Abstract
Introduction:
Oral cancer ranks third among all cancers in the Indian population with approximately 45% of call cancer cases in India being diagnosed as oral cancer, among which 20%–50% of the cases are observed to be associated with human papillomavirus (HPV) infection.
Aim:
This study aims to detect and evaluate the presence of p16 in oropharyngeal squamous cell carcinoma (OPSCC) patients using immunohistochemistry (IHC).
Materials and Methods:
This study was based on samples collected from 21 patients with primary OPSCC who were diagnosed and treated during the period of December 2017–March 2018. Inclusion criteria were complete clinicopathologic data, adequate clinical follow-up, and availability of sufficient paraffin-embedded tumor material. HPV immunoreactivity was further investigated by means of IHC using p16 as a marker.
Results:
IHC results revealed p16 positivity in six OPSCC cases. There was no statistically significant association of the p16 positivity of HPV with the age, gender, or site.
Conclusion:
Our results suggest that IHC-based detection of p16 provides a suboptimal prognostic information if not combined with detection of HPV DNA. Although p16 expression and HPV DNA infection are correlated with HPV-associated OPSCCs, neither of the tests alone is the optimal method for HPV status detection.
Keywords: Human papillomavirus, immunohistochemistry, oropharyngeal squamous cell carcinoma, p16
Introduction
Cancer cell possesses artifice to withstand a hostile host environment (e.g., tissue barriers, the immune system, and the cell death) and utilizes host resources to grow and escalate. Head-and-neck squamous cell carcinoma (HNSCC) includes a heterogeneous group of malignant neoplasm arising from the nonkeratinizing epithelium of the upper aerodigestive tract; HNSCC ranks sixth in overall neoplasms of all areas.[1] The oral cavity and the oropharynx being the most common sites for occurrence of HNSCC, with 300,000 new cases been reported worldwide annually.[1,2] Approximately, 45% of cancer cases in India are diagnosed as oral cancer, among which 20%–50% of the cases are observed to be associated with human papillomavirus (HPV) infection.[3]
HPV is considered to be one of the etiological factors for oral squamous cell carcinoma (OSCC) besides tobacco smoking and genetic factors.[4] It is a DNA oncovirus that presents tropism for epithelial cell causing infection of skin and mucous membranes.[2] There are 120 subtypes of Hpv, HPV6, and HPV11 which are considered to be low risk, and HPV16 and HPV18 are considered to be high risk. HPV16 and HPV18 are mainly linked to the pathogenesis of oral and oropharyngeal squamous cell carcinoma (OPSCC).[4,5]
p16 is a cyclin-dependent kinase inhibitor (CDKI) encoded by the CDKN2A locus; p16 is a cellular protein which is involved in cell cycle regulation and arrests the cell cycle in G1 stage.[5,6] p16 acts as a useful prognostic indicator of HPV status; p16 prognostic value for survival is fully equivalent to HPV DNA detection.[5] p16 survival outcomes are best documented to the tonsillar crypts of the oropharyngeal tissues with the subsequent capacity to integrate with the epithelial DNA.[1,4,5,7] This study aims to detect and evaluate the presence of p16 in OPSCC patients using immunohistochemistry (IHC).
Materials and Methods
This single-center prospective study was escorted between December 2017 and March 2018 in a peripheral cancer hospital. This study enrolled 21 patients with histologically diagnosed OPSCC, which includes 11 male and 10 female patients. The patients were aged from 32 to 76 years, and the mean age of the patients included was 51.3 years. Patients who had undergone or undergoing any treatment such as chemotherapy or radiotherapy were not considered into the study. For observation, ten normal healthy oropharyngeal tissue samples of six male and four female patients were taken.
The patients included in this study were nontobacco users. Biopsies were performed in the oropharyngeal region using local anesthesia, and tissue samples were collected in 10% buffered formalin; the tissues were then embedded in paraffin and sliced into 3–4-μm thick sections for histopathological diagnosis and immunohistochemical analysis.
