Abstract
Chimeric antigen receptor (CAR)-engineered T cells are efficacious in controlling advanced leukemia and lymphoma, however, they fail in the treatment of solid cancer, which is thought to be due to insufficient T cell activation. We revealed that the immune response of CAR T cells with specificity for carcinoembryonic antigen (CEA) was more efficacious against CEA+ cancer cells when simultaneously incubated with an anti-CD30 immunotoxin or anti-CD30 CAR T cells, although the targeted cancer cells lack CD30. The same effect was achieved when the anti-CD30 single-chain variable fragment (scFv) was integrated into the extracellular domain of the anti-CEA CAR. Improvement in T cell activation was due to interfering with the T cell CD30-CD30L interaction by the antagonistic anti-CD30 scFv HRS3; an agonistic anti-CD30 scFv or targeting the high-affinity interleukin-2 (IL-2) receptor was not effective. T cells with the anti-CD30/CEA CAR showed superior immunity against established CEA+ CD30− tumors in a mouse model. The concept is broadly applicable since anti-CD30/TAG72 CAR T cells also showed improved elimination of TAG72+ CD30− cancer cells. Taken together, targeting CD30 on CAR T cells by the HRS3 scFv within the anti-tumor CAR improves the redirected immune response against solid tumors.
Keywords: CAR, CD30, CEA, adoptive cell therapy, carcinoembryonic antigen, chimeric antigen receptor, CAR T cell therapy
Graphical Abstract
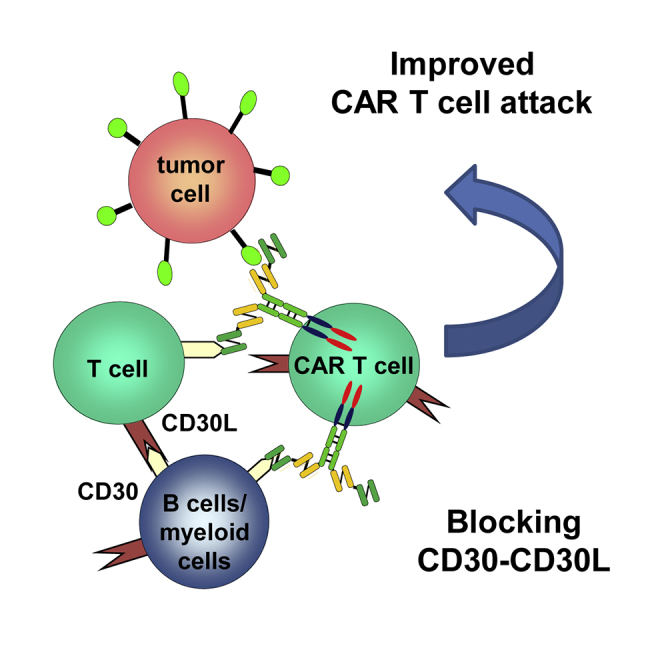
Hombach et al. found that simultaneous targeting of CD30 on T cells and a tumor-associated antigen (TAA) on solid cancer cells by a bispecific CAR with an integrated anti-CD30-binding domain improved the activation and anti-tumor activity of redirected CAR T cells against solid cancer cells.
Introduction
The success of adoptive cell therapy with chimeric antigen receptor (CAR)-modified T cells depends on the CAR T cell persistence and lasting activation; in the case of targeting B cell leukemia and lymphomas,1, 2 on-target off-tumor depletion of healthy B cells3 likely contributes to the sustained CAR T cell activation and, finally, to lasting control of the disease. In contrast to targeting hematologic malignancies, the efficacy of CAR T cell therapy against solid tumors is, so far, poor, and the T cells rapidly enter anergy.4 Tremendous efforts are currently made to sustain CAR T cell activation upon antigen engagement and to counteract physiologic response repression in order to improve the therapeutic efficacy against solid cancer.
T cell activation by the CAR or physiologically through the T cell receptor (TCR)/CD3 is orchestrated by a cascade of signaling events, which are inducing effector functions and, with some delay, are counteracting through numerous inhibitory ligands to limit the process. Early upon activation, CD30 is transiently expressed on the surface of certain T cell subsets,5 followed by a plethora of other activation-associated receptors. CD30 is also known as a tumor-associated antigen expressed by Hodgkin’s lymphoma cells and cells of other B and T cell malignancies;5, 6, 7 CD30 is, therefore, used as a target for CAR T cell therapy.8
Physiologically, CD30 is involved in the initiation of a productive immune response of mature T and B cells and in the initiation of hematopoiesis, which became obvious upon cytokine-mediated entry of bone marrow-derived CD34+ stem cells into maturation.9 The role of CD30 during these processes is pleiotropic and not yet fully understood.10 While primarily recognized as a Th2 marker defining interleukin-4 (IL-4) responsiveness of T cells,11, 12 CD30 is also expressed by Th1 cells upon activation,13, 14 reflecting a more general role during hematopoietic activation. Once initiated, the activation process is tightly regulated by reciprocal signaling through the CD30 ligand CD153 (CD30L). Transiently expressed by cells of the innate and adaptive immune systems, CD30L interacts with freshly activated CD30+ T cells to reduce the level of activation, indicating the CD30-CD30L interaction as an early checkpoint in regulating the lymphocyte response. To prolong T cell activation early after initiation, we asked whether CD30 targeting positively affects CAR T cell immunity in general and in particular during an attack against solid cancer cells that lack CD30.
To address the issue, we targeted CD30 during the initiation of a CAR-redirected T cell response by an anti-CD30 immunotoxin or anti-CD30 CAR T cells. Assuming that the CD30-CD30L interaction drives freshly activated T cells to limit their immune response, we designed a CAR that, in addition to a cancer recognition domain, also harbors a CD30-targeting domain to disrupt the CD30-CD30L interaction on freshly activated lymphocytes. T cells with such a combined tumor-targeting and T cell-instructing CAR showed improved cytotoxic activity against CD30-negative, non-hematopoietic cancer cells in vitro and in a mouse model. The data draw a novel concept in adoptive cell therapy based on providing two capacities by a single CAR, one being cancer cell targeting and the other being T cell de-repressing. This is all in order to improve anti-tumor immunity.
Results
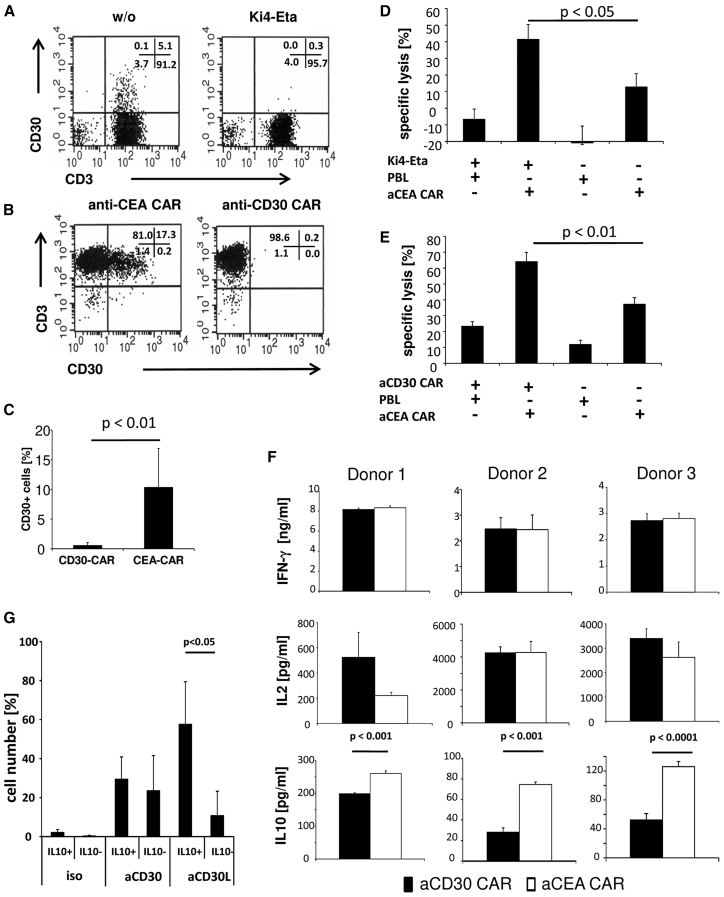
We asked whether CD30 targeting during CAR-redirected T cell activation impacts the tumor-specific immune response. To address the issue, we took advantage of the anti-CD30 immunotoxin Ki4-Eta15 and the CD30-specific CAR,16 which both were previously characterized with respect to their targeting specificity and capacity to eliminate CD30+ cells. Incubating activated human blood lymphocytes with the anti-CD30 immunotoxin eliminated the entire subset of CD30+ cells (Figure 1A). The same effect was achieved by co-incubating the lymphocytes with cytolytic T cells redirected by the anti-CD30 CAR (Figures 1B and 1C).
