells to Oxaliplatin
(A) The heatmap showed 22 differentially expressed miRNAs in hypoxic versus normoxic CRC cells by miRNA microarray (FC > 2-fold and p < 0.05). (B) miRNA microarray results showed miR-338-5p expression levels significantly decreased in hypoxic CRC cells. (C) qPCR validation showing miR-338-5p expression level was significantly decreased in hypoxic CRC cells. Overexpression of miR-338-5p induced by mimic transfection significantly (D) enhanced the growth-inhibitory effect of oxaliplatin in hypoxic CRC cells by CCK assay; (F) increased hypoxic CRC cell apoptosis level to oxaliplatin by DNA fragmentation assays; and (H) decreased Bcl2 level or increased cleaved caspase-3 level by western blot analysis. Downregulation of miR-338-5p induced by inhibitor transfection significantly (E) decreased normoxic CRC cell growth under oxaliplatin treatment by CCK assay; (G) decreased normoxic CRC cell apoptosis level to oxaliplatin by DNA fragmentation assays; and (I) increased Bcl2 level or decreased cleaved caspase-3 level by western blot analysis. *p < 0.05, **p < 0.01. Each bar represents the mean ± SD of three independent experiments.
To verify the results obtained by microarray profiling, we performed qRT-PCR analysis of miR-338-5p expression in these cell lines. Consistent with the microarray data (Figure 2B), qRT-PCR analysis confirmed decreased expression of miR-338-5p in hypoxic cells in comparison to the normoxic cells (Figure 2C). To determine whether hypoxia decreases miR-338-5p expression to alter the sensitivity of CRC cells to OXA, we either transfected miR-338-5p mimics into hypoxic CRC cells (Figure S4) or added inhibitors into normoxic CRC cells (Figure S4). The results showed that miR-338-5p mimics could reverse the drug resistance to 5-FU, DOX, and CTX, which was induced by hypoxia (Figure S5). Moreover, we found that an increased level of miR-338-5p made the CRC cells more sensitive to OXA treatment under hypoxia, which was confirmed by a left shift of the growth inhibition curve (Figure 2D); increased apoptosis (Figure 2F); reduced Bcl2 level; and enhanced the cleavage of caspase-3 (Figure 2H). Conversely, the inhibition of miR-338-5p prevented OXA-induced apoptosis of CRC cells in normoxic condition, as evidenced by a right shift of the growth inhibition curve (Figure 2E); significantly reduced apoptosis (Figure 2G); induced Bcl2 expression; and decreased levels of cleaved caspase-3 (Figure 2I). These data indicate that the modulation of miR-338-5p expression alters the sensitivity to OXA in both the hypoxic and normoxic CRC cells.
miR-338-5p-Targeting IL-6 Is Required to Induce Hypoxia-Mediated CRC Drug Resistance
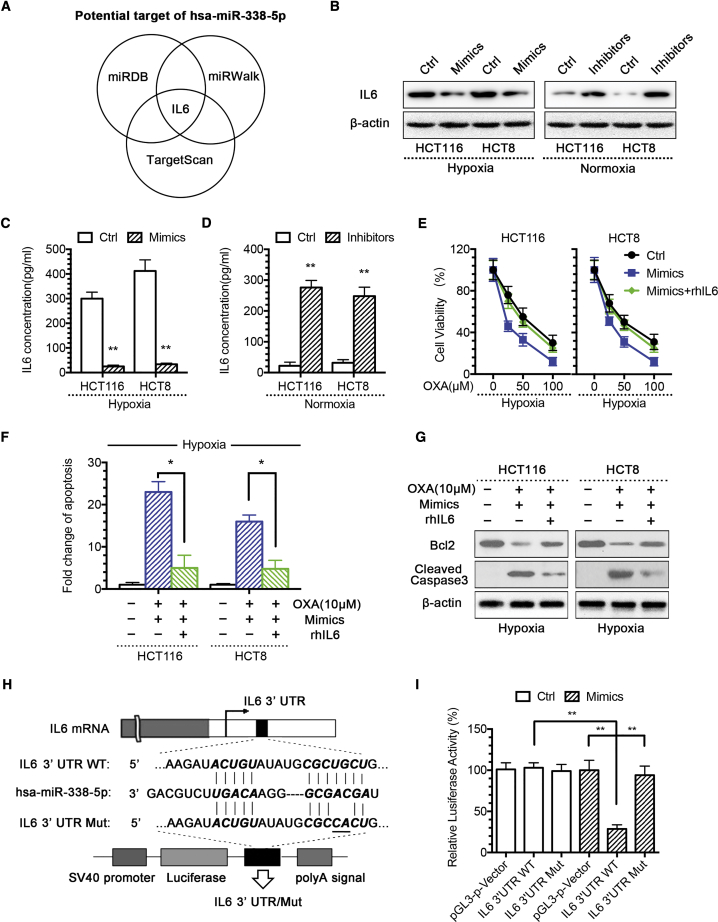
To identify the target gene of miR-338-5p, we used several algorithms to predict the mRNA targets of miRNAs, including the TargetScan (http://www.targetscan.org), miRWalk (http://mirwalk.umm.uni-heidelberg.de), and miRDB (http://mirdb.org). Based on the presence of miR-338-5p recognition sites on the 3′ UTRs, we found that IL-6 is one of the common candidate target genes revealed by all three algorithms (Figure 3A). Western blotting results showed that IL-6 protein expression level decreased in miR-338-5p mimic-transfected hypoxic CRC cells and increased in miR-338-5p inhibitor-transfected normoxic CRC cells (Figure 3B), and ELISA results showed that mimics could inhibit IL-6 release in hypoxic CRC cells (Figure 3C). In comparison, miR-338-5p inhibitors promoted IL-6 release in normoxic CRC cells (Figure 3D). The above data pointed out that IL-6 is a potential target of miR-338-5p. To confirm the function of IL-6 in hypoxia-mediated CRC drug resistance, we treated CRC cells with IL-6 recombination proteins or IL-6 antibody. Our results showed that, while increasing miR-338-5p resensitized hypoxic CRC cells to OXA treatment, IL-6 could reverse such effect (Figure 3H) and reduce the apoptosis (Figures 3E and 3G). In contrast, blocking IL-6-resensitized miR-338-5p inhibitor induced OXA resistance in normoxic CRC cells (Figure S6).
