Abstract
Disturbances in motivation are prominent in the clinical presentation of people with schizophrenia and might reflect a disturbance in reward processing. Recent advances in affective neuroscience have subdivided reward processing into distinct components, but there are two limitations of the prior work in schizophrenia. First, studies typically focus on only one component rather than on the unfolding of reward processing across multiple stages. Second, studies have not considered the impact of certainty effects, which represent an important contextual factor that impacts processing. We examined whether individuals with schizophrenia show the typical certainty effects across three phases of reward processing: cue evaluation, feedback anticipation, and feedback receipt. Electroencephalography from 74 healthy controls and 92 people with schizophrenia was recorded during a cued gambling task under conditions in which cues indicated forthcoming reward outcomes that were certain or uncertain. Controls demonstrated the expected certainty effects across each stage. Initial cue evaluation (cue P300) was intact in the schizophrenia group, but people with schizophrenia showed diminished certainty effects during feedback anticipation (stimulus-preceding negativity [SPN]) and receipt (feedback reward positivity [fRewP] and feedback P300). During feedback receipt, event-related potentials in people with schizophrenia were similar to controls for the uncertain context, but larger than controls for the certain context. Essentially, people with schizophrenia appeared to process certain feedback as though it were uncertain. These findings show, for the first time, that the fundamental distinction between certain and uncertain contexts is altered in schizophrenia at a neural level.
Keywords: schizophrenia, certainty effects, valence effects, reward processing, event-related potentials (ERPs)
General Scientific Summary
Distinguishing between certainty and uncertainty is a critical component of reward processing, because predicting the future requires consideration of the probabilities of potential outcomes. This study showed, for the first time across multiple reward-processing stages, that people with schizophrenia show a fundamental impairment in distinguishing between certain and uncertain contexts when anticipating and receiving reward feedback. People with schizophrenia appeared to process certain feedback as though it had occurred in an uncertain context.
Disturbances in motivation are prominent in the clinical presentation of people with schizophrenia (Green, Horan, Barch, & Gold, 2015; Reddy et al., 2017; Ventura et al., 2015). People with schizophrenia often fail to initiate and persist in goal-directed activities, which manifests in the marked functional impairment and social isolation that characterize this condition (Green, Horan, & Lee, 2015; Green et al., 2018; Horan & Blanchard, 2003; Juckel & Morosini, 2008). Guided by affective neuroscience-based models (Barch & Dowd, 2010; Barch, Pagliaccio, & Luking, 2016; Strauss, Waltz, & Gold, 2014), researchers have begun to investigate whether these motivational difficulties reflect a disturbance in reward processing using measures of neural functioning, such as event-related brain potentials (ERPs). While these investigations have provided new key insights, the current study addresses two issues that limit our understanding of reward processing in schizophrenia. First, reward processing is a complex, multifaceted construct composed of distinct stages, including feedback anticipation associated with cues signaling forthcoming reward and reward outcome. However, ERP research in schizophrenia has typically focused on a single component of reward processing rather than capturing the unfolding dynamics of reward processing as they occur in daily life. Second, reward processing is powerfully moderated by contextual factors. One fundamental factor is whether the reward signaling cues we encounter are certain and fully predict the receipt of rewards or are uncertain and provide only partial information about reward receipt. Although extensive human and non-human animal research indicates that reward processing differs in several fundamental ways between certain versus uncertain contexts, no studies have examined this basic distinction in schizophrenia. The current ERP study was designed to address both of these limitations by comparing schizophrenia and healthy comparison groups on a novel paradigm that evaluated multiple reward processing stages under conditions in which reward predicting cues were either certain or uncertain.
ERPs Corresponding to Reward Processing Stages
Reward processing comprises a dynamic set of component processes that unfold across a series of temporal stages and is often examined during paradigms that manipulate reward/loss contingencies (Barch et al., 2016; O’Doherty, Cockburn, & Pauli, 2017; Wallis, 2007). As displayed in Table 1, the current investigation examined three stages of reward processing during certain and uncertain contexts. Cue evaluation refers to the initial responsiveness to whether a predicted outcome is certain or uncertain. Feedback anticipation refers to the preparation for and anticipation of a future outcome. Feedback receipt refers to the immediate response to a reward or punishment. In uncertain contexts the hedonic value of an outcome (feedback receipt) informs the responsiveness to cues (cue evaluation), which guides preparation for the future outcomes (feedback anticipation). The majority of research in healthy individuals examines only one stage of reward processing during certain or uncertain contexts, which limits interpretation about the temporal unfolding of reward processing.