Figure 1.
CD30 Targeting Enhances Antigen-Specific Cytolysis by Anti-CEA CAR T Cells
(A–C) Targeting of CD30 by anti-CD30 immunotoxin or anti-CD30 CAR T cells resulted in the depletion of CD30+ T cells. Peripheral blood T cells were activated by CD3/CD28 stimulation, and they were incubated for 48 h in the presence or absence of the anti-CD30 immunotoxin Ki4-Eta (1 μg/mL) (A) or T cells engineered with first-generation anti-CD30 and anti-CEA CARs, respectively (B). CD30 expression by T cells in the presence of anti-CD30 immunotoxin (A) or anti-CD30 CAR T cells (B and C) was determined by flow cytometry, and the mean values of CD30+ cells of 5 healthy donors in the presence of anti-CD30 or anti-CEA CAR T cells were determined (C). (D and E) Target cell lysis of CEA+ tumor cells upon depletion of CD30+ lymphocytes. (D) Anti-CEA CAR T cells (2.5 × 103 anti-CEA CAR T cells/well) were co-cultivated for 48 h with CEA+ LS174T or CEA− Colo320 tumor cells (each 5 × 104 cells/well) in the presence of 1 μg/mL anti-CD30 Ki4-Eta immunotoxin. (E) Anti-CD30 (3 × 103/well) and anti-CEA CAR T cells (7.5 × 103/well) were co-cultivated with CEA+ LS174T or CEA− Colo320 tumor cells (each 5 × 104 cells/well) for 48 h as described above. Viability was determined by the XTT assay and target cell lysis was calculated. Data represent the mean of replicates ± SD. A representative experiment is shown. (F) CD30 targeting by CAR T cells reduces IL-10, but not IFN-γ or IL-2 secretion. Peripheral blood lymphocytes were engineered with first-generation anti-CD30 or anti-CEA CARs, and they were incubated for 48 h in microtiter wells (5 × 104 cells/well, 5 × 103 CAR T cells/well) that were coated with agonistic anti-CD3 and anti-CD28 mAbs (each 1 μg/mL). Supernatants were recovered and analyzed for IFN-γ, IL-2, and IL-10 secretion by ELISA. Data represent the means of technical replicates of three different healthy donors ± SD. (G) IL-10-secreting cells express high CD30L. T cells were activated as described in the Materials and Methods, cultivated for 72 h, and stimulated for 12 h with anti-CD3 and anti-CD28 mAbs (1 μg/mL each). IL-10 secretion was determined by the IL-10 secretion assay, and cells were additionally stained with anti-CD3, anti-CD30, and anti-CD30L mAbs. Cells were analyzed by flow cytometry and gates set for IL-10+ and IL-10− cells. Data represent mean values of 3 healthy donors ± SD. Significant differences were calculated by the Student’s t test.
To explore whether CD30 targeting impacts the CAR-redirected T cell attack against CD30-negative target cells, anti-carcinoembryonic antigen (CEA) CAR T cells were incubated with CEA+CD30− cancer cells in the presence of either anti-CD30 immunotoxin or anti-CD30 CAR T cells. The elimination of CAR-targeted CD30− cancer cells was more efficient when CD30+ cells from the T cell pool were targeted by Ki4-Eta or anti-CD30 CAR T cells (Figures 1D and 1E). Since the targeted cancer cells did not express CD30, but eliminating CD30+ CAR T cells improved the anti-tumor cell attack, we conclude that CD30+ T cells have a crucial role in repressing the CAR T cell immunity against cancer cells. The effect was independent of how the CD30+ T cells were eliminated, i.e., by a CD30-specific immunotoxin or CAR T cells.
We asked whether CD30 targeting in a CAR T cell population impacts the secretion of pro-inflammatory cytokines like interferon (IFN)-γ and interleukin-2 (IL-2), which mediate the immune control of tumors,17 or of anti-inflammatory cytokines like IL-10, which is central for T regulatory type-1 (Tr1) cells and immune homeostasis.18, 19 In the presence of anti-CD30 CAR T cells, the anti-CEA CAR T cell response was accompanied by a decrease in IL-10 release, while the IFN-γ and IL-2 releases were not altered (Figure 1F). Reduced IL-10 levels in the presence of anti-CD30 CAR T cells may be due to the repressive CD30-CD30L interaction. Accordingly, we found high CD30L levels in IL-10-secreting T cells, while the CD30 levels were similar in IL-10+ and IL-10− T cells (Figure 1G). The data suggested that reduced IL-10 secretion was due to interfering with the regulatory CD30-CD30L interaction.
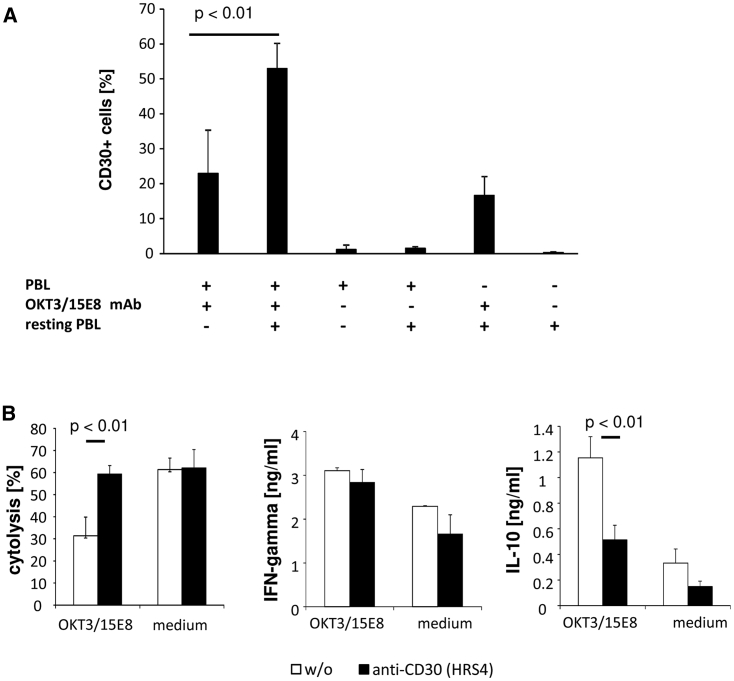
The expressions of CD30 and its ligand CD30L are tightly regulated in time upon T cell activation. CD30L appears rapidly upon CD3/CD28 signaling on the T cell surface, peaking within 24 h of the onset of activation followed by an increase in CD30,20 with a maximum after 48 h,21 implying that the CD30L-CD30 interaction limits the primary immune response. We accordingly observed high CD30 levels after secondary CD3/CD28 stimulation of previously activated T cells in the presence of resting autologous or allogeneic lymphocytes (Figure 2A). Moreover, CD30 upregulation after combined primary and secondary stimulations depended on the presence of primarily activated T cells (Figure 2A).
Figure 2.
Blocking of Enhanced CD30 Expression Improved a CAR T Cell Attack
(A) Simultaneous primary and secondary T cell activations by CD3 and CD28 signaling resulted in enhanced CD30 expression by T cells. T cells from healthy donors (n = 4) were activated by adding the agonistic anti-CD3 and anti-CD28 mAbs (each 100 ng/mL) and further cultivated in the presence of 500 U/mL IL-2. After 7 days, cells were re-stimulated in microtiter plates in the presence or absence of resting peripheral blood lymphocytes (PBLs) (each 5 × 104 cells/well) by solid-phase bound anti-CD3 and anti-CD28 mAbs (coating concentration each 1 μg/mL). For comparison, T cells were co-cultivated without stimulation; resting PBLs of two donors were cultivated in the absence of pre-activated T cells. Data represent mean values ± SD. (B) Blocking of CD30 during primary and secondary T cell stimulations resulted in enhanced target cell lysis by anti-CEA CAR T cells. Anti-CEA CAR T cells and non-modified T cells for control (each 1.25 × 104 cells/well) were co-cultivated with CEA+ LS174 tumor cells (2.5 × 104 cells/well) in the presence of freshly isolated autologous PBLs (1.25 × 104 cells/well) in microtiter plates that were coated with 1 μg/mL anti-CD3 and anti-CD28 mAbs. To block CD30 signaling, saturating amounts of the blocking anti-CD30 mAb HRS4 were added. For control, the assay was performed without anti-CD3/CD28 stimulation. Viability of target cells was determined by the XTT assay and cytolysis was calculated. IL-10 and IFN-γ secretion into supernatants was determined by ELISA. A representative experiment of 3 is shown. Data represent mean values ± SD. Significant differences were determined by the Student’s t test.