Immunohistochemistry
p16 IHC was performed on 4-μm paraffin-embedded tissue section following deparaffinization done with xylene, rehydration was done with (100%, 80%, and 60%) alcohol and distilled water, and antigen retrieval was carried out using ethylenediaminetetraacetic acid buffer (PH 9.0) for p16. A primary monoclonal mouse anti-human p16INK4a antibody (BioGenex, USA) and a secondary antibody detection kit (Dako Omnis, California, USA) were used. Positive staining was visualized, and sections were counterstained with hematoxylin, dehydrated in serial graded (50%, 70%, 90%, and 100%) water–ethanol solutions, and mounted. Positive controls of cervical cancer were used for p16. Based on staining intensity scaled from 0 to 3, the p16 immunohistochemical expression is rated.
Statistical analysis
Statistical analysis was performed using spss software version 13.0 (SPSS UK Ltd., Working, UK). Chi-squared test was done to measure the significance, and P < 0.05 is considered to be statistically significant.
Ethical considerations
The work was approved by the appropriate ethical committee related to the institutional review board of a tertiary care center in Visakhapatnam, India, on November 15, 2017, and every patient consigned to an informed consent for the work.
Results
The association of p16 and IHC with patient demographics, site of tissue, and histopathology grading is summarized in Table 1. The mean age of p16-positive cases (46 years) is low as compared to negative cases (53.53 years). In the 21 patients with OPSCC included in the study, 11 patients were male and 10 patients were female. HPV-positive cases were predominantly seen in the base of the tongue, whereas HPV-negative cases were mostly associated with soft palate. Histopathologically, 21 cases were nonkeratinized, of which 7 cases were well-differentiated SCC, 11 cases were moderately differentiated SCC, and 3 were poorly differentiated SCC [Figure 1].
Table 1.
Correlation of clinical variables with p16 immunoreactivity in oropharyngeal squamous cell carcinoma
| Characteristics | Number of cases (n=21) | p16 ihc (n=21) |
Chi-square test |
||
|---|---|---|---|---|---|
| Positive (6; 28.5%) | Negative (15: 71.4%) | χ2 | P | ||
| Age in years | |||||
| Mean | 51.3 | 46 | 53.53 | 2.17 | 0.70 |
| 20-29 | 0 | 0 | 0 | ||
| 30-39 | 3 | 1 | 2 | ||
| 40-49 | 6 | 3 | 3 | ||
| 50-59 | 6 | 1 | 5 | ||
| 60-69 | 5 | 1 | 4 | ||
| 70-79 | 1 | 0 | 1 | ||
| Gender | |||||
| Male | 11 | 2 | 9 | 1.2 | 0.26 |
| Female | 10 | 4 | 6 | ||
| Site | |||||
| Soft palate | 9 | 1 | 8 | 2.36 | 0.30 |
| Base of the tongue | 7 | 3 | 4 | ||
| Lateral wall of the pharynx | 5 | 2 | 3 | ||
| Grading | |||||
| Well differentiated | 7 | 2 | 5 | 2.71 | 0.25 |
| Moderately differentiated | 11 | 2 | 9 | ||
| Poorly differentiated | 3 | 2 | 1 | ||
*P<0.05 is considered to be statistically significant. IHC: Immunohistochemistry
Figure 1.
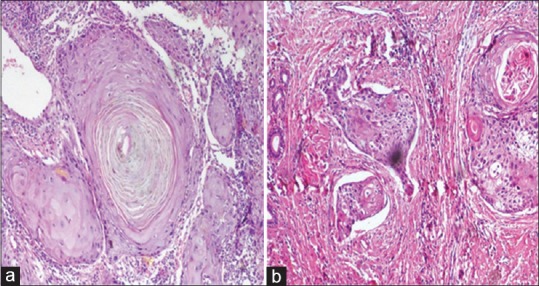
Hematoxylin- and eosin-stained section reveals (a) well-differentiated squamous cell carcinoma and (b) moderately differentiated squamous cell carcinoma with epithelial islands in the connective tissue
The results of p16 for 21 cases included in our study reveal p16 positivity in six cases. The p16 IHC analysis shows 0 staining in 15 cases, 2 + staining for 2 cases, and 3 + staining for 4 cases [Figure 2]. There was no staining for p16 in normal tissue sample.