Figure 3.
miR-338-5p Targeting IL-6 Is Required for Hypoxia-Mediated CRC Drug Resistance
(A) hsa-miR-338-5p was the intersection miRNA of miRDB, miRWalk, and TargetScan databases that targeted IL-6. (B) IL-6 protein expression level was found regulated directly by miR-338-5p, as reflected by the decreased IL-6 expression in hypoxic CRC cells after transient transfection of miR-338-5p mimic and increased IL-6 expression in normoxic CRC cells after miR-338-5p inhibitor transfection. IL-6 concentration of culture supernatants was (C) decreased by miR-338-5p mimic in hypoxic CRC cells and (D) increased by miR-338-5p inhibitor in normoxic CRC cells. Recombination human IL-6 (rhIL-6) significantly (E) decreased the growth-inhibitory effect of OXA combined with miR-338-5p in hypoxic CRC cells by CCK-8 assay; (F) decreased hypoxic CRC cell apoptosis level by DNA fragmentation assays; and (G) restored Bcl2 level or decreased cleaved caspase-3 level by western blot analysis. (H) The wild-type and mutant variant of the putative miR-338-5p target sequences of the IL-6 gene. (I) Two copies of the wild-type and mutant miR-338-5p target sequences were fused with a luciferase reporter and transfected into control oligonucleotide- and miR-338-5p mimic-infected HEK293T cells. miR-338-5p significantly suppressed the luciferase activity of the wild-type IL-6 3′ UTR. *p < 0.05, **p < 0.01. Each bar represents the mean ± SD of three independent experiments.
To check whether miR-338-5p directly targets IL-6, the IL-6 3′ UTR segment containing the miR-338-5p-binding sites was cloned to the luciferase reporting system. The plasmid lacking the miR-338-5p-binding site was used as a negative control for luciferase activity (Figure 3H). The reporter vector was transfected to the HEK293T cells along with the miR-338-5p mimics. The results showed that miR-338-5p inhibited luciferase activity in the construct with the IL-6 3′ UTR segment containing the miR-338-5p-binding site. No luciferase activity change was found when the cells were transfected with the negative control (Figure 3I).
Taken together, these results demonstrate that miR-338-5p-targeting IL-6 is required for hypoxia-mediated CRC drug resistance.
HIF-1α/miR-338-5p/IL-6 Feedback Loop May Be Responsible for Hypoxia-Mediated CRC Drug Resistance
In the abovementioned experiments, we observed that hypoxia could decrease miR-338-5p expression to alter the sensitivity of CRC cells to OXA. However, since HIF-1α is important for the regulation of coordinated adaptive responses under hypoxic condition, it is important to determine whether HIF-1α regulates miR-338-5p expression and, consequently, the level of IL-6.
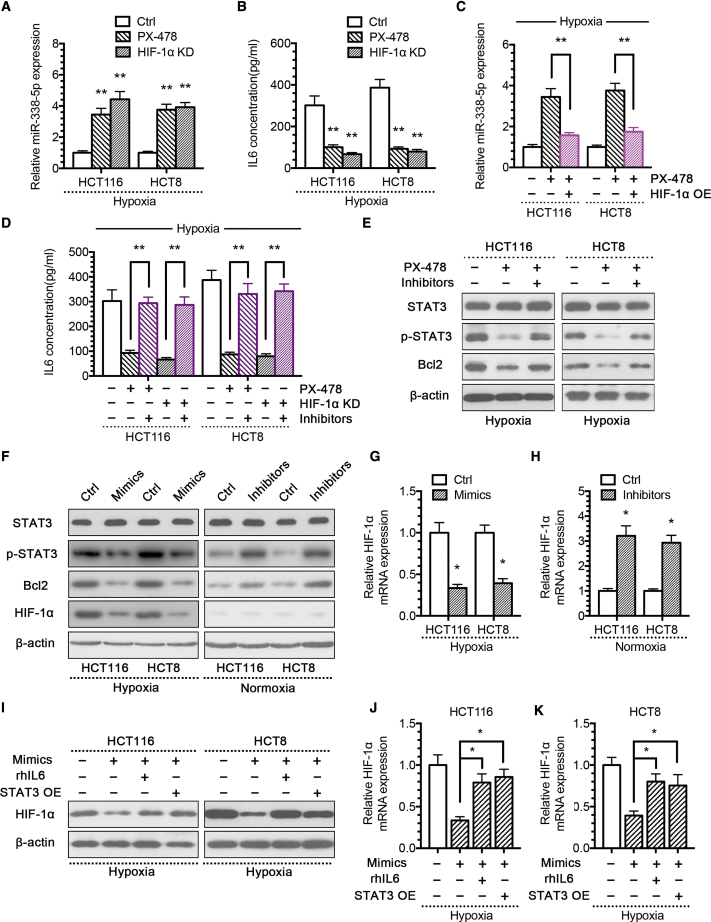
To confirm the functional role of HIF-1α in modulating miR-338-5p, we constructed an HIF-1α knockdown (HIF-1α KD) small hairpin RNA (shRNA) plasmid (Figure S7) and HIF-1α overexpression (HIF-1α OE) plasmid (Figure S7). Subsequent experiments showed that reducing HIF-1α expression by HIF-1α inhibitor PX-478 or HIF-1α KD plasmid increased the expression of miR-338-5p under hypoxia (Figure 4A) and decreased IL-6 level (Figure 4B). Conversely, overexpression of HIF-1α reduced the expression of miR-338-5p under normoxia condition (Figure S8). Re-expression of HIF-1α in hypoxic CRC cells treated by PX-478 again reduced miR-338-5p expression significantly (Figure 4C), indicating that HIF-1α inhibited miR-338-5p expression directly. As a result, the inhibition of miR-338-5p in hypoxic CRC cells rescued the diminished IL-6 expression after reducing the HIF-1α expression (Figure 4D), and it re-activated the STAT3/Bcl2-signaling pathway (Figure 4E). In contrast, miR-338-5p mimics reduced IL-6 expression activated by HIF-1α (Figure S8). Of note, the overexpression of miR-338-5p not only inhibited HIF-1α protein level but also the mRNA level under hypoxia; however, the inhibition of miR-338-5p only increased the HIF-1α mRNA level, but not the protein level, under normoxia (Figures 4F–4H). Those results suggested that miR-338-5p could inhibit HIF-1α translation through p-STAT3, but not at the post-translation level, because under normoxic conditions HIF-1α is ubiquitinated by the von Hippel-Lindau protein and degraded through the proteasomal pathway. That might be the reason why miR-338-5p increased only the mRNA level, but not the protein level, of HIF-1α.
Figure 4.