We asked whether CD30 induction upon secondary stimulation impairs the cytotoxic immune response of CAR T cells. We recorded the lysis of CEA+ LS174T tumor cells by anti-CEA CAR T cells in the presence of added resting lymphocytes and agonistic CD3/CD28 stimulation to induce CD30. As summarized in Figure 2B, the cytotoxic activity of anti-CEA CAR T cells decreased under conditions of high CD30 levels. The cytotoxic activity was restored in the presence of saturating amounts of the anti-CD30 antibody HRS4, which interferes with CD30-CD30L binding.22 Concomitantly, IL-10 levels decreased whereas IFN-γ secretion was not affected. The data sustained our hypothesis that blocking the CD30-CD30L interaction during activation improves T cell effector functions.
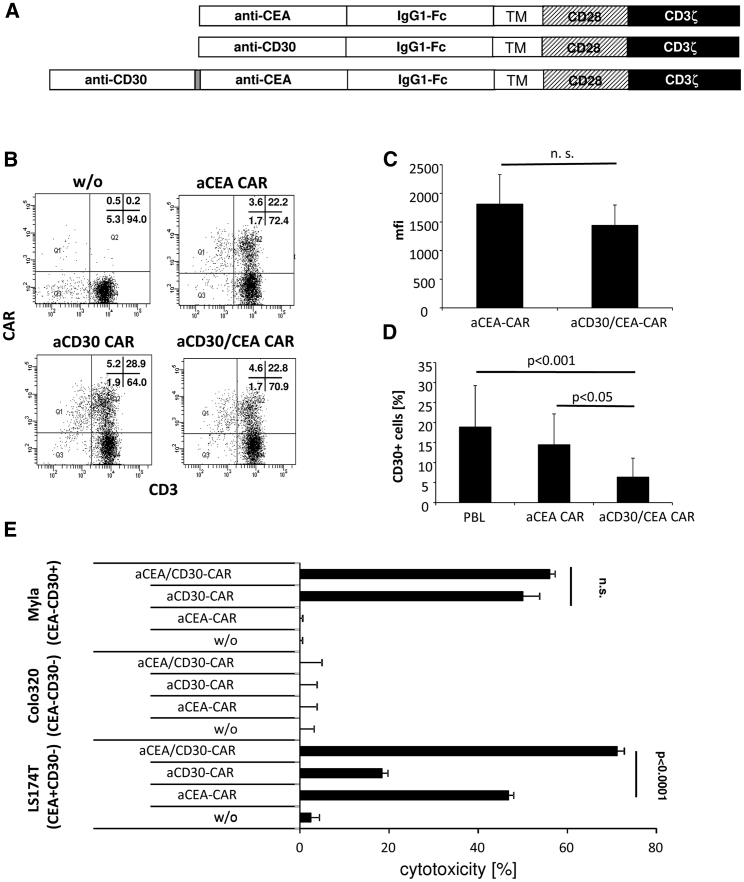
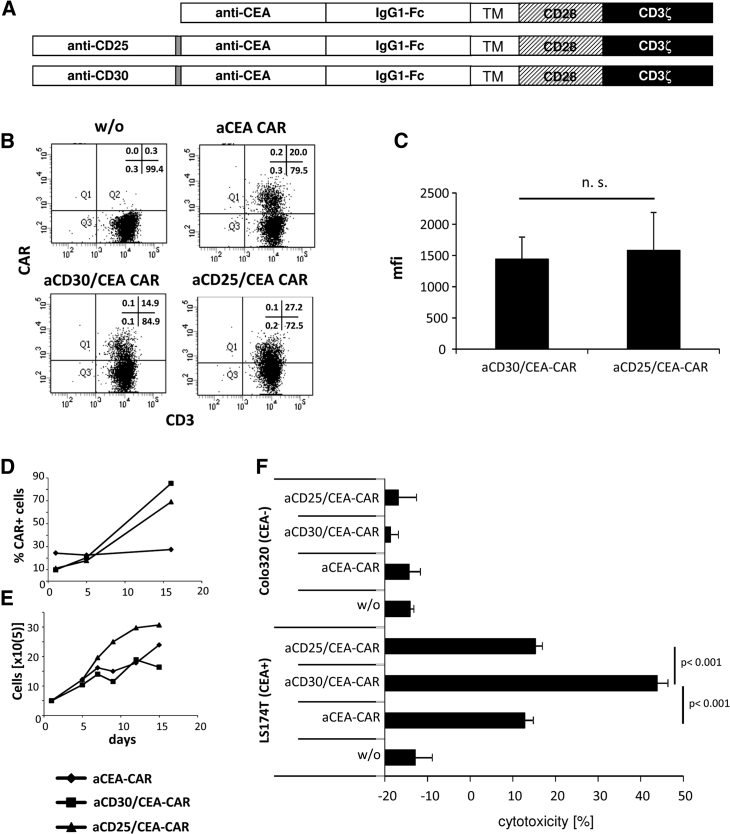
We asked whether targeting a cancer cell-associated antigen like CEA can be combined with CD30 targeting and an anti-CD30/CEA bispecific CAR in order to improve CAR-mediated lysis of CEA+CD30− cancer cells. The combined anti-CD30/CEA CAR, as schematically shown in Figure 3A, was expressed on the T cell surface, although at slightly lower levels than the individual anti-CEA CAR (Figures 3B and 3C). The number of CD30+ T cells decreased to a similar extent when engineered with the anti-CD30/CEA CAR (Figure 3D) or incubated with anti-CD30 CAR T cells (cf. Figure 1B). T cells engineered with the anti-CD30/CEA CAR eliminated both CD30+ CEA− and CD30− CEA+ tumor cells. Whereas T cells with the respective monospecific CAR lysed only tumor cells with the respective antigen (Figure 3E), the bispecific CAR T cells lysed CD30+ and CEA+ tumor cells, indicating that the anti-CD30/CEA CAR provided specificity for both antigens. Of note, CEA+ tumor cells were more efficiently eliminated by T cells with the anti-CD30/CEA CAR than by anti-CEA CAR T cells without the CD30-targeting domain. In contrast, T cells with the anti-CD30 and the anti-CD30/CEA CAR, respectively, eliminated CD30+ tumor cells with similar efficiencies. We concluded that targeting CD30 by the combined anti-CD30/CEA CAR improves the T cell elimination of CEA+ cancer cells.
Figure 3.
Dual Targeting of CD30 and CEA by Bispecific CAR T Cells Reduced CD30 Expression by T Cells and Enhanced Lysis of CEA+ Cancer Cells
(A) Schematic representation of the anti-CD30 and anti-CEA CARs. TM, transmembrane domain derived from CD28. (B and C) Expression of mono- and bispecific anti-CEA CARs by T cells. Peripheral blood T cells from healthy donors were retrovirally transduced to express the anti-CD30, anti-CEA, and anti-CD30/CEA CARs, as described in the Materials and Methods. The CAR on T cells was recorded by staining with anti-CD3 and anti-human IgG Fc antibodies, and analysis was by flow cytometry. Representative dot plots of T cells from one donor are shown (B), and mean fluorescence intensities (mfis) of 6 healthy donors were determined (C). Data represent the mean values ± SD. (D) CD30-specific CAR T cells downregulate CD30 expression. Non-transduced and anti-CEA or anti-CD30/CEA CAR T cells, respectively, of 8 different donors were cultivated in the presence of 500 U/mL IL-2. Cells were assayed for CD30 by staining with a fluorescein isothiocyanate (FITC)-conjugated anti-CD30 mAb 1–3 days after transduction, and they were analyzed by flow cytometry. Data represent the mean values ± SD. (E) Anti-CD30/CEA CAR T cells lyse specifically CD30+ and CEA+ target cells. Anti-CEA, anti-CD30, and anti-CD30/CEA CAR T cells (1.25 × 104/well) were co-cultivated for 48 h with CEA−CD30+ MyLa, CEA−CD30− Colo320, or CEA+CD30− LS174T tumor cells (each 2.5 × 104/well). Viability of target cells was determined by the XTT assay and cytolysis was calculated. Data represent mean values of technical replicates ± SD of a representative experiment. Significant differences were determined by the Student’s t test.
