Abstract
Glycative stress, caused by the accumulation of cytotoxic and irreversibly-formed sugar-derived advanced glycation end-products (AGEs), contributes to morbidity associated with aging, age-related diseases, and metabolic diseases. In this review, we summarize pathways leading to formation of AGEs, largely from sugars and glycolytic intermediates, and discuss detoxification of AGE precursors, including the glyoxalase system and DJ-1/Park7 deglycase. Disease pathogenesis downstream of AGE accumulation can be cell autonomous due to aggregation of glycated proteins and impaired protein function, which occurs in ocular cataracts. Extracellular AGEs also activate RAGE signaling, leading to oxidative stress, inflammation, and leukostasis in diabetic complications such as diabetic retinopathy. Pharmaceutical agents have been tested in animal models and clinically to diminish glycative burden. We summarize existing strategies and point out several new directions to diminish glycative stress including: plant-derived polyphenols as AGE inhibitors and glyoxalase inducers; improved dietary patterns, particularly Mediterranean and low glycemic diets; and enhancing proteolytic capacities of the ubiquitin-proteasome and autophagy pathways that are involved in cellular clearing of AGEs.
Keywords: Advanced glycation end-products, RAGE, Diabetes, Glyoxalase, Cataract, Ubiquitin-proteasome system, Autophagy, Age-related macular degeneration, Diabetic retinopathy
1. Introduction: AGE formation in biology
Glycative stress is etiologically related to accelerated aging and increased risk for multiple diseases such as diabetes, cancer, kidney, ocular, and cardiovascular diseases [1,2]. During glycative stress exogenous or endogenous advanced glycation end-products (AGEs) are aberrantly deposited, promoting protein modifications and dysregulating signaling and protein quality control pathways. These changes in the proteome and signaling lead to inflammation, perturbed tissue physiology, and ultimately to the onset of diseases [3].
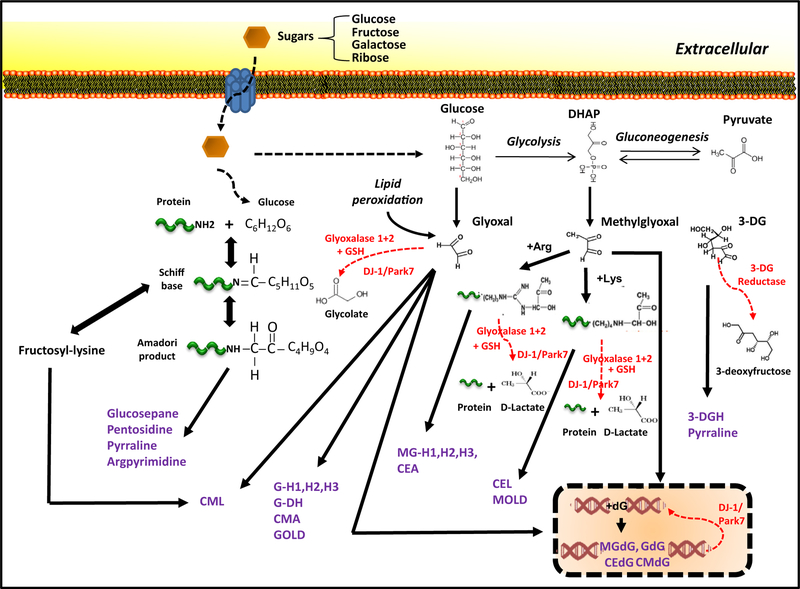
AGEs comprise a heterogeneous group of compounds that arise via a non-enzymatic reaction called glycation, a spontaneous post-translational modification in which a carbonyl group of reducing sugars is covalently coupled to proteins, lipids and nucleic acids (Fig. 1). First, highly reactive dicarbonyl metabolites are generated from sugars in circulation or formed during the glycolytic process [4]. These precursors undergo the Maillard reaction, a condensation between reducing sugars (e.g. glucose, fructose, ribose) and the free amino groups of proteins, leading to the formation of reversible unstable Schiff bases that finally may evolve into more stable Amadori products. Amadori products have the tendency to undergo oxidation re-arrangement, dehydration, and cyclization, and over time, form the dicarbonyl intermediates that react with arginine and lysine residues to irreversibly generate crosslinked AGEs.
Fig. 1.
Formation and detoxification of advanced glycation end-products (AGEs) from dietary sugars. AGEs are derived by different pathways including Maillard reaction with the generation of Schiff bases and Amadori products and reactions that involve oxaldehydes (glyoxal, methylglyoxal, 3-deoxyglucosone) derived, in part, from glycolytic intermediates. Fructosyl-lysine, a fructosamine, is an Amadori product that can further go on to form CML. Detoxifying routes (highlighted in red) and AGEs (highlighted in purple) are shown. DHAP: dihydroxyacetone phosphate; GSH: reduced glutathione; CML: Nε-(carboxymethyl)-lysine; CMA: Nε-(carboxymethyl)-arginine; GOLD: glyoxal-derived lysyl dimer; 3-DG: 3-deoxyglucosone; GH-1,2,3: glyoxal-derived hydroimidazolone; MGH-1,2,3: methylglyoxal-derived hydroimidazolone; CEL: Nε-(carboxyethyl)-lysine; CEA: Nε-(carboxyethyl)-arginine; MOLD: methylglyoxal-derived lysine dimer; dG: deoxyguanosine residue on DNA; GdG: 3-(2′-deoxyribosyl)-6,7-dihydro-6,7-dihydroxyimidazo-[2,3-b]purin-9(8)one; CMdG: N2-carboxymethyl-deoxyguanosine; MGdG: 3-(2′-deoxyribosyl)-6,7-dihydro-6,7-dihydroxyimidazo-[2,3-b]purin-9(8)one; CEdG: N2-(1-carboxyethyl)-deoxyguanosine.
Different sources contribute to the accumulation of AGEs in multiple human tissues. Firstly, there is an uptake of exogenous AGEs from dietary sources. The contribution of exogenous AGEs to circulating AGE levels has been estimated at approximately 30% of the total AGEs accumulated in the body [5]. Only 10% of consumed AGEs are intestinally absorbed and one-third of these AGEs are quickly excreted in the urine within 48 h, thus removed from the circulation [6–8]. Sugar intake also plays a central role. Thus the consumption of high sugar content diets accelerates the formation of reactive dicarbonyls. Levels of dicarbonyls compounds are usually lower than 150 nM in human plasma but deviations of these homeostatic values due to the nutrition may lead to detrimental consequences [9]. Food manufacturing processes or cooking methods can also dramatically increase the formation of dietary AGEs and consequently increase AGE deposition in the body. To avoid the glycation damage generated by circulating AGEs, efficient removal of AGEs via the renal system plays a vital role to preserve the health of the tissues and organs [7].
Apart of the exogenous sources, increased cellular AGEs may come from formation of dicarbonyls during glycolysis, a defective functionality of detoxification pathways, and/or insufficient degradation of AGEs (Figs. 1, 2). In mammalian cells, dicarbonyl concentrations range between 1 and 4 μM, approximately 10 fold the plasma level [7]. Multiple detoxification systems are required to avoid AGE-mediated cytotoxicity [9,10].
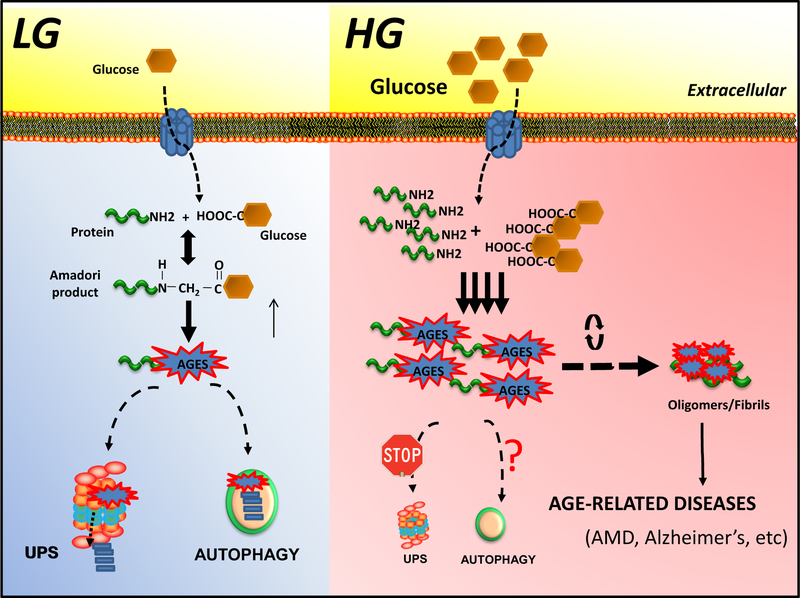
Fig. 2.
Dietary glycative-stress induced accumulation of AGEs. Under low glycemia diets (LG, left), intracellular proteolytic pathways are involved in the clearance of AGEs to preserve cellular function. During normal aging or under high glycemia diets (HG, right), proteolytic capacities are insufficient and AGEs accelerate the formation of aggregates/oligomers, leading to the onset of age-related diseases.
In the present review we will systematically discuss the intracellular molecular pathways that are available, at least in young tissues, to reduce glycative damage and how these capacities are compromised during aging and age-related diseases. Current therapeutic approaches and their limitations will be also discussed. We propose that dietary manipulations by targeting these molecular routes provide ways to maintain health as well as therapeutic alternatives for prevention of glycative stress.
2. Detoxification pathways: keeping the AGE concentration low
The formation of AGEs in vivo occurs mainly through the reaction of dicarbonyl derivatives. The main reason is that, although less abundant than glucose, these dicarbonyls are far more reactive [11]. The major glycating moieties formed during normal carbohydrate metabolism are methylglyoxal (MGO), glyoxal, or 3-deoxyglucosone (3-DG) [4,7].
Although MGO can be also found in food, the main source seems be the glycolysis intermediates [7,10,12,13] (Fig. 1). In physiological conditions, the concentration of MGO is maintained in the range of nM in human plasma but the levels of MGO may dramatically increase in pathological conditions leading to impaired tissue functionality [9]. For example, the level of MGO in the blood of diabetic patients (29.3 ± 5.5 μg/mL) has been shown to be > 3-fold higher than in controls (8.5 ± 0.5 μg/mL) [14]. MGO-derived AGEs have been causatively associated with a plethora of aging-related diseases including Alzheimer’s disease, Parkinson’s disease, arthritis, renal dysfunction and vascular failure [15].
Dicarbonyls are also derived from lipid metabolism. For example, glyoxal is a dicarbonyl that can be generated directly from glucose oxidation and lipid peroxidation (Fig. 1). Glyoxal has been reported to induce DNA damage [16] and may be found in high concentration in oxidized dietary oils (as high as 33.3 ± 5.2 nmol/g in sardine oil) [17]. Along with MGO, glyoxal can be detoxified by the enzymatic glyoxalase system. This ubiquitous protective detoxification route converts MGO into lactate and glyoxal into glycolate and utilizes reduced glutathione (GSH), Glyoxalase I (GLO1), and Glyoxalase II (GLO2) [18,19] (Fig. 1).
