Abstract
Objective
Bariatric surgery acutely improves glucose control, an effect that is generally sustained for years in most patients. The acute postoperative glycemic reduction is at least partially mediated by enhanced incretin secretion and islet function, and occurs independent of caloric restriction, whereas the sustained improvement in glucose control is associated with increased insulin sensitivity. However, studies in humans with bariatric surgery suggest that these elevations are not static but undergo coordinated regulation throughout the postoperative time course. The studies described here test the hypothesis that incretin secretion, islet function, and peripheral insulin sensitivity undergo temporal regulation following bariatric surgery as a means to regulate glucose homeostasis.
Methods
Incretin secretion, islet function, and insulin sensitivity in mice with vertical sleeve gastrectomy (VSG) were compared to sham-operated controls that were pair-fed for 90d, matching food consumption and body-weight between groups.
Results
Glucose clearance and insulin secretion were enhanced in VSG mice compared to controls during mixed-meal tolerance tests (MMTT) at 12 and 80 days postoperatively, as were prandial GLP-1, GIP, and glucagon levels. Insulin sensitivity was comparable between groups 14d after surgery, but significantly greater in the VSG group at day 75, despite similar body-weight gain between groups. Glucose stimulated insulin secretion was greater in VSG mice compared to controls in vivo (I.P. glucose injection) and ex vivo (islet perifusion) indicating a rapid and sustained enhancement of β-cell function after surgery. Notably, glycemia following a MMTT was progressively higher over time in the control animals but improved in the VSG mice at 80d despite weight regain. However, meal-stimulated incretin secretion decreased in VSG mice from 10 to 80 days postoperative, as did meal-stimulated and I.P. glucose-stimulated insulin secretion. This occurred over the same time period that insulin sensitivity was enhanced in VSG mice, suggesting postoperative islet output is tightly regulated by insulin demand.
Conclusions
These data demonstrate a dynamic, multifactorial physiology for improved glucose control after VSG, whereby rapidly elevated insulin secretion is complimented by later enhancements in insulin sensitivity. Critically, the glucose lowering effect of VSG is demonstrably larger than that of caloric-restriction, suggesting these adaptations are mediated by surgical modification of gastrointestinal anatomy and not weight-loss per se.
Keywords: Bariatric surgery, Islet function, Insulin secretion, Insulin sensitivity, Incretin
Highlights
-
•
β-cell glucose sensitivity is enhanced 90d after VSG compared to controls, coincident with improved glucose tolerance.
-
•
Prandial GLP-1 and GIP are elevated 12d following VSG but return to preoperative levels 80d after VSG.
-
•
Insulin sensitivity is enhanced 75d after surgery, but not 14d after surgery, in mice with VSG compared to controls.
-
•
Mixed-meal glucose control is improved from 12d to 80d in VSG mice, but worsens in controls despite similar body-weight.
1. Introduction
Recent clinical trials demonstrate that bariatric procedures like vertical sleeve gastrectomy (VSG) alleviate diabetic hyperglycemia in 40–50% of patients [1]. There is a rapid glycemic benefit of bariatric surgery that occurs prior to weight loss in many patients [2], and is sustained for extended time periods as body-weight decreases [3]. The acute, weight-independent benefit of surgery has been attributed to multiple mechanisms [4] including enhanced insulin sensitivity [5], [6], islet function [7], [8], and incretin secretion [9], [10]. While each of these factors can contribute to the improvements in glucose control after surgery [6], [11], [12], it is unclear whether the regulation of these key glucoregulatory factors is modified over the postoperative time-course independent of caloric restriction. This fundamental question has been inadequately assessed in preclinical experiments, while human studies are often confounded by the difficulty of matching caloric restriction or weight-loss in non-surgical groups for extended periods.
Here we describe experiments with a previously reported mouse model of bariatric surgery [9] to assess glycemic regulation, incretin secretion, insulin sensitivity, and islet function over 90 days in mice with VSG and sham-operated controls pair-fed to match caloric intake. This paradigm allowed comparison of acute (i.e. 10–14 day) and chronic (i.e. 70–90 day) effects of VSG, independent of changes in energy balance. Our data demonstrate that the acute postoperative enhancement of intrinsic islet function, described previously [7], persists over time. Furthermore, this surgical effect is superior to that seen with caloric restriction or weight loss alone, supporting mechanisms invoked by VSG in addition to those of weight-reduction. Critically, the early enhancements to incretin secretion and islet function diminish over time as insulin sensitivity increases, indicating modulation of these factors after surgery.
2. Materials and methods
2.1. Animals
Wild-type mice (C57BL/6J) were bred in-house at the Duke Molecular Physiology Institute. At age 8w (∼19 g) animals were placed on high fat diet (HFD; 45% kcal lipid, Research Diets; D12451) for 56d. Animals were divided into separate groups (VSG or sham-surgery) that were balanced for body-weight.