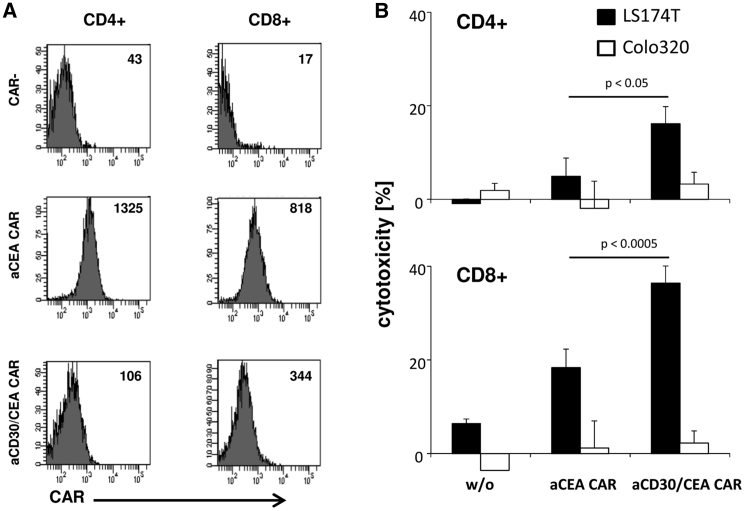
To exclude that the improvement in eliminating CEA+ cancer cells by anti-CD30/CEA CAR T cells is due to superior expansion of a T cell subset, we isolated CD4+ and CD8+ CAR T cells, respectively, by flow sorting (Figure 4A). Both CD4+ and CD8+ cells expressed the anti-CD30/CEA CAR at slightly lower levels compared with the anti-CEA CAR. However, the elimination of targeted CEA+ tumor cells by T cells with the anti-CD30/CEA CAR was substantially more efficient than by T cells with the anti-CEA CAR; the observation holds for CD4+ and CD8+ T cells (Figure 4B). The data confirmed that both CD4+ and CD8+ T cells redirected by the anti-CD30/CEA CAR exhibited improved lysis of CD30− target cells that was not due to higher CAR levels on the cell surface of anti-CD30/CEA CAR T cells.
Figure 4.
Bispecific CD4+ and CD8+ CAR T Cells Showed Improved Lysis of CEA+ Target Cells
(A) Anti-CEA and anti-CD30/CEA CAR T cells were labeled with antibodies specific for CD4, CD8, and human IgG1, which detects the CAR. Labeled cells were flow sorted in CD4+ or CD8+ cells with or without CAR, and the mfi of CAR expression was determined. (B) Purified CD4+ and CD8+ CAR T cell populations (3.75 × 103/well) were co-cultivated for 48 h with CEA− Colo320 or CEA+ LS174T tumor cells (each 2.5 × 104/well). Viability of target cells was determined by the XTT assay and cytolysis was calculated. Data represent mean values of technical replicates ± SD; one representative experiment of two is shown. Significant differences were determined by the Student’s t test.
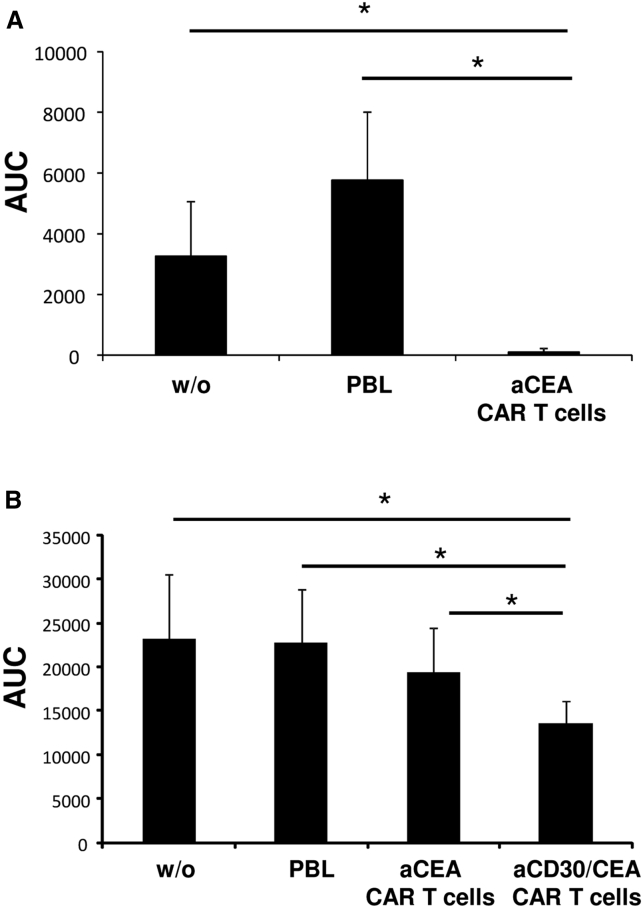
The in vitro data were confirmed by CAR T cell killing of cancer cells in vivo. Higher numbers of CEA CAR T cells efficiently suppressed tumor growth of the CEA+ colorectal cancer line LS174T (Figure 5A); however, they were not effective at a lower dose against the more aggressive growing CEA+ cancer cell line C15A3 (Figure 5B). At low doses, tumor establishment of C15A3 cancer cells was more efficiently prevented by T cells with the combined anti-CD30/CEA CAR than by T cells with the anti-CEA CAR (Figure 5B), again demonstrating improved anti-tumor cell activities of the anti-CD30/CEA CAR T cells.
Figure 5.
Anti-CD30/CEA CAR T Cells Show Superior Cancer Cell Killing In Vivo
(A and B) T cells with and without CAR (A, 5 × 106 cells/mouse, 20% CAR+; B, 2.5 × 105 cells/mouse, 30% CAR +) were co-injected with LS174T (A, 2.5 × 106/mouse) or C15A3 tumor cells (B, 106 cells/mouse) into Rag−/− cg−/− mice. Tumor volume was determined every 2–3 days until day 32. Areas under the curve (AUCs) were calculated and the mean values of 6 mice were determined. Significant differences were determined by Student's t test.
To address whether the CAR with anti-CD30 domain improved the elimination of cancer cells also when targeting other tumor-associated antigens, we engineered an anti-CD30/TAG72 CAR for targeting TAG72+ cancer cells (Figure S1A). The monospecific anti-TAG72 CAR served for comparison. T cells with the anti-CD30/TAG72 CAR showed reduced CD30 levels compared with anti-TAG72 CAR T cells (Figure S1B). Moreover, TAG72+ tumor cells were eliminated with substantially higher efficiency by anti-CD30/TAG72 CAR T cells than by anti-TAG72 CAR T cells (Figure S1C). Taken together, redirecting CAR T cells by a CAR specific for the T cell activation antigen CD30 and a tumor-associated antigen improved the elimination of CD30− cancer cells.
CD30 signaling is associated with T cell activation; we asked whether targeting of any activation-induced antigen on T cells in addition to targeting a cancer-associated antigen results in improved cytotoxic activities of CAR T cells. We therefore replaced the anti-CD30 single-chain variable fragment (scFv) by a scFv for CD25, since the high-affinity IL-2 receptor CD25 is highly upregulated by T cells upon activation (Figure 6A). The anti-CD25/CEA CAR was expressed in a similar fashion as the anti-CD30/CEA CAR (Figures 6B and 6C). As summarized in Figure 6D, CAR T cells with the anti-CD25/CEA and anti-CD30/CEA CAR persisted with higher frequencies compared with anti-CEA CAR T cells after stimulation. Strikingly anti-CD25/CEA CAR T cells expanded more rapidly than T cells with the anti-CEA and anti-CD30/CEA CAR. The data implied a relatively low fratricide activity and/or a high level of stimulation of anti-CD25/CEA CAR T cells, due to the abundance of the targeted CD25. Noteworthy, the cytotoxic activity of T cells with the anti-CD25/CEA CAR against CEA+ LS174T cells was in the same range as T cells with the anti-CEA CAR; however, the activity of the anti-CD30/CEA CAR T cells was substantially higher (Figure 6F). We concluded that the increase in cytotoxic activity of CAR T cells is specifically associated with targeting CD30; targeting any activation-associated protein like CD25 does not have the same effect but accelerated CAR T cell expansion in the long term.
Figure 6.
