Abstract
Objective
Skeletal muscle insulin signaling is a major determinant of muscle growth and glucose homeostasis. Protein kinase B/Akt plays a prominent role in mediating many of the metabolic effects of insulin. Mice and humans harboring systemic loss-of-function mutations in Akt2, the most abundant Akt isoform in metabolic tissues, are glucose intolerant and insulin resistant. Since the skeletal muscle accounts for a significant amount of postprandial glucose disposal, a popular hypothesis in the diabetes field suggests that a reduction in Akt, specifically in skeletal muscle, leads to systemic glucose intolerance and insulin resistance. Despite this common belief, the specific role of skeletal muscle Akt in muscle growth and insulin sensitivity remains undefined.
Methods
We generated multiple mouse models of skeletal muscle Akt deficiency to evaluate the role of muscle Akt signaling in vivo. The effects of these genetic perturbations on muscle mass, glucose homeostasis and insulin sensitivity were assessed using both in vivo and ex vivo assays.
Results
Surprisingly, mice lacking Akt2 alone in skeletal muscle displayed normal skeletal muscle insulin signaling, glucose tolerance, and insulin sensitivity despite a dramatic reduction in phosphorylated Akt. In contrast, deletion of both Akt isoforms (M-AktDKO) prevented downstream signaling and resulted in muscle atrophy. Despite the absence of Akt signaling, in vivo and ex vivo insulin-stimulated glucose uptake were normal in M-AktDKO mice. Similar effects on insulin sensitivity were observed in mice with prolonged deletion (4 weeks) of both skeletal muscle Akt isoforms selectively in adulthood. Conversely, short term deletion (2 weeks) of skeletal muscle specific Akt in adult muscles impaired insulin tolerance paralleling the effect observed by acute pharmacological inhibition of Akt in vitro. Mechanistically, chronic ablation of Akt induced mitochondrial dysfunction and activation of AMPK, which was required for insulin-stimulated glucose uptake in the absence of Akt.
Conclusions
Together, these data indicate that chronic reduction in Akt activity alone in skeletal muscle is not sufficient to induce insulin resistance or prevent glucose uptake in all conditions. Therefore, since insulin-stimulated glucose disposal in skeletal muscle is markedly impaired in insulin-resistant states, we hypothesize that alterations in signaling molecules in addition to skeletal muscle Akt are necessary to perturb glucose tolerance and insulin sensitivity in vivo.
Keywords: Insulin signaling, Glucose uptake, Muscle metabolism, Akt, Glucose homeostasis, Mitochondria dysfunction
Highlights
-
•
Deletion of skeletal muscle Akt2 alone does not reduce downstream insulin signaling or alter glucose homeostasis.
-
•
Inhibition of both skeletal muscle Akt isoforms prevents downstream signaling and results in muscle atrophy.
-
•
Chronic ablation of Akt in skeletal muscle does not block insulin-stimulated glucose uptake in vivo.
-
•
Prolonged Akt deficiency activates AMPK, which is required for insulin-stimulated glucose uptake in muscle lacking Akt.
1. Introduction
Insulin is a potent anabolic hormone that is critical for the systemic regulation of protein, lipid, and glucose metabolism. In response to a meal, insulin promotes muscle growth and leads to a robust increase in skeletal muscle glucose uptake accounting for 30–35% of total glucose disposal. The remaining glucose is taken up by the liver, brain, adipose, and other tissues in lesser amounts. Under hyperinsulinemic-clamp conditions, the role of skeletal muscle is more pronounced, accounting for ∼80% of total glucose disposal [2], [3], [4], [5], [6]. Notably, in insulin-resistant and type II diabetic individuals, there is a significant reduction in insulin-stimulated glucose uptake into skeletal muscle, contributing to feeding-associated hyperglycemia [7]. As a result, there has been considerable interest in defining the downstream signaling mechanisms responsible for coordinating insulin's control of skeletal muscle glucose uptake with the goal of identifying a cellular defect in insulin signaling that is responsible for the reduction of insulin-stimulated glucose uptake in vivo.
Over the last several decades, accumulating evidence has implicated the serine/threonine kinase Akt (protein kinase B) as a central regulator of insulin action [8]. Insulin binds to the insulin receptor resulting in trans-auto phosphorylation and activation of insulin receptor substrate (IRS) proteins. IRS proteins recruit phosphoinositide-3-kinase (PI3K), which phosphorylates PIP2 to PIP3 resulting in downstream activation of Akt. Akt exists in three isoforms in mammals Akt 1–3 (also known as PKB α, β, γ), encoded by three different genes. Akt1 is ubiquitously expressed in all tissues, Akt2 is selectively expressed in metabolic tissues such as muscle, adipose and liver, and Akt3 is enriched in brain and testis [9], [10]. In support of Akt's tissue-specific expression pattern, whole-body knockout mice of Akt1 are growth retarded but have no metabolic abnormalities [11], [12]. Akt2 null mice are glucose intolerant and display systemic insulin resistance [13], [14]. Akt3 knockout mice have reduced brain size but normal glucose homeostasis [10]. Combined deletion of Akt isoforms results in perinatal or early postnatal lethality suggesting some degree of compensation between the Akt isoforms [9]. In addition, deletion of Akt1 in the liver of Akt2 whole-body or liver-specific knockout mice leads to more profound glucose intolerance and insulin resistance then Akt2 alone [15]. Given Akt's essential role in insulin signaling, several groups have reported a defect in insulin-stimulated Akt activity in skeletal muscle from mice and humans with insulin resistance and type II diabetes correlating with a reduction in insulin-stimulated glucose uptake [16], [17], [18], [19], [20], [21], [22], [23], [74]. In support of this observation, Akt2 whole-body knockout mice show a reduction in total glucose disposal and a reduction in skeletal muscle specific glucose uptake at submaximal insulin concentrations [13]. Acute treatment with small molecule inhibitors of PI3K such as wortmannin or Akt inhibitors decrease insulin-stimulated glucose uptake in muscle [24], [25]. Downstream of Akt, AS160 (TBC1D4) and its paralog TBC1D1 have been identified as Akt substrates involved in glucose uptake and Glut-4 translocation [26], [27], [28], [29], [30]. Therefore, a prevailing hypothesis in the field suggests that a reduction in insulin-stimulated Akt activation in skeletal muscle decreases glucose uptake and Glut-4 translocation leading to hyperglycemia and systemic insulin resistance.
Attempts to selectively reduce skeletal muscle insulin signaling have shown no effect on insulin resistance as mice lacking the insulin receptor (IR) and/or insulin like growth factor 1- receptor (IGF1-R) have normal glucose tolerance and insulin sensitivity [31]. Paradoxically, loss of IR/IGF1-R in skeletal muscle upregulates Akt and AMPK activity, which correlates with increased glucose uptake failing to functionally reduce skeletal muscle insulin signaling. As a result, a common hypothesis in the T2DM field, that insulin resistance results from a defect in insulin mediated activation of Akt in skeletal muscle per se, is not yet experimentally tested. To directly assess the role of skeletal muscle Akt on muscle growth and glucose homeostasis, we deleted Akt2, which accounts for ∼90% of the Akt in muscle or both Akt1 and Akt2 specifically in the skeletal muscles of mice from birth or in adulthood. While mice bearing an Akt2 deletion alone show no effect on muscle growth and whole-body glucose metabolism because of the compensatory role of Akt1, congenital deletion of both the Akt isoforms results in a drastic reduction in muscle mass accompanied with a mild defect in glucose tolerance. Surprisingly, despite the complete loss of downstream Akt signaling, M-AktDKO muscle display normal insulin-stimulated glucose uptake and glucose transporter expression that is independent of the canonical Akt-pThr642 AS160 pathway. We performed similar experiments in mice lacking both Akt isoforms in adult skeletal muscle using a tamoxifen inducible system. Here, we demonstrate that Akt has a time-dependent effect on insulin sensitivity as short-term Akt deletion results in insulin resistance, while a more chronic deletion leads to the normalization of insulin sensitivity similar to congenital M-AktDKO mice. Mechanistically, we determined that prolonged loss of Akt results in mitochondrial dysfunction and activation of AMPK, which we show is required for insulin-stimulated glucose uptake in the absence of Akt. Together, these data provide in vivo evidence that Akt is required for insulin/IGF-1-mediated skeletal muscle growth in vivo. Additionally, these data suggest Akt is not an obligate intermediate for insulin-stimulated glucose uptake in skeletal muscle in vivo under all conditions. Lastly, this work uncovered a molecular crosstalk between Akt and AMPK for the non-canonical regulation of skeletal muscle glucose uptake by insulin.
2. Results
2.1. Skeletal muscle Akt1 functionally compensates for the absence of Akt2 to preserve insulin signaling
To examine the specific role of Akt in skeletal muscle growth and function, mice harboring the human skeletal actin (HSA)-CRE recombinase transgene were crossed with mice containing either Akt2loxp/loxp and/or Akt1loxp/loxp;Akt2loxp/loxp to generate mice with skeletal muscle-specific congenital deletion of Akt2 (M-Akt2KO), the predominant Akt expressed in both mouse and human muscle [9], [32], or both Akt1 and Akt2 (M-AktDKO). Combined cardiac and skeletal muscle-specific deletion of Akt1 alone, which accounts for less than 10% of the Akt expressed in skeletal muscle, has been previously reported and does not affect muscle growth or glucose homeostasis [12], [33]. M-Akt2KO and M-AktDKO mice were born at the expected Mendelian ratio. Gastrocnemius and soleus muscles isolated from M-Akt2KO and M-AktDKO male mice were examined for the presence Akt2 protein by western blotting. Akt2 and total Akt protein were significantly reduced in muscle fibers from both M-Akt2KO and M-AktDKO mice confirming that Akt2 accounts for a majority of the Akt expressed in skeletal muscle (Figure 1A). Isolated muscles also contain vascular cells, fibroblasts, blood cells, and satellite cells which are rich in Akt1, suggesting that the low levels of protein that were detected in the M-AktDKO muscles are likely ascribable to the contamination of these other cell types (Figure 1A). Western blot of other metabolic tissues confirmed the specificity of the HSA-Cre transgene as there was no reduction of Akt protein in liver, heart, or adipose tissue in the M-AktDKO mice (Supplementary Figure 1A). Akt3, which is highly expressed in brain tissue, is not detectable in skeletal muscle or upregulated in the absence of Akt1 and Akt2 (Supplemental Figure 1B).