The third major dicarbonyl is 3-deoxyglucosone (3-DG). 3-DG can be formed from non-oxidative rearrangement and hydrolysis of the Amadori product and by fructose-3-phosphate, an intermediate of the polyol pathway [7]. High levels of 3-DG (as high as 2622 mg/L in balsamic vinegar) may be also found in food and high-glucose conditions [7,13]. Unlike MGO or glyoxal, levels of 3-DG cannot be minimized through the glyoxalase pathway, thus requiring the action of other protective to maintain homeostatic levels of 3-DG. In this case, 3-DG reductases, specific aldoketo reductases, play critical roles in detoxification [7,20] (Fig. 1).
Some AGE precursors, such as fructosyl-lysine, are also derived from the direct degradation of the Amadori products. Fructosyl-lysine cannot be removed via the glyoxalase system. However, the presence of fructosamine 3-kinase (FN3K) phosphorylates fructosyl-lysine residues causing them to be shed from proteins. Thus it acts as a protective mechanism in kidney, blood and brain efficiently deglycating intracellularly protein bound and free fructosyl-lysines [21]. Recently, the DJ-1/Park7 family has been characterized as a deglycase enzyme, with abilities to repair early glycation intermediates and glycated guanine residues in nucleic acids and nucleotides [22–24] (Fig. 1). The detoxification pathway of DJ-1/Park7 is similar to the glyoxalase system, albeit glutathione-independent, and with a broadened range of substrates, including glycated amino acids and proteins [22,25] (Fig. 1).
Finally, these intermediates evolve into the final products, AGEs, which can be classified into 3 different groups based on their chemical properties: fluorescent cross-linking AGEs due to aromatic chemical structures (e.g. pentosidine), non-fluorescent cross-linking AGEs (e.g. glucosepane) and non-fluorescent non-crosslinking AGEs (e.g. Nε-(carboxymethyl)-lysine (CML), pyrraline or Nε-(carboxyethyl)-lysine (CEL)) [26]. Generally, AGEs are irreversible adducts [27].
Anti-glycation pathways generally only operate on intermediates of glycation. Once generated, excessive AGEs accumulate and eventually become insoluble causing intense changes in the subcellular metabolism. However, there are intracellular degradative mechanisms that help clear these toxic adducts. Both the ubiquitin-proteasome system (UPS) and autophagy have been reported to contribute to the removal of AGEs (Fig. 2) [28–31]. They may operate autonomously or cooperatively. At present, there is little information about the molecular processes and factors involved in the targeting of AGEs to these degradative routes. In addition, it is remains unclear how these two proteolytic systems work together in the intracellular detoxification of modified AGEs. The autophagic system might play a role in the degradation of high-molecular complexes that cannot be or are not removed by the proteasome [32]. Alternatively, autophagic clearance of AGEs might be a compensatory mechanism due to the partial inhibition of UPS activity mediated by products of glycation [28,33,34].
The importance of AGE detoxification pathways is well-illustrated in the context of normal aging, even in the absence of hyperglycemia. For example, glycotoxins have been shown to accumulate in aging diseases like Alzheimer’s disease, renal failure, and cardiac disease [35]. AGE accumulation in these diseases is also found on pathologically linked proteins, like Alzheimer’s disease associated tau or Parkinson’s disease association α-synuclein [36,37]. It has been postulated that the age-dependent accumulation of glycotoxins might be partially consequence of a gradual decrease of proteolytic capacities during normal aging [29]. In addition, the loss of glyoxalase activity with age would exacerbate the formation of reactive dicarbonyls resulting in a deleterious and additive effect on age-related defective AGE clearance [7,38–41].
3. Biology of glycation related disease: intracellular and extracellular
The effects of AGEs are manifested in a cell-type and context-dependent fashion. In this respect, it is important to distinguish between cell intrinsic (intracellular) consequences of AGEs versus cell extrinsic (extracellular) consequences (mediated at least in part by RAGE signaling, see Section 3.2.1 below), albeit many tissues and cell types may experience stresses from both sources.
3.1. Intracellular AGE accumulation
All cells in the body are exposed to some degree of glycative stresses, which arise from normal metabolic and glycolytic functions, within the cell and from the circulation. These glycative stresses increase with aging and with hyperglycemia. As indicated above, cells utilize a number of repair pathways to balance the formation and degradation of AGE precursors and AGEs (Figs. 1, 2).
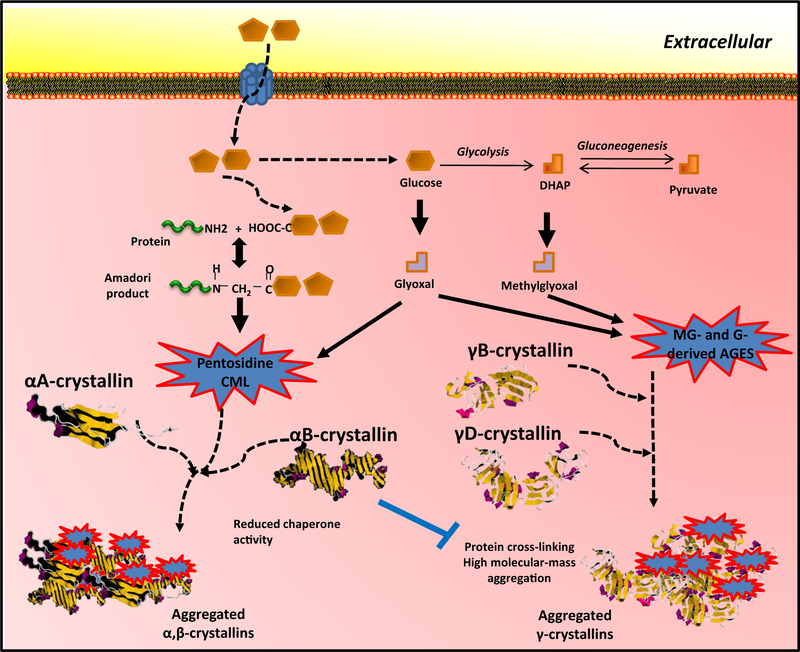
The abnormal accumulation of AGEs in a cell may lead to a variety of cell responses. These include repair or removal of the damage, and/or apoptotic cell death. The consequences of glycation in any given protein can also be variable. Many proteins are rendered functionally inactive by AGE modification. A typical example is the αA-crystallin protein, which is a target for glycation by pentosidine or CML on lysine 11 and 78 (Fig. 3) [42,43]. Consequently, intracellular AGE-modified αA-crystallin undergoes conformational changes leading to loss of its critical chaperone functions (Fig. 3), which then leads to widespread protein aggregation, protein insolubility, proteinopathy and eventually cataract formation [44–46]. Other proteins, like γB-crystallin undergo aggregation in response to N-terminal glycation, directly contributing to cataractogenesis (Fig. 3) [46–48]. Interestingly for αA-crystallin, glycation of lysine residues competes with acetylation of the same residue, which alters enzymatic properties [44].
Fig. 3.
Glycation of crystallins in cataractogenesis. Direct glycation of α-crystallins and γ-crystallins induces a loss of chaperone activity and aggregation, as examples of intracellular and RAGE-independent glycative damage. DHAP: dihydroxyacetone phosphate.
Other cell types undergo more complex pathological responses to intracellular AGE accumulation. These responses may have intercellular aspects and consequences. Retinal pigmented epithelial cells are postmitotic support cells of the retinal photoreceptors, and are among the most metabolically active cells in the body. Accumulation of AGEs in RPE cells affects the ubiquitin-proteolysis system, as described above [28,49]. Impaired proteolysis in RPE cells leads to accumulation of undigested photoreceptor outer segments, resulting in lipofuscin accumulation, an additional source of glyoxal and MGO [50,51]. In turn, RPE cells produce and secrete lipid-rich particles, which contribute to drusen formation and development of age-related macular degeneration (AMD) [50]. In more extreme cases, RPE cells undergo cell death or de-differentiation causing the more severe form of dry AMD, geographic atrophy. Cell death in response to AGEs can be caspase-dependent or independent and may occur in conjunction with mitochondrial dysfunction, heat-shock response, and ER-stress [49,52–54]. Apoptosis downstream of AGEs can be prevented in some contexts by the ER-stress inhibitor 4-phenylbutyric acid, suggesting that ER-stress may be coordinating multiple responses to AGE accumulation [53,55].
Brain tissues are also impacted by intracellular AGE accumulation. Alzheimer’s disease associated tau is stabilized by glycation on lysine residues, since the protein is no longer properly targeted for ubiquitin-dependent proteolysis or otherwise undergoes structural stability, potentially contributing to Alzheimer’s pathogenesis [56,57]. Similarly, α-Synuclein is stabilized by glycation at the N-terminal region, altering its subcellular localization, and thus promoting accumulation of toxic oligomers, potentially contributing to Parkinsonian diseases [37].
3.2. Extracellular AGE accumulation
The extracellular milieu contains a diverse array of AGEs that originate from the diet, metabolic processes, or from cellular breakdown products. Common circulating AGEs are serum albumins, apolipoproteins, and free glycated amino acids. Glycation of serum albumin causes structural changes to the protein, negatively affecting its antioxidant functions and causes binding to and activation of RAGE, described below [58].
Extracellular matrix proteins are a common site of glycation, and a significant source of circulating AGEs. Glycation of extracellular matrix proteins is also a common feature of aging and diabetes, and an important contributor to the thickening of basement membranes observed in diabetic complications [59]. Collagen proteins are a frequent target of glycation, with the most abundant skin collagen AGE being the crosslinking glucosepane [60]. Skin AGEs have been evaluated as prospective biomarkers for diabetic complications. In these studies, MG-H1, CML, glucosepane, and a glucosepane precursor surrogate, furosine, were shown to be strong risk factors for progression of diabetic retinopathy and neuropathy [60,61]. These associations were still significant upon correction for glycated hemoglobin A1C, indicating unique roles for these AGEs apart from just acting as surrogates of hyperglycemia, possibly as a basis for metabolic memory observed in diabetic complications. Lens capsule proteins are also glycated upon aging, which contributes to cataract formation and fibrosis [62].
3.2.1. RAGE-dependent signaling
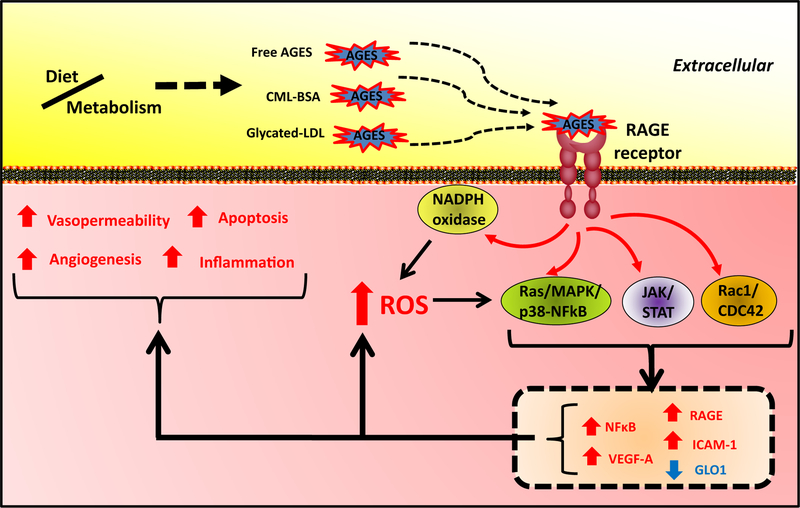
The RAGE immunoglobulin-family transmembrane protein is a major receptor for extracellular AGEs, as well as other classes of proteins and molecules. Among the best characterized ligands of RAGE are CML protein adducts, which are elevated in hyperglycemia and other stress conditions [63]. Activation of the RAGE receptor leads to recruitment of DIAPH1 to the C-terminal cytoplasmic tail of RAGE, which leads to activation of at least three prominent signaling pathways: Ras/MAPK/NF-κB, JAK/STAT, and Rac1/Cdc42 (Fig. 4) [64–66]. These signal transduction pathways initiate production of reactive oxygen species (ROS) via NADPH oxidase and cause increased expression of pro-inflammatory modulators (e.g. IL-6, TNF-α), pro-angiogenic factors (e.g. VEGF-A, VCAM1), and RAGE itself, while downregulating Glyoxalase 1, hence setting up a feed-forward amplification (Fig. 4) [67,68].