HIF-1α/miR-338-5p/IL-6 Feedback Loop May Be Responsible for Hypoxia-Mediated CRC Drug Resistance
(A) qPCR analysis of miR-338-5p expression in hypoxic CRC cells treated with HIF-1α inhibitor PX-478 (25 μM) or HIF-1α KD plasmid. (B) IL-6 expression level of culture supernatants from hypoxic CRC cells treated with HIF-1α inhibitor PX-478 or HIF-1α KD plasmid. (C) qPCR analysis of miR-338-5p expression in hypoxic CRC cells treated with PX-478, with or without HIF-1α OE plasmid. (D) IL-6 expression level of culture supernatants from hypoxic CRC cells treated with PX-478 or HIF-1α KD plasmid, with or without miR-338-5p inhibitor. (E) Western blot analysis of the indicated proteins from hypoxic CRC cells treated with PX-478, with or without miR-338-5p inhibitor. (F) Western blot analysis of the indicated proteins from hypoxic CRC cells treated with miR-338-5p mimics and normoxic CRC cells treated with miR-338-5p inhibitor. (G) qPCR analysis of HIF-1α expression in hypoxic CRC cells with miR-338-5p mimics. (H) qPCR analysis of HIF-1α expression in normoxic CRC cells treated with miR-338-5p inhibitor. (I) Western blot analysis of HIF-1α expression from hypoxic CRC cells treated with miR-338-5p mimics, with or without rhIL-6/STAT3 OE plasmid. qPCR analysis of HIF-1α expression from hypoxic CRC (J) HCT116 and (K) HCT8 cells treated with miR-338-5p mimics, with or without rhIL-6 or STAT3 OE plasmid. *p < 0.05, **p < 0.01. Each bar represents the mean ± SD of three independent experiments.
To confirm that miR-338-5p indeed regulated HIF-1α expression through the IL-6/STAT3 pathway, we constructed the STAT3 overexpression (STAT3 OE) plasmid (Figure S7). We found that either IL-6 recombination protein or STAT3 OE prevented miR-338-5p from inhibiting HIF-1α expression (Figures 4I–4K). These results suggest the existence of a feedback loop that is activated by HIF-1α, which mediates the repression of miR-338-5p and subsequent activation of IL-6 and STAT3, therefore maintaining the activation of HIF-1α.
Expression of the HIF-1α/miR-338-5p/IL-6 Feedback Loop in Human CRC Specimens
To better understand the clinical relevance of an altered HIF-1α/miR-338-5p/IL-6 feedback loop, we investigated the expression levels of HIF-1α, miR-338-5p, and IL-6 in 38 pairs of human CRC specimens (adjacent normal and tumor) by qPCR, as well as the serum level of IL-6 in healthy volunteers’ sera (n = 16) versus CRC patients’ sera (n = 38) by ELISA. All patients had a histological diagnosis of CRC and received radical resection. None of the patients included in the study had received neoadjuvant therapy before surgery.
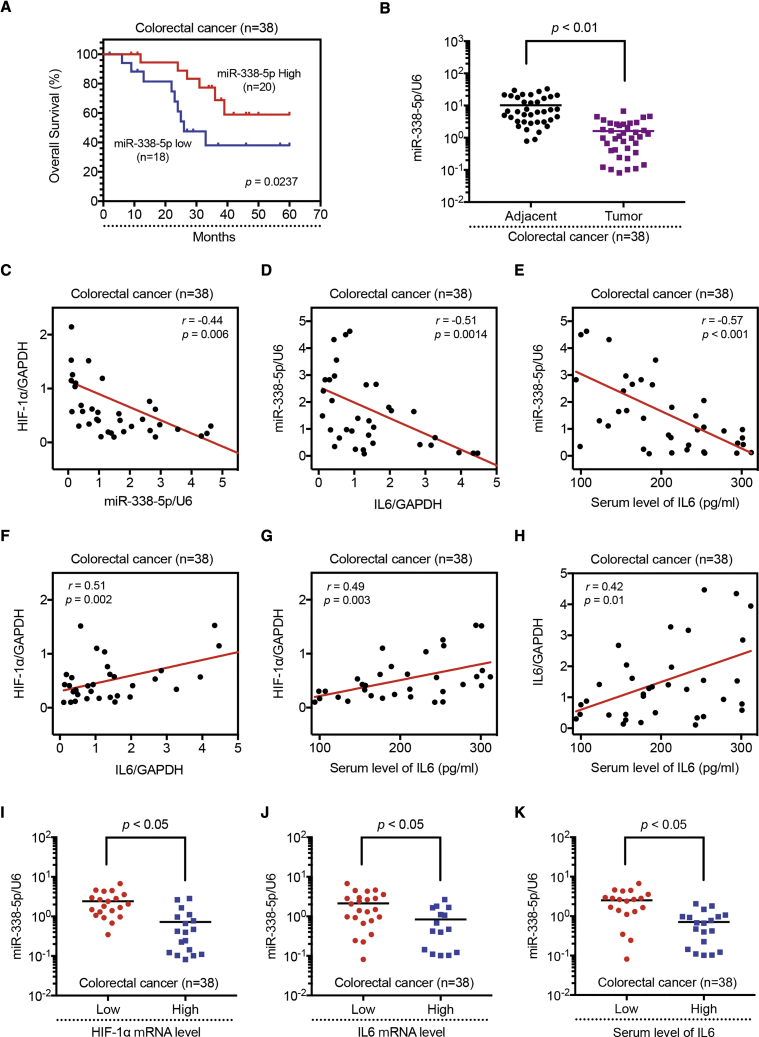
Kaplan-Meier curve analysis showed that the overall survival of the patients with low miR-388-5p expression was obviously longer than that of patients with high miR-338-5p expression (Figure 5A). Also, we found that the miR-338-5p expression was reduced in the tumor tissues as compared with their adjacent tissues (Figure 5B), but the expression levels of HIF-1α and IL-6 were both found increased in the tumor tissues (Figure S9). Moreover, IL-6 serum level of CRC patients was higher than that from the healthy volunteers (Figure S9). We also observed that expression of HIF-1α negatively correlated with miR-338-5p expression (Figure 5C); likewise, expression of miR-338-5p negatively correlated with IL-6 expression level (Figures 5D and 5E), whereas expression of HIF-1α positively correlated with IL-6 expression level (Figures 5F and 5G). Furthermore, IL-6 mRNA expression level in the tumor tissue was positively correlated with its serum level (Figure 5H). Finally, we detected a negative correlation between HIF-1α mRNA levels and the expression of miR-338-5p (Figure 5I) and a negative association of IL-6 mRNA level and miR-338-5p expression (Figures 5J and 5K). In summary, these results show that the HIF-1α/miR-338-5p/IL-6 loop is also active in primary human CRC and miR-338-5p has a negative correlation with HIF-1α and IL-6.
