Abstract
Background
The association between body mass index (BMI) and recurrence of anorectal abscess remains controversial. This study investigated the exact relationship between BMI and anorectal abscess recurrence or anal fistula formation following initial surgery.
Material/Methods
This was a retrospective registry-based study conducted at the First Affiliated Hospital of Guizhou University of Chinese Medicine. Patients treated for anorectal abscess from 01/2015 to 03/2016 were included. Clinical data and time to recurrence were recorded. The Cox regression model was used to estimate the association between BMI and recurrence.
Results
A total of 790 patients were operated on during the study period. The average age of the participants was 38.3±11.6 years, and 83.2% were male. Median follow-up was 27 (range, 1–38) months. Compared with the low BMI (range, 15.7–22.8 kg/m2) patients, the high BMI (range, 26.0–40.6 kg/m2) patients showed higher risk of recurrence (HR=1.75, 95% CI: 1.15–2.67). In the non-adjusted model, high BMI was found to be positively correlated with recurrence (HR=1.62, 95% CI: 1.10–2.40, P=0.02), and a stronger association was found in the fully adjusted model (HR=1.75, 95% CI: 1.15–2.67, P=0.01). BMI was also used as a continuous variable for sensitivity analysis, and a similar trend was observed (P=0.01 for trend).
Conclusions
Elevated BMI is an independent risk factor of anorectal abscess recurrence and for increased risk of abscess recurrence or anal fistula formation.
MeSH Keywords: Body Mass Index, Recurrence, Risk Factors
Background
Anorectal abscesses are collections of pus within the dermis and deeper skin tissues, most often due to cryptoglandular infection or associated inflammatory bowel disease. Perianal abscesses are the most common type of anorectal abscess [1,2]. Patients with anorectal abscess may have discomfort and even severe pain. The incidence of anorectal abscess varies among countries and areas. It affects an estimated18 000 patients in the UK every year [3] and the annual incidence is 16.1 per 100 000 in Sweden [4].
A meta-analysis found that fistula surgery with abscess drainage can largely reduce the recurrence of abscess/fistula or reoperation [5], but the patients need to be carefully selected for this procedure [2]. Approximately 29% to 70% of patients with anorectal abscess will develop concomitant fistula, and 33% of these patients are diagnosed with anal fistula months or years after drainage [1,6–12].
Previous studies investigating the association between body mass index (BMI) and anorectal abscess recurrence yielded elusive or even ambiguous results. The findings from recent studies suggest that >25 kg/m2 BMI is a risk factor of anorectal abscess recurrence [4,13–15]. Schwandner et al. [15] showed that the recurrence rate was lower in non-obese patients compared with obese ones (14% vs. 28%). Adamo et al. [4] showed that the recurrence rates were similar between obese and non-obese patients during the first 3 years after surgery, but that the risk increased in obese patients after 3 years, but a cohort study reported no correlation [16]. Furthermore, the impact of other risk factors of recurrence such as sex, hypertension, and diabetes on the observed associations is still unclear [4,14,16].
Therefore, the aim of the present study was to investigate the exact relationship between BMI and anorectal abscess recurrence or anal fistula formation following initial surgery. These results could help improve management of patients with anorectal abscess.
Material and Methods
Patients
This was a retrospective registry-based study conducted at the First Affiliated Hospital of Guizhou University of Chinese Medicine from January 2015 to March 2016. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guizhou University of Chinese Medicine. The need for individual consent was waived by the committee because of the retrospective nature of the study.
The inclusion criteria were: 1) diagnosed with perianal abscess; 2) operated on at the First Affiliated Hospital of Guizhou University of Chinese Medicine; and 3) 15–70 years of age. The exclusion criteria were: 1) history of perianal abscess and anal fistula; 2) perianal abscess with anal fistula; 3) combined with diseases like inflammatory bowel disease, tuberculosis, HIV, recto-vaginal fistula, necrotizing myofasciitis, sacral osteomyelitis, tumor, blood system diseases, and cases of perianal abscess due to trauma, and internal hemorrhoid operation, as they are probably more susceptible to recurrence [16,17]; or 4) pregnant.