2.2. Surgery
Descriptions of surgical techniques have been reported previously [7], [9]. In brief, mice received liquid diet for 2d (Ensure Plus, 28% lipid, 14% protein, 58% carbohydrate; Abbot) and then fasted overnight the day before surgery. A midline incision was made below the xyphoid process, the stomach and spleen were delivered through the incision, the spleen was separated from the stomach, and the suspensory ligament incised. The stomach contents were extruded, a stainless steel Ligaclip (LS-400, Ethicon) was placed along the angle of His, and 70–80% of the gastric remnant was excised. The Ligaclip was secured with three sutures (6-0 Ethilon, Ethicon), followed by closure of the body wall and skin (4-0 Ethilon, Ethicon). Sham operated control mice received laparotomy, with temporary removal of the stomach before abdominal closure.
2.3. Postoperative glucose and insulin tolerance testing
The experimental design is illustrated in Figure 1A. Mice were returned to HFD at 5d, and individual body-weight and food consumption were measured daily for 14d postoperatively, then weekly until day 84. Sham operated mice received a daily food ration equivalent to that consumed by the VSG group on the previous day. In vivo experiments were performed as follows: I.P. glucose tolerance tests (1.5 g/kg glucose injection; IPGTT) on postoperative days 10 and 70, mixed meal tolerance tests (300uL Ensure Plus gavage; MMTT) on postoperative days 12 and 80, and insulin tolerance tests (0.5 U/kg Humalog; ITT) on postoperative day 14 and 75; our methods for these procedures have been reported previously [9]. Tail-vein blood glucose was measured in real time (Bayer Contour Glucometer), and blood samples collected in EDTA tubes for peptide assays as follows: insulin (CrystalChem, 90080), total GLP-1 (Meso Scale Discovery, K150JVC-1), GIP (CrystalChem, 81517), glucagon (Mercodia, 10-1271-01). Animals were sacrificed 84d postoperative for islet isolation
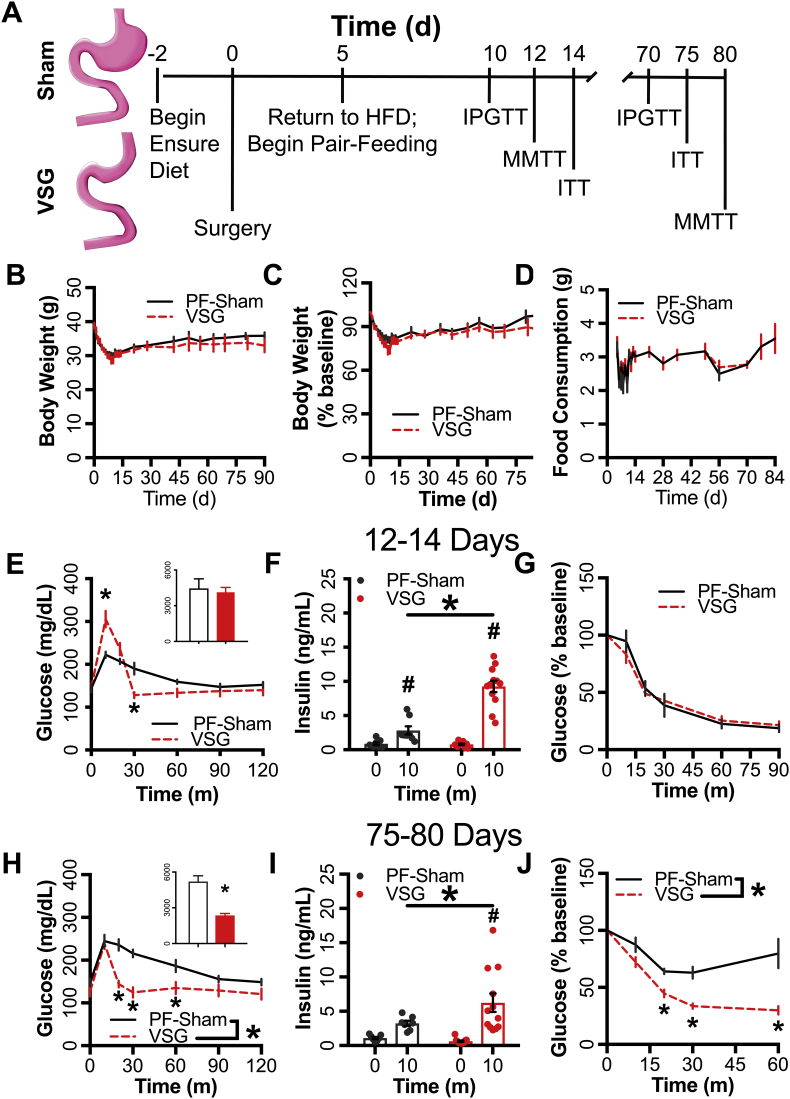
Figure 1.