Fig. 4.
RAGE signaling in glycative stress. Interactions of exogenous or endogenous AGEs in circulation with RAGE in plasma membrane trigger different signaling routes responsible for cytopathological responses, including apoptosis, inflammation, generation of reactive oxygen species (ROS), angiogenesis, and vasopermeability.
The importance of RAGE-dependent signaling in downstream disease pathogenesis from AGEs has also been explored in animal models in which the gene encoding RAGE, Ager, has been deleted, or where expression of dominant-negative RAGE proteins, either naturally occurring soluble RAGE forms or C-terminal truncated forms of RAGE, have been expressed [69–71]. Knock out of RAGE protects against diabetic complications like glomerulosclerosis, albuminuria, and atherosclerosis [69–71]. These studies have demonstrated critical roles for RAGE in diabetes, cardiovascular disease, and neurodegenerative diseases [67]. Cell types that express high levels of RAGE and are therefore more likely to exhibit RAGE-dependent diseases include vascular endothelial cells, pericytes, microglial cells, astrocytes, and neurons [67]. Interestingly, RAGE knockout mice are resistant to many deleterious consequences of AGE accumulation, live normal lifespans, and do not have any reported developmental or organ malfunctions [67].
Microvascular complications of diabetes commonly depend on RAGE signaling. In the case of diabetic retinopathy, either in mouse models of type 1 or type 2 diabetes, deletion of Ager protected against loss of retinal permeability, acellular capillaries, microglial activation, and Müller cell gliosis in the eye [72]. Surprisingly, pericyte loss, a common microvascular complication of diabetes was not rescued by deletion of Ager, demonstrating the importance of other RAGE-independent signals or a cell intrinsic function of AGE accumulation in retinal pericytes [72]. Similar results were observed after expression of secreted RAGE protein in diabetic mice [73]. In these experiments, systemic delivery of soluble RAGE improved retinal blood-barrier function in diabetic mice, whereas RAGE overexpression in the vasculature exacerbated blood-barrier function. Another target of RAGE signaling, ICAM-1 (Fig. 4), increased with diabetes and RAGE overexpression, and led to leukostasis, characterized by adherent leukocytes within the retinal vasculature. Leukostasis was decreased by soluble RAGE, even in diabetic mice [73].
An important caveat to experiments in which RAGE signaling is blocked is that loss of RAGE causes increased expression of Glyoxalase 1 and reduces levels of glyoxal and MGO [72,74]. Therefore, the exact mechanism βy which RAGE contributes to disease pathogenesis needs to be evaluated systematically, since RAGE on its own is pro-glycative. Several targets of RAGE signaling can be queried genetically or pharmacologically, allowing more mechanistic interpretations. For example, NADPH oxidase is inhibited by diphenylene iodonium and apocynin or more specifically by rationally designed inhibitors [75], or can be genetically targeted by deletion of gp91phox. Loss-of-function NADPH oxidase studies demonstrated that RAGE activation of NADPH oxidase is partly responsible for complications of diabetic nephropathy, including proteinuria and mesangial expansion via ROS-dependent pathways [76]. Macrophages deleted for the NADPH subunit gp91phox did not activate tissue factor, a pro-coagulant inflammatory response, to ovalbumin-AGEs [77]. RAGE signaling can also lead to ER-stress either directly or via ROS signaling and activation of ER stress can potentiate RAGE-dependent responses [78]. This potential cross-talk suggests pharmaceutical targeting of ER-stress may be helpful to ameliorate AGE stress.
3.2.2. Other extracellular AGE binding proteins
Extracellular AGEs have their own distinct functions within the ECM, independently of RAGE engagement. Extracellular AGEs can bind to and activate multiple extracellular or membrane-tethered proteins, including Galectin-3, OST-48 (also known as AGE-R1), PRKCSH (also known as 80K-H and AGE-R2), and SR-A [79].
AGE binding to Galectin-3 appears to have opposing outcomes, possibly depending on the forms of Galectin-3 and cell type specific responses [80]. In the diabetic eye, deletion of Galectin-3 resulted in reduced blood-retina barrier dysfunction, reduced VEGF response, and reduced endothelial tight junction integrity [81]. However, in the nearby RPE, which expresses high levels of RAGE, overexpression of Galectin-3 reduced the VEGF response to AGE exposure [82]. A similar contradictory finding was reporter in the kidney, where diabetic Galectin-3 knockout mice showed enhanced diabetic nephropathy, associated with glomerulopathy, proteinuria, and mesangial expansion [83]. This study showed that Galectin-3 knockout mice had increased levels of RAGE and increased accumulation of renal AGEs, suggesting that Galectin-3 functions to oppose RAGE [83]. Other potential AGE binding proteins were downregulated in Galectin-3 knockout mice, particularly OST-48 and MSR-A (macrophage scavenger receptor A), indicating a functional balancing act of pro-RAGE and anti-RAGE AGE binding proteins.
OST-48 remains poorly characterized, although it can function in an anti-RAGE mechanism via inhibition of NADPH oxidase activation. This inhibition operates at multiple levels, including preventing the NADPH oxidase subunit p47phox translocation to the cell membrane via inhibition of RAGE signaling pathways [84]. However, in the context of liver damage caused by a high AGE diet, overexpression of OST-48 was associated with increased liver damage, hepatomegaly, and increased adiposity. Even in the absence of a high AGE diet, overexpression of OST-48 increased the hepatic AGE content [85]. Macrophage scavenger receptor group A, SR-A, also appears to function as a negative and positive regulator of RAGE signaling, depending on context. Diabetic mice lacking SR-A have exacerbated diabetic retinopathy [86], yet are resistant to diabetic nephropathy [87].
The observations noted above indicate that the pro-inflammatory, pro-angiogenic, and oxidatively damage RAGE-dependent cellular response to AGEs are subject to a great deal of context-dependent factors. RAGE knockout mice have not yet revealed critical homeostatic functions [67]. Nevertheless, the evolution of RAGE signaling pathway was selected for during mammalian evolution, largely via adaptive selection [88], and it is likely that the diverse array of RAGE isoforms and other AGE receptors are designed to carefully balance the response to accumulation of AGEs and their physiological causes.
4. Pharmaceutical treatment of AGE accumulation
Accumulation of AGEs is a hallmark of many of the chronic diseases associated with high morbidity and mortality, and remains a viable target for intervention. Several classes of drugs have been developed and tested in various pre-clinical and clinical models to determine their efficacy in treating diseases. Drugs can be categorized into those that target: i) hyperglycemia, the most upstream cause of AGE accumulation, ii) AGEs themselves, and iii) downstream ramifications of AGE accumulation. Examples of each of these categories are discussed below.
4.1. Drugs to diminish hyperglycemia
The most direct way to target accumulation of AGEs is to prevent their formation. Decades of attempting to treat diabetes pharmacologically have yielded many important drugs and drug targets that attempt to normalize blood glucose concentrations. These range from drugs that prevent glucose absorption in the intestine (e.g. acarbose) to drugs that enhance glucose excretion from the kidneys (e.g. gliflozins), to those that promote insulin secretion from pancreatic beta cells (e.g. meglitinides) to those that directly and indirectly target glucose metabolic responses (e.g. metformin, thiazolidinediones). Metformin decreases hepatic glucose production and enhances peripheral insulin sensitivity, whereas thiozolidinediones diminish glucose catabolism by limiting transcription of enzymes involved in glucose metabolism.
Several randomized clinical trials have been performed with a variety of drugs that target glycemic control, many of them using AGEs as primary or secondary end-points. There are several recurring themes that emerge from these studies, which are summarized in [89]. One finding is that serum AGEs frequently show declines in association with improved glycemia, and the nature of the drug does not seem to be as critical as the glycemic effect. For example, metformin and pioglitazone (a thiazolidinedione) both lowered serum pentosidine levels compared to controls (diet therapy, sulfonylurea ± insulin) [90]. Similarly, metformin and repaglinide (a meglitinide) both lowered serum 3-DG versus controls [91]. The effects on 3-DG were dependent on the glucose-lowering effects of the drugs, despite different mechanisms of action [91]. These two comparison studies argue that metformin’s function in lowering serum AGEs is not due to its potential function as an inhibitor of early AGE formation [92].
Another recurring theme from clinical trials in glucose-lowering drugs is that different classes of AGEs and AGE precursors appear to be altered in different studies – sometimes leading to directly conflicting findings from the literature. The study comparing metformin to repaglinide above showed reductions in serum 3-DG, whereas a study also in type 2 diabetics showed an effect of metformin on reducing plasma MGO but no effect on plasma 3-DG [93]. Differences in methodologies, patient baseline characteristics, and treatment plans may also explain the opposing results. In general, there is a lack of consistent findings between studies [89].
A different limitation in human studies has been the practical decision to sample AGEs in serum or plasma. Steady-state levels of AGEs in circulation depend on dietary intake, rates of urinary clearance, and the general homeostatic regulation of AGE formation vs. breakdown. For this reason, intracellular AGE accumulation may be a more reliable indicator of long-term formation of stable AGEs, but less helpful for evaluating efficacy of glucose-lowering drugs. Indeed, studies that measure levels of AGE precursors and stable protein-bound AGEs in the context of improved glycemia have generally shown better trends for AGE precursors (e.g. MGO, 3-DG, fructosyl-lysine) than protein-bound AGEs (e.g. CEL, CML) [89]. Most of the published clinical studies have limitations in terms of duration of glucose-lowering treatments and the difficulty in knowing whether levels of serum AGE products will correlate tightly with levels of stable AGE products in tissues relevant to diabetic complications like the kidney, cardiovascular system, nerve cells, and eye.
4.2. Drugs to limit formation of AGEs
Multiple classes of drugs are capable of quenching AGE precursors, preventing the formation of AGE-precursors. The prototypical molecules with these capabilities are arginine analogs such as aminoguanidine and pyridoxamine, which, along with arginine itself, can effectively scavenge AGE precursors or Amadori product-derived AGE precursors [94]. A separate class of molecules is epitomized by alagebrium (ALT-711), which break AGE cross-links. Other anti-AGE drugs work by targeting the structure of glycation target proteins, making them less prone to glycation (e.g. limonene [95]).
Pre-clinical studies with AGE inhibitors have been largely positive, with reports of amelioration of diabetic nephropathy, retinopathy, neuropathy, and CVD phenotypes in multiple animal models [96]. In two related clinical trials, low- and high-dose aminoguanidine treatments improved some aspects of diabetic nephropathy, including the glomerular filtration rate and urinary proteinuria, while also providing protection against diabetic retinopathy [97,98]. The clinical trials were not successful in meeting the goal of preventing a doubling of serum creatinine. More significantly, some patients receiving high-dose aminoguanidine developed glomerulonephritis and other patients reported adverse events [97]. Finally, at the high doses necessary, aminoguanidine could potentially sequester essential carbonyls like vitamin B6 from the body. Ultimately, clinical trials with aminoguanidine were discontinued.