Enhanced Lysis of CEA+ Tumor Cells Depends on Simultaneous CD30, but Not CD25, Targeting
(A) Schematic representation of anti-CD30/CEA and anti-CD25/CEA CARs. (B and C) T cells express the anti-CD30/CEA and anti-CD25/CEA CAR with similar efficiency on the cell surface. T cells from the peripheral blood were grafted with anti-CD30/CEA and anti-CD25/CEA CARs, as described in the Materials and Methods, and CAR expression was recorded by flow cytometry utilizing anti-CD3 and anti-human IgG antibodies, respectively. Numbers of CD3+CAR+ T cells and mean fluorescence intensities (mfis) of CAR expression were determined. A typical dot plot (B) and mean values of CAR expression (mfis) of 5 healthy donors are shown (C). (D and E) Anti-CD30/CEA and anti-CD25/CEA CAR T cells persist similarly but expand differentially during prolonged cultivation. Anti-CEA, anti-CD30/CEA, and anti-CD25/CEA CAR T cells were cultivated in the presence of 100 U/mL IL-2 and recorded for viable CAR+ T cells by flow cytometry (D). The total number of viable cells was recorded by cell counting (E). Dead cells were excluded by trypan blue exclusion. A representative experiment of two is shown. (F) T cells with the anti-CD25/CEA, anti-CD30/CEA, and anti-CEA CAR, respectively, and non-modified T cells (w/o) for control (5 × 103 CAR T cells/well), were co-cultivated for 48 h with CEA+ LS174T and CEA− Colo320 tumor cells (each 2.5 × 104 cells/well). Viability of target cells was determined by the XTT assay and cytolysis was calculated. Data represent mean values of technical replicates of a representative experiment of two ± SD. Statistical analysis was performed using the Student’s t test.
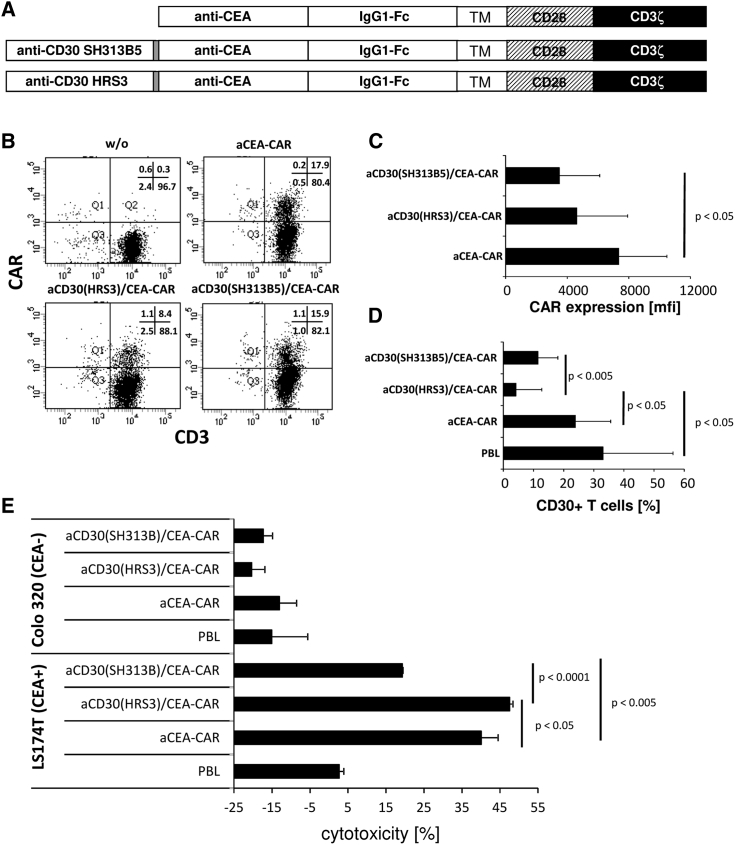
To address the mode of action of the anti-CD30 scFv in the context of the CEA-targeting CAR, we replaced the HRS3-derived anti-CD30 scFv with the anti-CD30 scFv SH313B5 (Figure 7A). The SH313B5 scFv has CD30L-like activities, as it inhibits the amplification of CD30+ Karpas 299 cells23 in a similar fashion as soluble CD30L.24 The anti-CD30/CEA CARs and, for comparison, the anti-CEA CAR were expressed by T cells. The anti-CD30(SH313B5)/CEA CAR was expressed with similar efficiency as the anti-CD30(HRS3)/CEA CAR by T cells but at lower levels than the anti-CEA CAR (Figures 7B and 7C). More importantly, the anti-CD30(SH313B5)/CEA CAR was not as efficient as the anti-CD30(HRS3)/CEA CAR in removing CD30+ lymphocytes (Figure 7D). Co-incubation of CEA+ LS174T cancer cells with T cells expressing the SH313B5 scFv CAR showed reduction, while T cells with the HRS3 scFv CAR increased the cytolytic activity against cognate target cells (Figure 7E). Since the HRS3 antibody belongs to the group of non-agonistic anti-CD30 antibodies, whereas SH313B5 is an agonistic anti-CD30 antibody,25 we assumed that CD30 blocking, rather than activating, results in improved target cell lysis.
Figure 7.
Enhanced Lysis of CEA+ Tumor Cells Depends on the CD30-Blocking HRS3-scFv, but Not the CD30-Activating SH31355B scFv, Domain within the anti-CD30/CEA CAR
(A) Schematic representation of anti-CD30/CEA CARs. (B and C) T cells express the anti-CD30(HRS3)/CEA and anti-CD30(SH313B5)/CEA CAR with similar efficiency on the cell surface. T cells from the peripheral blood were grafted with anti-CD30/CEA CARs, and CAR expression was recorded by flow cytometry utilizing anti-CD3 and anti-human IgG antibodies, respectively. Numbers of CD3+CAR+ T cells and mean fluorescence intensities (mfis) of CAR expression were determined. A typical dot plot (B) and mean values of CAR expression (mfis) (C) of 5 healthy donors are shown. (D) Anti-CD30(HRS3)/CEA and anti-CD30(SH313B5)/CEA CAR T cells suppress CD30 expression with different efficiencies. Peripheral blood T cells were grafted with CARs as described above, and they were analyzed 24 h after transduction for the expression of CD30 by flow cytometry, utilizing anti-CD3 and anti-CD30 mAbs, respectively. Data represent mean values ± SD of 5 healthy blood donors. (E) Anti-CD30(SH313B)/CEA CAR and anti-CD30(HRS3)/CAR T cells mediated an anti-CEA CAR T cell attack with different efficiencies. Anti-CD30/CEA CAR T cells (7.5 × 103 CAR T cells/well) were co-cultivated for 48 h with CEA+ LS174T or CEA− Colo320 tumor cells (each 2.5 × 104 cells/well). T cells with the anti-CEA CAR and cells without CAR (PBLs), respectively, served as controls. Viability of target cells was determined by the XTT assay and cytolysis was calculated. Data represent the mean values of technical replicates of a representative experiment of three ± SD. Significant differences were determined by the Student’s t test.
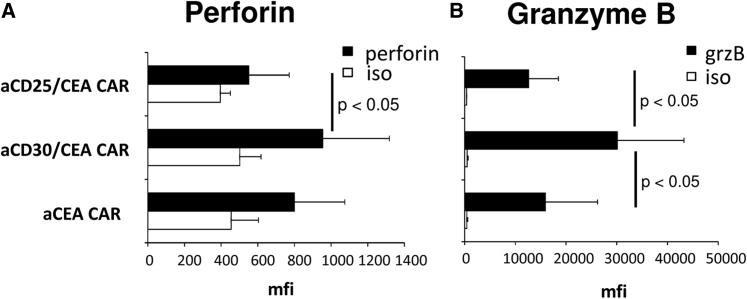
CD30 is reported to downregulate cytotoxic effector molecules in T cells upon CD30L binding.26 We asked whether targeting CD30 by the antagonistic anti-CD30 scFv HRS3 in the context of the anti-CEA CAR impacts the levels of perforin and granzyme B. As summarized in Figures 8A and 8B, the levels of perforin and, in particular, granzyme B were substantially enhanced in T cells with the anti-CD30/CEA CAR, but not in T cells with either the anti-CD25/CEA or the anti-CEA CAR. The data demonstrated that blocking the negative regulator CD30 on T cells improved the cytolytic capacity of CAR-redirected T cells.
Figure 8.
Anti-CD30/CEA CAR T Cells Express Higher Amounts of Cytotoxic Effector Molecules
(A and B) T cells with the anti-CD30/CEA (n = 9), anti-CD25/CEA (n = 5), and anti-CEA CAR (n = 9), respectively, were stained for perforin (A) and granzyme B (grzB) (B) expressions or for control with an isotype-matched antibody of irrelevant specificity (iso). Cells were analyzed by flow cytometry and mfis were determined. Data represent the mean values ± SD. Significant differences were determined using the Student’s t test.