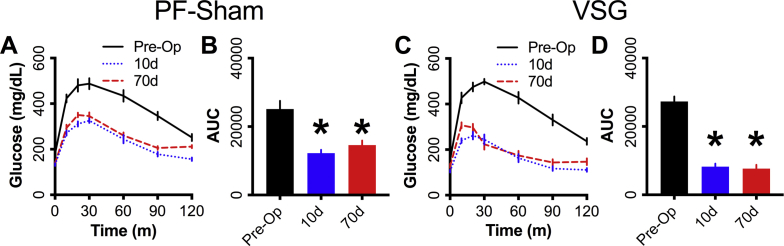
VSG enhanced glucose homeostasis compared to caloric restriction. (A) Schematic of the experimental paradigm employed. (B) Body-weight and (C) body weight as a percentage of baseline weight for 90d following surgery (PF-Sham n = 8, VSG n = 12). (D) Food consumption from the start of pair-feeding at 5d to 84d following surgery (PF-Sham n = 8, VSG n = 12). Panels E–G represent data from animals tested 12-14d following surgery; panels H–J represent data from the same animals tested 75-80d following surgery. (E,H) Blood glucose and integrated AUC (inset) during a MMTT along with (F,I) circulating insulin at 0m and 10m (PF-Sham n = 8, VSG n = 12). (G,J) Blood glucose during an insulin tolerance test (% of baseline glucose; PF-Sham n = 8, VSG n = 12). PF-Sham animals are shown with grey solid lines or grey circles; VSG animals are shown in red dashed lines or red circles. Data represent mean ± SEM; * represent p < 0.05 between PF-Sham and VSG groups; # represent p < 0.05 between time points within groups.
2.4. Islet isolation and perifusion
Primary mouse islet isolation and perifusion experiments are described elsewhere [7]. In brief, islet perifusion experiments were performed using a Biorep Perifusion system. After isolation, islets were cultured overnight in RPMI with 10% FBS at 37 °C. After overnight incubation 50 islets were loaded into each chamber, equilibrated for 48m in KRPH buffer + 2 mM glucose at a flow rate of 200uL/m, then treated with 2, 8, 12, and 16 mM glucose. Samples were assayed for insulin (Perkin-Elmer, AL-204).
2.5. Ca2+ flux
Calcium oscillation traces and calculations were performed in whole islets exposed to 7- and 10-mM glucose as previously published [7], [13].
2.6. Statistics
Comparisons between surgical groups at the early and late time points were made using standard two-way ANOVA followed by Holms-Sidak multiple comparisons test. Integrated glucose AUC (iAUC) was calculated using individual t0 blood glucose as the baseline, and compared between groups using unpaired t-tests. Longitudinal comparisons of preoperative, early, and later time points within groups were made using a mixed effect model (REML) two-way ANOVA followed by Holms-Sidak multiple comparison test. All data are presented as mean ± standard error and analyzed using GraphPad Prism.
3. Results
3.1. VSG improves prandial glucose metabolism independent of caloric restriction
3.1.1. Effects of VSG on MTT glucose metabolism 12d after surgery
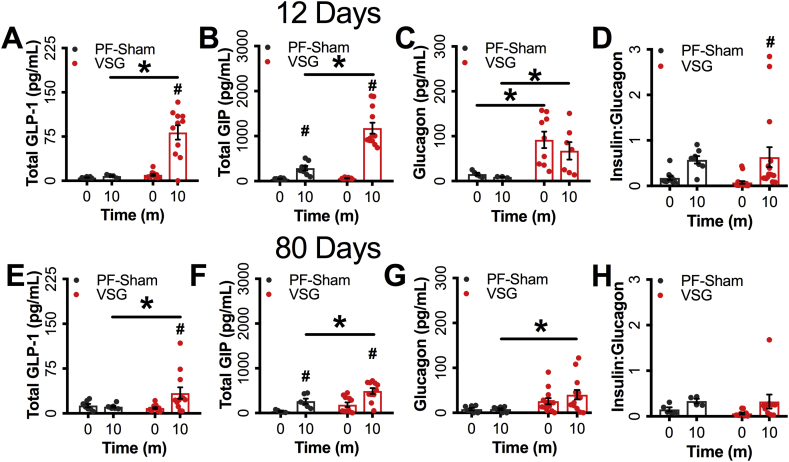
Mice achieved a mean preoperative body-weight of 38.42 g ± 0.56 before surgery (Figure 1B). Body-weight (raw or as a percent 0d body-weight) did not differ significantly between groups at the early (0-14d) or late (75-80d) periods (Figure 1B,C). Food intake was matched between the groups by design (Figure 1D). Consistent with previous reports of early responses to VSG [6], [7], [10], blood glucose 10m into the MMTT rose to a higher peak in VSG animals than controls, but had a more rapid decline to baseline levels within 30 min (Figure 1E). Despite differing glycemic profiles, the glucose iAUC was similar between groups (Figure 1E) at this early (12d) time-point. However, VSG mice had elevated prandial insulin (∼2.28-fold), GLP-1 (∼8-fold), GIP (∼2-fold), and glucagon (7-fold) levels relative to PF-Sham controls (Figure 1F, Supplementary Figs. 1A–C); insulin:glucagon ratio was elevated from t0 to 10m in the VSG but not the PF-Sham group (Supplementary Fig. 1D). Blood glucose did not differ between groups during the early (14 day) ITT (Figure 1E), similar to previous reports [7].