Figure 5.
Expression of HIF-1α/miR-338-5p/IL-6 Feedback Loop in Human CRC Specimens
(A) Kaplan-Meier analysis of overall survival in patients with variable miR-338-5p expression, according to the data from the selected CRC tissues samples (p = 0.0237). (B) Relative expression levels of miR-338-5p were detected in adjacent and tumor tissues (n = 38) via qRT-PCR. Abundance of miR-338-5p was normalized to U6 RNA. Expression levels of miR-338-5p and (C) HIF-1α mRNA, (D) IL-6 mRNA, and (E) IL-6 of serum were inversely correlated among all CRC specimens (n = 38), as indicated by two-tailed Pearson’s correlation analysis, respectively. Expression levels of HIF-1α mRNA and (F) IL-6 mRNA and (G) IL-6 of serum were positively correlated among all CRC specimens (n = 38), respectively. (H) Expression levels of IL-6 mRNA and IL-6 of serum were positively correlated among all CRC specimens (n = 38). miR-338-5p expression levels were inversely correlated with (I) HIF-1α mRNA, (J) IL-6 mRNA, and (K) IL-6 of serum level expression in CRC specimens. Results are representative of three experiments.
Modulation of HIF-1α/miR-338-5p/IL-6 Feedback Loop Expression Alters the Sensitivity of CRC Cells to OXA in a CRC Xenograft Model
To analyze the relevance of the HIF-1α/miR-338-5p/IL-6 feedback loop in vivo, we established a colon tumor xenograft model by subcutaneously implanting isogenic HCT116 cells, expressing either control (ctrl) vector or miR-338-5p, into athymic nude mice. After 2 weeks, mice were divided into two groups. One group was treated with OXA (10 mg/kg) every 5 days/week, administered by intraperitoneal (i.p.) injection, and the other group was treated with the solvent control. During the treatment with OXA, the tumor growth was monitored for 4 weeks until the mice were euthanized.
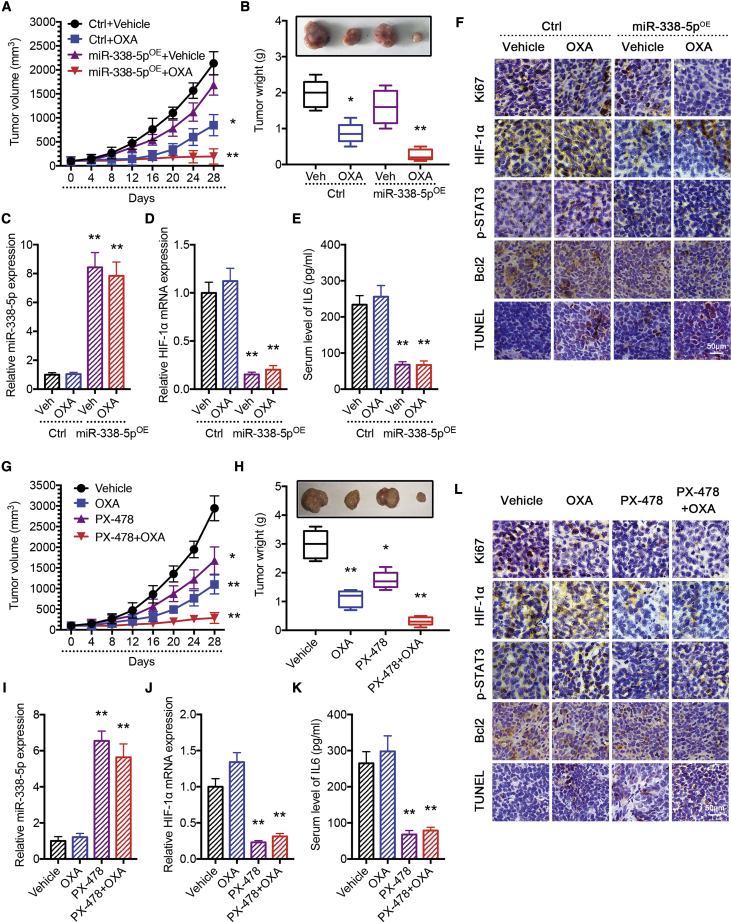
We found that tumors grew at similar rates independent of miR-338-5p expression. However, there were significant differences in the responses of tumors to OXA therapy. Tumors from miR-338-5p-expressing cells exhibited significantly enhanced response to OXA (Figure 6A), resulting in much reduced tumor size and weight (Figure 6B). As shown in Figure S10, none of the test subjects lost body weight or died in any of the four groups at the doses tested, suggesting minimal toxicities. Moreover, the qPCR results confirmed elevated expression of miR-338-5p in the overexpressing groups, and HIF-1α mRNA was selectively inhibited in the miR-338-5p-overexpressing CRC tumors (Figures 6C and 6D). Likewise, IL-6 serum levels were decreased in the miR-338-5p-expressing CRC tumor groups (Figure 6E). Using immunohistochemistry (IHC) analyses, we found that overexpression of miR-338-5p significantly improved the growth-inhibitory effect of OXA in CRC cells, which was demonstrated by reduced Ki67 level, enhanced apoptosis via TdT-mediated dUTP nick-end labeling (TUNEL), decreased phosphorylation of STAT3, and depressed expressions of HIF-1α and Bcl2 in tumors of the miR-338-5p-expressing group compared to the control vector-expressing group (Figure 6F). The positive rates based on IHC are summarized in Figure S11.
Figure 6.
Modulation of HIF-1α/miR-338-5p/IL-6 Feedback Loop Expression Alters the Sensitivity of CRC Cells to Oxaliplatin in a CRC Xenograft Model
Overexpression of miR-338-5p increased the effectiveness of OXA in the inhibition of tumor growth in vivo. (A) Xenograft tumor growth curves. (B) Representative tumor pictures and tumor weights. (C) qPCR analysis of miR-338-5p expression in vivo. Overexpression of miR-338-5p reduced (D) HIF-1α mRNA expression and (E) serum level of IL-6 in the CRC xenograft model. (F) Immunohistochemical analysis of Ki67, HIF-1α, p-STAT3, Bcl2, and TUNEL in tumors. Inhibition of HIF-1α increased the effectiveness of OXA in the inhibition of tumor growth in vivo. (G) Xenograft tumor growth curves. (H) Pictures and weights of tumors. (J) qPCR analysis of HIF-1α mRNA expression in vivo. Inhibition of HIF-1α (I) increased miR-338-5p expression and (K) reduced serum level of IL-6 in the CRC xenograft model. (L) Immunohistochemical analysis of Ki67, HIF-1α, p-STAT3, Bcl2, and TUNEL in tumors. *p < 0.05, **p < 0.01. Each bar represents the mean ± SD of three independent experiments.