Discussion
Successful CAR T cell therapy of malignant diseases requires a sustained cytotoxic anti-tumor response by activated T cells. The currently non-satisfying results in trials for the treatment of solid tumors point to the necessity to further improve and prolong the T cell response upon primary antigen engagement and activation through the CAR. Based on the growing evidence for the role of CD30 in modulating the immune response,27, 28 the approach described here combines targeting of a cancer cell antigen with the interference with the immune regulator CD30 in order to amplify the cytotoxic anti-tumor reaction. This is achieved by a dual targeting CAR that blocks CD30 along with targeting a tumor-associated antigen. T cells with a CD30-blocking and tumor-targeting CAR shape a specific immune landscape by at least two non-exclusive mechanisms: (1) CAR T cells are stimulated through CD30+ cells in the tumor tissue independently of cancer cells, and (2) eliminating CD30+ T cells prevents the inhibition of the cytotoxic T cell response. Blocking CD30 is required to improve the T cell response, since an agonistic anti-CD30 antibody proved ineffective in this respect; any agonistic anti-CD30 antibody targeting the same domain as HRS3 may likewise be suitable. The combined immune-blocking and/or cancer-targeting anti-CD30/CEA CAR thereby represents a new generation of smart CARs, which shape in cis the T cell immunity toward a more favorable and lasting response while targeting in trans the cancer cells in a tumor-specific fashion.
There is growing evidence that CD30 has a regulatory role during the secondary immune response. This is underlined by the correlation of CD30+ immune cells with worse prognosis in solid cancer, including colorectal cancer29 and melanoma,27 which goes along with a repressed cellular immune response.27, 28 CD30 seems to be more globally involved in counter-regulating the cellular response, since it is expressed by both Th2 as well as Th1 T cells;30 repetitive CD30 signaling promotes the development of Th2 T cells.31 On the other hand, blocking CD30-CD30L signaling by soluble CD30 or by a CD30L-blocking antibody inhibited the differentiation toward Th2 cells.31 Accordingly, we found a more pro-inflammatory response and an improved CAR T attack directed by the combined CAR with the antagonistic anti-CD30 monoclonal antibody (mAb), interfering with CD30L-CD30 signaling, than with the agonistic anti-CD30 mAb.
During T cell activation, CD30 and its ligand CD30L are transiently expressed in an orchestrated fashion, with CD30L peaking earlier than CD30.21 We moreover found that, in the presence of resting allogeneic or autologous T cells, secondary anti-CD3/CD28 stimulation increased the number of CD30+ T cells, suggesting a role for CD30 in downregulating the acute reaction and shifting an ongoing T cell response toward Th2 cells during the second wave of T cell activation. This is supported by the particular cross-talk between CD30L on malignant cells with activated CD30+ T cells; CD30L+ acute myeloid leukemia (AML) blasts upregulate CD30 and induce IL-4 secretion by T cells,20, 32 along with inhibiting T cell proliferation33 and downregulating their cytotoxic effector molecules.26 Accordingly, we found higher levels of granzyme B in T cells after blocking CD30 or eliminating CD30+ cells by anti-CD30 CAR T cells. The increase in granzyme B levels was specific, since CAR T cell targeting CD25, which also results in T cell stimulation, did not produce higher levels of the cytotoxic effector molecules.
Consequently, the cytotoxic activity of CAR T cells is enhanced upon depleting CD30+ lymphocytes by a CD30-specific immunotoxin or CD30-specific CAR T cells. The effect can be combined with tumor targeting, as shown for targeting CEA or TAG72 on colorectal cancer cells through the anti-CD30/CEA or anti-CD30/TAG72 CAR, moreover demonstrating the generality of the strategy. Taken together, the data indicate superior anti-tumor capacities of redirected CAR T cells upon blocking CD30, which can be achieved by a combined CD30-blocking and cancer-targeting CAR.
Materials and Methods
Cell Lines and Reagents
HEK293T cells are cells that express the SV40 large T antigen.34 The colon carcinoma cell lines Colo320 (ATCC CCL 220.1) and LS174T (ATCC CL-188) were obtained from ATCC (Rockville, MD, USA). The colon cancer cell line LS-C with high TAG72 expression was kindly provided by Dr. S.H. Itzkowitz,35 Mount Sinai School of Medicine, New York, USA, and the CD30+ lymphoma line MyLa was kindly provided by professor R. Dummer, Inselspital, Zurich, Switzerland. All cell lines were cultured in RPMI 1640 medium and 10% (v/v) fetal bovine serum (FBS) (Life Technologies, Paisly, UK).
Anti-CD3 mAb OKT3 and anti-CD28 mAb 15E8 were purified from OKT3 hybridoma (ATCC CRL 8001) and 15E8 hybridoma (kindly provided by Dr. R. van Lier, Red Cross Central Blood Bank, Amsterdam, the Netherlands) supernatants, respectively, by affinity chromatography. The anti-HRS3 idiotypic antibody 9G10 and the anti-CD30 mAbs HRS3 and HRS4 were described earlier.25, 36 HRS4 and HRS3 mAbs share reactivity with a set of internal image anti-idiotypic mAbs,37, 38 indicating recognition of identical epitopes. The phycoerythrin (PE)-conjugated F(ab')2 goat anti-human immunoglobulin G (IgG) antibody was purchased from Southern Biotechnology. Fluorochrome-conjugated anti-CD3, anti-CD4, anti-CD8, and anti-CD30 mAbs were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). The allophycocyanin (APC)-conjugated anti-CD30L mAb was purchased from R&D Systems (Minneapolis, MN, USA). Respective fluorochrome-conjugated isotype controls were purchased from BD Biosciences (San Diego, CA, USA). Matched antibody pairs for capture and detection of human IFN-γ, IL-2, and IL-10, respectively, were purchased from BD Biosciences. Recombinant IL-2 was obtained from Endogen (Woburn, MA, USA). Immunofluorescence was analyzed using a FACS-Canto cytofluorometer equipped with the Diva software (Becton Dickinson, Mountain View, CA, USA).
Preparation of Human T Cells
Peripheral blood lymphocytes were obtained from healthy donors by Ficoll density centrifugation. T cells were activated initially by OKT3 and 15E8 mAbs (100 ng/mL each) and IL-2 (500 U/mL) and further cultivated in the presence of IL-2 (500 U/mL).
CARs
Engineering of CARs with specificity for the CEA, TAG72, and CD30 with the modified CD28-CD3ζ signaling and the modified IgG1-CH2/3 extracellular spacer domains39 as well as the retroviral modification of T cells were previously described in detail.37, 40, 41 The generation of anti-CD25 and agonistic anti-CD30 scFvs was described elsewhere.23, 42 CARs containing the anti-CD30 scFv were generated by linking the scFv-binding domains with a (glycin4serin)4 linker.43
T Cell Modification
Human peripheral blood T cells were retrovirally transduced for CAR expression.44 T cells were stimulated and transduced on day 2 or 3 by γ-retrovirus containing supernatants or by co-culture with virus-producing 293T cells as described.9 Retroviruses were produced by 293T cells upon transient transfection with the DNA of the gibbon ape leukemia virus (GALV) encoding and the gag/pol-encoding helper plasmid and the plasmid encoding the respective CAR. CAR expression was monitored by flow cytometry using an antibody against the common extracellular IgG1 Fc domain.
Flow Cytometry and Cell Sorting
For flow cytometry analysis and cell sorting, CAR-engineered T cells were stained with fluorochrome-labeled antibodies specific for IgG1, CD3, CD4, and CD8, respectively, and they were recorded by a FACSCanto II flow cytometer equipped with the FACSDiva software (BD Biosciences). CD4+ and CD8+ CAR T cells were purified by flow sorting using a FACSAria III cell sorter (BD Biosciences). Doublets were discriminated using forward scatter (area) versus forward scatter (width) and side scatter (area) versus side scatter (width) gating.
Activation of CAR T Cells
CAR T cells (0.32 × 104–5 × 104 cells/well) were co-cultivated for 24–48 h in 96-well round-bottom plates with tumor cells (each 2.5–5 × 104 cells/well). Specific cytotoxicity of CAR T cells against antigen-positive target cells was monitored by an XTT-based colorimetric assay45 using the Cell Proliferation Kit II (Roche, Mannheim, Germany). Viability of tumor cells was calculated as the mean values of six wells containing only tumor cells subtracted by the mean background level of wells containing medium only. Non-specific formation of formazane due to the presence of T cells was determined from triplicate wells containing T cells in the same number as in the corresponding experimental wells. The number of viable tumor cells in experimental wells was calculated as follows: viability (%) = [OD(experimental wells – corresponding number of T cells)]/[OD(tumor cells without T cells − medium)] × 100, where OD is optical density. Cytotoxicity (%) was defined as 100 – viability (%).
