Abstract
Background:
India has the highest tuberculosis (TB) burden, accounting for one-fifth of the global incidence and two-third of the cases in Southeast Asia with an estimated 1.9 million new cases every year. Identifying and treating latent TB infection (LTBI) can reduce the risk of development of active disease by up to 90%, thereby decreasing a major burden to the prevalence of the disease, and thus reducing potential sources in future.
Aim:
Early diagnosis of LTBI by tuberculin skin test (TST) and a newer interferon-gamma release assay (IGRA).
Materials and Methods:
Seventy-seven clinically asymptomatic household contacts (≤18 years) of confirmed pulmonary TB patients were enrolled to compare the performance of TST and IGRA to diagnose LTBI. At baseline, all participants underwent testing for IGRA and TST.
Results:
TST showed positivity of 22%, while IGRA demonstrated positivity of 40% in the diagnosis of latent TB. Kappa value at 95% confidence interval was 0.4753, indicates a moderate agreement between the two tests. This indicates that IGRA is a better predictor of latent TB. Maximum positive percentage was in the age group of 16–18 years in both the tests followed by 1–5 years.
Aim:
Early diagnosis of LTBI by tuberculin skin test (TST) and a newer interferon-gamma release assay (IGRA).
Keywords: Interferon-gamma release assay, latent tuberculosis infection, tuberculin skin test, tuberculosis
Introduction
Tuberculosis (TB) is one of the major causes of morbidity and mortality in tropical countries. The World Health Organization estimates, approximately one-third of the population in the world is affected by TB.[1] India has the highest TB burden, accounting for one-fifth of the global incidence and two-thirds of the cases in Southeast Asia with an estimated 1.9 million new cases every year.[2] India, the world's second most populous country, accounts for a quarter of the world's annual incidence of TB. Every year around 2 million people develop TB in India and 300,000 die of it.[3] Children account for 5%–15% of total TB cases worldwide. In majority of the population, infection with Mycobacterium tuberculosis is contained by the host defenses and infection may either remain latent or get cleared. However, latent infection can become active at any time, hence identifying and treating these latent TB infections (LTBIs) can reduce the risk of development of active disease by up to 90%, thereby decreasing the major burden to the prevalence of the disease and thereby reducing potential sources in future.[4]
The contacts of TB patients are the vulnerable population who are at risk of developing TB. A recent study in India showed that 8.7% of household contacts were diagnosed with TB.[5] Particular attention should be paid to contacts with the highest susceptibility to TB infection and subsequent development of active disease, especially in the pediatric age group and the immunosuppressed people.[6] As the success of global TB elimination depends on achieving national TB control programme goals in high TB burden countries, it opens up a debate on whether LTBI should be treated along with active TB cases.[7] LTBI is defined as “a state of persistent immune response to stimulation by M. tuberculosis antigens with no evidence of clinically manifest active TB.” The global burden of LTBI is not known, as there is no definitive test for its diagnosis. The current modalities for diagnosing LTBI are tuberculin skin test (TST) and since the discovery of the ESAT6 antigen, Interferon-Gamma Release Assays (IGRAs).[8] As there is no gold standard test for diagnosing LTBI, sensitivity and specificity of these tests cannot be determined. But diagnosing LTBI in children, as compared to adults can have more beneficiary effect or results, as children are most likely to have been infected recently as compared to adults who might have been infected decades earlier. This will increase the predictability rate of TST and IGRA as well. Household contacts are at higher risk of developing LTBI when compared to the general population.[9] There are comparatively few studies reported in literature on diagnosis of LTBI in children and hence this study was conducted to investigate the predictive value of TST and IGRA in diagnosis of LTBI in pediatric household contacts of confirmed pulmonary TB cases.
Materials and Methods
This cross-sectional study was carried out in the Department of Microbiology at RRMCH, Bengaluru, India which is a large tertiary care center located in Bengaluru, South India, with a containment area of several adjacent regions. The study was approved by the Institute Ethics Review Board. Written informed surrogate consent was obtained from parents or legal guardians of all the participants of this study.