To further analyze the relevance of the HIF-1α/miR-338-5p/IL-6 feedback loop in vivo, we used the HIF-1α inhibitor PX-478. Tumor-harboring mice were divided into 4 groups (i.e., vehicle, OXA, PX-478, and PX-478 + OXA groups). Tumor growth was monitored for 4 weeks until the mice were euthanized. Tumor growth curves showed that PX-478 significantly enhanced the response of CRC cells to OXA (Figure 6G), which resulted in decreased tumor size and weight (Figure 6H). Again, none of the test subjects lost body weight or died in any of the four groups at the doses tested (Figure S10). Moreover, the qPCR results revealed that the inhibition of HIF-1α by PX-478 could enhance miR-338-5p level (Figures 6I and 6J) and reduce serum level of IL-6 by ELISA (Figure 6K). Using IHC, we found that PX-478 significantly increased the effect of OXA, which was evidenced by reduced Ki67 level, enhanced apoptosis by TUNEL signal, decreased phosphorylation of STAT3, and depressed expressions of HIF-1α and Bcl2 in tumor cells (Figure 6L). Their IHC scores are summarized in Figure S11.
Taken together, we confirmed that modulation of the HIF-1α/miR-338-5p/IL-6 feedback loop expression could indeed alter the sensitivity of CRC cells to OXA in vivo.
Discussion
In this study, we identified a hypoxia-triggered feedback loop involving HIF-1α-mediated repression of miR-338-5p that contributes to the drug resistance in CRC (Figure S12). We believe this is the first study illustrating the chemosensitizing function of miR-338-5p in CRC in vivo.
Hypoxia-induced HIF-1α accumulation allows cancer cells to survive under hypoxic conditions via modulation in the tumor growth, metastasis, drug resistance, and radioresistance,21, 22, 23, 24 and, therefore, it represents a compelling therapeutic target for cancer treatment. HIF-1α is generally considered an oncoprotein induced by intratumoral hypoxia.25 Increasing evidence from experimental and clinical studies has revealed that adaptations to hypoxia contribute to the development of resistance in colorectal carcinoma to chemotherapy, and HIF-1α overexpression is associated with poor prognosis in CRC patients.26 Consistent with previous studies, we showed that, under the hypoxia condition, CRC exhibited resistance to OXA, 5-Fu, DOX, and CTX, which was mediated by the activation of the HIF-1α/miR-338-5p/IL-6 feedback loop in CRC cells.
miRNA has been considered an important transcriptional mediator in biological activities, including tumorigenesis. In a few tumors, the abnormalities conferred by miR-338-5p are clearly identified.20, 27 For example, Park et al.28 showed that miR-338-5p could increase radiation-induced apoptosis and enhance radiosensitivity by downregulating survivin expression in esophageal squamous cell carcinoma cell lines, and another report found that miR-338-5p sensitizes glioblastoma cells to radiation through the regulation of genes involved in the DNA damage response.29 As same as miR-338-5p, miR-338-3p (another isoform of miR-338) was shown to function as a tumor suppressor in gastric cancer by targeting PTP1B.30 However, the activity of miR-338-5p in CRC is rarely reported, and the function of miR-338-5p in CRC remains unclear.
Our study demonstrated that miR-338-5p acts as a chemosensitizer through the enhancement of apoptosis to reverse the drug resistance in CRC under the hypoxia condition. First, we found that miR-338-5p was downregulated in CRC under the hypoxia condition by miRNA microarray, and upregulating miR-338-5p inhibited the proliferation of CRC cells and promoted their apoptosis significantly. Furthermore, the bioinformatics analysis predicted and our luciferase reporter assay determined that IL-6 is a direct target of miR-338-5p, which is required for hypoxia-mediated CRC drug resistance. Thereafter, we determined that repression of miR-338-5p was required for IL-6-induced STAT3 activation during hypoxia-mediated CRC drug resistance and reducing the HIF-1α expression could increase the expression of miR-338-5p under the hypoxia condition. Noteworthily, the inhibition of miR-338-5p activated STAT3 to upregulate HIF-1α. All these data confirmed that the HIF-1α/miR-338-5p/IL-6 feedback loop is involved in CRC drug resistance under the hypoxia condition.
To better understand the clinical implication of an altered HIF-1α/miR-338-5p/IL-6 feedback loop, we investigated the expression levels of HIF-1α, miR-338-5p, and IL-6 in human CRC specimens. An active HIF-1α/miR-338-5p/IL-6 feedback loop was necessary for drug resistance in colon cancer cell lines, and it was observed in CRC patient samples. In addition, miR-338-5p was found to have a negative correlation with HIF-1α and IL-6. These data suggested that the HIF-1α/miR-338-5p/IL-6 feedback loop might have prognostic value in CRC.
IL-6 has been shown to be involved in many anti-tumor immunity-signaling pathways of the tumor microenvironment; it must have the interactive responses of miR-338-5p with other molecules via IL-6 in anti-tumor immunity. However, in our study, we only used the 2D in vitro cell culture model and nude mice model to show the HIF-1α/miR-338-5p/IL-6 feedback circuit underlying hypoxia-mediated drug resistance in colorectal carcinoma, and we hope we will address the mechanism between miR-338-5p/IL-6 and anti-tumor immunity in the future.
Recently, specific inhibitors for HIF-1α have been identified to inhibit the mRNA expression, protein synthesis, protein stability, and transactivation of HIF-1α.31 Three inhibitors of HIF-1α have entered clinical study, including 2-Methoxyestradiol, BAY 87-2243, and PX-478. PX-478 seems to be selective in lowering HIF-1α, which is the only agent that was clearly shown to inhibit HIF-1α transcription and translation.32 In fact, previous study showed that hypoxia conferred resistance to anti-EGFR therapy in KRAS mutant CRC, and a combination treatment with cetuximab and PX-478 could reverse such resistance.33 Here we showed that PX-478 increased miR-338-5p expression and repressed the HIF-1α/miR-338-5p/IL-6 feedback loop. This was confirmed in the xenograft model, and we found that PX-478 was efficient at increasing OXA sensitivity in CRC via suppressing the HIF-1α/miR-338-5p/IL-6 feedback loop in vivo. Our study thus provided the rationale to combine PX-478 and OXA for the treatment of CRC.