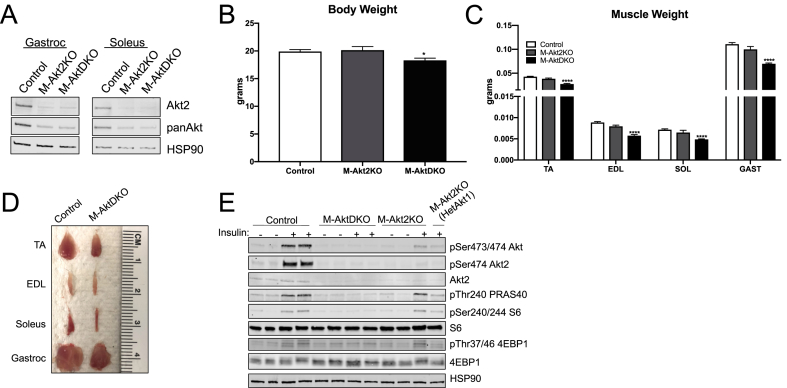
Figure 1.
Skeletal muscle Akt1 functionally compensates in the absence of Akt2 to preserve insulin signaling in skeletal muscles. (A) Western Blot of Akt2 and total Akt in soleus and gastrocnemius muscle from mice with specific deletion of Akt2 and both Akt1 and Akt2. (B) Body weight of control and experimental mice (n = 34 for control, 5 for M-Akt2KO and 12 for M-AktDKO mice). (C) Muscle weight from different muscle depots of control and experimental mice (n = 22 for control, n = 8 for M-Akt2KO and n = 24 for M-AktDKO mice). (D) Representative image of skeletal muscles isolated from control and M-AktDKO mice. (E) Western Blot for phosphorylation of Akt, Akt2, PRAS40, S6, 4EBP1 and HSP90 in Gastrocnemius muscle from control and experimental mice either unstimulated or stimulated with insulin (2 U/kg) for 20 min following an overnight fast. (*p < 0.05, ****p < 0.0001 vs. control).
Surprisingly, M-Akt2KO mice had normal body weight despite the fact that Akt2 accounts for approximately 90% of the total muscle Akt. On the other hand, M-AktDKO mice displayed a significant reduction (∼15%) in body weight (Figure 1B). Next, the mass of several different muscle groups in the hind leg were measured to determine if Akt signaling is required for muscle growth in vivo. M-AktDKO muscle, unlike M-Akt2KO, exhibit ∼40% reduction in the muscle mass from multiple muscle depots (Figure 1C,D). This reduction in muscle mass was accompanied by a significant reduction in the cross-sectional area as measured by WGA staining (Supplemental Figure 2A). Notably, there were no significant differences in tibia length in M-AktDKO mice compared to control (data not shown). Consistent with the data generated in male mice, female M-AktDKO mice have reduced body weight and muscle mass (Supplemental Figures 3A and 3B).
To determine the effects of Akt deletion on the insulin signaling pathway, control, M-Akt2KO, and M-AktDKO mice were injected with insulin and muscles were harvested 20 min later and subjected to western blot analysis. Consistent with normal muscle size, M-Akt2KO mice activated well-known targets of Akt such as PRAS40 and mTORC1 (as determined by S6 and 4EBP1 phosphorylation) in response to a bolus of insulin. These canonical targets of Akt were phosphorylated to a similar extent as in muscle from control animals. This signaling occurred despite a dramatic reduction in pAkt473/474 (an antibody that recognizes all Akt isoform phosphorylation by insulin) and pAkt474 (an Akt2-phospho specific antibody), suggesting residual Akt1 is sufficient to signal to downstream Akt-dependent pathways in the absence of Akt2 (Figure 1E). Consistent with this notion, insulin failed to activate direct Akt substrates including PRAS40, and mTORC1 signaling in M-AktDKO mice despite normal activation of the insulin receptor (Figure 1E, Supplemental Figure 1C). Moreover, M-Akt2KO mice heterozygous for Akt1 led to a ∼50% reduction in downstream signaling as compared to both control and M-Akt2KO mice (Figure 1E). These data indicate that Akt1 functionally compensates in the absence of Akt2, and minimal amounts of phosphorylated Akt are required to activate downstream signaling cascades in skeletal muscle. In addition, deletion of both Akt1 and Akt2 is required to prevent the activation of downstream Akt substrates by insulin in skeletal muscle.
2.2. M-AktDKO mice display modest abnormalities in glucose homeostasis
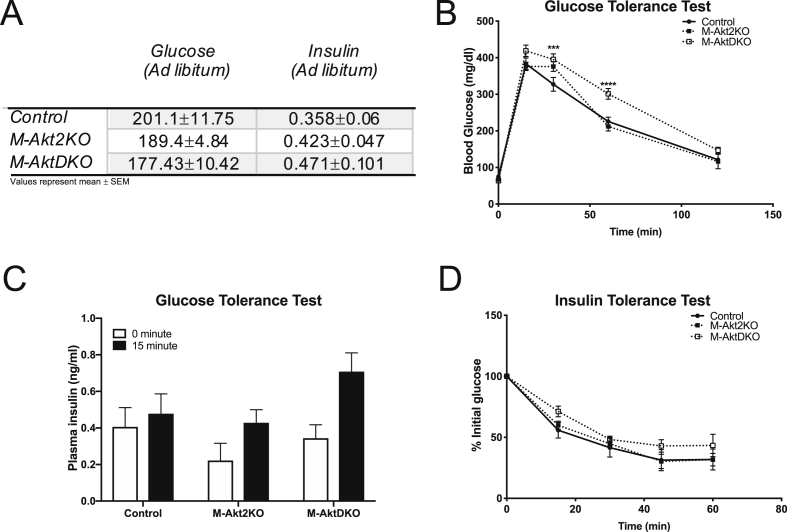
Several groups have reported defects in insulin-stimulated Akt activity in the insulin resistant and diabetic human and rodent skeletal muscle that correlate with a reduction in insulin-stimulated glucose uptake [16], [17], [18], [19], [20], [21], [22], [23], [74]. To determine if loss of Akt signaling specifically in skeletal muscle alters glucose uptake and systemic metabolism, several metabolic measurements in M-Akt2KO and M-AktDKO mice were performed. Consistent with normal insulin signaling in the skeletal muscle of M-Akt2KO mice, M-Akt2KO mice have normal ad libitum blood glucose and insulin levels and exhibit normal glucose tolerance and insulin sensitivity (Figure 2A–D). Despite a dramatic reduction in skeletal muscle insulin signaling in the M-AktDKO mice, M-AktDKO mice have normal glucose and insulin levels (Figure 2A). When subjected to a glucose or insulin tolerance test, M-AktDKO displayed mild glucose intolerance and near normal insulin tolerance (Figure 2B–D). Similar effects on glucose homeostasis were observed in female M-Akt2KO and M-AktDKO mice as compared to male animals (Supplemental Figures 4A and 4B).
Figure 2.
M-AktDKO mice display modest abnormalities in glucose homeostasis. (A) Blood glucose and plasma insulin level (n = 11 for control, 5 for M-Akt2KO and 7 for M-AktDKO mice) in ad libitum state. (B) Intraperitoneal glucose tolerance test (2 g/kg) (n = 17 for control, 6 for M-Akt2KO and n = 11 for M-AktDKO mice). (C) Plasma Insulin level in fasted or 15 min after insulin injection (n = 5–13). (D) Insulin tolerance test (0.75 U/kg) (n = 10 for control, 5 for M-Akt2KO and 7 for M-AktDKO mice) (***p < 0.001, ****p < 0.0001 vs. control).
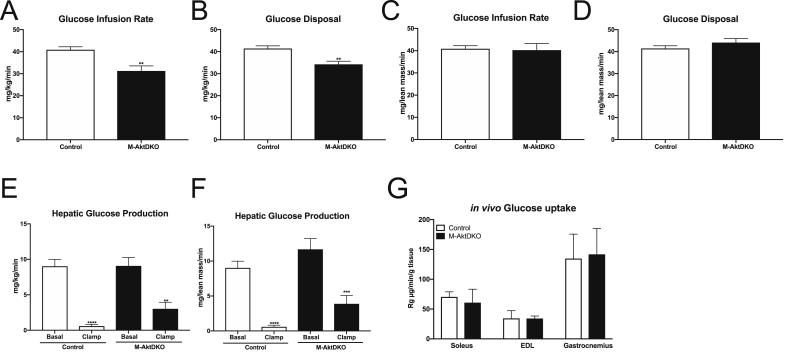
To assess insulin sensitivity directly, hyperinsulinemic-euglycemic clamps in control and M-AktDKO mice were performed. During the hyperinsulinemic portion of the clamp, M-AktDKO mice displayed a small but significant reduction in the glucose infusion rate (Figure 3A) and total insulin-dependent glucose disposal (Figure 3B). When normalized to total lean mass as determined by MRI, no apparent difference in glucose infusion or whole body turnover were observed (Figure 3C,D). In addition, insulin's ability to suppress hepatic glucose production was similar in both control and M-AktDKO mice (Figure 3E,F). Surprisingly, the rate of C14-2-deoxyglucose measured in the gastrocnemius, EDL, and soleus muscles during the hyperinsulinemic-euglycemic portion of the clamp suggested that the rate of glucose uptake per gram of muscle is comparable to control mice in all three skeletal muscle depots examined (Figure 3G). In summary, complete inhibition of skeletal muscle Akt signaling results in modest defects in whole-body glucose disposal as compared to M-Akt2KO mice, which have normal whole-body glucose homeostasis and insulin sensitivity. Moreover, the reduced glucose utilization in M-AktDKO mice is mainly attributable to the reduction in total muscle mass rather than a cell-autonomous defect in skeletal muscle glucose uptake.
Figure 3.
M-AktDKO mice have reduced whole-body glucose turnover and normal insulin-stimulated uptake in skeletal muscle. Hyperinsulinemic-euglycemic clamps were performed in control and M-AktDKO mice following 5 h fasting using a 2.5 mU/kg/min infusion of insulin. (A) Steady state glucose infusion rate, (B) rate of glucose disposal, (C) glucose infusion rate normalized to lean mass, (D) glucose disposal rate normalized to lean mass, (E) hepatic glucose production during basal and insulin portion of the clamp, (F) hepatic glucose production normalized to lean mass, and (G) in vivo glucose uptake in skeletal muscle during hyperinsulinemic-euglycemic clamp (n = 4–9). (**p < 0.01, ***p < 0.001 vs. controls, ****p < 0.0001 vs. control).