In this study, newly diagnosed sputum-positive pulmonary TB cases were first identified from the Outpatient Department of TB and Chest Disease department and Directly Observed Treatment Short-course (DOTS) center of our institute and various other surrounding DOTS centers of Bengaluru region. Simultaneously, their close clinically asymptomatic household contacts were enrolled in the study from January 2018 to 2019. For the present study, clinically asymptomatic household contacts of pulmonary TB patients were defined as an extended group of family members who were ≤18 years, residing together with the pulmonary TB index case in the same household for more than 3 months and having a common sleeping area at night.
In an index case, pulmonary TB was confirmed by sputum smear microscopy and Cartridge-Based Nucleic Acid Amplification Test as per the National TB Guidelines.[10] Household contacts of these patients who were <18 years without any signs and symptoms of active TB were included in the study.
On their first visit, blood samples were collected for STANDARD ETB-Feron enzyme-linked immunosorbent assay (ELISA) (E B-Feron from SD Biosensor, Republic of Korea), and subsequently, the tuberculin was administered intradermally into the middle one-third of volar surface of forearm. The TST was done using standardized 2 tuberculin units of purified protein derivative (PPD), RT23 (from Arkray Healthcare Pvt Ltd, Surat, India) and the results were interpreted after 48 h up to 72 h by measuring the size of the induration (mm). The cutoff for TST was 5 mm induration.[11]
The STANDARD E TB-Feron assay was performed as per the manufacturer's instructions. For this assay, three specialized blood collection tubes, namely: the nil control, TB antigen tube, and mitogen tube available with the test kit were utilized. Peripheral venous whole blood (3 ml) was collected through venipuncture from each participant directly into these three tubes of 1 ml capacity each. These tubes were gently shaken to ensure thorough mixing of the blood with the content of the tubes. The TB antigen tube contained M. tuberculosis-specific antigens ESAT-6, CFP-10, and TB 7.7 for stimulating the cytokines in blood samples. The mitogen contained phytohemagglutinin served as positive control. Tubes were incubated at 37°C (within 6 h of venipuncture) for 16–24 h. Following incubation, plasma was harvested from the blood samples through centrifugation (2000–3000 relative centrifugal force) for 15 min. The gel plugs present inside the tubes facilitated separation of the plasma from the blood cells. The plasma was collected and stored in properly labeled Cryo Vials at −70°C till future use. The level of interferon-γ (IFN-γ) in plasma samples was estimated through ELISA kits (with recombinant human IFN-γ standard) provided with assay packaging. The kit manufacturer's protocol was followed. Proper thawing of the plasma samples was carried out, and ELISA was performed in an automated processor in batches. Test results were interpreted as positive, negative, or indeterminate as per kit manufacturer's instructions.
Statistical analysis
Data are presented as means ± standard deviations for continuous variables and as frequencies (with percentages) for categorical variables. Continuous variables were compared using independent samples t-tests, and categorical variables were compared using Pearson's Chi-square test or Fisher's exact test. The Kappa statistic (k) was used to evaluate the concordance between TST and IGRA.
Results
A total of 77 clinically asymptomatic household contacts of 36 microscopically confirmed sputum-positive TB index patients were enrolled in the study. The contacts belonged to age group of 2.5–18 years. Age distribution of the contacts is as shown in Figure 1. Male-to-female ratio was 0.63. Maximum numbers of contacts were in the age group of 6–10 years with more occurrences at the mean age of 9.6 years.
Figure 1.