3.1.2. Effects of VSG on MTT glucose metabolism 80d after surgery
Glucose tolerance and insulin concentrations (Figure 1H,I) were significantly enhanced during the MMTT in the VSG group 80d after surgery, as were prandial GLP-1 (∼2-fold), GIP (70%), and glucagon (∼4-fold) concentrations, while insulin:glucagon ratio was unchanged (Supplementary Figs. 1E–H). However, in contrast to the early time period, blood glucose in response to IP insulin was significantly lower in VSG mice compared to controls 75d after surgery, consistent with worsening insulin sensitivity in the control group with weight regain, that did not occur in the VSG animals (Figure 1J).
3.1.3. Longitudinal comparison of effects of VSG on MTT glucose metabolism
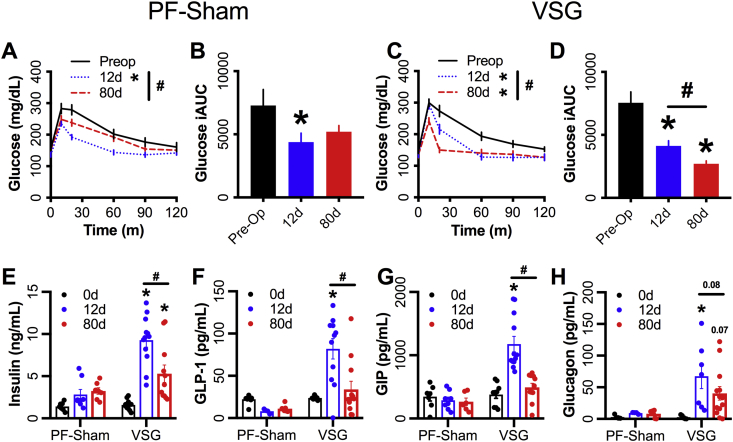
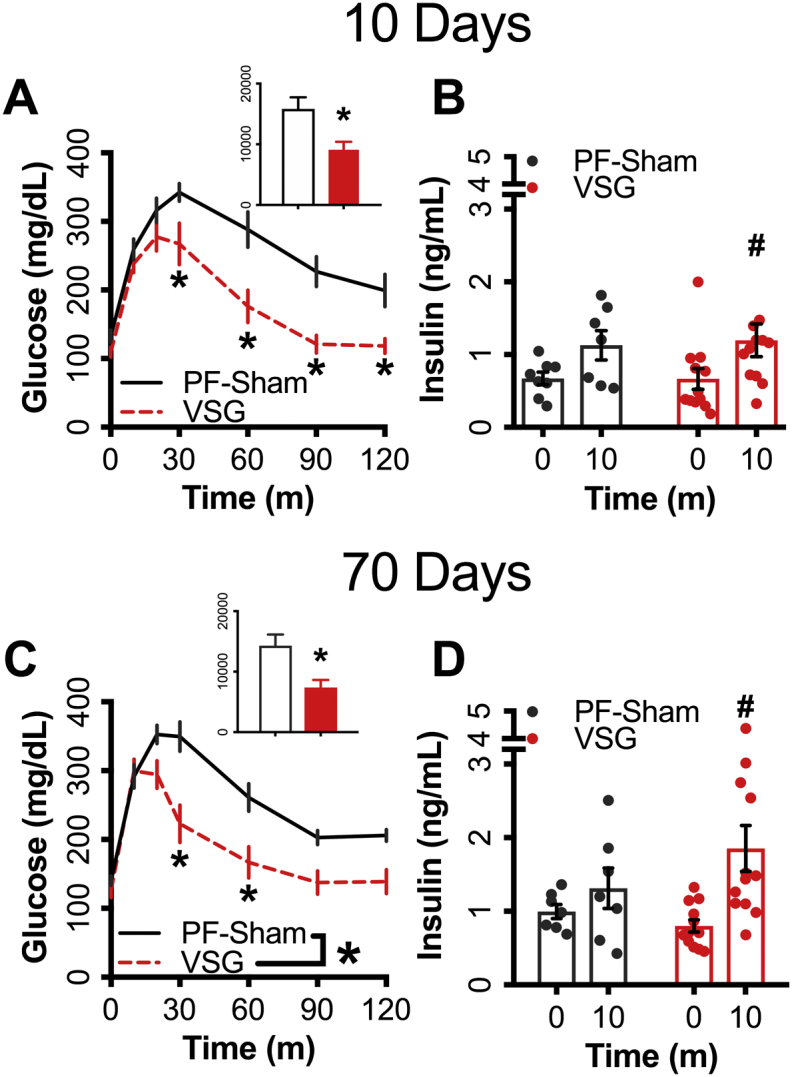
Both the VSG and PF-Sham mice had reduced MMTT glucose iAUC 10d after surgery compared to the within-subject, preoperative values (∼45% and ∼39% respectively) (Figure 2A–D). Both groups had gradual weight regain, moving back towards their own preoperative body-weight over 80 days. The PF-Sham glucose excursion returned to the preoperative values by 80d (Figure A,B), whereas the VSG group showed further reduction in glucose iAUC (∼41%) between 12 and 80d (Figure 2C,D) despite similar weight regain (Figure 1B,C). Twelve days after surgery, mice with a VSG show significantly increased prandial insulin (∼5-fold), GLP-1 (∼3.5-fold), GIP (3-fold), and glucagon (∼24-fold) secretion 10m after a mixed nutrient gavage (Figure 2E–H) compared to their preoperative levels. By day 80, prandial GLP-1, GIP, and glucagon secretion were all significantly reduced to levels comparable to preoperative values (Figure 2F–H). Insulin secretion was significantly reduced from day 12–80; however, the value was significantly higher than those seen preoperatively (Figure 2E). The PF-Sham group evinced no significant changes in these parameters throughout the postoperative time course (Figure 2E–H).