Figure 2.
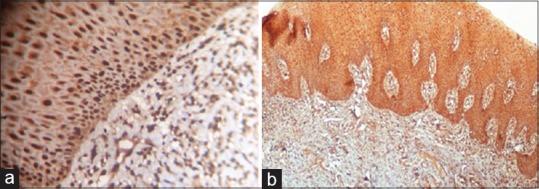
Representative immunohistochemistry staining of p16 in oropharyngeal squamous cell carcinoma tumor specimens. (a) Dispersed p16 staining (b) Diffuse p16 staining
Chi-square test was done using the results of p16 IHC and the categorical variables of the patients included in the study, and this statistical analysis showed an insignificant correlation.
Discussion
HPV and its correlation with oropharyngeal carcinoma are debatable and strangely unique in different parts of the globe. There has been a strong incidence and prevalence in the Caucasian population or the Western world than in the Eastern world.[4,8] According to a study in Kazakhstan in the year 2018, the Central Asian region has shown a high hpv positivity in opscc than in oscc; significantly, nonsmokers were more frequently seen in this HPV-positive group.[9] Some studies have shown that hpv positivity rates in OPSCC were lower in Asian countries when compared to the Western world.[9,10] A cohort study in Japan showed that HPV infection plays a minor role in oral oncogenesis in the Eastern part of the world.[11] In developing nations, tobacco is the main etiological factor for cause of head-and-neck cancers, whereas in developed countries with decreased tobacco use, increased awareness, and early detection of cancer with a higher infusion of medical awareness and treatment facilities, there is still increased incidence of oropharyngeal carcinoma (especially in nonsmokers and nondrinkers), which tends to show the spread of high-risk HPV (16 and 18) and propose HPV infection as an independent etiological risk factor for OPSCC and OSCC, similar to alcohol and tobacco.[4,12,13] HPV infection interaction with tobacco and alcohol acts in a synergistic mechanism in the oral and oropharyngeal regions, and the pathogenesis is yet unclear.[14,15]
In India, since the last two decades, 84 studies were published (data obtained from PubMed database using keywords: HPV and oral cancer in India) related to HPV correlating with oral cancer. In which, 32 studies have shown deep association of HPV with OPSCC and OSCC, of which 14 studies seemed to be significantly positive; these studies have shown that the prevalence of HPV in India varies across depending on the lifestyle differences and geographic regions; there is an increased HPV association in OPSCC and OSCC in South India than the Western and Eastern parts of India.[3,16,17,18] Apart from geographic location, the incidence of HPV-induced OPSCC also varies with the genotype of virus. polymerase chain reaction (PCR) was a commonly used test, and it has been proved to be more sensitive in detection of HPV in OPSCC, than IHC and in situ hybridization (ISH).[8,19,20]
HPV is an epitheliotropic, nonenveloped virus of Papillomaviridae family with double-stranded circular DNA genome.[3,21] Autonomous, specific, and strong carcinogenic effect of HPV is difficult to justify. Unprotected and erratic sexual behavior has a high risk of incidence of HPV, especially in younger age group.[3,6,8,13,22] HPV transmission to the mucosa is less understood and less defined; theories have proposed multiple pathways for HPV transmission, including perinatal transmission, or sexual transmission by oral-genital contact.[13] More than 99% of cervical cancer cases harbor HPV,[4,6] but in the oral cavity, 30%–75% of oropharyngeal cancers have been tested positive for HPV with rates in tonsillar cancer being the highest, followed by cancers of the tongue and soft palate.[1,23]
The early genes, E1, E2, E3, E4, E5, E6, and E7, are responsible for the control of viral transcription, replication, and cellular transformation and are regulated by LCR gene. The genotypic differences, especially in the gene region of E6 and E7, differentiate HPV into high- and low-risk types.[24] The mechanism of action of HPV is by integration of HPV into host genome and upregulates the expression of E6 and E7 oncogenes. The interaction of E7 oncoprotein with RB gene results in release of the transcription factor E2F from the RB-E2F complex; E7 also inactivates CDKIs and activates cyclins E and A and also leads to release of p16 gene from its transcriptional inhibition, resulting in overexpression of p16 in virtually all HPV-transformed cells in oropharyngeal lesions.[6,21,25]