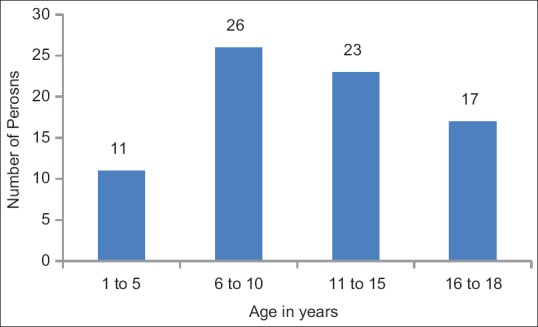
Age distribution of contacts
Baseline characteristics of household contacts of patients with pulmonary TB are as shown in Table 1. TST was positive in 22% and ETB-Feron was positive in 40% of the contacts. Positive percentage was highest in the age group of 16–18 years followed by 1–5 years. However, difference in positive percentage between TST and ETB-Feron was highest in the age group of 1–5 years followed by 11–15 years. Positive percentage is as shown in Table 2.
Table 1.
Baseline characteristics of household contacts (n=77)
| Variables | Household contacts, n (%) |
|---|---|
| Age (years)±range | 10.74±4.50 |
| Gender | |
| Male | 30 |
| Female | 47 |
| TST | |
| Positive | 17 (22) |
| Negative | 60 (78) |
| TB-Feron | |
| Positive | 31 (40) |
| Negative | 46 (60) |
| Indeterminate | 00 |
TST: Tuberculin skin test; IGRA: Interferon Gamma Release Assay; TB: Tuberculosis
Table 2.
Positive percentages of tuberculin skin test and ETB-Feron
| Age group | Number of persons | TST |
Positive percentage TST | ETB-Feron |
Positive percentage ETB-Feron | Difference in result of positive percentage between TST and ETB-Feron | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| 1-5 | 11 | 2 | 9 | 18 | 5 | 6 | 45 | 27 |
| 6-10 | 26 | 5 | 21 | 19 | 8 | 18 | 31 | 12 |
| 11-15 | 23 | 5 | 18 | 22 | 10 | 13 | 43 | 22 |
| 16-18 | 17 | 5 | 12 | 29 | 8 | 9 | 47 | 18 |
| Total | 77 | 17 | 60 | 31 | 46 | |||
TST: Tuberculin skin test
There was moderate agreement between the two tests, i.e., TST and IGRA with a Kappa value of 0.4753 (95% confidence interval) making the IGRA (E TB-FERON) test statistically significant. Agreement between the two tests is as shown in Table 3.
Table 3.
Agreement between tuberculin skin test and ETB-Feron assay
| Result | TST |
Total | Agreement | κ, 95% CI | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| ETB-Feron | |||||
| Positive | 15 | 16 | 31 | 0.5543 | 0.4753 (0.687-0.263) |
| Negative | 2 | 44 | 46 | ||
| Total | 17 | 60 | 77 | ||
CI: Confidence interval; TST: Tuberculin skin test
Discussion
It is a well-known fact that, infection with M. tuberculosis does not eventually lead to development of active TB. The risk for an individual with LTBI in his lifetime to progress to active TB is estimated to be 5%–10%.[12] This risk is especially high among pediatric population and possible immune-compromised states.[13]
The preventive treatment for this population is risky and costly as well. Moreover, the treatment should be aimed selectively at the high-risk population who will progress to active TB disease. Only they would benefit from treatment for LTBI. A critical component toward this end is timely diagnosis.[14]
There is no gold standard to diagnose LTBI. TST and IGRA provide reasonable options, but they both require a healthy immune response from the individuals. Although both these tests are not perfect in measuring progression to active disease, reviews show that IGRAs do well in this direction as compared to TST.[14]
Although TST was developed by Charles Mantoux in the year 1907, it has its significance as the best prognostic marker of active TB and the best diagnostic marker of LTBI since 1890. In cases of extrapulmonary TB, TST is one of the recommended and trusted tests along with others. Although TST is the oldest and cheapest test available to diagnose LTBI, it has many disadvantages such as cross-reaction of antigens used in PPD with Bacillus Calmette–Guerin (BCG) and several nontuberculous mycobacteria (NTM). Hence, it has lower specificity in high TB burden settings like India where there is high BCG coverage and also its sensitivity is compromised in immunosuppressed individuals, in advanced TB cases and the malnourished.[15]
In contrast, antigens used in IGRA are TB-specific antigens which do not cross-react with any BCG substrains or NTM species. Only one blood sample is necessary and a second visit is avoided as in TST. However, IGRA is expensive; requires blood samples and sophisticated laboratories.[16]