2.3. Insulin stimulates muscle glucose uptake in M-AktDKO mice ex vivo
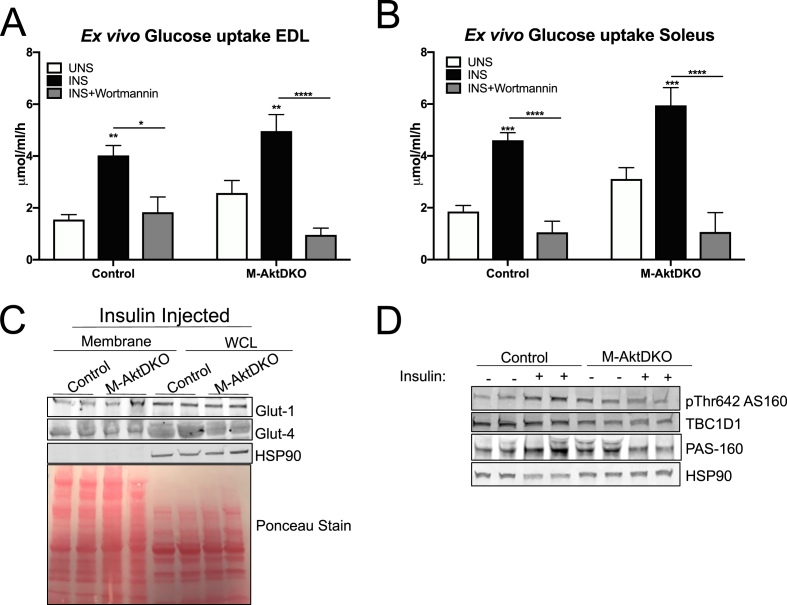
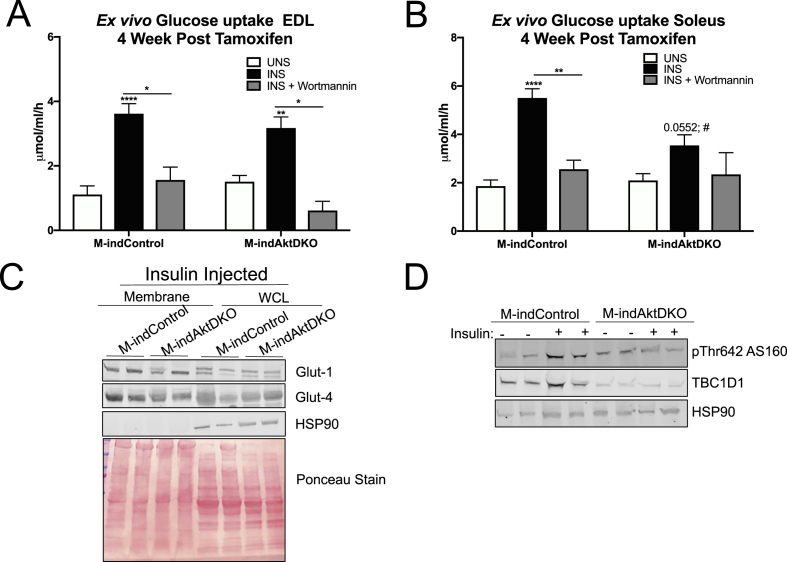
Given the mild effects on glucose homeostasis in M-AktDKO, we further evaluated the cell autonomous effect of insulin on skeletal muscle glucose uptake ex vivo. Consistent with the in vivo findings, insulin-stimulated glucose uptake from both EDL and soleus muscle from M-AktDKO were similar to control muscle (Figure 4A,B). Notably, this effect was dependent on intact PI3K signaling, as pretreatment with wortmannin completely prevented insulin's ability to stimulate glucose uptake in both the EDL and soleus muscles isolated from control and M-AktDKO mice (Figure 4A,B). This induction of glucose uptake by insulin was associated with normal levels of the glucose transporters, Glut-1 and Glut-4, as biochemical analysis indicated similar Glut-1 and Glut-4 protein levels in whole cell lysates and plasma membrane fractions from muscle of insulin-injected control and M-AktDKO mice (Figure 4C; Supplemental Figures 5A–C). Additionally, no significant change in either triglyceride or glycogen levels in ad libitum fed state in quadricep muscles of M-AktDKO were observed (Supplemental Figures 6A and 6B).
Figure 4.
Skeletal muscle from M-AktDKO mice exhibit normal PI3K-dependent insulin stimulated glucose uptake. (A–B) Ex vivo insulin-stimulated glucose uptake was measured in EDL (A) or soleus (B) from control and M-AktDKO mice with/without pretreatment of wortmannin (1 μM) (n = 7–13). (C) Western blot of Glut1 and Glut4 protein from plasma membrane fraction of hind limb and whole cell Lysate (WCL) of gastrocnemius muscles from control and M-AktDKO mice stimulated with insulin (2 U/kg) for 20 min. (D) Western Blot analysis of phosphorylated AS160, PAS-160 and TBC1D1 in gastrocnemius muscle from control and M-AktDKO mice fasted overnight and treated with BSA or insulin (2 U/kg) for 20 min (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control).
To gain further mechanistic insight into the regulation of glucose uptake, we investigated the phosphorylation and expression status of AS160 (TBC1D4) and TBC1D1, both of which are implicated in the regulation of Glut-4 translocation by insulin in muscle and adipose tissue [29]. In control muscles, insulin treatment stimulated phosphorylation of the 160 kDa band recognized by a phospho-Akt substrate antibody (PAS-160) and Thr642 of AS160 (Thr649 in mouse), a well-described Akt substrate known to regulate Glut-4 translocation (Figure 4D; Supplemental Figures 5D and 5E). These effects of insulin on PAS-160 and pThr 642 AS160 were not observed in M-AktDKO, consistent with AS160 being a direct phosphorylation substrate of Akt (Figure 4D; Supplemental Figures 5D and 5E). In contrast, the protein expression of AS160's paralog, TBC1D1, was significantly reduced in M-AktDKO mice independent of insulin treatment, an effect also observed in the IR/IGF1-R skeletal muscle double knockout mice (Figure 4D; Supplemental Figure 5F) [31]. Collectively, these genetic loss-of-function experiments indicate that Akt signaling in skeletal muscle is not an obligate intermediate for insulin-stimulated glucose uptake in vivo under all conditions. Moreover, these data suggest the functional contribution of a PI3K-dependent, Akt-independent pathway for the regulation of skeletal muscle glucose uptake and Glut-4 translocation by insulin.
2.4. Inducible deletion of Akt in adult muscle leads to time dependent effects on insulin sensitivity
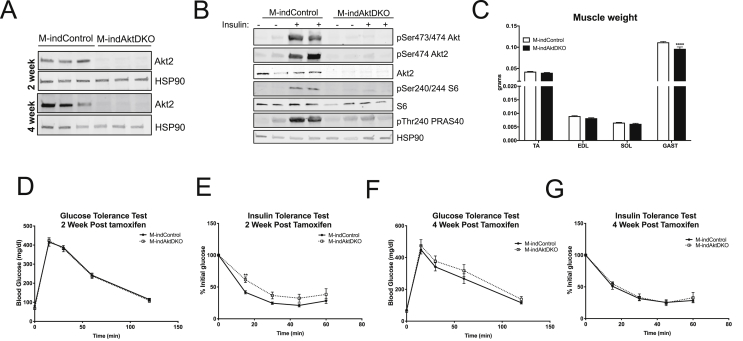
The ability of insulin to stimulate glucose uptake and induce Glut-4 translocation in M-AktDKO mice seems contradictory to the published studies that demonstrate an inhibitory effect of acute, small molecule inhibition of Akt on insulin-stimulated glucose uptake in skeletal muscle [24], [34]. One explanation for this apparent discrepancy between acute inhibition vs long-term genetic deletion of Akt could be the presence of a developmental compensatory pathway that is upregulated in the absence of Akt. To address this possibility directly, we generated an inducible model of total Akt deficient skeletal muscle by crossing mice that contain Akt1loxp/loxp;Akt2loxp/loxp to mice containing the CRE recombinase-estrogen receptor fusion protein under the control of the human skeletal actin promoter (HSA-ESR-CRE) to induce an acute deletion of Akt1 and Akt2 (M-indAktDKO) in adult skeletal muscle [35]. Adult 8–12 week old mice containing floxed Akt alleles lacking the HSA-ESR-CRE transgene (M-indControl) or containing the transgene (inducible knockouts) were injected for 5 consecutive days with tamoxifen and experiments were performed 2 and 4 weeks later, following the end of tamoxifen injection. Western blot analysis in quadricep muscles from M-indAktDKO male mice examined at 2 week and 4 week post tamoxifen demonstrates complete loss of Akt2 at both these time points (Figure 5A). Further, similar to M-AktDKO mice, inducible deletion of both Akt isoforms in adult skeletal muscle prevented the activation of canonical Akt targets such PRAS40 and mTORC1 in response to insulin (Figure 5B). In addition, M-indAktDKO have a small but significant reduction in the gastrocnemius muscle mass as compared to the normal weight of the TA, EDL, and soleus muscles (Figure 5C).
Figure 5.
Inducible deletion of Akt in adult skeletal muscle have time responsive effect on glucose tolerance and insulin sensitivity. (A) Western Blot for Akt2 in Quadriceps of M-indAktKO 2 and 4 week post tamoxifen. (B) Western blot for phosphorylation of Akt, Akt2, pPRAS40, phosphorylation of S6, S6 and HSP90 in Gastrocnemius muscle from M-indControl and M-indAktDKO animals either unstimulated or stimulated with insulin (2 U/kg) for 20 min after overnight fasting. (C) Muscle weight from different muscle depots of M-indControl and M-indAktDKO mice (n = 50–60 for M-indControl and n = 20–24 for M-indAktDKO mice). (D–E) Intraperitoneal glucose tolerance test (2 g/kg) (n = 22 for M-indControl and n = 20 for M-indAktDKO) and Insulin tolerance test (0.75 U/kg) (n = 22 for M-indControl and n = 19 for M-indAktDKO) 2 week post tamoxifen. (F–G) Intraperitoneal glucose tolerance test (2 g/kg) (n = 11 for M-indControl and n = 7 for M-indAktDKO mice) and Insulin tolerance test (0.75 U/kg) (n = 11 for M-indControl n = 8 for M-indAktDKO mice) 4 week post tamoxifen. (**p < 0.01, ****p < 0.0001 vs. control).