Data collection
The clinical data (age, BMI, sex, occupation, race, marital status, smoking habits, drinking habits, fatigue, diarrhea, diabetes, hypertension, and other variables) were retrieved from the medical records. All blood tests were performed routinely. The participants’ height and weight were measured routinely before surgery. BMI was calculated as weight (kilogram) divided by height (meter) squared (kg/m2). The patients were grouped into BMI tertiles: 20.8 (15.7–22.8) kg/m2 was regarded as low BMI (n=183), 24.2 (22.9–25.8) kg/m2 was regarded as intermediate BMI (n=197), and 28.0 (26.0–40.6) kg/m2 was regarded as high BMI (n=202). The covariates were selected a priori based on previous studies on the association between risk factors and anorectal abscess recurrence. According to these studies, risk factors for anorectal abscess recurrence were age, sex, diabetes, smoking habits, BMI, time from disease onset to surgery, and abscess type [4,11,13,15–19].
Operative technique
During the study period, all patients were operated on using the same technique. Patients on continuous epidural anesthesia were placed in the lithotomy position after a preoperative cleansing enema. The anal canal was carefully examined by trained and experienced surgeons to identify the type, extent, and components of the abscess, and to examine for the presence of internal openings. Incision and drainage with fistulotomy (I&D with F) were performed for anorectal abscesses with easily detected internal openings. If the opening was not easy to detect, incision and drainage (I&D) was performed alone. Loculations were freed by blunt digital dissection. Care was taken to preserve the internal anal sphincter. Stool softener was prescribed to all patients for 1 week, with no diet restriction. After the operation, patients were instructed to take a sitz bath twice daily until the wound was healed.
Follow-up
The follow-up data collection was based on the patient’s medical records, which include outpatient, inpatient, and telephone follow-ups. The investigators responsible for data extraction were trained together to ensure uniformity. Follow-up ended on March 1, 2018.
Recurrence
The retrospective study of recurrence was based on the patients’ medical records and follow-up interviews. Abscess recurrence and an anal fistula formation were deemed as recurrence. The presence of any 1 of symptoms such as dull perianal pain, swelling, secretion, itchiness, fever, or tenderness was regarded as a sign of recurrence [11,16].
Statistical analysis
Continuous data are presented as mean ± standard deviation (normal distribution) or medians (range) (non-normal distribution), and categorical data are presented as n (%). The t test (normal distribution), Mann-Whitney U test (non-normal distribution), and chi-square test (categorical variables) were performed to detect significant differences between groups. We presented the unadjusted results, the results of minimally adjusted analyses, and the results of the fully adjusted analyses. The covariance adjustment was determined by the following principle: when added to this model, the corresponding odds ratio had to change by a minimum of 10% [20]. To assess the reliability of the results, we performed a sensitivity analysis. When BMI and recurrence showed an evident ratio in the smoothed curve, the likelihood ratio test and bootstrap resampling method were used [21]. Outcome comparisons among different BMI groups were conducted using the Kaplan-Meier method and log-rank test. The Empower Dataweb System (http://www.empowerstats.com/dataweb/, X&Y Solutions, Inc., Boston, MA) was used for data input, with all the analyses completed with R software (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A <0.05 P value (two-sided) was considered to indicate statistical significance.
Results
Baseline characteristics of participants
From January 2015 to March 2016, 790 patients with anorectal abscess were operated on, and of these, 582 patients were included based on the inclusion/exclusion criteria. The average age of the participants was 38.3±11.6 years, and 83.2% were male. The median follow-up was 27 (range, 1–38) months. The cumulative recurrence rate was 27.5%. The recurrences rates in the low, intermediate, and high BMI tertiles were 21.9%, 26.9%, and 33.2%, respectively (P=0.045). Recurrences within 3, 6, and 12 months after surgery accounted for 33.1%, 55.0%, and 70.6% of the total recurrence rate, respectively. The characteristics of the patients are listed in Table 1. Only 9 patients had a BMI <18 kg/m2 and the sample size was too small for reliable analyses in this group.