The E6 protein released from HPV-16 and HPV-18 induces the loss of G1 checkpoint activation very early and is able to bind with p53. The E6-mediated degradation of p53 is dependent on a cellular protein, E6-associated protein (E6-AP). Thus, the E3 ligase in the p53 degradation cascade is not simply E6-AP but must be an E6-AP/E6 complex which results in the degradation of p53. As a result, these infected cells are also resistant to p53-induced growth arrest and apoptosis, making them immortal.[21,26]
p16 is used for detection of HPV as it is a surrogate biomarker of HPV-induced carcinomas.[6,7,14,20,25] Studies comparing the results of HPV + in OPSCC revealed using three methods (IHC, ISH, and PCR) explained that cases which were HPV positive by PCR\ISH were also p16 positive by IHC.[14,19,20] The detection of p16 by IHC showed a very high level of sensitivity but less level of specificity.[6,20,25] p16 or HPV E6\E7 mRNA expression was thought to be the parameter to describe the activity of viral oncogenes but a finding that exactly explained that the p16\HPV DNA (+) events were the results of HPV inactive infection.[6] Consequently, HPV status was decided by HPV infection and transactivation. The HPV DNA PCR test can detect HPV infection but cannot detect its activation. Although p16 expression and HPV DNA infection are correlated with HPV-associated OPSCCs, neither of the tests alone is the optimal method for HPV status detection.
In this study, the result revealed HPV-positive correlation with p16 in 6 of the 21 cases (29%), and histopathologically, poorly differentiated cases have shown 66% positive for p16. Females represented in higher number in p16-positive group when compared with males.[25] In this case series, 40% of female patients have shown positivity for p16, whereas 18% of male patients have shown p16 positivity.
Conclusion
The association of HPV to the human body has been the core of debate ever since the beginning of this century. The resulting carcinomatous link in humans involving cervical and in a subset of oropharyngeal carcinomas is highly established. The key to a better HPV disease-free life can help associated morbidity to reduce. Given that the expression of p16 can be affected by genetic or epigenetic changes, our results suggest that IHC-based detection of p16 provides a suboptimal prognostic information if not combined with detection of HPV DNA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smitha T, Mohan CV, Hemavathy S. Prevalence of human papillomavirus16 DNA and p16INK4a protein in oral squamous cell carcinoma: A systematic review and meta-analysis. J Oral Maxillofac Pathol. 2017;21:76–81. doi: 10.4103/jomfp.JOMFP_248_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and – Unrelated oral squamous cell carcinomas in the united states. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 5.Singh V, Husain N, Akhtar N, Khan MY, Sonkar AA, Kumar V, et al. P16 and p53 in HPV-positive versus HPV-negative oral squamous cell carcinoma: Do pathways differ? J Oral Pathol Med. 2017;46:744–51. doi: 10.1111/jop.12562. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Sun R, Lin H, Hu WH. P16INK4A as a surrogate biomarker for human papillomavirus-associated oropharyngeal carcinoma: Consideration of some aspects. Cancer Sci. 2013;104:1553–9. doi: 10.1111/cas.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil S, Rao RS, Amrutha N, Sanketh DS. Analysis of human papilloma virus in oral squamous cell carcinoma using p16: An immunohistochemical study. J Int Soc Prev Community Dent. 2014;4:61–6. doi: 10.4103/2231-0762.131269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–42. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adilbay D, Adilbayev G, Kidirbayeva G, Shipilova V, Sadyk Z, Koyanbekova G, et al. HPV infection and P16 expression in oral and oropharyngeal cancer in Kazakhstan. Infect Agent Cancer. 2018;13:2. doi: 10.1186/s13027-018-0175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toman J, Von Larson S, Umeno H, Kurita T, Furusaka T, Hasegawa H, et al. HPV-positive oropharyngeal cancer via p16 immunohistochemistry in Japan. Ann Otol Rhinol Laryngol. 2017;126:152–8. doi: 10.1177/0003489416681582. [DOI] [PubMed] [Google Scholar]