Given the inducible nature of this mouse model, the temporal effect of Akt deletion on insulin sensitivity can be monitored over time. Two weeks post tamoxifen injections, M-indAktDKO mice display normal glucose tolerance with a significant reduction in insulin sensitivity as measured by an insulin tolerance test (Figure 5D,E). However, at 4 weeks post tamoxifen, M-indAktDKO displayed normal rates of glucose and insulin tolerance (Figure 5F,G) suggesting a more prolonged inhibition of Akt in adult skeletal muscle does not affect insulin sensitivity, mimicking the insulin sensitivity of the congenital deletion of Akt. Next, we determined if this inducible deletion of Akt (4 weeks post tamoxifen) in adult skeletal muscle affects skeletal muscle glucose uptake by insulin. Similar to the congenital M-AktDKO mice, insulin stimulated glucose uptake in a PI3K-dependent manner in both control and M-indAktDKO mice in the EDL muscle (Figure 6A). In soleus muscles, glucose uptake trended to increase in response to insulin in M-indAktDKO; however, this was significantly reduced compared to control mice (Figure 6B). Consistent with congenital knockout of Akt, the levels of glucose transporters at the plasma membrane and in whole cell lysates from the muscle of insulin-injected mice were normal in M-indAktDKO (Figure 6C; Supplemental Figures 7A–C) with a non-significant trend for 50% higher glycogen levels in quadriceps muscles from M-indAktDKO mice 4-week post tamoxifen (Supplemental Figure 8). In addition, the effect on glucose transport was independent of Thr642 (Thr649 in mouse) AS160, as this site was only induced in control mice by insulin (Figure 6D; Supplemental Figure 7D). On the other hand, there was a significant reduction in TBC1D1 protein content in M-indAktDKO mice (Figure 6D; Supplemental Figure 7E), recapitulating the effects of congenital deletion of Akt. Collectively, these data indicate relatively acute deletion of Akt in skeletal muscle results in abnormalities in insulin tolerance while a more chronic inhibition of Akt, either from birth or in adulthood, has minimal effects on systemic insulin sensitivity and skeletal muscle glucose uptake.
Figure 6.
M-indAktDKO mice display a fiber type specific effect on glucose uptake. (A–B) Ex vivo insulin-stimulated 2-deoxyglucose uptake in EDL (A) or soleus (B) from M-indControl and M-indAktDKO mice with/without pretreatment of wortmannin (1 μM) (n = 20 for control and insulin, n = 4 for wortmannin). (C) Western blot of Glut1 and Glut4 protein and HSP90 in Plasma membrane fraction of hind limb and whole cell lysate of gastrocnemius muscles from M-indControl and M-indAktDKO mice stimulated with insulin (2 U/kg) for 20 min after overnight fast. (D) Western Blot analysis of phosphorylated AS160, TBC1D1 and HSP90 in gastrocnemius muscle from M-indControl and M-indAktDKO mice fasted overnight and treated with BSA or insulin (2 U/kg) for 20 min (*p < 0.05, **p < 0.01, ****p < 0.0001 vs. control; #p < 0.05 vs control insulin injected).
2.5. Chronic deletion of Akt induces mitochondrial dysfunction and activates AMPK, which is required for insulin-stimulated glucose uptake
AMP activated protein kinase (AMPK) is a sensor of cellular energy state and is activated under conditions of reduced mitochondrial energy production [36]. AMPK is known to be an important regulator of glucose transport and utilization in the contracting muscle [37], [38], [39], [40]. Similar to Akt, a reduction in AMPK activity is observed in skeletal muscle from insulin-resistance humans and rodents [36], [41], [42], [43], [44]. Classic studies suggest that the AMPK pathway for glucose uptake is independent of the canonical insulin signaling pathway in muscle, as contraction and/or AICAR treatment (both potent activators of glucose transport) do not stimulate Akt activity [24], [25], [38], [45]. However, activators of AMPK such as exercise and AICAR are known to potentiate insulin-stimulated glucose uptake in skeletal muscle [46], [47], [48]. In addition, inhibition of AMPK with compound C can prevent insulin-stimulated glucose uptake under conditions of cellular stress in muscle cells [49], [50], [51].
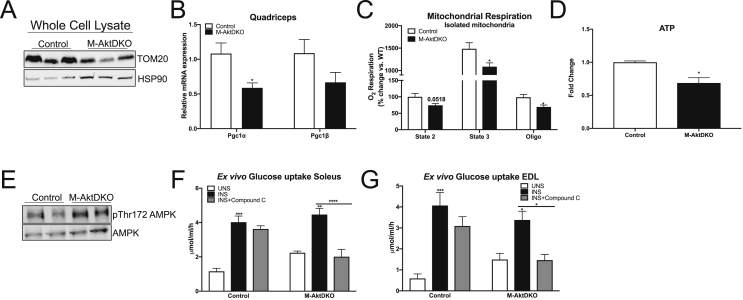
To determine if the activation of AMPK was contributing to the regulation of glucose uptake by insulin in the absence of Akt, we assessed the quantity and function of mitochondria following chronic Akt ablation in muscle. TOM20 protein content was reduced in whole cell lysates from the gastrocnemius muscle of M-AktDKO mice, suggesting reduction in total mitochondrial content (Figure 7A; Supplemental Figure 9A). Additionally, PGC1alpha, a known transcriptional co-activator involved in mitochondrial biogenesis, was significantly reduced in muscle from M-AktDKO mice (Figure 7B). To determine if mitochondrial function was also reduced independent of content, we purified mitochondria from control and M-AktDKO mice and measured oxygen consumption utilizing a high-capacity respirometer. A significant reduction in the complex I-dependent mitochondrial respiration (State 2/3) rate was observed in mitochondria of M-AktDKO mice as compared to control (Figure 7C). These functional changes resulted in a reduction in total levels of ATP in the skeletal muscle of M-AktDKO mice (Figure 7D). These changes in cellular energetics coincided with the activation of AMPK in M-AktDKO mice, as confirmed by an increase in the phosphorylation status of Thr 172 (Figure 7E; Supplemental Figure 9B). Consistent with a functional increase in AMPK activity, pretreatment with a AMPK inhibitor, compound C, prevented insulin-stimulated glucose uptake in M-AktDKO mice but not in control EDL and soleus muscles ex vivo (Figure 7F,G). The absence of an effect of compound C in control muscle is consistent with previous reports in isolated muscle [38]. Given the known off-target effect of compound C to inhibit other kinases [52], we performed additional ex vivo glucose uptake assays in muscles from control and M-AktDKO using a recently described AMPK inhibitor, SBI-0206965, which has improved selectively and potency toward AMPK compared to compound C [53]. Consistent with our compound C data, SBI-0206965 was found to suppress insulin-stimulated glucose uptake specifically in M-AktDKO muscles without affecting control muscles (Supplementary Figure 9C). Collectively, these data support the notion that prolonged ablation of Akt activates AMPK, which in turn becomes an important determinant of insulin-stimulated glucose uptake in skeletal muscle.
Figure 7.
Akt deletion induces mitochondrial dysfunction and activates AMPK, which is required for insulin-stimulated glucose uptake. (A) Western blot analysis for TOM20 and HSP90 from whole cell lysates of gastrocnemius muscle. (B) mRNA expression from quadricep muscle (n = 7 control and n = 7 M-AktDKO). (C) Oroborus high-capacity respirometer analysis of isolated mitochondria from control and M-AktDKO gastrocnemius muscle (n = 7 control and n = 7 M-AktDKO). (D) relative ATP levels in gastrocnemius muscle of control and M-AktDKO mice (n = 4 control and n = 5 M-AktDKO). (E) Western blot analysis of phosphorylated AMPK and AMPK from control and M-AktDKO gastrocnemius muscle. (F–G) Ex vivo insulin-stimulated 2-deoxyglucose uptake in soleus (F) or EDL (G) from control and M-AktDKO mice with/without pretreatment of compound C (20 μM) (n = 6 for control, 7 for insulin and 7 for compound C). (*p < 0.05, **p < 0.01, ***p < 0.001****p < 0.0001 vs. control).
3. Discussion
In humans, skeletal muscle is the primary site of insulin-dependent glucose disposal; therefore, resistance of skeletal muscle to insulin action is considered an early step in the development of type 2 diabetes [7], [54]. Mechanistically, several studies have reported defects in insulin signaling via Akt in skeletal muscle during the progression of metabolic disease and have correlated these defects with a failure of insulin to stimulate glucose uptake and glycogen synthesis (reviewed in [54]). Despite this popular belief, there is no in vivo evidence to support the causal role of reduced skeletal muscle insulin signaling via Akt to the development of systemic glucose intolerance. Mice with skeletal muscle specific deletion of the IR and/or IGF1-R have normal glucose tolerance and insulin sensitivity [31]. The potential explanation for this discrepancy was hyper-activation of Akt and normal activation of Akt substrates (due to significant upregulation of Akt protein content) which correlated to an increased basal and insulin-stimulated glucose uptake. Although reducing IR/IGF1-R clearly prevents direct insulin binding in skeletal muscle, downstream signaling via Akt is still active in this model. In addition, knockout of the insulin receptor substrates proteins in muscle using the muscle creatine kinase (MCK)-CRE results in lethality at 2–3 weeks of age due to cardiac abnormalities precluding detailed assessment of glucose homeostasis without the complication of IRS deletion in the heart [55]. Lastly, inhibition of class I PI3K's regulatory subunit p85, reduces Akt activity and glucose uptake; however, these mice lack the minor isoforms of p85 throughout the whole body and all p85 isoforms in heart since the MCK-Cre was used [56]. Therefore, in the present study, we directly tested the common hypothesis that inhibition of insulin signaling via Akt in skeletal muscle per se causes systemic metabolic dysfunction. Our data demonstrate that a significant reduction in phosphorylated Akt in muscle alone does not affect whole-body metabolism as M-Akt2KO exhibit normal glucose tolerance and insulin sensitivity. We also made the unexpected observation that insulin can stimulate glucose uptake in the absence of Akt, suggesting Akt is not an obligate intermediate in insulin's actions to control glucose uptake in vivo under all conditions. This is inconsistent with the prevailing molecular model of insulin-regulated glucose transport and the molecular basis for insulin resistance.