Table 1.
Characteristics of the subjects by BMI tertiles.
| Characteristics | BMI tertiles (kg/m2) | P | ||
|---|---|---|---|---|
| Low (15.7–22.8) | Intermediate (22.9–25.8) | High (26.0–40.6) | ||
| N | 183 | 197 | 202 | |
| Age (years) | 36.7±12.7 | 39.5±11.3 | 38.7±10.6 | 0.047 |
| Time from disease onset to surgery (days) | 11.9±33.5 | 7.8±10.5 | 7.8±14.5 | 0.099 |
| Leukocyte count (×109/L) | 10.54±3.99 | 10.76±3.99 | 11.09±3.40 | 0.361 |
| Red blood cell count (×109/L) | 4.91±0.58 | 5.14±0.56 | 5.24±0.52 | <0.001 |
| Platelet count (×109/L) | 218.60±63.49 | 216.03±60.14 | 232.20±67.81 | 0.026 |
| Fibrinogen (g/L) | 4.30±1.40 | 4.52±1.39 | 4.55±1.38 | 0.158 |
| Sex, n (%) | <0.001 | |||
| Male | 130 (71.0%) | 172 (87.31%) | 182 (90.1%) | |
| Female | 53 (29.0%) | 25 (12.7%) | 20 (9.9%) | |
| Age (years), n (%) | 0.196 | |||
| <40 | 107 (58.5%) | 97 (49.2%) | 109 (54.0%) | |
| ≥40 | 76 (41.5%) | 100 (50.8%) | 93 (46.0%) | |
| Ethnicity, n (%) | 0.867 | |||
| Ethnic Han | 167 (91.3%) | 179 (91.3%) | 187 (92.6%) | |
| Minority | 16 (8.7%) | 17 (8.7%) | 15 (7.4%) | |
| Married, n (%) | 129 (70.5%) | 156 (79.2%) | 166 (82.2%) | 0.018 |
| Occupation, n (%) | 0.817 | |||
| Technical occupation | 141 (77.5%) | 149 (75.6%) | 163 (80.7%) | |
| Manual occupation | 15 (8.2%) | 21 (10.7%) | 13 (6.4%) | |
| Unemployment | 20 (11.0%) | 20 (10.2%) | 21 (10.4%) | |
| Retired | 6 (3.3%) | 7 (3.6%) | 5 (2.5%) | |
| Smoking habits, n (%) | 72 (39.3%) | 111 (56.4%) | 100 (49.5%) | 0.004 |
| Drinking habits, n (%) | 81 (44.3%) | 116 (58.9%) | 111 (55.0%) | 0.013 |
| Seafood-based diet, n (%) | 5 (2.7%) | 11 (5.6%) | 13 (6.4%) | 0.222 |
| Spice food diet, n (%) | 98 (53.6%) | 97 (49.2%) | 98 (48.5%) | 0.571 |
| Diabetes, n (%) | 13 (7.1%) | 18 (9.1%) | 14 (6.93) | 0.661 |
| Excessive fatigue, n (%) | 80 (43.7%) | 79 (40.1%) | 88 (43.6%) | 0.716 |
| Hypertension, n (%) | 10 (5.5%) | 17 (8.6%) | 23 (11.4%) | 0.117 |
| Diarrhea, n (%) | 4 (2.2%) | 6 (3.1%) | 14 (6.9%) | 0.042 |
| Time from disease onset to surgery, n (%) | 0.767 | |||
| <7 days | 101 (55.2%) | 116 (58.9%) | 115 (56.9%) | |
| ≥7 days | 82 (44.8%) | 81 (41.1%) | 87 (43.1%) | |
| Abscess type | 0.827 | |||
| Perianal | 142 (77.6%) | 159 (80.7%) | 151 (74.8%) | |
| Ischiorectal | 19 (10.4%) | 19 (9.6%) | 24 (11.9%) | |
| Intersphincteric | 12 (6.6%) | 8 (4.1%) | 11 (5.5%) | |
| Supralevator | 6 (3.3%) | 9 (4.6%) | 11 (5.5%) | |
| Submucosal | 4 (2.2%) | 2 (1.0%) | 5 (2.5%) | |
| Surgical approach | 0.859 | |||
| I&D | 118 (64.5%) | 123 (62.4%) | 125 (61.9%) | |
| I&D with F | 65 (35.5%) | 74 (37.6%) | 77 (38.1%) | |
BMI – body mass index; I&D – incision and drainage; I&D with F – incision and drainage with fistula treatment. Data are presented as mean ±SD for continuous variables and percentage for categorical variables.