However, there are still opportunities for repurposing aminoguanidine in a clinical setting. Combining different classes of anti-AGE compounds, aminoguanidine and limonene, allowed effective anti-glycation activity using an almost 20× reduction in aminoguanidine concentration, potentially bypassing the possible side effects observed prior [99]. Since aminoguanidine functions by scavenging carbonyls, requiring high concentrations for efficacy, but requires intact kidney function for clearance, optimizing dosing of the drug is most critical for any future success.
Pyridoxamine is a vitamin B6 metabolite that has been exploited widely as an AGE inhibitor. Its mode of action appears similar to aminoguanidine, in that it can quench reactive dicarbonyls and can reverse Amadori intermediates. Pyridoxamine has been tested clinically for its ability to diminish diabetic complications, either alone or in combination with other vitamins. Clinical results have been mixed. In a combined phase 2 trial, pyridoxamine treatment improved serum creatinine levels in type 1 and type 2 diabetics with nephropathy, while also slightly lowering serum CML and CEL levels [100]. The improvement in serum creatinine was independent of changes in urinary albumin excretion, which was unchanged. However, the phase 2 studies revealed adverse reactions in the treatment group, and a phase 3 trial, scheduled to be completed in early 2018 has been terminated (https://clinicaltrials.gov/ct2/show/NCT02156843). An early shorter-term and small scale interventional trail failed to show reductions in serum pentosidine or other oxidative stress markers following 8-weeks of treatment with pyridoxamine and benfotiamine (a fat soluble form of thiamine) [101]. Considering the mixed results obtained with glucoselowering drugs (as described above), it would be helpful to re-analyze these clinical trials using a broader set of AGE markers, with a bias toward AGE precursor levels.
Alagebrium appears to have performed better than aminoguanidine and pyridoxamine in the clinical setting. Mechanistically, alagebrium was proposed to function as a breaker of AGE cross-links, which was supported by findings that alagebrium could cleave AGE cross-linked αA-crystallin from diabetic lenses in vitro [102]. However, alagebrium was not able to break collagen cross-links from diabetic skin and tail samples [103]. It remains an open question exactly how alagebrium functions as an AGE inhibitor, although pre-clinical studies support this function. Alagebrium has also been found to effectively scavenge MGO in physiological conditions [104]. Alagebrium has been tested clinically specifically in the context of vascular dysfunction associated with hypertension or left ventricular hypertrophy, with mostly mixed success and a small number of side-effects [105–108]. Nevertheless, largely owing to financial decisions, alagebrium is neither being pursued for further clinical trials, nor is it being distributed.
4.3. Drugs to modulate AGE responses
As discussed above, RAGE-dependent signaling is responsible for many of the pathogenic consequences of AGE accumulation, and itself is largely dispensable for normal development and aging. Therefore, inhibition of the RAGE pathway constitutes an attractive target for treating diseases associated with AGE accumulation. Physiologically, several RAGE inhibitors are generated via alternative splicing to generate RAGE molecules without intracellular domains. On their own, these secreted and non-secreted RAGE isoforms act as competitive inhibitors of RAGE signaling and can ameliorate several complications of diabetes. However neither soluble RAGE nor blocking antibodies against RAGE can cross the blood-brain barrier, limiting their clinical use in AGE-associated neuropathological scenarios.
Several RAGE inhibitor drugs have been generated, and some have advanced in clinical studies, including PF-04494700 (or TTP488, Azeliragon), which is currently in Phase 3 trials for treatment of dementia in Alzheimer’s patients [109,110]. PF-04494700 acts extracellularly by preventing the binding of a number of ligands to RAGE, including CML, Aβ42, S100B, and HMGB1 and is able to cross the blood-brain barrier [111]. Another extracellular-acting RAGE inhibitor, FPS-ZM1, was capable of blocking RAGE signaling mediated by Aβ42 as well as by BSA-AGEs [112,113]. Other drugs are being developed that target the intracellular domain of RAGE that interacts with the RAGE-dependent signaling modulator, DIAPH1, although these studies are still in the pre-clinical stages [114].
Some known anti-diabetic compounds appear to exert some of their efficacy via targeting downstream events in the RAGE signaling cascade. Metformin, which acts to both lower blood glucose and is a weak dicarbonyl scavenger, also dampens RAGE signaling via AMPK-dependent blockage of ROS formation [115]. Compounds that target the well-characterized pro-inflammatory pathways downstream of NF-κB may also be well-suited to block some of the deleterious signals downstream of RAGE. Similarly, compounds that target ER-stress may effectively ameliorate pro-apoptotic and pro-inflammatory responses downstream of AGEs. A different approach to modulate RAGE signaling may be via microRNAs, some of which are being developed as potential therapeutics. For example, miR-5591–5p is downregulated by RAGE signaling and promoted cell survival and repair when overexpressed [116]. Another microRNA that is downregulated by AGEs, miR-126 is a negative regulator of IL-6 and TNF-α and prevents ROS and inflammation [117].
4.4. Current status of drug discovery
Drug discovery in the field of AGE inhibitors is still very active. Optimization of existing compounds has been a particularly fruitful approach. Studies optimizing alagebrium or that resemble heterocyclic Schiff bases have yielded new drug leads that may overcome some of the challenges of existing compounds and function more effectively to remove AGEs [118,119]. Other promising approaches include bi-functional drugs, which can target multiple steps in AGE formation. An example is a hybrid molecule of glutathione diester and mercaptoethylguanidine [120]. Upon penetration of the cell membrane, the hybrid is cleaved into glutathione and mercaptoethylguanidine. The glutathione acts as an anti-oxidant and ensures maximal glyoxalase function, while the mercaptoethylguanidine acts similarly to amino-guanidine as a dicarbonyl scavenger.
5. New directions and approaches
In considering treatments for complex multifactorial diseases associated with AGE accumulation, it is likely that a multifaceted approach will be most effective. Environmental factors and modifications should be considered and implemented. Diet, nutrition, and exercise can have significant benefit to patient outcomes and should always be considered as primary interventions.
5.1. Plant bioactives as AGE inhibitors
Diet and nutrition are capable of modulating numerous pathways that are capable of converging on AGE homeostasis. For example, dozens of naturally occurring compounds, including pyridoxamine (discussed above), can function as direct AGE inhibitors. Most of the compounds described as activating Glyoxalase 1 via Nrf2-dependent pathways are also food-derived bioactives (trans-resveratrol, sulforaphane, etc....). Plant-derived polyphenols have been shown to have glucose lowering activities and in some cases direct AGE inhibitory functions [27]. A phenolic acid, 3,5-di-O-caffeoyl-epi-quinic acid, from Erigeron annuus was a potent AGE inhibitor that reduced BSA-AGE crosslinking and prevented opacification of lenses ex vivo [121]. A trio of aromatic compounds from Pueraria lobate, including puerarin, PG-3, and (+/−)-puerol B, was capable of inhibiting AGE formation in vitro, with the isoflavone puerarin having an IC50 more than eight-fold lower than aminoguanidine [122].
Despite the promising functions of plant-derived bioactives, it is not clear whether these foods or bioactives, on their own, will deliver clinical advantage. It is unlikely that food polyphenols could be consumed in high enough quantities or frequently enough to have clinically meaningful results, and it is equally unclear whether naturally occurring compounds will have a sufficiently safe and desirable profile when taken in therapeutic doses. Nevertheless a promising direction of identifying bioactives with anti-AGE or glucose-lowering properties is the idea of utilizing them in combination with bioactives targeting different steps in the pathway.
5.2. Natural compounds targeting detoxification pathways
An alternative strategy to those described above is to enhance the naturally occurring detoxification pathways for AGEs and AGE precursors. As discussed above, the glyoxalase system is the primary mechanism for detoxifying reactive dicarbonyls. Aging and disease are associated with reduced levels of Glyoxalase 1 and reduced levels of the critical cofactor glutathione [7,39,40]. Therefore, compounds that increase Glyoxalase 1 activity, either through increased transcription or post-translationally, or that increase levels of the reduced form of glutathione should reduce de novo formation of AGEs, similarly to reducing hyperglycemia.
Other detoxification systems are involved in reducing levels of dicarbonyls, including aldo-keto reductases (which are particularly efficient in metabolizing 3-deoyxglucosone) and aldehyde reductases. These enzyme systems are NADPH-dependent and can conceivably have their capacity augmented to enhance AGE detoxification. Given the broadness of this class of enzymes and their wide range of substrates, they have not been widely considered as drug targets to treat AGE-related diseases.
An intriguing possibility derives from understanding the functions of the Nrf2 antioxidant pathway. In response to oxidative stress, Nrf2 is stabilized, translocates into the nucleus, and transcriptionally activates numerous cytoprotective genes that encode detoxification enzymes and molecules with antioxidant properties. These genes include those involved in glutathione synthesis (γ-glutamylcysteine ligase encoded by GCLC and GCLM), aldo-keto reductases (AKR1A1), glyoxalase 1, and other antioxidant enzymes [123,124]. One might surmise that compounds known to act as Nrf2 agonists, like the naturally occurring broccoli-sprout derived isothiocyanate, sulforaphane, would effectively lead to AGE detoxification. Studies in cell culture support this hypothesis, whereby treatment with 2 μM sulforaphane led to a 2-fold increase in Glyoxalase activity [124]. However, because of high cost and low bioavailability, sulforaphane is not well-suited for human studies. An alternative formulation was tested in a small human clinical study of overweight and obese patients [125]. This formulation consisted of trans-resveratrol, which had been shown to activate Glyoxalase activity in an Nrf2-dependent fashion [126] and hesperetin, a vasodilating flavonoid also capable of activating Glyoxalase 1 via Nrf2-dependent activation [125]. Both trans-resveratrol and hesperetin were well-tolerated by diet and had sufficient bioavailability to activate Glyoxalase1 leading to lower plasma MGO and MGO-derived AGES, improved insulin sensitivity, and improved vascular function [125].
Other compounds have been identified that are capable of activating Glyoxalase 1 in an Nrf2-dependent pathway, including butylated hydroxyanisole [127] and allyl isothiocyanate [124]. However, there are concerns about long-term global Nrf2 activation, including negative effects in the kidney and increased hypertension and mortality due to Nrf2-mediated stimulation of the renal-angiotensin system [128]. Another limitation could be the enhanced tumorigenesis in different disease models upon Nrf2 activation [129]. Furthermore, global upregulation of Glyoxalase 1 is not without consequence. Transgenic mice overexpressing Glyoxalase 1 have increased anxiety, which appears to be due to reduced levels of MGO in the brain, which acts as a GABA receptor agonist [130]. Therefore, limiting the range of Glyoxalase 1-inducing function may be critical for maximizing benefit over side-effects. In this regard, restoration of normal physiological Glyoxalase 1 activity and GSH levels should be prioritized.