Although ∼90% of the Akt protein in skeletal muscle is represented by the Akt2 isoform, M-Akt2KO mice are indistinguishable from control mice both in terms of growth and metabolism (Figure 1, Figure 2). The robust activation of all the well-known Akt targets in spite of the dramatic reduction in pAkt protein in these mice clearly indicates the capacity of residual Akt1 to compensate under these conditions. This suggests only a small fraction of total muscle Akt phosphorylation is required to convey skeletal muscle insulin signaling in vivo. This observation is consistent with data in L6 myotubes where insulin had a maximal effect on Glut-4 translocation at doses where ~10% of Akt was phosphorylated supporting the notion that only a small fraction of Akt needs to be phosphorylated for its substrates to be maximally activated [75], [76]. In contrast, M-AktDKO mice exhibit a reduction in body mass and a further reduction in muscle mass (Figure 1) similar to the phenotype observed in MIGIRKO mice (combined deletion of IR and IGF-1R in skeletal muscle) [31], [57]. Moreover, M-AktDKO mice display mild glucose intolerance and a reduction in whole-body glucose turnover despite normal insulin-stimulated glucose uptake and glucose transporter expression in skeletal muscle. This effect is recapitulated in mice lacking Akt in the adult muscle for 4 weeks, as M-indAktDKO mice have normal insulin sensitivity in vivo in addition to normal insulin stimulated glucose uptake in EDL muscle (Figure 5, Figure 6). Of note, short-term inhibition of Akt in adult muscle resulted in insulin resistance as assessed by insulin tolerance test (Figure 5), which is consistent with the acute effects of small molecule Akt inhibitors on insulin-stimulated glucose uptake [24]. Collectively, these data indicate that Akt has a time-dependent effect on muscle insulin sensitivity such that acute inhibition of Akt signaling either pharmacological or genetically is necessary for insulin sensitivity, while a more chronic reduction of Akt activity leads to the activation of a non-canonical pathway for insulin-stimulated glucose uptake in Akt-deficient skeletal muscle.
It is worth noting that despite normal insulin sensitivity in vivo, M-indAktDKO mice have a partial inhibition of glucose uptake in the soleus but not EDL muscle ex vivo. This is dissimilar to M-AktDKO mice, which display normal insulin-responsive glucose uptake regardless of muscle depot. The reason for this fiber type specific effect in the M-indAktDKO mice is unknown. Analysis from mixed muscle of M-indAktDKO mice indicate normal levels of Glut-1 and Glut-4 at the plasma membrane and in whole cell lysates of insulin injected mice (Figure 6C). In addition, total levels of Glut-4 were indistinguishable in the soleus muscle from control and M-indAktDKO mice (Supplemental Figure 7). However, we cannot exclude the possibility that in the soleus muscle of M-indAktDKO mice there is a specific requirement for Akt for Glut-4 translocation that may contribute to this partial decrease in glucose uptake. Nevertheless, this modest reduction in glucose uptake in the soleus is not sufficient to alter systemic glucose or insulin tolerance.
AMPK is a heterotrimeric serine/threonine kinase, activated in response to low cellular energy and physical exercise. This increase in AMPK activity is suggested to regulate glucose uptake in response to exercise in an insulin-independent manner [36]. However, several lines of evidence imply that prior AMPK activation regulates skeletal muscle insulin sensitivity independently of changes in proximal insulin Akt signaling [46], [58]. Therefore, we hypothesized that AMPK activity is required for insulin-stimulated glucose uptake independent of the canonical Akt-AS160 pathway. Our data reveal that chronic ablation of Akt induces mitochondrial dysfunction and subsequent activation of AMPK. In the absence of Akt, AMPK becomes a dominant regulator of insulin sensitivity in skeletal muscle. This is supported by the fact that acute inhibition of AMPK with compound C or SBI-0206965 selectively prevented insulin-stimulated glucose uptake in M-AktDKO but not in control muscles (Figure 7 and Supplemental Figure 9C). Notably, a series of physiological studies have also demonstrated the role of glycogen availability on AMPK activity [59], [60], [61]. However, since glycogen levels were not reduced in either M-AktDKO or M-indAktDKO mice (Supplemental Figures 6 and 8), we believe that enhanced AMPK activity in M-AktDKO muscles is a result of mitochondrial dysfunction rather than glycogen depletion.
Activation of Akt and AMPK cause redistribution of the insulin-sensitive glucose transporter isoform Glut-4 from intracellular storage vesicles to the plasma membrane. The canonical model suggests that Akt serves as an essential signaling molecule to regulate the process by phosphorylating and inhibiting the Rab-GTPase-activating protein, AS160, triggering the activation of Rab small GTPases that are required for the translocation of Glut-4 to the plasma membrane [29]. Similarly, Akt phosphorylates TBC1D1 (paralog of AS160), promoting Glut-4 translocation to the plasma membrane [62]. Phosphorylation of AS160 is important for the trafficking of Glut-4 to the plasma membrane as mice with a AS160/TBC1D4-Thr649Ala knock-in mutation show a partial inhibition of glucose uptake in skeletal muscle yet normal rates of glucose uptake in adipose tissue [28]. In both the congenital and inducible models of Akt deficiency, pThr642 AS160 is not phosphorylated by insulin in the absence of Akt (Figure 4, Figure 6D). Despite a lack of pThr642 phosphorylation, insulin was capable of stimulating glucose uptake and this correlated with normal amounts of Glut-4 expression and localization at the plasma membrane (Figure 4, Figure 6). On the other hand, both M-AktDKO and M-indAktDKO mice exhibit a significant reduction in the protein expression of TBC1D1, which is a negative regulator of Glut-4 translocation (Figure 4, Figure 6D) [31]. Therefore, this reduction in TBC1D1 may contribute to increased glucose uptake in M-AktDKO; however, this reduction in total TBC1D1 protein alone is unlikely to be the only mechanism since we do not see further reduction TBC1D1 protein content upon insulin stimulation in Akt deficient muscles. In addition to the Akt-dependent sites on AS160, AMPK activity is required for the phosphorylation of AS160 at Ser 711 in response to insulin and Ser 231 of TBC1D1 in response to contraction [36]. Mechanistically, activation of AMPK by prior contraction enhances insulin sensitivity in part through Ser711 phosphorylation on AS160 [46]. Future studies will be focused on defining the role of additional known and unknown phosphorylation sites on AS160 and TBC1D1 to define the distal mechanisms linking this non-canonical activation of AMPK following chronic Akt deficiency to the stimulation of glucose uptake and Glut-4 translocation by insulin.
In addition to the Rab GTPase-activating proteins AS160/TBC1D1, the Rho GTPases, in particular Rac1, are involved in insulin-stimulated glucose uptake and Glut-4 translocation in skeletal muscle [63]. Rac1 is activated by insulin in a PI3K-dependent manner [64]. Several studies have reported both pharmacological inhibition and genetic deletion of Rac1 attenuates insulin-stimulated Glut-4 translocation and glucose uptake in response to insulin [64], [65], [66]. Mechanistically, Rac1 is thought to be involved in insulin-stimulated actin remodeling via PAK-dependent and independent pathways [67], [68]. Work from Sylow and Klip et al. have suggested that Rac1 and Akt mediate insulin-stimulated glucose uptake via parallel signaling mechanisms, as inhibition of Rac1 inhibits glucose uptake without affecting Akt activity [64], [66]. Sylow et al. report that inhibition of Akt with MK2206 prevents insulin-stimulated Rac1 activation in both EDL and soleus muscle and combined chemical inhibition of Akt and Rac1 had an additive effect on reducing insulin-stimulated glucose uptake [64]. However, when investigating the Akt-Rac1 interplay in the insulin-resistant whole-body Akt2 knockout mice, the authors report a tissue specific effect on Rac1 activity by Akt, as insulin fails to stimulate Rac1 in the EDL but not soleus muscles from Akt2 knockout mice [64]. In addition, acute knockdown experiments for Akt2 performed by the Satoh group have suggested Rac1 lies downstream of the PI3K-Akt signaling in the regulation of glucose uptake by insulin in gastrocnemius muscle [69], [70], [71]. Therefore, the molecular hierarchy and potential interplay between Akt and Rac1 in skeletal muscle remain ill-defined. It will be of interest to further define the functional role for Rac1 signaling acting downstream or in parallel to Akt and AMPK in the regulation of insulin-stimulated glucose uptake in both normal and Akt-deficient muscle.
Collectively, the present study confirms that Akt signaling is required for the anabolic effects of insulin on muscle growth in vivo. In addition, we demonstrate that following chronic Akt ablation, AMPK activity is induced in response to energetic stress, which serves as an important regulator of insulin sensitivity in skeletal muscle. Since insulin-stimulated glucose disposal in skeletal muscle is markedly impaired in insulin-resistant states, these data imply that alterations in Akt signaling alone are not sufficient to inhibit insulin-stimulated glucose uptake in skeletal muscle in vivo. The cellular crosstalk between Akt and AMPK in the regulation of muscle insulin sensitivity is of significant interest and future work will be aimed at defining its role in the physiological responses to insulin in skeletal muscle during normal and insulin-resistant conditions.
3.1. Experimental procedures
3.1.1. Mice
The Akt2loxP/loxP mice and Akt1loxP/loxP, Akt2loxP/loxP were previously described [15]. Floxed mice were crossed with mice carrying the Cre recombinase driven by a skeletal muscle actin promoter, ACTA1-Cre (Jackson Laboratory, stock number 006149) to generate knockout mice and littermate controls that lack the HSA transgene. Male mice between the age of 8–12 weeks were used for all experiments, except for those as indicated. For skeletal muscle Akt deficiency in adult mice, floxed mice were crossed to mice containing the Cre recombinase-estrogen receptor fusion protein under the control of human ACTA-1 (HSA-ESR-CRE) (Jackson Laboratory, stock number 031934), allowing tamoxifen-inducible Cre-mediated recombination. Floxed AKT mice (8–12 weeks of age) for the indicated genotype that lack the HSA-ESR-CRE transgene (M-indControl) or mice positive for the transgene (M-indAktDKO) were injected with tamoxifen IP (100 mg/kg) for 5 consecutives days to induce knockout. Experiments were performed 2 and 4 weeks later following the 5th day of tamoxifen. All mice experiments were reviewed and approved by the University of Pennsylvania IACUC in accordance with NIH guidelines.
3.1.2. Metabolic measurement
Ad libitum fed mice were used for measurements of blood glucose using a glucometer (OneTouch Ultra, Lifescan). Insulin measurements (Ultra-Sensitive Rat Insulin ELISA kit, Crystal Chem, Inc.) were performed on plasma collected from mice ad libitum fed or after an overnight fast and 15 min following a glucose injection.