Univariable analyses
The univariable analyses (Table 2) showed that BMI and I&D with F were associated with recurrence. The Kaplan-Meier curves showed that patients in the upper BMI tertile (26.0–40.6) kg/m2 had a higher cumulative incidence of recurrence (Figure 1).
Table 2.
Univariable analyses for the risk of recurrence.
| HR (95% CI) | P-value | |
|---|---|---|
| Sex | ||
| Male | 1.0 | |
| Female | 0.75 (0.48, 1.17) | 0.21 |
| Age (years) | 1.00 (0.99, 1.02) | 0.64 |
| Age (years) | ||
| <40 | 1.0 | |
| ≥40 | 1.18 (0.86, 1.61) | 0.30 |
| BMI (kg/m2) | 1.04 (1.00, 1.08) | 0.03 |
| BMI tertiles (kg/m2) | ||
| Low (15.7–22.8) | 1.0 | |
| Intermediate (22.9–25.8) | 1.29 (0.85, 1.94) | 0.23 |
| High (26.0–40.6) | 1.62 (1.10, 2.40) | 0.02 |
| Ethnicity | ||
| Ethnic Han | 1.0 | |
| Minority | 1.18 (0.70, 2.02) | 0.53 |
| Marital status | ||
| No | 1.0 | |
| Yes | 1.01 (0.70, 1.46) | 0.97 |
| Occupation | ||
| Technical | 1.0 | |
| Manual | 1.26 (0.75, 2.11) | 0.39 |
| Unemployment | 0.96 (0.57, 1.62) | 0.88 |
| Retired | 0.74 (0.27, 2.01) | 0.56 |
| Smoking habits | ||
| No | 1.0 | |
| Yes | 1.08 (0.79, 1.47) | 0.63 |
| Drinking habits | ||
| No | 1.0 | |
| Yes | 1.20 (0.87, 1.64) | 0.26 |
| Diet with seafood | ||
| No | 1.0 | |
| Yes | 1.35 (0.71, 2.56) | 0.36 |
| Excessive fatigue | ||
| No | 1.0 | |
| Yes | 1.11 (0.82, 1.52) | 0.50 |
| Diet with spicy food | ||
| No | 1.0 | |
| Yes | 0.98 (0.72, 1.33) | 0.89 |
| Diarrhea | ||
| No | 1.0 | |
| Yes | 0.88 (0.39, 2.00) | 0.77 |
| Diabetes | ||
| No | 1.0 | |
| Yes | 1.10 (0.64, 1.91) | 0.72 |
| Hypertension | ||
| No | 1.0 | |
| Yes | 1.29 (0.78, 2.14) | 0.32 |
| Time from disease onset to surgery(days) | 1.00 (0.99, 1.01) | 0.67 |
| Time from disease onset to surgery(days) | ||
| <7 | 1.0 | |
| ≥7 | 1.03 (0.75, 1.40) | 0.86 |
| Abscess type | ||
| Perianal | 1.0 | |
| Ischiorectal | 1.18 (0.74, 1.90) | 0.49 |
| Intersphincteric | 0.42 (0.15, 1.14) | 0.09 |
| Supralevator | 1.00 (0.47, 2.14) | 0.99 |
| Submucosal | 1.28 (0.47, 3.46) | 0.63 |
| Surgical approach | ||
| I&D | 1.0 | |
| I&D with F | 0.21 (0.13, 0.33) | <0.0001 |
| Leukocyte count (×109/L) | 1.02 (0.98, 1.06) | 0.32 |
| Red blood cell count (×109/L) | 0.94 (0.71, 1.23) | 0.64 |
| Platelet count (×109/L) | 1.00 (1.00, 1.00) | 0.19 |
| Fibrinogen (g/L) | 1.06 (0.95, 1.18) | 0.30 |
HR – hazard ratio; CI – confidence interval; BMI – body mass index; I&D – incision and drainage; I&D with F – incision and drainage with fistula treatment.