In our study, TST positive percentage was 22% and standard ETB-Feron positivity was 40%. There are studies which have compared agreement between TST and IGRA, rather than sensitivity and specificity, because there is no gold standard test for diagnosis of LTBI. The Kappa values, which are used to assess the agreement between the two tests, are highly inconsistent ranging from −0.03 to 0.87. These studies had study participants who were active TB cases or HIV cases with symptoms, treated TB cases, immigrant individuals from high TB burden settings, and most importantly, all were adults >18 years.[17,18,19] Our study is unique in that, we have taken pediatric age group who are more vulnerable for the development of active TB disease and are close contacts of TB cases. Our Kappa value is 0.4753; this value shows moderate agreement between the two tests. This indicates that IGRA is better than TST in identification or diagnosis of LTBI. These results need to be further supported by clinical follow-up of these participants over the next 2 years for development of active TB.
In a study by Sharma et al.,[7] where comparison was done between TST and IGRA in diagnosis of LTBI from India, they found that IGRA Gold in-tube QuantiFERON-TB-Gold in-tube assay was marginally superior to TST with IGRA positivity of 60% as compared to 52% by TST. Another study by Mohamed et al.[16] also had similar findings. Furthermore, they compared TST results at different measurements, i.e., at 5 mm, 10 mm, and 15 mm. However, as per Centers for Disease Control and Prevention guidelines, 5 mm is the cutoff for children who are recent contacts of TB patients, which was followed in our study.[11] In the present study, TB-Feron positivity was 40% compared to 22% by TST which is statistically significant. The possible explanation for this is, only children <18 years were included in the study; they would have diminished TST response owing to altered or immature cellular immunity in this age group. It is well known that regular BCG vaccination and mucosal exposure to several nonpathogenic environmental Mycobacteriae suppresses TST response and increases Mycobacterium-specific IFN-γ response in peripheral blood.[20] There are studies which support findings similar to our study, that IGRAs are better predictors of LTBI when compared to TST.[21,22,23] In a meta-analysis by Sun et al., who compared seven studies that assessed IGRA specificity and showed pooled specificity for IGRA was 100% as compared to 56% by TST.[24] IGRA performs differently in high TB settings when compared to low burden settings; this explains the differences seen in various studies.[16]
A key finding in our study is IGRA+TST- discordance among children who were close household contacts of sputum-positive pulmonary TB patients. IGRA+TST- discordance has also been reported in many other studies where recent household TB contacts, elderly, recent immigrants from TB endemic areas, immunocompromised population, and in pregnancy.[25,26,27,28,29] The proposed theory for this discordance is that, TST is a delayed type of hypersensitivity reaction by Th1 immune response, wherein IFN-γ, tumor necrosis factor-α, interleukin (IL)-2, and other Th1 cytokines are also involved in causing skin induration. However, IGRA measures only IFN-γ produced in the blood in response to stimulus by TB-specific antigens. Lower IFN-gamma and IL-2 may prevent immune system from triggering a delayed type of hypersensitivity reaction, resulting in false-negative TST.[29]
In the present study, there is an increase in the positivity percentage of TST with increasing age; however, this is not true with IGRA. However, IGRA has shown maximum positivity percentage in the age group of 16–18 years (47%) followed by 1–5 years (45%), 11–15 (43%) years, and 6–10 years (31%). In a study by Eom et al.,[9] TST was positive in 42.6% and IGRA 45.7% and also there was an increase in the positive percentage with age in both TST as well as IGRA. This study had included elderly patients. Many other studies also support the findings of our study that IGRAs are better predictors of LTBI than TST in close household contacts of sputum-positive pulmonary TB patients.[9]
Conclusion
IGRA (ETB-Feron) is a better predictor of latent TB infection. Follow-up of these contacts is required to know the development of active TB disease in them in near future in order to corroborate this.