In summary, our results uncovered an HIF-1α/miR-338-5p/IL-6 feedback circuit underlying hypoxia-mediated drug resistance in colorectal carcinoma, and they provided evidence that each participating member could potentially serve as a novel target to reverse such resistance. In addition, we demonstrated the clinical potential of the HIF-1α inhibitor PX-478 in circumventing drug resistance in colorectal carcinoma.
Materials and Methods
Cell Lines and Reagents
The human colon cancer cell lines HCT116 and HCT8 were obtained from the Cell Bank of the Chinese Academy of Sciences. Cells were cultured in a 5% CO2 atmosphere at 37°C in DMEM (Gibco, Carlsbad, CA, USA) or RPMI (Gibco), supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). Hypoxic conditions were achieved by placing the cells in a 1% O2, 94% N2, and 5% CO2 multigas incubator (Sanyo, Osaka, Japan).
PX-478 was purchased from Selleck (Houston, TX, USA). OXA, 5-FU, DOX, and CTX were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant human IL-6 protein (rhIL-6) was purchased from R&D Systems (Minneapolis, MN, USA). miR-338-5p mimics, negative control mimics, miR-338-5p inhibitors, and negative control inhibitors were all purchased from Exiqon (Woburn, MA, USA). cDNA encoding miR-338-5p precursor was cloned into pMCS-CMV lentiviral vector purchased from GeneChem, Shanghai, China. HIF-1α knockdown shRNA plasmid (HIF-1α KD) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HIF-1α overexpression plasmid (HIF-1α OE) and STAT3 overexpression plasmid (STAT3 OE) were purchased from Addgene (Cambridge, MA, USA). 293T packaging cells were co-transfected with pPackH1 packaging plasmid mix (SBI, Mountain View, CA, USA) and the lentiviral vectors using Fugene HD (Promega, Madison, WI, USA). Viruses were harvested 48 h later and used to infect target cells. In some experiments, CRC cells were treated with neutralizing antibodies to IL-6 (anti-IL-6; Abcam) at 1:400, following the manufacturer’s instructions.
Tissue Samples
Human CRC and their corresponding non-tumorous (negative control [NC]) colon samples were collected at the time of surgical resection at Putuo Hospital, Shanghai University of Traditional Chinese Medicine, China, from January 2010 to December 2011. Written informed consent was obtained from the patients, in accordance with the institutional guidelines, before sample collection, and the study was approved by the Committees for the Ethical Review of Research at the Putuo Hospital, Shanghai University of Traditional Chinese Medicine, China. The methods were performed in accordance with the approved guidelines. All patients had a histological diagnosis of CRC and received radical resection. None of the patients included in the study had received neoadjuvant therapy before surgery. Samples were immediately snap frozen in liquid nitrogen and stored at −80°C.
Cell Viability and Apoptosis Assays
Colon cancer cells were plated in 96-well plates and treated with various chemotherapeutic agents for the indicated time. After 48 h, cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, MD, USA). The absorbance at 490 nm of each well was read on a spectrophotometer (Bio-Rad, Hercules, CA, USA) Cell viability was calculated as a ratio of OD values of drug-treated samples to those of controls. For apoptosis, an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Invitrogen, USA) was used according to the manufacturer’s instructions.
miRNA Microarray Analysis
Total RNA from HCT116 and HCT8 under hypoxic or normoxic conditions was isolated with Trizol reagent (Invitrogen, Carlsbad, CA, USA), and miRNA fraction was further purified using a mirVana miRNA isolation kit (Ambion, Austin, TX, USA). The isolated miRNAs were labeled with Hy3 using the miRCURY Array Labeling kit (Exiqon, Vedbaek, Denmark) and hybridized on a miRCURY LNA miRNA Array (version [v.]8.0, Exiqon). Microarray images were acquired using a Genepix 4000B scanner (Axon Instruments, Union City, CA, USA) and processed and analyzed with Genepix Pro 6.0 software (Axon Instruments) and Excel.
qRT-PCR
To prepare total RNA from tissues, the frozen tissues were ground into finely ground particles after 5-mm3 sections of each sample were cut, and then the tissue particles were subjected to extraction of RNA with TRIzol (Invitrogen). Total RNA was extracted from cultured CRC cells with Trizol (Invitrogen). The concentration of total RNA was quantitated by measuring the absorbance at 260 nm. Expression of mature miRNAs was assayed using stem-loop RT followed by real-time PCR analysis. All reagents for stem-loop RT were obtained from Applied Biosystems (Foster City, CA, USA).
The relative amount of each miRNA was normalized to U6 small nuclear RNA (snRNA). The relative expression levels of each cell line of each group were measured using the 2−ΔΔCt method. Briefly, the average ΔCt of each group was calculated by the following formula: ΔCt = average miR-338-5p Ct − average of HK (housekeeping) gene (U6 snRNA)’s Ct. ΔΔCt was calculated by ΔΔCt = ΔCt of control group − ΔCt of the treated group. The fold change for miR-338-5p expression level in cell lines was calculated using 2−ΔΔCt. The results are presented as fold change for each miRNA to its control, and the miR-338-5p expression level in tissues was calculated using 2−ΔCt. The primers of miR-338-5p and U6 snRNA used for stem-loop RT-PCR were purchased from QIAGEN (Valencia, CA, USA). For SYBR Green qPCR amplifications, reaction was performed in a 20-mL reaction volume containing SYBR Green PCR Master Mix (Applied Biosystems). The relative expression levels of each cell line of each group were measured using the 2−ΔΔCt methods as before, and the relative expression level of tissues was measured using 2−ΔCt.
Luciferase Activity Assay
The wild-type and mutated miR-338-5p putative targets on IL-6 3′ UTR were cloned into pGL3-promoter vector. The cells (2 × 104) were co-transfected with 500 ng pGL3-IL-6-wild-type (WT) and pGL3-IL-6-mutant (Mut) constructs with miR-338-5p mimics. Each sample was co-transfected with 50 ng pRL-SV40 plasmid expressing renilla luciferase to monitor the transfection efficiency. A luciferase activity assay was performed 48 h after transfection with the dual luciferase reporter assay system (Promega, WI, USA). The relative luciferase activity was normalized to the renilla luciferase activity.