Figure 2.
Prandial glucose control is increased in VSG mice but decreased in calorically restricted controls during weight-regain following surgery. Glucose curves and integrated AUC during a mixed-meal gavage are shown for (A,B) PF-Sham animals or (C,D) VSG operated animals. Circulating levels of (E) insulin, (F) total GLP-1, (G) GIP, and (H) glucagon 10m post mixed-meal gavage. Preoperative values are shown with a black solid line, black bar or black circles, 12d MMTT values are shown with a blue dotted line, blue bar, or blue circles, 80d MMTT values are shown with a red dashed line, red bar, or red circles. Data represent mean ± SEM; * represents p-value < 0.05 compared to preoperative value; # represents p-value < 0.05 between 12d and 80d MMTT; p-values > 0.05 but <0.1 are indicated.
3.2. Glucose clearance is enhanced in response to I.P. glucose in VSG mice
To test the effects of VSG on glucose regulation independent of GI factors, VSG and control mice received IPGTTs. Compared to preoperative results, glucose iAUC during the IPGTT was reduced in VSG (∼69%) and control (51%) mice 10d after surgery, an effect that was sustained 70d postoperative in both groups (Supplementary Figs. 2A–D). However, at both the early and late postoperative time points, mice with VSG had lower glucose excursions and glucose iAUC than controls (Figure 3A,C). Glucose stimulated insulin levels (10m) were significantly increased compared to fasting insulin levels in the VSG mice at both 10d and 70d after surgery, while there was no change in plasma insulin in the controls (Figure 3B,D).
Figure 3.
Glucose clearance during an I.P. glucose challenge is enhanced by VSG. Panels A–B represent data from animals tested 10d following surgery; panels C–D represent data from the same animals tested 70d following surgery. (A,C) Blood glucose and integrated AUC (inset) along with (B,D) circulating insulin concentration from 0m and 10m during an IPGTT (PF-Sham n = 7; VSG n = 12). PF-Sham animals are shown with grey solid lines or grey circles; VSG animals are shown in red dashed lines or red circles. Data represent mean ± SEM; * represents p-value < 0.05 between PF-Sham and VSG groups; # represent p < 0.05 between time points within groups.
3.3. VSG induces sustained elevations in islet glucose sensitivity
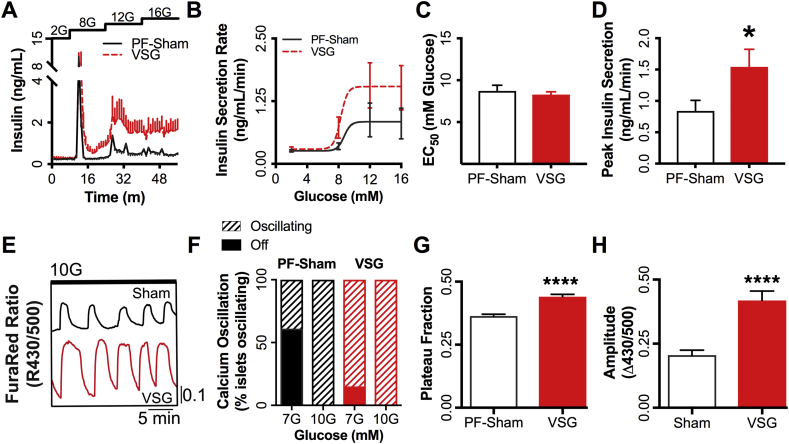
Our previous work has shown that VSG rapidly enhances islet glucose sensitivity (i.e. glucose-stimulated insulin secretion and Ca2+ oscillations) independent from differences in energy balance [7]. However, it is unknown whether this effect persists chronically after surgery. Islets isolated from VSG mice 84d after surgery displayed elevated glucose stimulated insulin secretion relative to controls in ex vivo islet perifusion (83% increased peak insulin secretion; Figure 4A–D). Relative to controls, the VSG islets had increased Ca2+ oscillatory amplitude (17%) and plateau fraction (29.5%), the time spent in the active state and a primary index of β-cell glucose sensitivity [14], [15] (Figure 4G,H). The enhanced glucose sensitivity of insulin secretion after VSG is also evident in the percentage of islets activated by 7 mM glucose. Among islets from PF-Sham mice, 40% increased intracellular calcium on exposure to 7 mM glucose compared to 85% for VSG (Figure 4H). Thus, the increased insulin secretion induced by VSG is associated with, and possibly due to, left-shifted glucose-stimulated β-cell Ca2+ flux.
Figure 4.