- 11.Kouketsu A, Sato I, Abe S, Oikawa M, Shimizu Y, Takahashi T, et al. Detection of human papillomavirus infection in oral squamous cell carcinoma: A cohort study of Japanese patients. J Oral Pathol Med. 2016;45:565–72. doi: 10.1111/jop.12416. [DOI] [PubMed] [Google Scholar]
- 12.Migaldi M, Pecorari M, Forbicini G, Nanni N, Grottola A, Grandi T, et al. Low prevalence of human papillomavirus infection in the healthy oral mucosa of a Northern Italian population. J Oral Pathol Med. 2012;41:16–20. doi: 10.1111/j.1600-0714.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 13.Martín-Hernán F, Sánchez-Hernández JG, Cano J, Campo J, del Romero J. Oral cancer, HPV infection and evidence of sexual transmission. Med Oral Patol Oral Cir Bucal. 2013;18:e439–44. doi: 10.4317/medoral.18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhanapal R, Ranganathan K, Kondaiah P, Devi RU, Joshua E, Saraswathi TR, et al. High-risk human papilloma virus in archival tissues of oral pathosis and normal oral mucosa. Contemp Clin Dent. 2015;6:148–52. doi: 10.4103/0976-237X.156033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 16.Balaram P, Nalinakumari KR, Abraham E, Balan A, Hareendran NK, Bernard HU, et al. Human papillomaviruses in 91 oral cancers from Indian betel quid chewers – high prevalence and multiplicity of infections. Int J Cancer. 1995;61:450–4. doi: 10.1002/ijc.2910610403. [DOI] [PubMed] [Google Scholar]
- 17.D'Costa J, Saranath D, Dedhia P, Sanghvi V, Mehta AR. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998;34:413–20. doi: 10.1016/s1368-8375(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 18.Patel KR, Vajaria BN, Begum R, Desai A, Patel JB, Shah FD, et al. Prevalence of high-risk human papillomavirus type 16 and 18 in oral and cervical cancers in population from Gujarat, West India. J Oral Pathol Med. 2014;43:293–7. doi: 10.1111/jop.12147. [DOI] [PubMed] [Google Scholar]
- 19.Awan MS, Irfan B, Zahid I, Mirza Y, Ali SA. Comparison of polymerase chain reaction and immunohistochemistry assays for analysing human papillomavirus infection in oral squamous cell carcinoma. J Clin Diagn Res. 2017;11:XC10–3. doi: 10.7860/JCDR/2017/24742.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: P16 immunohistochemistry, consensus PCR HPV-DNA, and in situ hybridization. Infect Agent Cancer. 2012;7:4. doi: 10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe. 2014;15:266–82. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaiswal R, Pandey M. Human papilloma virus in oral carcinogenesis and its routes of transmission. World J Epidemiol Cancer Prev. 2012;1:1–9. [Google Scholar]
- 23.Chocolatewala NM, Chaturvedi P. Role of human papilloma virus in the oral carcinogenesis: An Indian perspective. J Cancer Res Ther. 2009;5:71–7. doi: 10.4103/0973-1482.52788. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Gupta S. Role of human papillomavirus in oral squamous cell carcinoma and oral potentially malignant disorders: A review of the literature. Indian J Dent. 2015;6:91–8. doi: 10.4103/0975-962X.155877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golusiński P, Pazdrowski J, Szewczyk M, Misiołek M, Pietruszewska W, Klatka J, et al. Is Immunohistochemical evaluation of p16 in oropharyngeal cancer enough to predict the HPV positivity? Rep Pract Oncol Radiother. 2017;22:237–42. doi: 10.1016/j.rpor.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]