5.3. Dietary patterns in disease prevention
Ultimately, our dietary pattern can be thought of as a large combinatorial approach to maintaining health and the treatment of disease. Effective dietary patterns have been identified for prolonging healthful life, the treatment of hypertension, metabolic disease, age-related macular degeneration and others. The impact of diet in treating a complex disease was epitomized in a recent study demonstrating that weight loss of > 10 kg at 12-months led to remission of diabetes in 73% of patients aged 20–65 years [131]. A Mediterranean diet also shows efficacy in treating diabetes, particularly in improving glycemic control [132,133]. Other dietary patterns have been beneficial in treating diabetes, although these effects have not been as well-reproduced through multiple cohorts as the Mediterranean diet.
Glycemic control is most frequently measured via fasting blood glucose or HbA1C concentrations. However, it would be expected that these same glycemic effects would lead to reduced levels of AGE or AGE precursors in the circulation and in tissues as has been demonstrated above for pharmaceutical treatments of diabetes. One might predict enhanced effects due to the ability of the diet to impact multiple pathways in AGE formation and reduce oxidative stresses. One study evaluated the Mediterranean diet with regards to serum CML and MGO levels, also evaluating RAGE and Glyoxalase 1 levels. Compared to a Western-style diet with higher levels of saturated fats, the Mediterranean diet led to decreased CML and MGO levels, reduced RAGE transcript levels, and increased Glyoxalase 1 transcript [134].
A feature of the Mediterranean diet, as described in the above study, is that it contains lower levels of dietary AGEs, whereas typical Western diets have higher levels of AGEs [5]. Dietary AGEs may play important roles in determining physiologic levels of circulating and tissue AGEs, and this is borne out in clinical studies evaluating the impact of high-AGE and low-AGE diets. In a small trial of type 2 diabetics, a low-AGE diet compared to an isocaloric normal diet over a 4-month duration led to decreased plasma insulin levels, decreased leptin levels, and decreased levels of serum MGO and CML, while also lowering RAGE signaling [135]. Similar improvements were noted in a small cohort of patients with chronic kidney disease [136], as well as a slightly larger follow-up trial of obese individuals with metabolic syndrome [137]. Improvements in insulin levels were observed in the absence of changes to glycemic control, suggesting that dietary AGEs, and not those produced metabolically, may be responsible for negative effects on oxidative stress, inflammation, and insulin resistance.
5.4. Glycemic index, AGEs, and chronic disease
The Mediterranean diet not only features higher amounts of n-3 polyunsaturated fats, monounsaturated fats, lean protein, and lower amounts of dietary AGEs, but critically, it is a low glycemic index diet. Although not necessarily a low carbohydrate diet, the quality of the carbohydrates in the Mediterranean diet differs from the typical Western diet in consisting of more whole-grain breads, cereals, and pastas and fewer processed starches and sugars. In the majority of studies evaluating a low glycemic index dietary pattern, there is improved glycemic control, reduced HbA1C levels, and lower risk for development of diabetes [138,139]. Accordingly, low glycemic index diets lead to decreased levels of circulating AGEs, particularly those of AGE precursors like fructosamine, and a reduced burden of chronic disease [138,140,141].
The influence of different dietary patterns and dietary glycemic index was evaluated in the context of age-related macular degeneration (AMD), a glycemia-associated chronic disease associated with accumulation of AGEs [142,143]. Adherence to a Mediterranean diet or a similar prudent dietary pattern was associated with a highly significant decrease in early and late macular degeneration [144,145]. Low glycemic index diets were similarly protective against AMD [146]. We developed an experimental animal model to evaluate the connection between dietary glycemic index and accumulation of AGEs in AMD. High glycemic index diets led to systemic accumulation of AGEs, as well as accumulation of AGEs in the eye tissues directly affected by AMD along with other highly sensitive tissues to glycation damage including brain or kidney [28,147,148]. We also identified the cross-linking AGE glucosepane as a potential plasma biomarker of AMD [148]. Plasma glucosepane may also be a biomarker of diabetes [149]. The accumulation of AGEs with a high glycemic index diet was not irreversible. In mice that were initially fed high glycemic index diets, but transferred to a lower glycemic index diet, tissue levels of multiple AGEs decreased in the eye and pathology was also limited [148]. Therefore targeting diet as a modifiable risk factor for AMD may be therapeutic, possibly via an AGE-lowering property.
5.5. Targeting novel clearance pathways of AGEs
If detoxifying systems are unable to reduce the levels of reactive intermediates, the accumulation and deposition of AGEs caused, for example, by a high glycemic diet, drives tissue malfunction. AGEs interfere in the subcellular proteome by crosslinking proteins, promoting oligomerization/fibril formation and inactivating vital cellular function leading to molecular onset of diseases [150] (Fig. 2). To avoid cellular dysfunction, proteolytic systems like the ubiquitin-proteasome system (UPS) and autophagy efficiently clear these toxic compounds, however these activities decline during normal aging [151]. Furthermore, AGEs directly inhibit the activity of the UPS, further potentiating proteostatic impairment [28,34,49] (Fig. 2). Dietary manipulation could be utilized in order to preserve these declining proteolytic capacities, thus reducing the deposition of AGEs in human tissues. For example, caloric restriction or short-term intermittent fasting has been shown to upregulate autophagy in several organisms leading to extension of the lifespan [152]. In addition, different bioactive compounds present in the diet such as flavonoids, vitamins, triterpenoids, or isothiocyanates have been proven to activate autophagy [153].
Animal studies have showed that low-glycemic nutritional options delaying sugar absorption might help to control the level of proteolytic systems [29]. A low glycemic diet reduces the formation of intermediates of AGEs, maintaining proteolytic capacities, which subsequently clear AGEs to homeostatic levels (Fig. 2). Therefore, we propose that the consumption of carbohydrates that result in smaller and/or more gradual rises in blood glucose (low glycemic foods) might be exploited as an effective intervention for the prevention of AGE-related diseases [154] (Fig. 2). Interestingly, trehalose, which is a low glycemic disaccharide, has been proven to remove neurotoxic protein by activation of autophagy, and may potentiate AGE remediation [155–158]. Alternatively, moderate exercise has been shown to induce autophagy activity [159]. All these strategies may represent non-toxic and low-cost suitable approaches to promote the intracellular clearance of AGEs in order to fight diseases related to glycative stress.
6. Conclusions and outlook
AGE accumulation is associated with a large number of chronic diseases and contributes significantly to morbidity and mortality. In this review, we discussed several mechanisms by which glycation can be countered, using the intrinsic glyoxalase and glyoxalase-like enzymes. Pharmacological inhibition of glycation, either upstream or downstream of AGE formation may eventually become a practical route for treating chronic glycation-associated diseases. Nevertheless, prevention of AGE accumulation through healthy low glycemic dietary patterns and through the beneficial activation of proteolytic capacities using exercise and dietary fasting will likely offer the best outcomes. There is still considerable opportunity for new dietary bioactive compounds to be used to reduce glycation via inhibition of AGE formation and enhancement of AGE detoxification. Reduction of AGE accumulation will not only preserve health of most organs and prevent diseases known to be associated with AGE cytotoxicity, but also will likely enhance the overall health span.
Acknowledgments
This work was funded by NIH RO1 EY13250, RO1 EY21212, RO1 EY26979, USDA contract 1950–510000-060–03A from U.S. Department of Agriculture-Agriculture Research Service (ARS), and USDA AFRI Grant 12212122.
Abbreviations:
- AGE
advanced glycation end-product
- GSH
reduced glutathione
- MGO
methylglyoxal
- 3-DG
3-deoxyglucosone
- CML
Nε-(carboxymethyl)-lysine
- CEL
Nε-(carboxyethyl)-lysine
- ROS
reactive oxygen species
- AMD
age-related macular degeneration
- RPE
retinal pigmented epithelial cells
Footnotes
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- [1].Piperi C, Adamopoulos C, Papavassiliou AG, Potential of glycative stress targeting for cancer prevention, Cancer Lett. 390 (2017) 153–159. [DOI] [PubMed] [Google Scholar]
- [2].Prasad C, Imrhan V, Marotta F, Juma S, Vijayagopal P, Lifestyle and advanced glycation end products (AGEs) burden: its relevance to healthy aging, Aging Dis. 5 (2014) 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rabbani N, Thornalley PJ, Glyoxalase Centennial conference: introduction, history of research on the glyoxalase system and future prospects, Biochem. Soc. Trans 42 (2014) 413–418. [DOI] [PubMed] [Google Scholar]
- [4].Thornalley PJ, Langborg A, Minhas HS, Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose, Biochem. J 344 (Pt 1) (1999) 109–116. [PMC free article] [PubMed] [Google Scholar]
- [5].Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H, Advanced glycation end products in foods and a practical guide to their reduction in the diet, J. Am. Diet. Assoc 110 (2010) 911–916.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].He C, Sabol J, Mitsuhashi T, Vlassara H, Dietary glycotoxins: inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration, Diabetes 48 (1999) 1308–1315. [DOI] [PubMed] [Google Scholar]
- [7].Rabbani N, Thornalley PJ, Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease, Biochem. Biophys. Res. Commun 458 (2015) 221–226. [DOI] [PubMed] [Google Scholar]
- [8].Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H, Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 6474–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rabbani N, Thornalley PJ, Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples, Nat. Protoc 9 (2014) 1969–1979. [DOI] [PubMed] [Google Scholar]
- [10].Rabbani N, Xue M, Thornalley PJ, Methylglyoxal-induced dicarbonyl stress in aging and disease: first steps towards glyoxalase 1-based treatments, Clin. Sci. (Lond.) 130 (2016) 1677–1696. [DOI] [PubMed] [Google Scholar]
- [11].Thornalley PJ, Dicarbonyl intermediates in the maillard reaction, Ann. N. Y. Acad. Sci 1043 (2005) 111–117. [DOI] [PubMed] [Google Scholar]
- [12].Nemet I, Varga-Defterdarovic L, Turk Z, Methylglyoxal in food and living organisms, Mol. Nutr. Food Res 50 (2006) 1105–1117. [DOI] [PubMed] [Google Scholar]