Islet function is increased 84 days after VSG. (A) Glucose stimulated insulin secretion, and (B) calculated insulin secretion rate dose response curve from ex vivo perifusion of islets isolated for PF-Sham animals (n = 6) and VSG animals (n = 8) treated with 2, 8, 12, and 16 mM glucose; (C) EC50 and (D) peak insulin secretion rate calculated from the dose response curve. (E) Representative Ca2+ traces in islets from PF-Sham (totaling 92 islets from 3 mice) or VSG animals (totaling 93 islets from 3 mice), (F) percentage of islets undergoing active Ca2+ oscillations when treated with either 7 mM glucose (7G) or 10 mM glucose (10G). Quantification of (G) calcium plateau fraction (the fraction of time spent in the active state of each oscillation, defined as >50% oscillation amplitude) and (H) oscillation period. PF-Sham animals are shown with a solid grey line or white/black bars; VSG animals are shown with a dashed red line or red bars. Data represent mean ± SEM; * represents p < 0.05 between PF-Sham and VSG groups; **** represents p < 0.0001 between PF-Sham and VSG groups.
4. Discussion
Bariatric surgical procedures like VSG rapidly improve glucose control, often prior to significant weight loss in humans [2], and this effect is sustained over years following surgery as body-weight first decreases rapidly then plateaus [3]. Numerous factors contribute to improved glucose control including enhanced insulin sensitivity, which is strongly associated with weight loss [5], [6], islet function [7], [8], and incretin secretion [9], [10]. While weight-independent mechanisms have been suggested to acutely improve glucose tolerance in clinical and preclinical studies, the contribution of chronically limited caloric intake to the sustained enhancement of glucose control has not been rigorously tested. Furthermore, the temporal modification of key glucose regulatory factors including incretin secretion, insulin output, and insulin sensitivity has not been well characterized independent of changes in caloric restriction. In this study, we assessed these parameters over a 3-month period, ∼15–20% of the murine lifespan, in mice with VSG or sham-operated controls with matched caloric intake. Mice with a VSG show a rapid improvement in glucose tolerance relative to controls that is sustained over a prolonged period even when body-weight and food intake do not differ between groups. Incretin and insulin secretion, but not insulin sensitivity, are elevated early following surgery (i.e. 10-14d), whereas insulin action increases later time points (i.e. 70-90d). The mechanism for enhanced insulin sensitivity at later time points is unclear, but changes in body composition and hepatic function have been implicated by previous studies [5]. Notably, despite similar trends in body-weight glycemic control worsens in pair-fed control mice while continuing to improve over time (i.e. between 12 and 80d) in mice with VSG. These results suggest a model in which VSG improves glucose control beyond changes in energy balance alone through an early increase in insulin secretion, which is complimented by a later enhancement of peripheral insulin sensitivity.
Evidence that bariatric surgeries elicit salutary effects on glucose control beyond simple weight loss and/or caloric restriction has largely come from relatively short studies [1], [2], [3], [5], [6], [7], [8], [10], [11], [16], [17], [18]. Weight-independent phenomena have been difficult to quantify in longer human studies given the superior weight-reducing efficacy of bariatric surgery compared to dietary interventions [3], [16]. To overcome this variable we extended comparisons of VSG and pair-fed control mice, previously reported in 2 week studies [7], to 90d following surgery. While the pair-feeding regimen successfully normalized food consumption and body-weight between groups, blood glucose during either IPGTT or MMTT was significantly lower in the VSG group compared to the PF-Sham controls throughout the 3-month experiment. Thus, these data demonstrate rapid enhancement of glucose homeostasis after VSG in mice that is sustained over an extended period independent of differences in body-weight between groups.
Both PF-Sham and VSG groups had significant reductions in body-weight and glucose iAUC during IPGTT and MMTT 10-12d after surgery compared to their preoperative values, although glucose tolerance was superior with VSG than dietary intervention. However, over the extended experimental time-course, both the VSG and the PF-Sham groups had steady weight gain, as previously reported in animal models of bariatric surgery [5], [19], and consistent weight regain in humans after reaching a nadir weight loss ∼ 1 year post-operative [1], [3]. With body-weight regain, the PF-Sham mice had predictable worsening of glucose control during the MMTT. On the other hand, the VSG mice with similar increases in body-weight had a further improvement in the glucose iAUC. The findings from the early and late glucose tolerance comparisons indicate that VSG improves glucose tolerance during states of both negative and positive energy balance. This result is the basis for our primary conclusion that surgery has effects on glucose regulation beyond those of caloric intake and body-weight.
We have previously reported that islets isolated from VSG operated mice have distinct functional and transcriptional characteristics without changes in size or cellular composition compared to controls 14d after surgery [7]. These changes in islet function were demonstrated ∼24 h after removal from the in vivo milieu and include an increase in glucose-stimulated insulin secretion (GSIS). This intrinsic enhancement of islet function (i.e. increased GSIS) persists 3 months after VSG in the present study and is likely to contribute to improved glucose tolerance in these mice. The greater GSIS ex vivo seems to be driven by proximal factors in stimulus-secretion coupling, as it is associated with a strong increase in Ca2+ oscillations. Previous work has linked the Ca2+ oscillatory plateau fraction, the percent of time an islet spends in a Ca2+ excited state, with β-cell glucose sensing [14], [15]. While the molecular underpinnings of this effect are unclear, it seems plausible that gut derived neuroendocrine factors act to restructure the islet transcriptional program, consistent with what we have previously described [7]. While a number of investigators have posited GLP-1 as a candidate factor for this effect, several studies indicate that the GLP-1 receptor is dispensable for improved glucose tolerance after VSG [9], [20]. We did not perform transcriptional analysis in this study due to allocation of islets to functional assessments. While we cannot comment on changes between the early and later effects of VSG on islet gene expression profiles, differences between these two periods seem likely as VSG mice appear to reduce in vivo GSIS from day 12–80 as insulin sensitivity increases. Furthermore, while some studies report changes in β-cell proliferation markers [5] and islet composition [21] after VSG in mice, we cannot comment on how these potential changes might affect islet function over time after surgery.