Financial support and sponsorship
The kit was supplied free of cost for evaluation by SD Biosensor, Korea.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Mrs Chandrakala C for statistical analysis of data and Mr. Ayub for the technical help.
References
- 1.World Health Organization. Global Tuberculosis Control: WHO Report 2015. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 2.Seth P. The solution of HIV/M. Tuberculosis co-infection in India. Open Infect Dis J. 2011;5(Suppl 1-M5):51–9. [Google Scholar]
- 3.Central TB Division. New Delhi Ministry of Health and family Welfare, Government of India; [Last assessed on 2018 Aug 12]. National Strategic Plan (2012-17) for TB-Directorate of Health Services. Available from: http://www.tbcindia.nic.in . [Google Scholar]
- 4.Boskovska K, Naceva-Fustic S, Simonovska L, Dilberovska M, Dacevski D, Popova G, et al. Comparison of IFN-γ levels in children with tuberculosis disease (TB) and latent tuberculosis infection (LTBI) Open Access Maced J Med Sci. 2018;6:2091–6. doi: 10.3889/oamjms.2018.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaparde K, Jethani P, Dewan PK, Nair SA, Deshpande MR, Satyanarayana S. Evaluation of TB case finding through systematic contact investigation, Chhattisgarh, India. Tuberc Res Treat. 2015;2015:670167. doi: 10.1155/2015/670167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: A systematic review and meta-analysis. Eur Respir J. 2013;41:140–56. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma SK, Vashishtha R, Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-burden setting. PLoS One. 2017;12:e0169539. doi: 10.1371/journal.pone.0169539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenyon TA, Creek T, Laserson K, Makhoa M, Chimidza N, Mwasekaga M, et al. Risk factors for transmission of mycobacterium tuberculosis from HIV-infected tuberculosis patients, Botswana. Int J Tuberc Lung Dis. 2002;6:843–50. [PubMed] [Google Scholar]
- 9.Eom JS, Kim I, Kim WY, Jo EJ, Mok J, Kim MH. Household tuberculosis contact investigation in a tuberculosis-prevalent country: Are the tuberculin skin test and interferon-gamma release assay enough in elderly contacts? Medicine (Baltimore) 2018;97:e9681. doi: 10.1097/MD.0000000000009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revised National Tuberculosis Control Programme. [Last accessed on 2018 Jun 24]. Available from: https://tbcindia.gov.in/index1.php?lang=1&level=2&sublinkid=4573&lid=317 .