3.1.3. Muscle lysate and western blotting
Muscles frozen in liquid nitrogen were powdered on dry ice, then transferred to cold RIPA buffer supplemented with protease inhibitor cocktail tablets (Roche), and phosphatase inhibitor cocktail I and II (Sigma). Cell lysates were homogenized using tissue homogenizer (Fisherbrand™ 150), incubated for 10 min on ice and centrifuged at 13,600 g for 30 min at 4 °C. Cleared lysates were then used to determine total protein levels (BCA Protein Assay, Pierce). After dilution with sample buffer, equal protein amounts were loaded onto SDS gels. The antibodies used for blotting are as follows: From Cell Signaling: Phospho-Akt (Ser473/474), pAMPK (Thr 172), AMPK, Akt (pan), Phospho-Akt2 (Ser474), P-(S/T) Akt Substrate, Akt2, Akt3, S6 Ribosomal Protein, Phospho-S6 Ribosomal Protein (Ser240/244), Phospho-PRAS40 (Thr 246), Phospho-4E-BP1 (Thr37/46), Phospho-Insulin Receptor (Tyr1150/1151), TOM20, Glut-4, Insulin receptor, Phospho-AS160 (Thr642), AS160, HSP90; From Millipore: Glut-4; From Abcam: Glut-1.
3.1.4. mRNA isolation and real time PCR
Total RNA was isolated from the frozen muscles using the RNeasy Plus kit from Qiagen. cDNA was generated using M–MuLV reverse transcriptase and quantitated for the relative expression of genes of interest by real time PCR using the SYBR Green Dye–based assay.
3.1.5. Glucose tolerance and insulin tolerance test
For glucose tolerance test, mice were fasted overnight and injected with glucose IP (2 g/kg). Blood glucose was monitored by tail bleeding at 0, 15, 30, 60, 120 min after glucose injection. For insulin tolerance test, mice were fasted for 5 h then injected IP with insulin (0.75 U/Kg body weight). Blood glucose levels were monitored by tail bleeding at 0, 15, 30, 45 and 60 min.
3.1.6. Hyperinsulinemic–euglycemic clamp
Hyperinsulinemic–euglycemic clamps were performed at the Mouse Phenotyping, Physiology and Metabolism Core of the Diabetes Research Center at the University of Pennsylvania School of Medicine as previously described [72]. Human insulin was infused at the rate of 2.5 mU kg/min and blood glucose was maintained between 120 mg/dL and 140 mg/dL by infusing 20% glucose at various rate. For in vivo glucose uptake, mice were administered [14C]2-deoxyglucose during the final 40 min of the hyperinsulinemic-euglycemic clamp and muscles were harvested to determine the [14C] levels incorporated.
3.1.7. Ex vivo muscle glucose uptake
Glucose uptake was measured in EDL and soleus strips as previously described [25]. Briefly, mice were fasted for 5 h following which EDL and soleus muscles were incubated with resting tension in the KHB buffer containing 0.1% bovine serum albumin (BSA), 32 mM mannitol, and 8 mM glucose with continuous gas supply (95% O2/5% CO2). For inhibitor experiments, 1 μM wortmannin, 20 μM of compound C, or 1 μM SBI-0206965 were added to the incubation media for the last 30 min of pre-incubation. Following pre-incubation, muscles were incubated under basal (BSA) or insulin-stimulated (15 mU/ml) for 15 min with the addition of [3H]-2-deoxyglucose (2DG) for the last 20 min. Following the wash step in the incubation media, muscles were finally frozen in liquid nitrogen and were used for measurement of 2DG transport.
3.1.8. Membrane fractionation
Plasma membrane fractionation of skeletal muscle was performed using previously described technique [73]. Briefly, hindlimb muscles were dissected, combined and minced in ice cold PBS with 10 mM EDTA and 0.05% trypsin for 5 min, washed 3 times with PBS plus 10 mM EDTA and then homogenized in M1 buffer (0.25 M Sucrose, 1 mM EDTA, 10 mM Tris–HCl (pH 7.4), with protease (Roche) and phosphatase inhibitors (Sigma) using a tissue grinder (Thomas Scientific). Homogenate was centrifuged at 1000 ×g to remove nuclei and unbroken cells and then at 16,000 ×g to pellet mitochondria and plasma membranes (F1). F1 was then resuspended in 0.5 mL of M1 buffer and loaded on 5–25% linear Ficoll gradient and centrifuged for 30 min at 28,000 rpm (SW. 55 rotor). The upper band containing the plasma membrane was removed, diluted and washed 3 times with M1 buffer and re-centrifuged at 16,000.
3.1.9. WGA staining
Muscles frozen in tissue freezing O.C.T. Compound (Electron Microscopy Sciences) in liquid nitrogen-cooled isopentane were cut into 10 mm cross-sections. Cross-sections were blocked in 4% BSA/PBS for 60 min, and incubated with WGA for 30 min at 37 °C. Slides were closed with Prolong without DAPI.
3.1.10. Mitochondrial respiration
Isolated mitochondria (150 μg) from gastrocnemius muscles were resuspended in MiR05 respiration medium (110 mM mannitol, 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 60 mM K lactobionate, 0.3 mM DTT, and 0.1% BSA [fatty acid free], adjusted to pH of 7.1 with KOH) and oxygen consumption was measured using an Oroboros Oxygraph-2k high-resolution respirometer. Real-time mitochondrial oxygen consumption rates were collected at 37 °C with constant stirring. To measure state 2 respiration, 10 mM pyruvate and 5 mM malate was added. For state 3 respiration, 2 mM ADP was added. To inhibit complex V after state 3 respiration, 1 μg/ml Oligomycin was added.
3.1.11. ATP measurement
ATP levels were measured by the Metabolomics Core of the Diabetes Research Center at the University of Pennsylvania as previously described [72]. Briefly, frozen tissue samples were weighed and ground at liquid nitrogen temperature with a Cryomill (Retsch, Newtown, PA); samples were then extracted by adding 40:40:20 acetnitrile:methanol:water at −20 °C with 0.5% formic acid to the powder and incubated at −20 °C for 10 min, followed by addition of 15% NH4HCO3 in H2O and centrifugation at 16,000 ×g for 30 min at 4 °C. The supernatant was transferred to another tube and centrifuged at 16,000 ×g for 30 min at 4 °C. The supernatant was taken for LC-MS analysis. LC separation was achieved using a Vanquish UHPLC system (Thermo Fisher Scientific, San Jose, CA) and an Xbridge BEH Amide column (150 × 2 mm, 2.5 μm particle size; Waters, Milford, MA) using two different solvents, Solvent A consisting of water: acetonitrile (95:5) with 20 mM ammonium acetate and 20 mM ammonium hydroxide at pH 9.4, and solvent B as acetonitrile to form the gradient at 0 min, 90% B; 2 min, 90% B; 3 min, 75% B; 7 min, 75% B; 8 min, 70% B, 9 min, 70% B; 10 min, 50% B; 12 min, 50% B; 13 min, 25% B; 14 min, 25% B; 16 min, 0% B, 21 min, 0% B; 21.5 min, 90% B; 25 min, 90% B and was allowed to run for 25 min at a flow rate of 150 μL/min. For all experiments, 5 μL of extract was injected with column temperature at 25 °C. The Q-Exactive Plus mass spectrometer was operated in both negative and positive mode scanning m/z 70–1000 with a resolution of 140,000 at m/z 200. MS parameters were as follows: sheath gas flow rate, 28 (arbitrary units); aux gas flow rate, 10 (arbitrary units); sweep gas flow rate, 1 (arbitrary unit); spray voltage, 3.3 kV; capillary temperature, 320 °C; S-lens RF level, 65; AGC target, 3E6 and maximum injection time, 500 ms. Data analyses were performed using MAVEN software which allowed for sample alignment, feature extraction and peak picking. Extracted ion chromatogram for each metabolite was manually examined to obtain its signal, using a custom-made metabolite library.
3.1.12. Muscle glycogen analysis
Quadricep muscles from ad libitum fed mice were ground in liquid nitrogen and total glycogen was extracted from 100 mg of muscle by digesting tissue samples in KOH followed by digestion with amyloglucosidase at 40 °C for 2 h. The resulting free glycosyl units were assayed spectrophotometrically using a hexokinase based glucose assay kit (Sigma) as described previously [72].
3.1.13. Muscle triglyceride measurement
Quadricep muscles from ad libitum control or M-AktDKO mice were homogenized in cell lysis buffer (CLB) and then diluted with CLB resulting in homogenate at 25 mg/ml 20 μl of the sample was then solubilized with 20 μl of 1% deoxycholate and incubated at 37 °C for 10 min. For triglyceride measurements, 200 μl of reagent (Infinity triglyceride reagent, Thermo Scientific) was added and incubated for 10 min at 37 °C. The triglyceride content was determined using a standard curve generated with triglyceride standard (Pointe Scientific) as described previously [72].
3.2. Statistical analysis
All data are presented as means ± S.E.M. The statistical analysis with one–way ANOVA was performed when more than two groups of data were compared, with two–way ANOVA when two conditions were involved, and with student's t–test when only two groups of data were concerned. P < 0.05 was deemed to be significant.
Author contributions
N.J. designed and performed experiments, analyzed data, and prepared the manuscript. T.S.L., W.J.Q. designed and performed experiments and analyzed data. M.G., R.G.G. provided technical assistance. J.A.B contributed to discussion and analyzed the data. P.M.T. conceived the hypothesis, designed and performed experiments, analyzed data, prepared the manuscript and directed the project.