Figure 1.
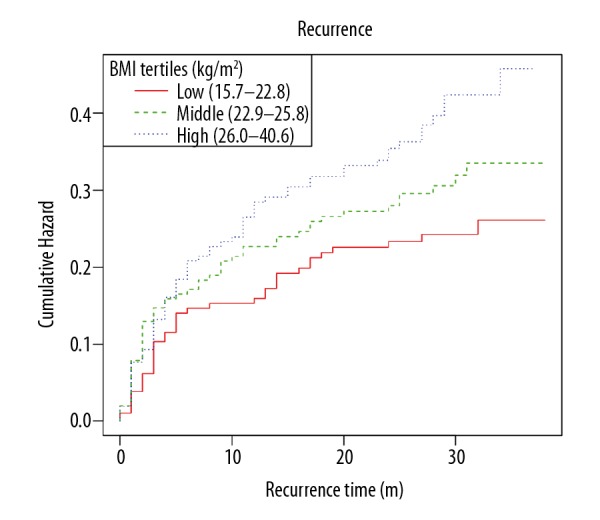
Kaplan-Meier curves of the cumulative event rate of recurrence stratified by the following baseline body mass index (BMI) tertiles: low BMI (15.70–22.80) kg/m2 (n=183), intermediate BMI (22.90–25.80) kg/m2 (n=197), and high BMI (26.00–40.60) kg/m2 (n=202).
Multivariable analysis
The relationship between BMI and recurrence was evaluated using the Cox proportional hazards regression model. As presented in Table 3, 3 models were constructed: (1) non-adjusted; (2) minimally adjusted for demographic factors (sex, age, ethnicity, occupation, and marital status); and (3) fully adjusted for all factors (sex, age, ethnicity, occupation, marital status, diabetes, hypertension, smoking habits, drinking habits, fatigue, diarrhea, time from disease onset to surgery, surgical approach, abscess type, red blood cell count, platelet count, fibrinogen, and leukocyte count). In the non-adjusted model, the high BMI group was found to be positively correlated with recurrence (HR=1.62, 95% CI: 1.10–2.40, P=0.02). In the minimally adjusted model, the results showed slight distinct changes (HR=1.69, 95% CI: 1.13–2.52, P=0.01). A more obvious association was found in the fully adjusted model (HR=1.75, 95% CI: 1.15–2.67, P=0.01). BMI was also used as a continuous variable for sensitivity analysis, and a similar increased trend was observed (P=0.01 for trend).
Table 3.