- [13].Degen J, Hellwig M, Henle T, 1,2-Dicarbonyl compounds in commonly consumed foods, J. Agric. Food Chem 60 (2012) 7071–7079. [DOI] [PubMed] [Google Scholar]
- [14].Lapolla A, Flamini R, Dalla Vedova A, Senesi A, Reitano R, Fedele D, Basso E, Seraglia R, Traldi P, Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method, Clin. Chem. Lab. Med 41 (2003) 1166–1173. [DOI] [PubMed] [Google Scholar]
- [15].Rabbani N, Thornalley PJ, Dicarbonyl proteome and genome damage in metabolic and vascular disease, Biochem. Soc. Trans 42 (2014) 425–432. [DOI] [PubMed] [Google Scholar]
- [16].Roberts MJ, Wondrak GT, Laurean DC, Jacobson MK, Jacobson EL, DNA damage by carbonyl stress in human skin cells, Mutat. Res 522 (2003) 45–56. [DOI] [PubMed] [Google Scholar]
- [17].Ichinose T, Nobuyuki S, Takano H, Abe M, Sadakane K, Yanagisawa R, Ochi H, Fujioka K, Lee KG, Shibamoto T, Liver carcinogenesis and formation of 8-hydroxy-deoxyguanosine in C3H/HeN mice by oxidized dietary oils containing carcinogenic dicarbonyl compounds, Food Chem. Toxicol 42 (2004) 1795–1803. [DOI] [PubMed] [Google Scholar]
- [18].Thornalley PJ, Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation, Biochem. Soc. Trans 31 (2003) 1343–1348. [DOI] [PubMed] [Google Scholar]
- [19].Sousa Silva M, Gomes RA, Ferreira AE, Ponces Freire A, Cordeiro C, The glyoxalase pathway: the firsthundred years... and beyond, Biochem. J 453 (2013) 1–15. [DOI] [PubMed] [Google Scholar]
- [20].Kanazu T, Shinoda M, Nakayama T, Deyashiki Y, Hara A, Sawada H, Aldehyde reductase is a major protein associated with 3-deoxyglucosone reductase activity in rat, pig and human livers, Biochem. J 279 (Pt 3) (1991) 903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van Schaftingen E, Collard F, Wiame E, Veiga-da-Cunha M, Enzymatic repair of Amadori products, Amino Acids 42 (2012) 1143–1150. [DOI] [PubMed] [Google Scholar]
- [22].Richarme G, Mihoub M, Dairou J, Bui LC, Leger T, Lamouri A, Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methyl-glyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues, J. Biol. Chem 290 (2015) 1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mihoub M, Abdallah J, Gontero B, Dairou J, Richarme G, The DJ-1 superfamily member Hsp31 repairs proteins from glycation by methylglyoxal and glyoxal, Biochem. Biophys. Res. Commun 463 (2015) 1305–1310. [DOI] [PubMed] [Google Scholar]
- [24].Richarme G, Liu C, Mihoub M, Abdallah J, Leger T, Joly N, Liebart JC, Jurkunas UV, Nadal M, Bouloc P, Dairou J, Lamouri A, Guanine glycation repair by DJ-1/Park7 and its bacterial homologs, Science 357 (2017) 208–211. [DOI] [PubMed] [Google Scholar]
- [25].Lee JY, Song J, Kwon K, Jang S, Kim C, Baek K, Kim J, Park C, Human DJ-1 and its homologs are novel glyoxalases, Hum. Mol. Genet 21 (2012) 3215–3225. [DOI] [PubMed] [Google Scholar]
- [26].Peyroux J, Sternberg M, Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes, Pathol. Biol. (Paris) 54 (2006) 405–419. [DOI] [PubMed] [Google Scholar]
- [27].Yeh WJ, Hsia SM, Lee WH, Wu CH, Polyphenols with antiglycation activity and mechanisms of action: a review of recent findings, J. Food Drug Anal 25 (2017) 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak DB, Handa JT, Brownlee M, Nagaraj R, Taylor A, Glycation-altered proteolysis as a pathobio-logic mechanism that links dietary glycemic index, aging, and age-related disease (in non diabetics), Aging Cell 11 (2012) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taylor A, Mechanistically linking age-related diseases and dietary carbohydrate via autophagy and the ubiquitin proteolytic systems, Autophagy 8 (2012) 1404–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takahashi A, Takabatake Y, Kimura T, Maejima I, Namba T, Yamamoto T, Matsuda J, Minami S, Kaimori JY, Matsui I, Matsusaka T, Niimura F, Yoshimori T, Isaka Y, Autophagy inhibits the accumulation ofadvanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules, Diabetes 66 (2017) 1359–1372. [DOI] [PubMed] [Google Scholar]
- [31].Eisermann DJ, Wenzel U, Fitzenberger E, Inhibition of chaperone-mediated autophagy prevents glucotoxicity in the Caenorhabditis elegans mev-1 mutant by activation of the proteasome, Biochem. Biophys. Res. Commun 484 (2017) 171–175. [DOI] [PubMed] [Google Scholar]
- [32].Grimm S, Ernst L, Grotzinger N, Hohn A, Breusing N, Reinheckel T, Grune T, Cathepsin D is one of the major enzymes involved in intracellular degradation of AGE-modified proteins, Free Radic. Res 44 (2010) 1013–1026. [DOI] [PubMed] [Google Scholar]
- [33].Moheimani F, Morgan PE, van Reyk DM, Davies MJ, Deleterious effects of reactive aldehydes and glycated proteins on macrophage proteasomal function: possible links between diabetes and atherosclerosis, Biochim. Biophys. Acta 1802 (2010) 561–571. [DOI] [PubMed] [Google Scholar]
- [34].Queisser MA, Yao D, Geisler S, Hammes HP, Lochnit G, Schleicher ED, Brownlee M, Preissner KT, Hyperglycemia impairs proteasome function by methylglyoxal, Diabetes 59 (2010) 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H, Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging, J. Gerontol. A Biol. Sci. Med. Sci 62 (2007) 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA, et al. , Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 7787–7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vicente Miranda H, Szego EM, Oliveira LMA, Breda C, Darendelioglu E, de Oliveira RM, Ferreira DG, Gomes MA, Rott R, Oliveira M, Munari F, Enguita FJ, Simoes T, Rodrigues EF, Heinrich M, Martins IC, Zamolo I, Riess O, Cordeiro C, Ponces-Freire A, Lashuel HA, Santos NC, Lopes LV, Xiang W, Jovin TM, Penque D, Engelender S, Zweckstetter M, Klucken J, Giorgini F, Quintas A, Outeiro TF, Glycation potentiates alpha-synuclein-associated neurodegeneration in synucleinopathies, Brain J. Neurol 140 (2017) 1399–1419. [DOI] [PubMed] [Google Scholar]
- [38].Kuhla B, Luth HJ, Haferburg D, Weick M, Reichenbach A, Arendt T, Munch G, Pathological effects of glyoxalase I inhibition in SH-SY5Y neuroblastoma cells, J. Neurosci. Res 83 (2006) 1591–1600. [DOI] [PubMed] [Google Scholar]
- [39].Mailankot M, Padmanabha S, Pasupuleti N, Major D, Howell S, Nagaraj RH, Glyoxalase I activity and immunoreactivity in the aging human lens, Biogerontology 10 (2009) 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fleming TH, Theilen TM, Masania J, Wunderle M, Karimi J, Vittas S, Bernauer R, Bierhaus A, Rabbani N, Thornalley PJ, Kroll J, Tyedmers J, Nawrotzki R, Herzig S, Brownlee M, Nawroth PP, Aging-dependent reduction in glyoxalase 1 delays wound healing, Gerontology 59 (2013) 427–437. [DOI] [PubMed] [Google Scholar]
- [41].Inagi R, Miyata T, Ueda Y, Yoshino A, Nangaku M, van C. Ypersele de Strihou, K. Kurokawa, Efficient in vitro lowering of carbonyl stress by the glyoxalase system in conventional glucose peritoneal dialysis fluid, Kidney Int. 62 (2002) 679–687. [DOI] [PubMed] [Google Scholar]
- [42].Abraham EC, Cherian M, Smith JB, Site selectivity in the glycation of alpha A- and alpha B-crystallins by glucose, Biochem. Biophys. Res. Commun 201 (1994) 1451–1456. [DOI] [PubMed] [Google Scholar]
- [43].Nagaraj RH, Linetsky M, Stitt AW, The pathogenic role of Maillard reaction in the aging eye, Amino Acids 42 (2012) 1205–1220. [DOI] [PubMed] [Google Scholar]
- [44].Nahomi RB, Oya-Ito T, Nagaraj RH, The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human alpha-crystallin, Biochim. Biophys. Acta 1832 (2013) 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kumar PA, Kumar MS, Reddy GB, Effect of glycation on alpha-crystallin structure and chaperone-like function, Biochem. J 408 (2007) 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Perry RE, Swamy MS, Abraham EC, Progressive changes in lens crystallin glycation and high-molecular-weight aggregate formation leading to cararact development in streptozotocin-diabetic rats, Exp. Eye Res 44 (1987) 269–282. [DOI] [PubMed] [Google Scholar]
- [47].Casey EB, Zhao HR, Abraham EC, Role of glycine 1 and lysine 2 in the glycation of bovine gamma B-crystallin. Site-directed mutagenesis of lysine to threonine, J. Biol. Chem 270 (1995) 20781–20786. [DOI] [PubMed] [Google Scholar]
- [48].Beswick HT, Harding JJ, Conformational changes induced in lens a - and g-crystallins by modification with glucose 6-phosphate, Biochem. J 246 (1987) 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bento CF, Marques F, Fernandes R, Pereira P, Methylglyoxal alters the function and stability of critical components of the protein quality control, PLoS One 5 (2010) e13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ferrington DA, Sinha D, Kaarniranta K, Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration, Prog. Retin. Eye Res 51 (2016) 69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR, Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 7275–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, Baehr W, Receptor for advanced glycation end products and age-related macular degeneration, Invest. Ophthalmol. Vis. Sci 45 (2004) 3713–3720. [DOI] [PubMed] [Google Scholar]
- [53].Chan CM, Huang DY, Huang YP, Hsu SH, Kang LY, Shen CM, Lin WW, Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction, J. Cell. Mol. Med 20 (2016) 1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yamabe S, Hirose J, Uehara Y, Okada T, Okamoto N, Oka K, Taniwaki T, Mizuta H, Intracellular accumulation of advanced glycation end products induces apoptosis via endoplasmic reticulum stress in chondrocytes, FEBS J. 280 (2013) 1617–1629. [DOI] [PubMed] [Google Scholar]
- [55].Chiang CK, Wang CC, Lu TF, Huang KH, Sheu ML, Liu SH, Hung KY, Involvement of endoplasmic reticulum stress, autophagy, and apoptosis in advanced glycation end products-induced glomerular mesangial cell injury, Sci. Rep 6 (2016) 34167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kontaxi C, Piccardo P, Gill AC, Lysine-directed post-translational modifications of tau protein in Alzheimer’s disease and related tauopathies, Front. Mol. Biosci 4 (2017) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kuhla B, Haase C, Flach K, Luth HJ, Arendt T, Munch G, Effect of pseudo-phosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation, J. Biol. Chem 282 (2007) 6984–6991. [DOI] [PubMed] [Google Scholar]
- [58].Rondeau P, Bourdon E, The glycation of albumin: structural and functional impacts, Biochimie 93 (2011) 645–658. [DOI] [PubMed] [Google Scholar]
- [59].Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW, Accumulation of Maillard reaction products in skin collagen in diabetes and aging, J. Clin. Invest 91 (1993) 2463–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Genuth S, Sun W, Cleary P, Gao X, Sell DR, Lachin J, Monnier VM, Skin advanced glycation end products glucosepane and methylglyoxal hydro-imidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes, Diabetes 64 (2015) 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM, Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes, Diabetes 54 (2005) 3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Raghavan CT, Smuda M, Smith AJ, Howell S, Smith DG, Singh A, Gupta P, Glomb MA, Wormstone IM, Nagaraj RH, AGEs in human lens capsule promote the TGFbeta2-mediated EMT of lens epithelial cells: implications for age-associated fibrosis, Aging Cell 15 (2016) 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kislinger T, Fu C, Huber B, Qu W, Taguchi A, DuYan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM, N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression, J. Biol. Chem 274 (1999) 31740–31749. [DOI] [PubMed] [Google Scholar]