IL-10 Secretion Assay
T cells from the peripheral were activated and cultivated for 72 h as described above. Cells were additionally stimulated for 12 h with OKT3 and 15E8 mAbs (each 1 μg/mL), and IL-10-secreting cells were identified by the IL-10 secretion assay (Miltenyi Biotec), according to the manufacturer’s recommendations. IL-10-secreting cells were additionally stained with anti-CD3, anti-CD30, and anti-CD30L mAbs and analyzed by flow cytometry.
Activation and CD30 Induction in T Cells
For the induction of high CD30 expression, T cells from the peripheral blood were activated and grafted with CARs, as described above, and further cultivated in the presence of IL-2 (500 U/mL). After cultivation for 7–9 days, cells were washed and co-cultivated at 37°C and 5% (v/v) CO2 with freshly isolated allogeneic or autologous peripheral blood lymphocytes (PBLs) (2.5–5 × 104 lymphocytes/well) in microtest plates (Nunc Polysorb, Thermo Fisher Scientific, Wiesbaden, Germany) that were coated overnight at 4°C with the agonistic anti-CD3 and anti-CD28 mAbs (each 1 μg/mL in PBS). After 48 h, cells were recovered and analyzed for CD30 expression by flow cytometry. Alternatively, supernatants were analyzed for cytokine concentrations. In another set of experiments, anti-CEA-CAR T cells and freshly isolated PBLs (5 × 104 total lymphocytes/well) were co-activated in the presence of CEA+ LS174T cells (2.5 × 104/well), and the specific lysis of tumor cells was determined. For the blocking of CD30-CD30L interaction, HRS4 mAb (10 μg/mL) was added, which reacts with epitopes on cluster group A of CD3022 and interferes with CD30-CD30L binding,22 as does HRS3.
ELISA
IFN-γ, IL-2, and IL-10 in culture supernatants were monitored by ELISA by binding to the solid-phase anti-IFN-γ, IL-2, and IL-10 capture antibody (each 1 μg/mL), respectively, and detection by the biotinylated anti-IFN-γ, anti-IL-2, or anti-IL-10 detection antibody (0.5 μg/mL). The reaction product was visualized by a peroxidase-streptavidin conjugate (1:10,000) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS; Roche).
CAR T Cell-Mediated Suppression of Tumor Growth
Rag2−/− cγ−/− mice (Charles River Laboratories, Sulzfeld, Germany) (4–8 animals/group) were subcutaneously inoculated with CEA+ C15A3 (106/animal) or LS174T cancer cells (2.5 × 106 cells/animal) and CAR T cells (2.5−5 × 106 cells/animal). T cells without CAR served as the control. Tumor volumes were recorded every 2–3 days. The area under the curve (AUC) was recorded as described,46 and significant differences were determined by the Student’s t test. Studies were approved by the ethical committee according to the local guidelines.
Statistics
Experimental results from independent representative experiments are reported as mean values + SD. Significance analyses were performed by the two-sided Student’s t test using Microsoft Excel and GraphPad Prism, respectively.
Author Contributions
A.A.H. and G.R. conducted the experiments. A.A.H. and H.A. designed the experiments, interpreted the data, and wrote the paper.
Conflicts of Interest
A.A.H. and H.A. are inventors of a patent application covering the anti-CD30 CAR design; the paper may have impact on the value of the patent application.
Acknowledgments
The authors thank Birgit Hops and Danuta Chrobok for excellent technical assistance. Our work was funded by grants from the German Federal Ministry of Education and Research through the CD20CAR Time project within the funding program Innovations for Individualized Medicine (Fkz 01EK1507A-C), the Else Kröner-Fresenius-Stiftung, the Deutsche Krebshilfe, and the Fortune Program of the Medical Faculty of the University of Cologne. The authors thank professor Stefan Barth, University of Cape Town, South Africa, for providing the Ki4-Eta immunotoxin and professor Stefan Dübel, Technische Universität Braunschweig, Germany, for providing us with the anti-CD30 scFv SH313-B5.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.06.007.
Supplemental Information
References
- 1.Porter D.L., Hwang W.-T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhoj V.G., Arhontoulis D., Wertheim G., Capobianchi J., Callahan C.A., Ellebrecht C.T., Obstfeld A.E., Lacey S.F., Melenhorst J.J., Nazimuddin F. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128:360–370. doi: 10.1182/blood-2016-01-694356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz R.H. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 5.Horie R., Watanabe T. CD30: expression and function in health and disease. Semin. Immunol. 1998;10:457–470. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 6.Pierce J.M.R., Mehta A. Diagnostic, prognostic and therapeutic role of CD30 in lymphoma. Expert Rev. Hematol. 2017;10:29–37. doi: 10.1080/17474086.2017.1270202. [DOI] [PubMed] [Google Scholar]
- 7.Muta H., Podack E.R. CD30: from basic research to cancer therapy. Immunol. Res. 2013;57:151–158. doi: 10.1007/s12026-013-8464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos C.A., Ballard B., Zhang H., Dakhova O., Gee A.P., Mei Z., Bilgi M., Wu M.F., Liu H., Grilley B. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Invest. 2017;127:3462–3471. doi: 10.1172/JCI94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hombach A.A., Görgens A., Chmielewski M., Murke F., Kimpel J., Giebel B., Abken H. Superior Therapeutic Index in Lymphoma Therapy: CD30(+) CD34(+) Hematopoietic Stem Cells Resist a Chimeric Antigen Receptor T-cell Attack. Mol. Ther. 2016;24:1423–1434. doi: 10.1038/mt.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarkowski M. Expression and a role of CD30 in regulation of T-cell activity. Curr. Opin. Hematol. 2003;10:267–271. doi: 10.1097/00062752-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Romagnani S., Del Prete G., Maggi E., Chilosi M., Caligaris-Cappio F., Pizzolo G. CD30 and type 2 T helper (Th2) responses. J. Leukoc. Biol. 1995;57:726–730. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T., Lee R.K., Nam S.Y., Al-Ramadi B.K., Koni P.A., Bottomly K., Podack E.R., Flavell R.A. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-gamma. J. Immunol. 1997;158:2090–2098. [PubMed] [Google Scholar]
- 13.Bengtsson A., Johansson C., Linder M.T., Halldén G., van der Ploeg I., Scheynius A. Not only Th2 cells but also Th1 and Th0 cells express CD30 after activation. J. Leukoc. Biol. 1995;58:683–689. doi: 10.1002/jlb.58.6.683. [DOI] [PubMed] [Google Scholar]
- 14.Hamann D., Hilkens C.M., Grogan J.L., Lens S.M., Kapsenberg M.L., Yazdanbakhsh M., van Lier R.A. CD30 expression does not discriminate between human Th1- and Th2-type T cells. J. Immunol. 1996;156:1387–1391. [PubMed] [Google Scholar]
- 15.Klimka A., Barth S., Matthey B., Roovers R.C., Lemke H., Hansen H., Arends J.W., Diehl V., Hoogenboom H.R., Engert A. An anti-CD30 single-chain Fv selected by phage display and fused to Pseudomonas exotoxin A (Ki-4(scFv)-ETA’) is a potent immunotoxin against a Hodgkin-derived cell line. Br. J. Cancer. 1999;80:1214–1222. doi: 10.1038/sj.bjc.6690488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hombach A., Heuser C., Sircar R., Tillmann T., Diehl V., Pohl C., Abken H. Characterization of a chimeric T-cell receptor with specificity for the Hodgkin’s lymphoma-associated CD30 antigen. J. Immunother. 1999;22:473–480. doi: 10.1097/00002371-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Aqbi H.F., Wallace M., Sappal S., Payne K.K., Manjili M.H. IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J. Leukoc. Biol. 2018;103:1219–1223. doi: 10.1002/JLB.5MIR0917-351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng H., Zhang R., Jin B., Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell. Mol. Immunol. 2015;12:566–571. doi: 10.1038/cmi.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glocker E.-O., Kotlarz D., Boztug K., Gertz E.M., Schäffer A.A., Noyan F., Perro M., Diestelhorst J., Allroth A., Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi F.M., Degan M., Mazzocut-Zecchin L., Di Francia R., Aldinucci D., Pinto A., Gattei V. CD30L up-regulates CD30 and IL-4 expression by T cells. FEBS Lett. 2001;508:418–422. doi: 10.1016/s0014-5793(01)03076-9. [DOI] [PubMed] [Google Scholar]