Relationship between BMI and risk of recurrence of an abscess or the formation of an anal fistula in different models.
| Exposure | Non-adjusted HR (95% CI) P |
Minimally adjusted HR (95% CI) P |
Fully adjusted HR (95% CI) P |
|---|---|---|---|
| BMI (kg/m2) | 1.04 (1.00, 1.08) 0.03 | 1.04 (1.01, 1.09) 0.03 | 1.05 (1.01, 1.10) 0.02 |
| BMI tertiles (kg/m2) | |||
| Low (15.7–22.8) | 1.0 | 1.0 | 1.0 |
| Intermediate (22.9–25.8) | 1.29 (0.85, 1.94) 0.23 | 1.25 (0.82, 1.89) 0.30 | 1.30 (0.85, 1.99) 0.23 |
| High (26.0–40.6) | 1.62 (1.10, 2.40) 0.02 | 1.60 (1.07, 2.39) 0.02 | 1.75 (1.15, 2.67) 0.01 |
| P for trend | 0.01 | 0.02 | 0.01 |
Non-adjusted model: model without adjustment. Minimally adjusted model: model with adjustment for demographics. Fully adjusted model: model with adjustment for sex, age, ethnicity, occupation, marital status, diabetes, hypertension, smoking habits, drinking habits, diarrhea, time from disease onset to surgery, surgical approach, abscess type, red blood cell count, platelet count, fibrinogen, and leukocyte count. HR – hazard ratio; CI – confidence interval; BMI – body mass index.
Stratified analysis for the type of surgery
I&D with F was associated with a lower recurrence of anorectal abscess (Table 2), but there was no difference in the stratified analysis between the 2 procedures (P for interaction was 0.49), indicating that the relationship between BMI and recurrence was stable (Table 4).
Table 4.
Subgroup analysis of the associations between BMI and risk of recurrence of an abscess or the formation of an anal fistula.
| Characteristics | HR (95% CI) | P for interaction |
|---|---|---|
| Surgical approach | 0.49 | |
| I&D | 1.05 (1.01, 1.10) | |
| I&D with F | 1.00 (0.89, 1.14) |
Stratification adjusted for all the variables (sex, age, ethnicity, occupation, marital status, diabetes, hypertension, smoking habits, drinking habits, excessive fatigue, diarrhea, time from disease onset to surgery, surgical approach, abscess type, red blood cell count, platelet count, fibrinogen, and leukocyte count) except the stratification variables themselves. HRs (95% CI) were derived from Cox proportional hazards regression models. HR – hazard ratio; CI – confidence interval; I&D – incision and drainage; I&D with F – incision and drainage with fistula treatment.
Discussion
The association between BMI and the recurrence of anorectal abscess remains controversial [4,14–16]. This study aimed to evaluate the relationship between BMI and the recurrence of anorectal abscess or anal fistula formation. There was a strong and significant association between elevated BMI and the recurrence rate. The high BMI group (range, 26.0–40.6 kg/m2) was found to be positively correlated with recurrence (HR=1.75, 95% CI: 1.15–2.67). To test the reliability of the results, BMI was also used as a continuous variable for sensitivity analysis, and a trend of increase was observed.
In this study, only 9 patients had BMI <18 kg/m2. Therefore, to ensure a sufficient number of patients in each group, BMI data were grouped by tertiles instead of using the traditional cut-off BMI values, and the association with anorectal abscess recurrence was observed. Our findings are consistent with a large-scale case-control study of 1342 patients [13], in which multivariable logistic regression models were used to evaluate the OR of BMI on recurrence, and showed that a BMI >25 kg/m2 was independently associated with the risk of anal fistula. A study of 220 patients also showed similar results: Schwandner et al. [15] showed that the recurrence rate was lower non-obese patients compared with obese (BMI ≥30 kg/m2) ones (14% vs. 28%). Nevertheless, there was insufficient adjustment for potential confounders since some factors that were closely related to recurrence (such as sex, time from disease onset to surgery, abscess type, and leukocyte count) were not adjusted for [11,16,17,19]. Hence, the conclusions of these previous studies are limited [13,15]. Another study reported that obesity was positively associated with risk of perianal abscess [4]. Due to different study populations and study designs, the risk magnitude varied extensively among these studies [4,13,15]. Indeed, Adamo et al. [4] showed that the recurrence rates were similar between obese and non-obese patients during the first 3 years after surgery, but that the risk increased in obese patients after 3 years. Nevertheless, a cohort study reported no association at all between BMI and recurrence [16].