Western Blot Analysis
Proteins were resolved in an SDS-PAGE gel and subjected to immunoblot analysis antibodies (Cell Signaling Technology, USA). All antibodies were used at 1 mg/mL working concentration in PBS with 5% dried milk. The membrane was further probed with horseradish peroxidase (HRP)-conjugated rabbit antimouse immunoglobulin G (IgG) (Santa Cruz Biotechnology, 1:2,000), and the protein bands were visualized using enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ, USA). Quantification of protein bands was performed using ImageJ software.
In Vivo Xenograft Model
HCT116 cells (1 × 106) expressing miR-338-5p or a control vector were injected into the flank of male athymic nude mice (4–5 weeks old). At 2 weeks after injection, OXA (10 mg/kg) or carrier was administered by i.p. injection every 5 days for 4 weeks. In another group, HCT116 cells (1 × 106) expressing miR-338-5p were injected into the flank of male athymic nude mice (4–5 weeks old). At 2 weeks after injection, OXA (10 mg/kg) or PX-478 (20 mg/kg) was administered by i.p. injection every 5 days/week for 4 weeks. Tumor volumes were measured at the beginning of the treatment and every 4 days until the mice were euthanized. The estimated tumor volumes (Vs) were calculated by the formula V = W2 × L × 0.5, where W represents the largest tumor diameter in centimeters and L represents the next largest tumor diameter. Tumors were dissected out and weighed. Harvested tumors were weighed and immediately fixed in formalin for IHC.
All proposals were approved and supervised by the institutional animal care and use committee of Putuo Hospital, Shanghai University of Traditional Chinese Medicine, China. All animal studies were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
IHC
Tissues were fixed in 10% formalin, embedded in paraffin, and sectioned (5-mm thickness). IHC of TUNEL, Ki67, Bcl2, p-STAT3, and HIF-1α was conducted as follows. Slides were deparaffinized and incubated for 10 min with 3% H2O2 in water to quench the endogenous peroxidase activity. The heat-induced antigen retrieval method was used for the detection of antigens. Tissues were incubated with 5% normal rabbit serum in Tris-buffered saline (TBS) (0.05 M Tris-HCl and 0.5 M NaCl [pH = 7.4]) for 30 min at room temperature, and they were incubated with primary antibodies in TBS for 60 min at 37°C. The indirect avidin-biotin-peroxidase method was applied, using the appropriate secondary antibodies, for 30 min at room temperature. The EnVision (K4007, Dako) signal enhancement system was used to develop the bound antibodies. Sections were counterstained with Harris hematoxylin, dehydrated, and mounted. For quantifications, 30 random images (400×) per experimental group were captured with a microscope (Leica, Wetzlar, Germany).
Statistical Analysis
Each experimental value was expressed as the mean ± SD. Statistical analysis was performed using a t test to evaluate the significance of differences between cell line groups, with significance accepted at *p < 0.05 and **p < 0.01. All data points represent the mean value of triplicate measurements. Statistical analysis of tissue samples was performed using the Spearman’s rank statistical test and the Mann-Whitney test to evaluate the significance of differences among groups.
Author Contributions
K.X., X.L., and P.Y. conceived and directed the project. K.X. designed the experiments. Y.Z., Z.Y., Y.Q., H.W., and G.F. carried out the experiments. J.W., W.L., and Y.C. were responsible for collecting tissue specimen. K.X., J.Z., and X.S. conducted the data analysis and interpreted the results. K.X., X.L., J.Z., and P.Y. wrote and edited the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
Special thanks to Dr. Teng Chen and Dr. Jianhua Xu for the help during the study. This project was sponsored by the National Nature Science Foundation of China (81873137), the Shanghai Rising-Star Program (17QA1403400), and the Shanghai Committee of Science and Technology (16411972600). This project was also sponsored by “the twelfth five year” key subject (Integrated Chinese and Western Medicine and General practice training of Traditional Chinese Medicine) of traditional Chinese medicine of State Administration of Traditional Chinese medicine and the Action Plan of Shanghai Municipality for Further Accelerating the Development of Traditional Chinese Medicine (ZY[2018-2020]-RCPY-2016).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.05.017.
Contributor Information
Ke Xu, Email: kexu2577@shutcm.edu.cn.
Xin Liang, Email: xin.liang@ecust.edu.cn.
Peihao Yin, Email: yinpeihao@shutcm.edu.cn.
Supplemental Information
References
- 1.Frederiksen L.J., Sullivan R., Maxwell L.R., Macdonald-Goodfellow S.K., Adams M.A., Bennett B.M., Siemens D.R., Graham C.H. Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clin. Cancer Res. 2007;13:2199–2206. doi: 10.1158/1078-0432.CCR-06-1807. [DOI] [PubMed] [Google Scholar]
- 2.Simões-Sousa S., Littler S., Thompson S.L., Minshall P., Whalley H., Bakker B., Belkot K., Moralli D., Bronder D., Tighe A. The p38α Stress Kinase Suppresses Aneuploidy Tolerance by Inhibiting Hif-1α. Cell Rep. 2018;25:749–760.e6. doi: 10.1016/j.celrep.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng L., Cheng Y., Tong X., Gan S., Ding Y., Zhang Y., Wang C., Xu L., Zhu Y., Wu J. Tumor Oxygenation and Hypoxia Inducible Factor-1 Functional Inhibition via a Reactive Oxygen Species Responsive Nanoplatform for Enhancing Radiation Therapy and Abscopal Effects. ACS Nano. 2018;12:8308–8322. doi: 10.1021/acsnano.8b03590. [DOI] [PubMed] [Google Scholar]
- 4.Luo Z., Tian H., Liu L., Chen Z., Liang R., Chen Z., Wu Z., Ma A., Zheng M., Cai L. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics. 2018;8:3584–3596. doi: 10.7150/thno.25409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillies R.J., Gatenby R.A. Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer Metastasis Rev. 2007;26:311–317. doi: 10.1007/s10555-007-9065-z. [DOI] [PubMed] [Google Scholar]