Previous longitudinal studies suggest plasticity in the surgical effects on factors that govern glucose metabolism [12]. Our data are consistent with this hypothesis and raise some possibilities regarding the regulation of this adaptation. In mice, there is a rapid and sustained enhancement of islet function after VSG, which is partially mediated by increases to both incretin levels and intrinsic islet glucose sensitivity [7]. In this study we observed that the rise in prandial insulin secretion and incretin levels is dampened over time as peripheral insulin sensitivity increases 3 months following VSG. The in vivo data reported here largely reflect first-phase insulin secretion, but not second-phase; thus, the mechanisms that govern these two distinct elements may be modified after surgery. It is possible that persistent elevations in intrinsic islet glucose sensitivity may explain the continued increase in prandial insulin secretion at day 80 even as incretin levels are diminished; the mechanisms underlying the heightened sensitivity of the islet to glucose is unknown, but the chronic elevation of glucagon may provide some explanation [22]. One interpretation of these responses is that attenuated prandial insulin secretion is necessary to counterbalance improved insulin action. We have recently reported that humans have reduced β-cell sensitivity to glucose and incretins 2 or more years following gastric bypass and have argued that this represents a mechanism to better match insulin secretion and sensitivity [23], [24]. A temporally dynamic model, with changing parameters controlling glucose tolerance is suggested by these and other studies in humans [12] but requires further clinical evaluation. A corollary of a more fluid model of glucose regulation after bariatric surgery is that the design and interpretation of experiments need to take into consideration timing of cross-sectional comparisons or longitudinal measures that would be expected to impact outcomes.
Data from human [6], [8], [12] and rodent studies [5], [7] support an important contribution of enhanced islet function to improve glucose control acutely following VSG. These observations, along with the present paper, have implications for the course of diabetes before and after bariatric surgery. Postoperative diabetes remission and relapse are not simple functions of weight loss or regain [25] but are more closely related to the duration of preoperative diabetes and/or insulin use. Thus, preoperative β-cell function [26], [27] and subsequent β-cell rescue after bariatric surgery may be necessary elements of diabetes remission. Furthermore, understanding the mechanisms by which different surgical modalities improve islet function and preoperative β-cell function assessments could prove useful in the choice of surgical procedure.
In summary, we report that at least some of the improvement in glucose tolerance following VSG in mice occurs independently of changes in energy balance, reflected here as matched weight and caloric intake. Better glucose tolerance is at least partially determined by enhanced insulin and incretin secretion soon after surgery but over time includes enhanced insulin sensitivity. These findings fit with a fluid model of glycemic regulation after surgery, with different regulatory components playing greater and lesser roles over time, ostensibly to maintain homeostasis. These findings provide a clear rationale for investigating factors, presumably related to gut function, that mediate responses of the islet and other key tissues involved in glucose metabolism.
Author contributions
JDD, TG, MJM, JC, JT, DAD conceptualized, designed experiments; JDD, JJN, SS, TG, JC performed experiments; JDD, MJM, JC, JT, DAD provided interpretation of data; JDD, SS, MJM, JC, JT, DAD wrote the manuscript.