At our center, 2 different surgical procedures are routinely used for the management of anorectal abscesses. If the internal opening of the anorectal abscess is easy to detect, I&D with F is routinely performed, but if it is not easy to detect, I&D is performed alone. The recurrence of an abscess and the formation of an anal fistula were both deemed as recurrence. A meta-analysis found that I&D with F could largely reduce the recurrence of abscess/fistula or reoperation [5]. In the present study, according to the univariable and multivariable analyses, I&D with F reduced the recurrence of anorectal abscess, but there was no difference in the stratified analysis between these 2 procedures.
The present study has some strengths. First, a generalized linear model was applied to assess the linear relationship between BMI and recurrence. Second, since this was an observation-based study and unavoidable confounding might have been involved, the analyses were adjusted for confounding factors to minimize the residual confounding, which was not performed in the previous studies. This helped identify high-risk patients and improve postoperative follow-up management.
Nevertheless, this study has several limitations. The assessment of recurrence was underpowered because of the relatively small number of events observed and the relatively short follow-up (37 months at the most); therefore, cautious evaluation is needed before applying our findings to other populations of different demographic and clinical backgrounds. Despite the control of important epidemiological and clinical covariates in the analyses, possible residual bias could not be ruled out. In addition, our study excluded some of the previously reported risk factors (e.g., anorectal abscess/fistula history and IBD) for recurrence, which may have biased our results. Those factors were excluded because of the large proportion of patients with missing data. Further well-designed and standardized observational studies are needed to determine the real association between BMI and recurrence of anorectal abscess.
Conclusions
An association was found between high BMI and a significantly higher risk of recurrence of anorectal abscess. Our findings may help identify high-risk patients and improve postoperative follow-up management.
Acknowledgments
The authors gratefully thank Dr. Changzhong Chen, Chi Chen, and Xin-Lin Chen (EmpowerStats X&Y Solutions, Inc., Boston, MA) for providing statistical methodology consultation.
Footnotes
Source of support: This work was supported by grants from the Shanghai Three-Year Action Plan to Further Accelerate the Development of Chinese Medicine (Grant No. ZY (2018-2020)-ccx-1007), National Natural Science Foundation of China, Grant No. 816032624), and Shanghai Municipal Science and Technology Commission Scientific Research Program (Grant No. 19401933000)
Conflict of interest
None.
References
- 1.Vogel JD, Johnson EK, Morris AM, et al. Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2016;59:1117–33. doi: 10.1097/DCR.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 2.Amato A, Bottini C, De Nardi P, et al. Evaluation and management of perianal abscess and anal fistula: A consensus statement developed by the Italian Society of Colorectal Surgery (SICCR) Tech Coloproctol. 2015;19:595–606. doi: 10.1007/s10151-015-1365-7. [DOI] [PubMed] [Google Scholar]
- 3.Pearce L, Newton K, Smith SR, et al. Multicentre observational study of outcomes after drainage of acute perianal abscess. Br J Surg. 2016;103:1063–68. doi: 10.1002/bjs.10154. [DOI] [PubMed] [Google Scholar]
- 4.Adamo K, Sandblom G, Brannstrom F, Strigard K. Prevalence and recurrence rate of perianal abscess – a population-based study, Sweden 1997–2009. Int J Colorectal Dis. 2016;31:669–73. doi: 10.1007/s00384-015-2500-7. [DOI] [PubMed] [Google Scholar]