- [64].Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, Schmidt AM, Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42, J. Biol. Chem 283 (2008) 34457–34468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM, Activation of the receptor for advanced glycation end products triggers a p21ras-dependent mitogen-activated protein kinase pathway regulated by oxidant stress, J. Biol. Chem 272 (1997) 1780–17814. [DOI] [PubMed] [Google Scholar]
- [66].Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY, Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells, J. Cell. Biochem 81 (2001) 102–113. [DOI] [PubMed] [Google Scholar]
- [67].Ramasamy R, Shekhtman A, Schmidt AM, The multiple faces of RAGE-opportunities for therapeutic intervention in aging and chronic disease, Expert Opin. Ther. Targets 20 (2016) 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ahmad S, Khan H, Siddiqui Z, Khan MY, Rehman S, Shahab U, Godovikova T, Silnikov V, Moinuddin, AGEs, RAGEs and s-RAGE; friend or foe for cancer, Semin. Cancer Biol 49 (2017) 44–55. [DOI] [PubMed] [Google Scholar]
- [69].Myint KM, Yamamoto Y, Doi T, Kato I, Harashima A, Yonekura H, Watanabe T, Shinohara H, Takeuchi M, Tsuneyama K, Hashimoto N, Asano M, Takasawa S, Okamoto H, Yamamoto H, RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low-molecular weight heparin, Diabetes 55 (2006) 2510–2522. [DOI] [PubMed] [Google Scholar]
- [70].Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D’Agati VD, Schmidt AM, RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy, Am. J. Pathol 162 (2003) 1123–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr., Chow WS, Stern D, Schmidt AM, Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts, Nat. Med 4 (1998) 1025–1031. [DOI] [PubMed] [Google Scholar]
- [72].McVicar CM, Ward M, Colhoun LM, Guduric-Fuchs J, Bierhaus A, Fleming T, Schlotterer A, Kolibabka M, Hammes HP, Chen M, Stitt AW, Role of the receptor for advanced glycation endproducts (RAGE) in retinal vasodegenerative pathology during diabetes in mice, Diabetologia 58 (2015) 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TC, Moore J, Yamamoto Y, Yamamoto H, Adamis AP, Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products, Invest. Ophthalmol. Vis. Sci 48 (2007) 858–865. [DOI] [PubMed] [Google Scholar]
- [74].Reiniger N, Lau K, McCalla D, Eby B, Cheng B, Lu Y, Qu W, Quadri N, Ananthakrishnan R, Furmansky M, Rosario R, Song F, Rai V, Weinberg A, Friedman R, Ramasamy R, D’Agati V, Schmidt AM, Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse, Diabetes 59 (2010) 2043–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Altenhofer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH, Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement, Antioxid. Redox Signal 23 (2015) 406–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS, Effects of NADPH oxidase inhibitor in diabetic nephropathy, Kidney Int. 67 (2005) 1890–1898. [DOI] [PubMed] [Google Scholar]
- [77].Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL, Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE, Am. J. Physiol. Endocrinol. Metab 280 (2001) E685–E694. [DOI] [PubMed] [Google Scholar]
- [78].Adamopoulos C, Mihailidou C, Grivaki C, Papavassiliou KA, Kiaris H, Piperi C, Papavassiliou AG, Systemic effects of AGEs in ER stress induction in vivo, Glycoconj. J 33 (2016) 537–544. [DOI] [PubMed] [Google Scholar]
- [79].Vlassara H, Protein glycation in the kidney: role in diabetes and aging, Kidney Int. 49 (1996) 1795–1804. [DOI] [PubMed] [Google Scholar]
- [80].Pugliese G, Iacobini C, Pesce CM, Menini S, Galectin-3: an emerging all-out player in metabolic disorders and their complications, Glycobiology 25 (2015) 136–150. [DOI] [PubMed] [Google Scholar]
- [81].Canning P, Glenn JV, Hsu DK, Liu FT, Gardiner TA, Stitt AW, Inhibition of advanced glycation and absence of galectin-3 prevent blood-retinal barrier dysfunction during short-term diabetes, Exp. Diabetes Res 2007 (2007) 51837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].McFarlane S, Glenn JV, A.M. L, Characterisation of the advanced glycation endproduct receptor complex in the retinal pigment epithelium, Br. J. Ophthalmol 89 (2005) 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Iacobini C, Menini S, Oddi G, Ricci C, Amadio L, Pricci F, Olivieri A, Sorcini M, Di Mario U, Pesce C, Pugliese G, Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: evidence for a protective role of galectin-3 as an AGE receptor, FASEB J. 18 (2004) 1773–1775. [DOI] [PubMed] [Google Scholar]
- [84].Cai W, Torreggiani M, Zhu L, Chen X, He JC, Striker GE, Vlassara H, AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-delta: implications for vascular disease, Am. J. Phys. Cell Physiol 298 (2010) C624–C634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhuang A, Yap FY, Bruce C, Leung C, Plan MR, Sullivan MA, Herath C, McCarthy D, Sourris KC, Kantharidis P, Coughlan MT, Febbraio MA, Hodson MP, Watt MJ, Angus P, Schulz BL, Forbes JM, Increased liver AGEs induce hepatic injury mediated through an OST48 pathway, Sci. Rep 7 (2017) 12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ma K, Xu Y, Wang C, Li N, Li K, Zhang Y, Li X, Yang Q, Zhang H, Zhu X, Bai H, Ben J, Ding Q, Li K, Jiang Q, Xu Y, Chen Q, A cross talk between class A scavenger receptor and receptor for advanced glycation end-products contributes to diabetic retinopathy, Am. J. Physiol. Endocrinol. Metab 307 (2014) E1153–E1165. [DOI] [PubMed] [Google Scholar]
- [87].Usui HK, Shikata K, Sasaki M, Okada S, Matsuda M, Shikata Y, Ogawa D, Kido Y, Nagase R, Yozai K, Ohga S, Tone A, Wada J, Takeya M, Horiuchi S, Kodama T, Makino H, Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation, Diabetes 56 (2007) 363–372. [DOI] [PubMed] [Google Scholar]
- [88].Wu X, Wu J, Thompson CW, Li Y, Adaptive evolution of the MHC class III-encoded receptor RAGE in primates and murine rodents, Int. J. Immunogenet 42 (2015) 461–468. [DOI] [PubMed] [Google Scholar]
- [89].Engelen L, Stehouwer CD, Schalkwijk CG, Current therapeutic interventions in the glycation pathway: evidence from clinical studies, Diabetes Obes. Metab 15 (2013) 677–689. [DOI] [PubMed] [Google Scholar]
- [90].Kanazawa I, Yamamoto M, Yamaguchi T, Sugimoto T, Effects of metformin and pioglitazone on serum pentosidine levels in type 2 diabetes mellitus, Exp. Clin. Endocrinol. Diabetes 119 (2011) 362–365. [DOI] [PubMed] [Google Scholar]
- [91].Engelen L, Lund SS, Ferreira I, Tarnow L, Parving HH, Gram J, Winther K, Pedersen O, Teerlink T, Barto R, Stehouwer CD, Vaag AA, Schalkwijk CG, Improved glycemic control induced by both metformin and repaglinide is associated with a reduction in blood levels of 3-deoxyglucosone in nonobese patients with type 2 diabetes, Eur. J. Endocrinol 164 (2011) 371–379. [DOI] [PubMed] [Google Scholar]
- [92].Beisswenger P, Ruggiero-Lopez D, Metformin inhibition of glycation processes, Diabetes Metab. 29 (2003) 6S95–103. [DOI] [PubMed] [Google Scholar]
- [93].Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold BS, Metformin reduces systemic methylglyoxal levels in type 2 diabetes, Diabetes 48 (1999) 198–202. [DOI] [PubMed] [Google Scholar]
- [94].Servetnick DA, Bryant D, Wells-Knecht KJ, Wiesenfeld PL, L-Arginine inhibits in vitro nonenzymatic glycation and advanced glycosylated end product formation of human serum albumin, Amino Acids 11 (1996) 69–81. [DOI] [PubMed] [Google Scholar]
- [95].Joglekar MM, Panaskar SN, Chougale AD, Kulkarni MJ, Arvindekar AU, A novel mechanism for antiglycative action of limonene through stabilization of protein conformation, Mol. BioSyst 9 (2013) 2463–2472. [DOI] [PubMed] [Google Scholar]
- [96].Borg DJ, Forbes JM, Targeting advanced glycation with pharmaceutical agents: where are we now? Glycoconj. J 33 (4) (2016) 653–670. [DOI] [PubMed] [Google Scholar]
- [97].Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, Foiles PG, Freedman BI, Raskin P, Ratner RE, Spinowitz BS, Whittier FC, Wuerth JP, Group AII, Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy, Am. J. Nephrol 24 (2004) 32–40. [DOI] [PubMed] [Google Scholar]
- [98].Freedman BI, Wuerth JP, Cartwright K, Bain RP, Dippe S, Hershon K, Mooradian AD, Spinowitz BS, Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II), Control. Clin. Trials 20 (1999) 493–510. [DOI] [PubMed] [Google Scholar]
- [99].Joglekar MM, Bavkar LN, Sistla S, Arvindekar AU, Effective inhibition of protein glycation by combinatorial usage of limonene and aminoguanidine through differential and synergistic mechanisms, Int. J. Biol. Macromol 99 (2017) 563–569. [DOI] [PubMed] [Google Scholar]
- [100].Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB, Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy, Am. J. Nephrol 27 (2007) 605–614. [DOI] [PubMed] [Google Scholar]
- [101].Nascimento MM, Suliman ME, Murayama Y, Nihi M, Hayashi SY, Stenvinkel P, Riella MC, Lindholm B, Effect of high-dose thiamine and pyridoxine on advanced glycation end products and other oxidative stress markers in hemodialysis patients: a randomized placebo-controlled study, J. Ren. Nutr 16 (2006) 119–124. [DOI] [PubMed] [Google Scholar]
- [102].Hollenbach S, Thampi P, Viswanathan T, Abraham EC, Cleavage of in vitro and in vivo formed lens protein cross-links by a novel cross-link breaker, Mol. Cell. Biochem 243 (2003) 73–80. [DOI] [PubMed] [Google Scholar]
- [103].Yang S, Litchfield JE, Baynes JW, AGE-breakers cleave model compounds, but do not break Maillard crosslinks in skin and tail collagen from diabetic rats, Arch. Biochem. Biophys 412 (2003) 42–46. [DOI] [PubMed] [Google Scholar]
- [104].Kim T, Spiegel DA, The unique reactivity of N-phenacyl-derived thiazolium salts toward alpha-dicarbonyl compounds, Rejuvenation Res. 16 (2013) 43–50. [DOI] [PubMed] [Google Scholar]
- [105].Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, Degroof RC, The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure, J. Card. Fail 11 (2005) 191–195. [DOI] [PubMed] [Google Scholar]
- [106].Zieman SJ, Melenovsky V, Clattenburg L, Corretti MC, Capriotti A, Gerstenblith G, Kass DA, Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension, J. Hypertens 25 (2007) 577–583. [DOI] [PubMed] [Google Scholar]
- [107].Hartog JW, Willemsen S, van Veldhuisen DJ, Posma JL, van Wijk LM, Hummel YM, Hillege HL, Voors AA, investigators B, Effects of alagebrium, an advanced glycation endproduct breaker, on exercise tolerance and cardiac function in patients with chronic heart failure, Eur. J. Heart Fail 13 (2011) 899–908. [DOI] [PubMed] [Google Scholar]