Acknowledgments
This work was supported by U.S. National Institutes of Health Pilot and Feasibility grant P30 19525 (P.M.T.), K01 111715 (P.M.T.), the Samuel Chiaffa Memorial Fund (P.M.T.), and start-up funds from the University of Pennsylvania (P.M.T.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.08.001.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplemental Figure 1. Normal activation of insulin receptor and liver and heart Akt signaling in M-AktDKO mice (A) Western blot analysis for the Akt2 and phosphorylated Akt at Ser 474 in metabolic tissues and heart. (B) Western blot for the protein level of Akt3 in muscle and brain. (C) Western blot for phosphorylated IR (Tyr1150/1151), total Insulin Receptor (IR), phosphorylated Akt at Ser473/Ser474 and total Akt2. Supplemental Figure 2. Deletion of Akt in skeletal muscle results in decreased myofibril size (A) WGA staining and measurement of cross-sectional area (CSA) in soleus and EDL muscle from control and M-AktDKO mice (****p < 0.0001 vs. control). Supplemental Figure 3. Female M-AktDKO mice display a significant reduction in muscle mass (A) Body weight (n = 19 for control, n = 4 for M-Akt2KO and n = 6 for M-AktDKO (B) Muscle mass of different muscles of female mice n = 24 for control and n = 2 for M-Akt2KO and n = 8 for M-AktDKO mice (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control). Supplemental Figure 4. Female M-AktDKO mice exhibit mild alterations in glucose homeostasis (A) Intraperitoneal glucose tolerance test (2 g/kg) (n = 23 for control and 5 for M-Akt2KO and M-AktDKO mice) (B) Insulin tolerance test (0.75 U/kg) (n = 13 for control and 4 for M-Akt2KO and M-AktDKO mice). Supplemental Figure 5. Glut-4 expression in EDL and soleus muscle in M-AktDKO mice and quantitation of western blots from Figure 4 (A) Glut4 protein expression measured by western blot in EDL and soleus muscles lysates of control and M-AktDKO mice. Quantification of western blot of (B) Glut-1 (C) Glut-4 (D) pAS160 (E) PAS-160 and (F) TBC1D1 from figure 4 (n = 4). Supplemental Figure 6. Muscle triglyceride and glycogen content (A) Triglyceride level and (B) glycogen content in quadricep muscles of ad libitum fed control or M-AktDKO mice. Supplemental Figure 7. Glut-4 expression in EDL and soleus muscle in M-indAktDKO mice and quantitation of western blots from Figure 6 (A) Western blot of Glut4 protein in EDL and soleus muscles lysates of M-indControl and M-indAktDKO mice and quantification of western blots of (B) Glut-4 (C) Glut-1 (D) phosphorylated AS160 and (E) TBC1D1 from Figure 6 (n = 2). Supplementary Figure 8. Glycogen content in Quadriceps muscles of M-indAktDKO mice 4 weeks post-tamoxifen (A) Glycogen content in ad libitum fed quadricep muscles from control or M-indAktDKO mice (n = 7 for M-indControl and 8 for M-indAktDKO). Supplementary Figure 9: Quantitation of western blots from Figure 7 and treatment with SBI-0206965, a small molecule AMPK inhibitor, in ex vivo muscle Quantification of western blot of (A) TOM-20 (n = 3) and (B) phosphorylated AMPK (n = 4) from figure 7 and (C) ex vivo insulin-stimulated 2-deoxyglucose uptake in control or M-AktDKO soleus muscle with/without pretreatment with SBI-0206965 (n = 3–4 for each condition; **p < 0.01vs. insulin).
References
- 2.DeFronzo R.A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization∖Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes m. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo R.A. Pathogenesis of type 2 diabetes mellitus. Medical Clinics of North America. 2004 doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Kelley D., Mokan M., Veneman T. Impaired postprandial glucose utilization in non-insulin-dependent diabetes mellitus. Metabolism. 1994;43(12):1549–1557. doi: 10.1016/0026-0495(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 5.Kelley D., Mitrakou A., Marsh H., Schwenk F., Benn J., Sonnenberg G. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. Journal of Clinical Investigation. 1988 doi: 10.1172/JCI113489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor R., Magnusson I., Rothman D.L., Cline G.W., Caumo A., Cobelli C. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. Journal of Clinical Investigation. 1996 doi: 10.1172/JCI118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl. 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteman E.L., Cho H., Birnbaum M.J. Role of Akt/protein kinase B in metabolism. Trends in Endocrinology and Metabolism TEM. 2002;13(10):444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 9.Peng X. ding., Xu P.Z., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes and Development. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Easton R.M., Cho H., Roovers K., Shineman D.W., Mizrahi M., Forman M.S. Role for Akt3/protein kinase B in attainment of normal brain size. Molecular and Cellular Biology. 2005;25(5):1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z.-Z., Tschopp O., Hemmings-Mieszczak M., Feng J., Brodbeck D., Perentes E. Protein kinase Bα/Akt1 regulates placental development and fetal growth. Journal of Biological Chemistry. 2003;278(34):32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 12.Cho H., Thorvaldsen J.L., Chu Q., Feng F., Birnbaum M.J. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. Journal of Biological Chemistry. 2001;276(42):38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 13.Cho H., Mu J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBbeta) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 14.Garofalo R.S., Orena S.J., Rafidi K., Torchia A.J., Stock J.L., Hildebrandt A.L. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. Journal of Clinical Investigation. 2003;112(2):197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu M., Wan M., Leavens K.F., Chu Q., Monks B.R., Fernandez S. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nature Medicine. 2012;18(3):388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krook A., Roth R.A., Jiang X.J., Zierath J.R., Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998;47(8):1281–1286. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- 17.Zierath J.R., Krook A., Wallberg-Henriksson H. Insulin action in skeletal muscle from patients with NIDDM. Molecular and Cellular Biochemistry. 1998;182(1–2):153–160. [PubMed] [Google Scholar]
- 18.Højlund K., Birk J.B., Klein D.K., Levin K., Rose A.J., Hansen B.F. Dysregulation of glycogen synthase COOH- and NH2-terminal phosphorylation by insulin in obesity and type 2 diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 2009;94(11):4547–4556. doi: 10.1210/jc.2009-0897. [DOI] [PubMed] [Google Scholar]
- 19.Brozinick J.T., Roberts B.R., Dohm G.L. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes. 2003;52(4):935–941. doi: 10.2337/diabetes.52.4.935. [DOI] [PubMed] [Google Scholar]
- 20.Cozzone D., Fröjdö S., Disse E., Debard C., Laville M., Pirola L. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia. 2008;51(3):512–521. doi: 10.1007/s00125-007-0913-8. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson H.K.R., Zierath J.R., Kane S., Krook A., Lienhard G.E., Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54(6):1692–1697. doi: 10.2337/diabetes.54.6.1692. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay F., Lavigne C., Jacques H., Marette A. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (ζ/λ) activities. Diabetes. 2001;50(8):1901–1910. doi: 10.2337/diabetes.50.8.1901. [DOI] [PubMed] [Google Scholar]
- 23.Shao J., Yamashita H., Qiao L., Friedman J.E. Decreased Akt kinase activity and insulin resistance C57BL/KsJ-Lepr(db/db) mice. Journal of Endocrinology. 2000;167(1):107–115. doi: 10.1677/joe.0.1670107. [DOI] [PubMed] [Google Scholar]
- 24.Lai Y.-C., Liu Y., Jacobs R., Rider M.H. A novel PKB/Akt inhibitor, MK-2206, effectively inhibits insulin-stimulated glucose metabolism and protein synthesis in isolated rat skeletal muscle. Biochemical Journal. 2012;447(1):137–147. doi: 10.1042/BJ20120772. [DOI] [PubMed] [Google Scholar]
- 25.Yeh J.I., Gulve E.A., Rameh L., Birnbaum M.J. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. Journal of Biological Chemistry. 1995;270(5):2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]
- 26.Ducommun S., Wang H.Y., Sakamoto K., MacKintosh C., Chen S. Thr649Ala-AS160 knock-in mutation does not impair contraction/AICAR-induced glucose transport in mouse muscle. American Journal of Physiology Endocrinology and Metabolism. 2012;302(9):E1036–E1043. doi: 10.1152/ajpendo.00379.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H.Y., Ducommun S., Quan C., Xie B., Li M., Wasserman D.H. AS160 deficiency causes whole-body insulin resistance via composite effects in multiple tissues. Biochemical Journal. 2013;449(2):479–489. doi: 10.1042/BJ20120702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S., Wasserman D.H., MacKintosh C., Sakamoto K. Mice with as160/TBC1D4-Thr649Ala Knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metabolism. 2011;13(1):68–79. doi: 10.1016/j.cmet.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto K., Holman G.D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. American Journal of Physiology-Endocrinology and Metabolism. 2008;295(1):E29–E37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitfield J., Paglialunga S., Smith B.K., Miotto P.M., Simnett G., Robson H.L. Ablating the protein TBC1D1 impairs contraction-induced sarcolemmal glucose transporter 4 redistribution but not insulin-mediated responses in rats. Journal of Biological Chemistry. 2017;292(40):16653–16664. doi: 10.1074/jbc.M117.806786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neill B.T., Lauritzen H.P.M.M., Hirshman M.F., Smyth G., Goodyear L.J., Kahn C.R. Differential role of insulin/IGF-1 receptor signaling in muscle growth and glucose homeostasis. Cell Reports. 2015;11(8):1220–1235. doi: 10.1016/j.celrep.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geddis A.V., Abdalla M.N., Leandry L.A., McClung H.L., Pasiakos S.M., Matheny R.W. AKT2 is the predominant Akt isoform expressed in human skeletal muscle. The FASEB Journal. 2017;31(1 Suppl.) doi: 10.14814/phy2.13652. lb144–lb144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan M., Easton R.M., Gleason C.E., Monks B.R., Ueki K., Kahn C.R. Loss of Akt1 in mice increases energy expenditure and protects against diet-induced obesity. Molecular and Cellular Biology. 2012;32(1):96–106. doi: 10.1128/MCB.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green C.J., Göransson O., Kular G.S., Leslie N.R., Gray A., Alessi D.R. Use of Akt inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/Akt in the insulin-dependent regulation of glucose and system A amino acid uptake. Journal of Biological Chemistry. 2008 Oct 10;283(41):27653–27667. doi: 10.1074/jbc.M802623200. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy J.J., Srikuea R., Kirby T.J., Peterson C.A., Esser K.A. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skeletal Muscle. 2012;2(1):8. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjøbsted R., Hingst J.R., Fentz J., Foretz M., Sanz M.N., Pehmøller C. AMPK in skeletal muscle function and metabolism. FASEB Journal. 2018 doi: 10.1096/fj.201700442R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii N., Aschenbach W.G., Musi N., Hirshman M.F., Goodyear L.J. Regulation of glucose transport by the AMP-activated protein kinase. Proceedings of the Nutrition Society. 2004;63(02):205–210. doi: 10.1079/PNS2004340. [DOI] [PubMed] [Google Scholar]