- 5.Malik AI, Nelson RL, Tou S. Incision and drainage of perianal abscess with or without treatment of anal fistula. Cochrane Database Syst Rev. 2010;(7):CD006827. doi: 10.1002/14651858.CD006827.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Cox SW, Senagore AJ, Luchtefeld MA, Mazier WP. Outcome after incision and drainage with fistulotomy for ischiorectal abscess. Am Surg. 1997;63:686–89. [PubMed] [Google Scholar]
- 7.Read DR, Abcarian H. A prospective survey of 474 patients with anorectal abscess. Dis Colon Rectum. 1979;22:566–68. doi: 10.1007/BF02587008. [DOI] [PubMed] [Google Scholar]
- 8.Vasilevsky CA, Gordon PH. The incidence of recurrent abscesses or fistula-in-ano following anorectal suppuration. Dis Colon Rectum. 1984;27:126–30. doi: 10.1007/BF02553995. [DOI] [PubMed] [Google Scholar]
- 9.Hamalainen KP, Sainio AP. Incidence of fistulas after drainage of acute anorectal abscesses. Dis Colon Rectum. 1998;41:1357–61. doi: 10.1007/BF02237048. discussion 1361–62. [DOI] [PubMed] [Google Scholar]
- 10.Schouten WR, van Vroonhoven TJ. Treatment of anorectal abscess with or without primary fistulectomy. Results of a prospective randomized trial. Dis Colon Rectum. 1991;34:60–63. doi: 10.1007/BF02050209. [DOI] [PubMed] [Google Scholar]
- 11.Hamadani A, Haigh PI, Liu IL, Abbas MA. Who is at risk for developing chronic anal fistula or recurrent anal sepsis after initial perianal abscess? Dis Colon Rectum. 2009;52:217–21. doi: 10.1007/DCR.0b013e31819a5c52. [DOI] [PubMed] [Google Scholar]
- 12.Oliver I, Lacueva FJ, Perez Vicente F, et al. Randomized clinical trial comparing simple drainage of anorectal abscess with and without fistula track treatment. Int J Colorectal Dis. 2003;18:107–10. doi: 10.1007/s00384-002-0429-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Yang G, Qiu J, et al. Risk factors for anal fistula: A case-control study. Tech Coloproctol. 2014;18:635–39. doi: 10.1007/s10151-013-1111-y. [DOI] [PubMed] [Google Scholar]
- 14.Ommer A, Herold A, Berg E, et al. German S3 guidelines: Anal abscess and fistula (second revised version) Langenbecks Arch Surg. 2017;402:191–201. doi: 10.1007/s00423-017-1563-z. [DOI] [PubMed] [Google Scholar]
- 15.Schwandner O. Obesity is a negative predictor of success after surgery for complex anal fistula. BMC Gastroenterol. 2011;11:61. doi: 10.1186/1471-230X-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano T, Asano M, Matsuda Y, et al. Prognostic factors for recurrence following the initial drainage of an anorectal abscess. Int J Colorectal Dis. 2010;25:1495–98. doi: 10.1007/s00384-010-1011-9. [DOI] [PubMed] [Google Scholar]
- 17.Sahnan K, Askari A, Adegbola SO, et al. Natural history of anorectal sepsis. Br J Surg. 2017;104:1857–65. doi: 10.1002/bjs.10614. [DOI] [PubMed] [Google Scholar]
- 18.White JR, Chang CC, So-Armah KA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation. 2015;132:1630–38. doi: 10.1161/CIRCULATIONAHA.114.014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohsiriwat V, Yodying H, Lohsiriwat D. Incidence and factors influencing the development of fistula-in-ano after incision and drainage of perianal abscesses. J Med Assoc Thai. 2010;93:61–65. [PubMed] [Google Scholar]
- 20.Lee PH, Burstyn I. Identification of confounder in epidemiologic data contaminated by measurement error in covariates. BMC Med Res Methodol. 2016;16:54. doi: 10.1186/s12874-016-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Wang X, Lu Y, et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol Int. 2013;7:901–9. doi: 10.1007/s12072-013-9457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