- 11.CDC. Targeted Tuberculin testing and treatment of latent tuberculosis infection. MMWR. 2000;49:23–5. [PubMed] [Google Scholar]
- 12.Biraro IA, Kimuda S, Egesa M, Cose S, Webb EL, Joloba M, et al. The use of interferon gamma inducible protein 10 as a potential biomarker in the diagnosis of latent tuberculosis infection in Uganda. PLoS One. 2016;11:e0146098. doi: 10.1371/journal.pone.0146098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasambira TS, Shah M, Adrian PV, Holshouser M, Madhi SA, Chaisson RE, et al. QuantiFERON-TB gold in-tube for the detection of mycobacterium tuberculosis infection in children with household tuberculosis contact. Int J Tuberc Lung Dis. 2011;15:628–34. doi: 10.5588/ijtld.10.0555. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Latent TB Infection: Updated and Consolidated Guidelines for Programmatic Management. World Health Organization. 2018. [Last accessed on 2019 Feb 09]. Available from: http://www.who.int/tb/publications/2018/latent-tuberculosis.infection/en/ [PubMed]
- 15.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: A systematic review. Lancet Infect Dis. 2004;4:761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M, et al. The tuberculin skin test versus quantiFERON TB gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6:e17984. doi: 10.1371/journal.pone.0017984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brock I, Weldingh K, Lillebaek T, Follmann F, Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med. 2004;170:65–9. doi: 10.1164/rccm.200402-232OC. [DOI] [PubMed] [Google Scholar]
- 18.Fietta A, Meloni F, Cascina A, Morosini M, Marena C, Troupioti P, et al. Comparison of a whole-blood interferon-gamma assay and tuberculin skin testing in patients with active tuberculosis and individuals at high or low risk of mycobacterium tuberculosis infection. Am J Infect Control. 2003;31:347–53. doi: 10.1016/s0196-6553(02)48240-5. [DOI] [PubMed] [Google Scholar]
- 19.Katial RK, Hershey J, Purohit-Seth T, Belisle JT, Brennan PJ, Spencer JS, et al. Cell-mediated immune response to tuberculosis antigens: Comparison of skin testing and measurement of in vitro gamma interferon production in whole-blood culture. Clin Diagn Lab Immunol. 2001;8:339–45. doi: 10.1128/CDLI.8.2.339-345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoft DF, Brown RM, Belshe RB. Mucosal bacille calmette-guérin vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-gamma responses. Clin Infect Dis. 2000;30(Suppl 3):S217–22. doi: 10.1086/313864. [DOI] [PubMed] [Google Scholar]
- 21.Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–73. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi K, Harada N, Mori T, Sekiya Y. Use of quantiFERON-TB gold to investigate tuberculosis contacts in a high school. Respirology. 2007;12:88–92. doi: 10.1111/j.1440-1843.2006.01000.x. [DOI] [PubMed] [Google Scholar]
- 23.Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the quantiFERON-TB gold in-tube test. Pediatrics. 2009;123:30–7. doi: 10.1542/peds.2007-3618. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Xiao J, Miao Q, Feng WX, Wu XR, Yin QQ, et al. Interferon gamma release assay in diagnosis of pediatric tuberculosis: A meta-analysis. FEMS Immunol Med Microbiol. 2011;63:165–73. doi: 10.1111/j.1574-695X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro-Rodrigues R, Kim S, Coelho da Silva FD, Uzelac A, Collins L, Palaci M, et al. Discordance of tuberculin skin test and interferon gamma release assay in recently exposed household contacts of pulmonary TB cases in Brazil. PLoS One. 2014;9:e96564. doi: 10.1371/journal.pone.0096564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinfurter P, Blumberg HM, Goldbaum G, Royce R, Pang J, Tapia J, et al. Predictors of discordant tuberculin skin test and quantiFERON®-TB gold in-tube results in various high-risk groups. Int J Tuberc Lung Dis. 2011;15:1056–61. doi: 10.5588/ijtld.10.0650. [DOI] [PubMed] [Google Scholar]
- 27.Saracino A, Scotto G, Fornabaio C, Martinelli D, Faleo G, Cibelli D, et al. QuantiFERON-TB gold in-tube test (QFT-GIT) for the screening of latent tuberculosis in recent immigrants to Italy. New Microbiol. 2009;32:369–76. [PubMed] [Google Scholar]
- 28.Kim EY, Lim JE, Jung JY, Son JY, Lee KJ, Yoon YW, et al. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis. 2009;9:207. doi: 10.1186/1471-2334-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathad JS, Bhosale R, Balasubramanian U, Kanade S, Mave V, Suryavanshi N, et al. Quantitative IFN-γ and IL-2 response associated with latent tuberculosis test discordance in HIV-infected pregnant women. Am J Respir Crit Care Med. 2016;193:1421–8. doi: 10.1164/rccm.201508-1595OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