- 38.Funai K., Cartee G.D. Inhibition of contraction-stimulated amp-activated protein kinase inhibits contraction-stimulated increases in pas-tbc1d1 and glucose transport without altering pas-as160 in rat skeletal muscle. Diabetes. 2009;58(5):1096–1104. doi: 10.2337/db08-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mu J., Brozinick J.T., Valladares O., Bucan M., Birnbaum M.J. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Molecular Cell. 2001;7(5):1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 40.Cokorinos E.C., Delmore J., Reyes A.R., Albuquerque B., Kjøbsted R., Jørgensen N.O. Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell Metabolism. 2017;25(5):1147–1159. doi: 10.1016/j.cmet.2017.04.010. e10. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg G.R., Michell B.J., van Denderen B.J.W., Watt M.J., Carey A.L., Fam B.C. Tumor necrosis factor α-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metabolism. 2006;4(6):465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Bandyopadhyay G.K., Yu J.G., Ofrecio J., Olefsky J.M. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55(8):2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- 43.Geng T., Liu Y., Xu Y., Jiang Y., Zhang N., Wang Z. H19 lncRNA promotes skeletal muscle insulin sensitivity in part by targeting AMPK. Diabetes. 2018;67(11):2183–2198. doi: 10.2337/db18-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sriwijitkamol A., Coletta D.K., Wajcberg E., Balbontin G.B., Reyna S.M., Barrientes J. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007 doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brozinick J.T., Birnbaum M.J. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. Journal of Biological Chemistry. 1998;273(24):14679–14682. doi: 10.1074/jbc.273.24.14679. [DOI] [PubMed] [Google Scholar]
- 46.Kjøbsted R., Munk-Hansen N., Birk J.B., Foretz M., Viollet B., Björnholm M. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes. 2017 doi: 10.2337/db16-0530. [DOI] [PubMed] [Google Scholar]
- 47.Hamada T., Arias E.B., Cartee G.D. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. Journal of Applied Physiology. 2006 doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- 48.Holloszy J.O. Exercise-induced increase in muscle insulin sensitivity. Journal of Applied Physiology. 2005 doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- 49.Ching J.K., Rajguru P., Marupudi N., Banerjee S., Fisher J.S. A role for AMPK in increased insulin action after serum starvation. American Journal of Physiology Cell Physiology. 2010 doi: 10.1152/ajpcell.00514.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith J.L., Patil P.B., Fisher J.S. AICAR and hyperosmotic stress increase insulin-stimulated glucose transport. Journal of Applied Physiology. 2005 doi: 10.1152/japplphysiol.01297.2004. [DOI] [PubMed] [Google Scholar]
- 51.Fisher J.S., Gao J., Han D.-H., Holloszy J.O., Nolte L.A. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. American Journal of Physiology Endocrinology and Metabolism. 2017 doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 52.Bain J., Plater L., Elliott M., Shpiro N., Hastie C.J., Mclauchlan H. The selectivity of protein kinase inhibitors: a further update. Biochemical Journal. 2007 doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dite T.A., Langendorf C.G., Hoque A., Galic S., Rebello R.J., Ovens A.J. AMP-activated protein kinase selectively inhibited by the type II inhibitor SBI-0206965. Journal of Biological Chemistry. 2018 doi: 10.1074/jbc.RA118.003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. Journal of Clinical Investigation. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long Y.C., Cheng Z., Copps K.D., White M.F. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Molecular and Cellular Biology. 2011;31(3):430–441. doi: 10.1128/MCB.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo J., Sobkiw C.L., Hirshman M.F., Logsdon M.N., Li T.Q., Goodyear L.J. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cell Metabolism. 2006;3(5):355–366. doi: 10.1016/j.cmet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 57.O'Neill Brian T., Lee Kevin Y., Klaus Katherine, Softic Samir, Krumpoch Megan T., Fentz Joachim, Stanford Kristin I., Robinson Matthew M., Cai Weikang, Kleinridders Andre, Pereira Renata O., Hirshman Michael F., Dale Abel Dom E., Ronald Kahn C. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. Journal of Clinical Investigation. 2016;126(May):3433–3446. doi: 10.1172/JCI86522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kjøbsted R., Treebak J.T., Fentz J., Lantier L., Viollet B., Birk J.B. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes. 2015 doi: 10.2337/db14-1402. [DOI] [PubMed] [Google Scholar]
- 59.Steinberg G.R., Watt M.J., McGee S.L., Chan S., Hargreaves M., Febbraio M.A. Reduced glycogen availability is associated with increased AMPKα2 activity, nuclear AMPKα2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Applied Physiology Nutrition and Metabolism. 2006 doi: 10.1139/h06-003. [DOI] [PubMed] [Google Scholar]
- 60.McBride A., Ghilagaber S., Nikolaev A., Hardie D.G. The glycogen-binding domain on the AMPK β subunit Allows the kinase to act as a glycogen sensor. Cell Metabolism. 2009 doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wojtaszewski J.F.P., Mourtzakis M., Hillig T., Saltin B., Pilegaard H. Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochemical and Biophysical Research Communications. 2002 doi: 10.1016/s0006-291x(02)02465-8. [DOI] [PubMed] [Google Scholar]
- 62.Taylor E.B., An D., Kramer H.F., Yu H., Fujii N.L., Roeckl K.S.C. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. Journal of Biological Chemistry. 2008;283(15):9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudich A., Klip A. Putting Rac1 on the path to glucose uptake. Diabetes. 2013 doi: 10.2337/db13-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sylow L., Kleinert M., Pehmøller C., Prats C., Chiu T.T., Klip A. Akt and Rac1 signaling are jointly required for insulin-stimulated glucose uptake in skeletal muscle and downregulated in insulin resistance. Cellular Signalling. 2014;26(2):323–331. doi: 10.1016/j.cellsig.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Ueda S., Kitazawa S., Ishida K., Nishikawa Y., Matsui M., Matsumoto H. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. The FASEB Journal. 2010 doi: 10.1096/fj.09-137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sylow L., Jensen T.E., Kleinert M., Højlund K., Kiens B., Wojtaszewski J. Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes. 2013;62(6):1865–1875. doi: 10.2337/db12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tunduguru R., Chiu T.T., Ramalingam L., Elmendorf J.S., Klip A., Thurmond D.C. Signaling of the p21-activated kinase (PAK1) coordinates insulin-stimulated actin remodeling and glucose uptake in skeletal muscle cells. Biochemical Pharmacology. 2014 doi: 10.1016/j.bcp.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moller L.L.V., Jaurji M., Nielsen I.L., Joseph G.A., Kjobsted R., Knudsen J.R. The role of p-21 activated kinases (PAKs) in glucose homeostasis and skeletal muscle glucose uptake. BioRxiv. 2019 [Google Scholar]
- 69.Nozaki S., Takeda T., Kitaura T., Takenaka N., Kataoka T., Satoh T. Akt2 regulates Rac1 activity in the insulin-dependent signaling pathway leading to GLUT4 translocation to the plasma membrane in skeletal muscle cells. Cellular Signalling. 2013 doi: 10.1016/j.cellsig.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 70.Takenaka N., Yasuda N., Nihata Y., Hosooka T., Noguchi T., Aiba A. Role of the guanine nucleotide exchange factor in Akt2-mediated plasma membrane translocation of GLUT4 in insulin-stimulated skeletal muscle. Cellular Signalling. 2014 doi: 10.1016/j.cellsig.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Takenaka N., Araki N., Satoh T. Involvement of the protein kinase Akt2 in insulin-stimulated Rac1 activation leading to glucose uptake in mouse skeletal muscle. PLoS One. 2019 doi: 10.1371/journal.pone.0212219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Titchenell P.M., Quinn W.J., Lu M., Chu Q., Lu W., Li C. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metabolism. 2016;23(6):1154–1166. doi: 10.1016/j.cmet.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKeel D.W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. Journal of Cell Biology. 1970 doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tonks K.T., Ng Y., Miller S., Coster A.C., Samocha-Bonet D., Iseli T.J. Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia. 2013 Apr;56(4):875–885. doi: 10.1007/s00125-012-2811-y. [DOI] [PubMed] [Google Scholar]
- 75.Fazakerley D.J., Krycer J.R., Kearney A.L., Hocking S.L., James D.E. Muscle and adipose tissue insulin resistance: malady without mechanism? Journal of Lipid Research. 2018 Jul 27 doi: 10.1194/jlr.R087510. pii: jlr.R087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoehn K.L., Hohnen-Behrens C., Cederberg A., Wu L.E., Turner N., Yuasa T. IRS1-independent defects define major nodes of insulin resistance. Cellular Metabolism. 2008 May;7(5):421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Normal activation of insulin receptor and liver and heart Akt signaling in M-AktDKO mice (A) Western blot analysis for the Akt2 and phosphorylated Akt at Ser 474 in metabolic tissues and heart. (B) Western blot for the protein level of Akt3 in muscle and brain. (C) Western blot for phosphorylated IR (Tyr1150/1151), total Insulin Receptor (IR), phosphorylated Akt at Ser473/Ser474 and total Akt2. Supplemental Figure 2. Deletion of Akt in skeletal muscle results in decreased myofibril size (A) WGA staining and measurement of cross-sectional area (CSA) in soleus and EDL muscle from control and M-AktDKO mice (****p < 0.0001 vs. control). Supplemental Figure 3. Female M-AktDKO mice display a significant reduction in muscle mass (A) Body weight (n = 19 for control, n = 4 for M-Akt2KO and n = 6 for M-AktDKO (B) Muscle mass of different muscles of female mice n = 24 for control and n = 2 for M-Akt2KO and n = 8 for M-AktDKO mice (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control). Supplemental Figure 4. Female M-AktDKO mice exhibit mild alterations in glucose homeostasis (A) Intraperitoneal glucose tolerance test (2 g/kg) (n = 23 for control and 5 for M-Akt2KO and M-AktDKO mice) (B) Insulin tolerance test (0.75 U/kg) (n = 13 for control and 4 for M-Akt2KO and M-AktDKO mice). Supplemental Figure 5. Glut-4 expression in EDL and soleus muscle in M-AktDKO mice and quantitation of western blots from Figure 4 (A) Glut4 protein expression measured by western blot in EDL and soleus muscles lysates of control and M-AktDKO mice. Quantification of western blot of (B) Glut-1 (C) Glut-4 (D) pAS160 (E) PAS-160 and (F) TBC1D1 from figure 4 (n = 4). Supplemental Figure 6. Muscle triglyceride and glycogen content (A) Triglyceride level and (B) glycogen content in quadricep muscles of ad libitum fed control or M-AktDKO mice. Supplemental Figure 7. Glut-4 expression in EDL and soleus muscle in M-indAktDKO mice and quantitation of western blots from Figure 6 (A) Western blot of Glut4 protein in EDL and soleus muscles lysates of M-indControl and M-indAktDKO mice and quantification of western blots of (B) Glut-4 (C) Glut-1 (D) phosphorylated AS160 and (E) TBC1D1 from Figure 6 (n = 2). Supplementary Figure 8. Glycogen content in Quadriceps muscles of M-indAktDKO mice 4 weeks post-tamoxifen (A) Glycogen content in ad libitum fed quadricep muscles from control or M-indAktDKO mice (n = 7 for M-indControl and 8 for M-indAktDKO). Supplementary Figure 9: Quantitation of western blots from Figure 7 and treatment with SBI-0206965, a small molecule AMPK inhibitor, in ex vivo muscle Quantification of western blot of (A) TOM-20 (n = 3) and (B) phosphorylated AMPK (n = 4) from figure 7 and (C) ex vivo insulin-stimulated 2-deoxyglucose uptake in control or M-AktDKO soleus muscle with/without pretreatment with SBI-0206965 (n = 3–4 for each condition; **p < 0.01vs. insulin).