- [108].Fujimoto N, Hastings JL, Carrick-Ranson G, Shafer KM, Shibata S, Bhella PS, Abdullah SM, Barkley KW, Adams-Huet B, Boyd KN, Livingston SA, Palmer D, Levine BD, Cardiovascular effects of 1 year of alagebrium and endurance exercise training in healthy older individuals, Circ. Heart Fail 6 (2013) 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sabbagh MN, Agro A, Bell J, Aisen PS, Schweizer E, Galasko D, PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzheimer disease, Alzheimer Dis. Assoc. Disord 25 (2011) 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, Thomas RG, Aisen PS, Alzheimer’s Disease Cooperative S, Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease, Neurology 82 (2014) 1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bongarzone S, Savickas V, Luzi F, Gee AD, Targeting the receptor for advanced glycation endproducts (RAGE): a medicinal chemistry perspective, J. Med. Chem 60 (2017) 7213–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV, A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease, J. Clin. Invest 122 (2012) 1377–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shen C, Ma Y, Zeng Z, Yin Q, Hong Y, Hou X, Liu X, RAGE-specific inhibitor FPS-ZM1 attenuates AGEs-induced neuroinflammation and oxidative stress in rat primary microglia, Neurochem. Res 42 (2017) 2902–2911. [DOI] [PubMed] [Google Scholar]
- [114].Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, DeVita RJ, Ramasamy R, Shekhtman A, Schmidt AM, Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction, Sci. Rep 6 (2016) 22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S, Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression, Horm. Metab. Res 44 (2012) 891–895. [DOI] [PubMed] [Google Scholar]
- [116].Li Q, Xia S, Yin Y, Guo Y, Chen F, Jin P, miR-5591–5p regulates the effect of ADSCs in repairing diabetic wound via targeting AGEs/AGER/JNK signaling axis, Cell Death Dis. 9 (2018) 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Li Y, Zhou Q, Pei C, Liu B, Li M, Fang L, Sun Y, Li Y, Meng S, Hyperglycemia and advanced glycation end products regulate miR-126 expression in endothelial progenitor cells, J. Vasc. Res 53 (2016) 94–104. [DOI] [PubMed] [Google Scholar]
- [118].Dhar A, Udumula MP, Medapi B, Bhat A, Dhar I, Malapati P, Babu MS, Kalra J, Sriram D, Desai KM, Pharmacological evaluation of novel alagebrium analogs as methylglyoxal scavengers in vitro in cardiac myocytes and in vivo in SD rats, Int. J. Cardiol 223 (2016) 581–589. [DOI] [PubMed] [Google Scholar]
- [119].Jahan H, Choudhary MI, Glycation, carbonyl stress and AGEs inhibitors: a patent review, Expert Opin. Ther. Pat 25 (2015) 1267–1284. [DOI] [PubMed] [Google Scholar]
- [120].Rakete S, Linetsky M, Dunbar A, Nagaraj RH, A novel inhibitor against advanced glycation endproduct formation in eye tissues, Invest. Ophthalmol. Vis. Sci 58 (2018) (ARVO E-Abstract 5310). [Google Scholar]
- [121].Jang DS, Yoo NH, Kim NH, Lee YM, Kim CS, Kim J, Kim JH, Kim JS, 3,5-Di-O-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis, Biol. Pharm. Bull 33 (2010) 329–333. [DOI] [PubMed] [Google Scholar]
- [122].Kim JM, Lee YM, Lee GY, Jang DS, Bae KH, Kim JS, Constituents of the roots of Pueraria lobata inhibit formation of advanced glycation end products (AGEs), Arch. Pharm. Res 29 (2006) 821–825. [DOI] [PubMed] [Google Scholar]
- [123].Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM, Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 3381–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, Park BK, Souma T, Moriguchi T, Yamamoto M, Thornalley PJ, Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation, Biochem. J 443 (2012) 213–222. [DOI] [PubMed] [Google Scholar]
- [125].Xue M, Weickert MO, Qureshi S, Kandala NB, Anwar A, Waldron M, Shafie A, Messenger D, Fowler M, Jenkins G, Rabbani N, Thornalley PJ, Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation, Diabetes 65 (8) (2016) 2282–2294. [DOI] [PubMed] [Google Scholar]
- [126].Cheng AS, Cheng YH, Chiou CH, Chang TL, Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells, J. Agric. Food Chem 60 (2012) 9180–9187. [DOI] [PubMed] [Google Scholar]
- [127].Nair S, Xu C, Shen G, Hebbar V, Gopalakrishnan A, Hu R, Jain MR, Lin W, Keum YS, Liew C, Chan JY, Kong AN, Pharmacogenomics of phenolic antioxidant butylated hydroxyanisole (BHA) in the small intestine and liver of Nrf2 knockout and C57BL/6J mice, Pharm. Res 23 (2006) 2621–2637. [DOI] [PubMed] [Google Scholar]
- [128].Zhao S, Ghosh A, Lo CS, Chenier I, Scholey JW, Filep JG, Ingelfinger JR, Zhang SL, Chan JSD, Nrf2 deficiency upregulates intrarenal angiotensin-converting enzyme-2 and angiotensin 1–7 receptor expression and attenuates hypertension and nephropathy in diabetic mice, Endocrinology 159 (2) (2018) 836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sporn MB, Liby KT, NRF2 and cancer: the good, the bad and the importance of context, Nat. Rev. Cancer 12 (2012) 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Distler MG, Plant LD, Sokoloff G, Hawk AJ, Aneas I, Wuenschell GE, Termini J, Meredith SC, Nobrega MA, Palmer AA, Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal, J. Clin. Invest 122 (2012) 2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R, Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial, Lancet 391 (10120) (2017) 541–551. [DOI] [PubMed] [Google Scholar]
- [132].Ajala O, English P, Pinkney J, Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes, Am. J. Clin. Nutr 97 (2013) 505–516. [DOI] [PubMed] [Google Scholar]
- [133].Esposito K, Maiorino MI, Ceriello A, Giugliano D, Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review, Diabetes Res. Clin. Pract 89 (2010) 97–102. [DOI] [PubMed] [Google Scholar]
- [134].Lopez-Moreno J, Quintana-Navarro GM, Delgado-Lista J, Garcia-Rios A, Delgado-Casado N, Camargo A, Perez-Martinez P, Striker GE, Tinahones FJ, Perez-Jimenez F, Lopez-Miranda J, Yubero-Serrano EM, Mediterranean diet reduces serum advanced glycation end products and increases antioxidant defenses in elderly adults: a randomized controlled trial, J. Am. Geriatr. Soc 64 (2016) 901–904. [DOI] [PubMed] [Google Scholar]
- [135].Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H, Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1, Diabetes Care 34 (2011) 1610–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J, Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1, J. Clin. Endocrinol. Metab 94 (11) (2009) 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Vlassara H, Cai W, Tripp E, Pyzik R, Yee K, Goldberg L, Tansman L, Chen X, Mani V, Fayad ZA, Nadkarni GN, Striker GE, He JC, Uribarri J, Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial, Diabetologia 59 (2016) 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Brand-Miller J, Hayne S, Petocz P, Colagiuri S, Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials, Diabetes Care 26 (2003) 2261–2267. [DOI] [PubMed] [Google Scholar]
- [139].Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB, Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis, Am. J. Clin. Nutr 100 (2014) 218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC, Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies, Am. J. Clin. Nutr 87 (2008) 627–637. [DOI] [PubMed] [Google Scholar]
- [141].Chiu CJ, Liu S, Willett WC, Wolever TM, Brand-Miller JC, Barclay AW, Taylor A, Informing food choices and health outcomes by use of the dietary glycemic index, Nutr. Rev 69 (2011) 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ni J, Yuan X, Gu J, Yue X, Gu X, Nagaraj RH, Crabb JW, Plasma protein pentosidine and carboxymethyllysine, biomarkers for age-related macular degeneration, Mol. Cell. Proteomics 8 (2009) 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Chiu CJ, Taylor A, Dietary hyperglycemia, glycemic index and metabolic retinal diseases, Prog. Retin. Eye Res 30 (2011) 18–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chiu CJ, Chang ML, Zhang FF, Li T, Gensler G, Schleicher M, Taylor A, The relationship of major American dietary patterns to age-related macular degeneration, Am J. Ophthalmol 158 (2014) 118–127.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Merle BM, Silver RE, Rosner B, Seddon JM, Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study, Am. J. Clin. Nutr 102 (2015) 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Weikel KA, Chiu CJ, Taylor A, Nutritional modulation of age-related macular degeneration, Mol. Asp. Med 33 (2012) 318–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Weikel KA, Fitzgerald P, Shang F, Caceres MA, Bian Q, Handa JT, Stitt AW, Taylor A, Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets, Invest. Ophthalmol. Vis. Sci 53 (2012) 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rowan S, Jiang S, Korem T, Szymanski J, Chang ML, Szelog J, Cassalman C, Dasuri K, McGuire C, Nagai R, Du XL, Brownlee M, Rabbani N, Thornalley PJ, Baleja JD, Deik AA, Pierce KA, Scott JM, Clish CB, Smith DE, Weinberger A, Avnit-Sagi T, Lotan-Pompan M, Segal E, Taylor A, Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration, Proc. Natl. Acad. Sci. U. S. A 114 (2017) E4472–E4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chiu CJ, Rabbani N, Rowan S, Chang ML, Sawyer S, Hu F, Willett WC, Thornalley PJ, Anwar A, Bar L, Kang JH, Taylor A, Studies of advanced glycation end products and oxidation biomarkers for type 2 diabetes, Biofactors 44 (3) (2018) 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Munch G, Thome J, Foley P, Schinzel R, Riederer P, Advanced glycation end-products in ageing and Alzheimer’s disease, Brain Res. Brain Res. Rev 23 (1997) 134–143. [DOI] [PubMed] [Google Scholar]
- [151].Cuervo AM, Autophagy and aging: keeping that old broom working, Trends Genet. 24 (2008) 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bergamini E, Cavallini G, Donati A, Gori Z, The role of autophagy in aging: its essential part in the anti-aging mechanism of caloric restriction, Ann. N. Y. Acad. Sci 1114 (2007) 69–78. [DOI] [PubMed] [Google Scholar]
- [153].Singletary K, Milner J, Diet, autophagy, and cancer: a review, Cancer Epidemiol. Biomark. Prev 17 (2008) 1596–1610. [DOI] [PubMed] [Google Scholar]
- [154].Whitcomb EA, Chiu CJ, Taylor A, Dietary glycemia as a determinant of health and longevity, Mol. Asp. Med 46 (2015) 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Rodriguez-Navarro JA, Rodriguez L, Casarejos MJ, Solano RM, Gomez A, Perucho J, Cuervo AM, Garcia de Yebenes J, Mena MA, Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation, Neurobiol. Dis 39 (2010) 423–438. [DOI] [PubMed] [Google Scholar]
- [156].Casarejos MJ, Solano RM, Gomez A, Perucho J, de Yebenes JG, Mena MA, The accumulation of neurotoxic proteins, induced by proteasome inhibition, is reverted by trehalose, an enhancer of autophagy, in human neuroblastoma cells, Neurochem. Int 58 (2011) 512–520. [DOI] [PubMed] [Google Scholar]
- [157].Kruger U, Wang Y, Kumar S, Mandelkow EM, Autophagic degradation of tau in primary neurons and its enhancement by trehalose, Neurobiol. Aging 33 (2012) 2291–2305. [DOI] [PubMed] [Google Scholar]
- [158].Lotfi P, Tse DY, di Ronza A, Seymour ML, Martano G, Cooper JD, Pereira FA, Passafaro M, Wu SM, Sardiello M, Trehalose reduces retinal degeneration, neuroinflammation and storage burden caused by a lysosomal hydrolase deficiency, Autophagy 14 (8) (2018) 1419–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B, Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis, Nature 481 (2012) 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]