Acknowledgments
Funding: JDD received fellowship support from the NIH/NIDDK (T32DK007012, F32DK115031). MJM received funding from ADA (1-16-IBS-212), the NIH/NIDDK (R01DK113103), the NIH/NIA (R21AG050135, R01AG062328), and a New Investigator Award from the Wisconsin Partnership Program. JT received funding from the NIH/NIDDK (5R01DK097550). JEC is supported by a career development award from the ADA (1-18-JDF-017) and is a Borden Scholar. DAD received funding from the NIH/NIDDK (RO1 DK57900). Dr. Jonathan D. Douros is the guarantor of this work, had access the data, and takes responsibility for the integrity of the data and analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.07.003.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Schauer P.R., Bhatt D.L., Kirwan J.P., Wolski K., Brethauer S.A., Navaneethan S.D. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. New England Journal of Medicine. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umeda L.M., Silva E.A., Carneiro G., Arasaki C.H., Geloneze B., Zanella M.T. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obesity Surgery. 2011;21:896–901. doi: 10.1007/s11695-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 3.Schauer P.R., Bhatt D.L., Kirwan J.P., Wolski K., Aminian A., Brethauer S.A. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. New England Journal of Medicine. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douros J.D., Tong J., D'Alessio D.A. The effects of bariatric surgery on islet function, insulin secretion, & glucose control. Endocrine Reviews. 2019 doi: 10.1210/er.2018-00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Gazala S., Horwitz E., Ben-Haroush Schyr R., Bardugo A., Israeli H., Hija A. Sleeve gastrectomy improves glycemia independent of weight loss by restoring hepatic insulin sensitivity. Diabetes. 2018;67:1079–1085. doi: 10.2337/db17-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorgensen N.B., Jacobsen S.H., Dirksen C., Bojsen-Moller K.N., Naver L., Hvolris L. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. American Journal of Physiology Endocrinology and Metabolism. 2012;303:E122–E131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 7.Douros J.D., Niu J., Sdao S.M., Gregg T., Fisher-Wellman K.H., Bharadwaj M.S. Sleeve gastrectomy rapidly enhances islet function independently of body weight. JCI Insight. 2019;4:126688. doi: 10.1172/jci.insight.126688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polyzogopoulou E.V., Kalfarentzos F., Vagenakis A.P., Alexandrides T.K. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52:1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 9.Douros J.D., Lewis A.G., Smith E.P., Niu J., Capozzi M., Wittmann A. Enhanced glucose control following vertical sleeve gastrectomy does not require a beta-cell glucagon-like peptide 1 receptor. Diabetes. 2018;67:1504–1511. doi: 10.2337/db18-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falken Y., Hellstrom P.M., Holst J.J., Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. Journal of Clinical Endocrinology Metabolism. 2011;96:2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 11.Bojsen-Moller K.N., Dirksen C., Jorgensen N.B., Jacobsen S.H., Serup A.K., Albers P.H. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014;63:1725–1737. doi: 10.2337/db13-1307. [DOI] [PubMed] [Google Scholar]
- 12.Nannipieri M., Baldi S., Mari A., Colligiani D., Guarino D., Camastra S. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. Journal of Clinical Endocrinology Metabolism. 2013;98:4391–4399. doi: 10.1210/jc.2013-2538. [DOI] [PubMed] [Google Scholar]
- 13.Gregg T., Poudel C., Schmidt B., Dhillon R., Sdao S., Truchan N. Pancreatic B cells from mice offset age-associated mitocondrial deficiency with reduced K-ATP channel activity. Diabetes. 2016;65:2700–2710. doi: 10.2337/db16-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.M.R.M., P.M. Correlations of rates of insulin release from islets and plateau fractions for beta-cells. Bulletin of Mathematical Biology. 1995;57:229–246. doi: 10.1007/BF02460617. [DOI] [PubMed] [Google Scholar]
- 15.Henquin J.C. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–751. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 16.Halperin F., Ding S.A., Simonson D.C., Panosian J., Goebel-Fabbri A., Wewalka M. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surgery. 2014;149:716–726. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashyap S.R., Daud S., Kelly K.R., Gastaldelli A., Win H., Brethauer S. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. International Journal of Obesity. 2010;34:462–471. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laferrere B., Teixeira J., McGinty J., Tran H., Egger J.R., Colarusso A. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. Journal of Clinical Endocrinology Metabolism. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers A.P., Jessen L., Ryan K.K., Sisley S., Wilson-Perez H.E., Stefater M.A. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson-Perez H.E., Chambers A.P., Ryan K.K., Li B., Sandoval D., Stoffers D.A. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes. 2013;62 doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garibay D., Lou J., Lee S.A., Zaborska K.E., Weissman M.H., Sloma E. Beta cell GLP-1r signaling alters alpha cell proglucagon processing after vertical sleeve gastrectomy in mice. Cell Reports. 2018;23:967–973. doi: 10.1016/j.celrep.2018.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capozzi M.E., Svendsen B., Encisco S.E., Lewandowski S.L., Martin M.D., Lin H. beta-Cell tone is defined by proglucagon peptides through cyclic AMP signaling. JCI Insight. 2019;4:126742. doi: 10.1172/jci.insight.126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi M., Gastaldelli A., D'Alessio D.A. Beta-cell sensitivity to glucose is impaired after gastric bypass surgery. Diabetes Obesity and Metabolism. 2017;20:872–878. doi: 10.1111/dom.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salehi M., Gastaldelli A., D'Alessio D. Beta-cell sensitivity to insulinotropic gut hormones is reduced after gastric bypass surgery. Gut. 2019 doi: 10.1136/gutjnl-2018-317760. Published Online First: 16 February 2019. [DOI] [PubMed] [Google Scholar]
- 25.Arterburn D.E., Bogart A., Sherwood N.E., Sidney S., Coleman K.J., Haneuse S. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obesity Surgery. 2012;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallipedhi A., Min T., Prior S.L., MacIver C., Luzio S.D., Dunseath G. Association between the preoperative fasting and postprandial C-peptide AUC with resolution of type 2 diabetes 6 months following bariatric surgery. Metabolism. 2015;64:1556–1563. doi: 10.1016/j.metabol.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Souteiro P., Belo S., Neves J.S., Magalhaes D., Silva R.B., Oliveira S.C. Preoperative beta cell function is predictive of diabetes remission after bariatric surgery. Obesity Surgery. 2017;27:288–294. doi: 10.1007/s11695-016-2300-3. [DOI] [PubMed] [Google Scholar]