- 6.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 7.Oh E.T., Kim J.W., Kim J.M., Kim S.J., Lee J.S., Hong S.S., Goodwin J., Ruthenborg R.J., Jung M.G., Lee H.J. NQO1 inhibits proteasome-mediated degradation of HIF-1α. Nat. Commun. 2016;7:13593. doi: 10.1038/ncomms13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y., Tozzi F., Chen J., Fan F., Xia L., Wang J., Gao G., Zhang A., Xia X., Brasher H. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan R., Paré G.C., Frederiksen L.J., Semenza G.L., Graham C.H. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol. Cancer Ther. 2008;7:1961–1973. doi: 10.1158/1535-7163.MCT-08-0198. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y.A., Chen Y.F., Bao Y., Mahara S., Yatim S.M.J.M., Oguz G., Lee P.L., Feng M., Cai Y., Tan E.Y. Hypoxic tumor microenvironment activates GLI2 via HIF-1α and TGF-β2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. USA. 2018;115:E5990–E5999. doi: 10.1073/pnas.1801348115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim V.N., Han J., Siomi M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 12.Lee H.C., Kim J.G., Chae Y.S., Sohn S.K., Kang B.W., Moon J.H., Jeon S.W., Lee M.H., Lim K.H., Park J.Y. Prognostic impact of microRNA-related gene polymorphisms on survival of patients with colorectal cancer. J. Cancer Res. Clin. Oncol. 2010;136:1073–1078. doi: 10.1007/s00432-009-0754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosakhani N., Sarhadi V.K., Borze I., Karjalainen-Lindsberg M.L., Sundström J., Ristamäki R., Osterlund P., Knuutila S. MicroRNA profiling differentiates colorectal cancer according to KRAS status. Genes Chromosomes Cancer. 2012;51:1–9. doi: 10.1002/gcc.20925. [DOI] [PubMed] [Google Scholar]
- 14.Hisamatsu T., McGuire M., Wu S.Y., Rupaimoole R., Pradeep S., Bayraktar E., Noh K., Hu W., Hansen J.M., Lyons Y. PRKRA/PACT Expression Promotes Chemoresistance of Mucinous Ovarian Cancer. Mol. Cancer Ther. 2019;18:162–172. doi: 10.1158/1535-7163.MCT-17-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Zhang Y., Ding J., Hu W., Tan C., Wang M., Tang J., Xu Y. miR-17-3p Downregulates Mitochondrial Antioxidant Enzymes and Enhances the Radiosensitivity of Prostate Cancer Cells. Mol. Ther. Nucleic Acids. 2018;13:64–77. doi: 10.1016/j.omtn.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iorio M.V., Croce C.M. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert M.S., Sharp P.A. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rokavec M., Öner M.G., Li H., Jackstadt R., Jiang L., Lodygin D., Kaller M., Horst D., Ziegler P.K., Schwitalla S. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long J., Luo J., Yin X. MiR-338-5p promotes the growth and metastasis of malignant melanoma cells via targeting CD82. Biomed. Pharmacother. 2018;102:1195–1202. doi: 10.1016/j.biopha.2018.03.075. [DOI] [PubMed] [Google Scholar]
- 20.Xing Z., Yu L., Li X., Su X. Anticancer bioactive peptide-3 inhibits human gastric cancer growth by targeting miR-338-5p. Cell Biosci. 2016;6:53. doi: 10.1186/s13578-016-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh E.T., Kim C.W., Kim S.J., Lee J.S., Hong S.S., Park H.J. Docetaxel induced-JNK2/PHD1 signaling pathway increases degradation of HIF-1α and causes cancer cell death under hypoxia. Sci. Rep. 2016;6:27382. doi: 10.1038/srep27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yong Y., Zhang C., Gu Z., Du J., Guo Z., Dong X., Xie J., Zhang G., Liu X., Zhao Y. Polyoxometalate-Based Radiosensitization Platform for Treating Hypoxic Tumors by Attenuating Radioresistance and Enhancing Radiation Response. ACS Nano. 2017;11:7164–7176. doi: 10.1021/acsnano.7b03037. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z., Wang Y., Dou C., Xu M., Sun L., Wang L., Yao B., Li Q., Yang W., Tu K., Liu Q. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma. Theranostics. 2018;8:4649–4663. doi: 10.7150/thno.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo W., Zhong J., Chang R., Hu H., Pandey A., Semenza G.L. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but Not HIF-2α. J. Biol. Chem. 2010;285:3651–3663. doi: 10.1074/jbc.M109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlus M.R., Hu C.J. Enhanceosomes as integrators of hypoxia inducible factor (HIF) and other transcription factors in the hypoxic transcriptional response. Cell. Signal. 2013;25:1895–1903. doi: 10.1016/j.cellsig.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimura H., Dhar D.K., Kohno H., Kubota H., Fujii T., Ueda S., Kinugasa S., Tachibana M., Nagasue N. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin. Cancer Res. 2004;10:8554–8560. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- 27.Yong F.L., Law C.W., Wang C.W. Potentiality of a triple microRNA classifier: miR-193a-3p, miR-23a and miR-338-5p for early detection of colorectal cancer. BMC Cancer. 2013;13:280. doi: 10.1186/1471-2407-13-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park M., Yoon H.J., Kang M.C., Kwon J., Lee H.W. MiR-338-5p enhances the radiosensitivity of esophageal squamous cell carcinoma by inducing apoptosis through targeting survivin. Sci. Rep. 2017;7:10932. doi: 10.1038/s41598-017-10977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besse A., Sana J., Lakomy R., Kren L., Fadrus P., Smrcka M., Hermanova M., Jancalek R., Reguli S., Lipina R. MiR-338-5p sensitizes glioblastoma cells to radiation through regulation of genes involved in DNA damage response. Tumour Biol. 2016;37:7719–7727. doi: 10.1007/s13277-015-4654-x. [DOI] [PubMed] [Google Scholar]
- 30.Sun F., Yu M., Yu J., Liu Z., Zhou X., Liu Y., Ge X., Gao H., Li M., Jiang X. miR-338-3p functions as a tumor suppressor in gastric cancer by targeting PTP1B. Cell Death Dis. 2018;9:522. doi: 10.1038/s41419-018-0611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y., Wang B., Shi Q., Wang X., Wang D., Zhu L. Brusatol inhibits HIF-1 signaling pathway and suppresses glucose uptake under hypoxic conditions in HCT116 cells. Sci. Rep. 2016;6:39123. doi: 10.1038/srep39123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., He Y., Tan Z., Lu J., Li L., Song X., Shi F., Xie L., You S., Luo X. Wild-type IDH2 promotes the Warburg effect and tumor growth through HIF1α in lung cancer. Theranostics. 2018;8:4050–4061. doi: 10.7150/thno.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Lei F., Rong W., Zeng Q., Sun W. Positive feedback between oncogenic KRAS and HIF-1α confers drug resistance in colorectal cancer. OncoTargets Ther. 2015;8:1229–1237. doi: 10.2147/OTT.S80017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.