- 21.Barbieri A., Dolcino M., Tinazzi E., Rigo A., Argentino G., Patuzzo G., Ottria A., Beri R., Puccetti A., Lunardi C. Characterization of CD30/CD30L(+) Cells in Peripheral Blood and Synovial Fluid of Patients with Rheumatoid Arthritis. J. Immunol. Res. 2015;2015:729654. doi: 10.1155/2015/729654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke A.C., Jung D., Ellis T.M. Characterization of the CD30L binding domain on the human CD30 molecule using anti-CD30 antibodies. Hybridoma. 2000;19:43–48. doi: 10.1089/027245700315789. [DOI] [PubMed] [Google Scholar]
- 23.Wezler X., Hust M., Helmsing S., Schirrmann T., Dübel S. Human antibodies targeting CD30(+) lymphomas. Hum. Antibodies. 2012;21:13–28. doi: 10.3233/HAB-2012-0258. [DOI] [PubMed] [Google Scholar]
- 24.Powell I.F., Li T., Jäck H.M., Ellis T.M. Construction and expression of a soluble form of human CD30 ligand with functional activity. J. Leukoc. Biol. 1998;63:752–757. doi: 10.1002/jlb.63.6.752. [DOI] [PubMed] [Google Scholar]
- 25.Engert A., Burrows F., Jung W., Tazzari P.L., Stein H., Pfreundschuh M., Diehl V., Thorpe P. Evaluation of ricin A chain-containing immunotoxins directed against the CD30 antigen as potential reagents for the treatment of Hodgkin’s disease. Cancer Res. 1990;50:84–88. [PubMed] [Google Scholar]
- 26.Muta H., Boise L.H., Fang L., Podack E.R. CD30 signals integrate expression of cytotoxic effector molecules, lymphocyte trafficking signals, and signals for proliferation and apoptosis. J. Immunol. 2000;165:5105–5111. doi: 10.4049/jimmunol.165.9.5105. [DOI] [PubMed] [Google Scholar]
- 27.Vallacchi V., Vergani E., Camisaschi C., Deho P., Cabras A.D., Sensi M., De Cecco L., Bassani N., Ambrogi F., Carbone A. Transcriptional profiling of melanoma sentinel nodes identify patients with poor outcome and reveal an association of CD30(+) T lymphocytes with progression. Cancer Res. 2014;74:130–140. doi: 10.1158/0008-5472.CAN-13-1672. [DOI] [PubMed] [Google Scholar]
- 28.Vallacchi V., Camisaschi C., Dugo M., Vergani E., Deho P., Gualeni A., Huber V., Gloghini A., Maurichi A., Santinami M. microRNA Expression in Sentinel Nodes from Progressing Melanoma Patients Identifies Networks Associated with Dysfunctional Immune Response. Genes (Basel) 2016;7:E124. doi: 10.3390/genes7120124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dle Beato T., Berghella A.M., Pellegrini P., Domenico A., Casciani C.U. The role of the soluble CD30 serum level in colorectal cancer: a possible marker for a patient subset which could benefit from IL-2 biotherapy. Cancer Biother. Radiopharm. 1997;12:297–304. doi: 10.1089/cbr.1997.12.297. [DOI] [PubMed] [Google Scholar]
- 30.Gerli R., Lunardi C., Vinante F., Bistoni O., Pizzolo G., Pitzalis C. Role of CD30+ T cells in rheumatoid arthritis: a counter-regulatory paradigm for Th1-driven diseases. Trends Immunol. 2001;22:72–77. doi: 10.1016/s1471-4906(00)01829-9. [DOI] [PubMed] [Google Scholar]
- 31.Del Prete G., De Carli M., D’Elios M.M., Daniel K.C., Almerigogna F., Alderson M., Smith C.A., Thomas E., Romagnani S. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J. Exp. Med. 1995;182:1655–1661. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi F.M., Degan M., Mazzocco F.T., Di Francia R., Aldinucci D., Poletto D., Vellenga E., Pinto A., Gattei V. Co-expression of CD30 ligand and interleukin 4 (IL-4) receptors by acute myeloid leukaemia blasts is associated with the expansion of IL-4-producing CD30+ normal T cells. Br. J. Haematol. 2002;117:59–69. doi: 10.1046/j.1365-2141.2002.03398.x. [DOI] [PubMed] [Google Scholar]
- 33.Su C.-C., Chiu H.-H., Chang C.-C., Chen J.-C., Hsu S.-M. CD30 is involved in inhibition of T-cell proliferation by Hodgkin’s Reed-Sternberg cells. Cancer Res. 2004;64:2148–2152. doi: 10.1158/0008-5472.can-03-1337. [DOI] [PubMed] [Google Scholar]
- 34.Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 35.Ogata S., Chen A., Itzkowitz S.H. Use of model cell lines to study the biosynthesis and biological role of cancer-associated sialosyl-Tn antigen. Cancer Res. 1994;54:4036–4044. [PubMed] [Google Scholar]
- 36.Pohl C., Renner C., Schwonzen M., Schobert I., Liebenberg V., Jung W., Wolf J., Pfreundschuh M., Diehl V. CD30-specific AB1-AB2-AB3 internal image antibody network: potential use as anti-idiotype vaccine against Hodgkin’s lymphoma. Int. J. Cancer. 1993;54:418–425. doi: 10.1002/ijc.2910540312. [DOI] [PubMed] [Google Scholar]
- 37.Hombach A., Heuser C., Gerken M., Fischer B., Lewalter K., Diehl V., Pohl C., Abken H. T cell activation by recombinant FcepsilonRI gamma-chain immune receptors: an extracellular spacer domain impairs antigen-dependent T cell activation but not antigen recognition. Gene Ther. 2000;7:1067–1075. doi: 10.1038/sj.gt.3301195. [DOI] [PubMed] [Google Scholar]
- 38.Pohl C., Renner C., Schwonzen M., Sieber M., Lorenz P., Pfreundschuh M., Diehl V. Anti-idiotype vaccine against Hodgkin’s lymphoma: induction of B- and T-cell immunity across species barriers against CD30 antigen by murine monoclonal internal image antibodies. Int. J. Cancer. 1992;50:958–967. doi: 10.1002/ijc.2910500623. [DOI] [PubMed] [Google Scholar]
- 39.Hombach A., Hombach A.A., Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17:1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 40.Weijtens M.E., Willemsen R.A., Hart E.H., Bolhuis R.L. A retroviral vector system ‘STITCH’ in combination with an optimized single chain antibody chimeric receptor gene structure allows efficient gene transduction and expression in human T lymphocytes. Gene Ther. 1998;5:1195–1203. doi: 10.1038/sj.gt.3300696. [DOI] [PubMed] [Google Scholar]
- 41.Hombach A., Wieczarkowiecz A., Marquardt T., Heuser C., Usai L., Pohl C., Seliger B., Abken H. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J. Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 42.Barth S., Huhn M., Wels W., Diehl V., Engert A. Construction and in vitro evaluation of RFT5(scFv)-ETA’, a new recombinant single-chain immunotoxin with specific cytotoxicity toward CD25+ Hodgkin-derived cell lines. Int. J. Mol. Med. 1998;1:249–256. doi: 10.3892/ijmm.1.1.249. [DOI] [PubMed] [Google Scholar]
- 43.Martyniszyn A., Krahl A.-C., André M.C., Hombach A.A., Abken H. CD20-CD19 Bispecific CAR T Cells for the Treatment of B-Cell Malignancies. Hum. Gene Ther. 2017;28:1147–1157. doi: 10.1089/hum.2017.126. [DOI] [PubMed] [Google Scholar]
- 44.Golumba-Nagy V., Kuehle J., Abken H. Genetic Modification of T Cells with Chimeric Antigen Receptors: A Laboratory Manual. Hum. Gene Ther. Methods. 2017;28:302–309. doi: 10.1089/hgtb.2017.083. [DOI] [PubMed] [Google Scholar]
- 45.Jost L.M., Kirkwood J.M., Whiteside T.L. Improved short- and long-term XTT-based colorimetric cellular cytotoxicity assay for melanoma and other tumor cells. J. Immunol. Methods. 1992;147:153–165. doi: 10.1016/s0022-1759(12)80003-2. [DOI] [PubMed] [Google Scholar]
- 46.Duan F., Simeone S., Wu R., Grady J., Mandoiu I., Srivastava P.K. Area under the curve as a tool to measure kinetics of tumor growth in experimental animals. J. Immunol. Methods. 2012;382:224–228. doi: 10.1016/j.jim.2012.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.