Table 1.
Event-Related Potential Components Associated with Reward Processing Across Three Stages: Cue Evaluation, Reward Anticipation, and Feedback Receipt
| Cue Evaluation | Feedback Anticipation | Feedback Receipt | ||
|---|---|---|---|---|
| cP300 | SPN | fRewP | fP300 | |
| Certain vs Uncertain | - | Uncertain > Certain | Uncertain > Certain | Uncertain > Certain |
| Valence Effect | Reward and Loss > Neutral | Reward and Loss > Neutral | Reward > Neutral > Loss | Reward and Loss > Neutral |
Note: Certain > uncertain indicates that ERPs are larger for certain events than for uncertain events. The impact of certainty on cP300 has not been studied independently of valence effects. The valence effect describes the pattern of findings for reward, loss, and neutral outcomes. For a recent review, see Glazer et al. (2018). cP300 = cue P300; SPN = stimulus-preceding negativity; fRewP = feedback reward positivity; fP300 = feedback P300
Each of the three stages of reward processing is associated with distinct ERP components, which are related to characteristic patterns for certainty effects (i.e., certainty vs. uncertainty) and valence effects (i.e., reward/loss vs. neutral conditions; see Table 1; for review, see Glazer, Kelley, Pornpattananangkul, Mittal, & Nusslock, 2018). For cue evaluation, the cue P300 (cP300) is a centro-medial ERP that is larger following cues indicating upcoming reward than for cues indicating upcoming neutral outcomes (Broyd et al., 2012; Novak, Novak, Lynam, & Foti, 2016; Novak & Foti, 2015), and this cP300 valence effect is intact in schizophrenia (Vignapiano et al., 2016). For feedback anticipation, the stimulus-preceding negativity (SPN) is a right-hemisphere dominant ERP that is associated with the anticipation of feedback (Brunia, Hackley, van Boxtel, Kotani, & Ohgami, 2011; Brunia, van Boxtel, & Böcker, 2011). SPN is larger for reward-related feedback than for neutral feedback (Hughes, Mathan, & Yeung, 2013). For reward anticipation, one study found that patients generally show a reduced SPN while viewing emotional and neutral images (Wynn, Horan, Kring, Simons, & Green, 2010). Notably, other work on the anticipation of reward or pleasure in schizophrenia using self-report and fMRI measures generally evidence impairments (Buck & Lysaker, 2013; Chan et al., 2010; Gard, Kring, Gard, Horan, & Green, 2007; Li et al., 2015). Hence, patients should show deficits in reward-related feedback anticipation.
Two ERPs are commonly associated with feedback receipt: the feedback reward positivity (fRewP) and the feedback P300 (fP300). fRewP is a medial-frontal ERP that is generally largest for reward outcomes, less for neutral outcomes, and even less for loss outcomes (see Proudfit, 2015). Whereas fRewP is generally related to outcome valence, fP300 is a centro-parietal ERP that tends to relate to outcome magnitude and expectancy (Hajcak, Holroyd, Moser, & Simons, 2005; Hajcak, Moser, Holroyd, & Simons, 2007; Pfabigan, Alexopoulos, Bauer, & Sailer, 2011; Wu & Zhou, 2009). Both reward and loss trials yield larger fP300 than neutral trials (Glazer et al., 2018). For feedback receipt in schizophrenia, a recent meta-analysis of fRewP indicates that fRewP appears intact (Martin et al., 2018). To our knowledge, no studies have examined valence effects for fP300 in patients.
Taken together, the limited data on valence effects of these ERPs suggest impairments in SPN and intact cP300 and fRewP, with no studies of the fP300. However, limitations of these studies of valence effects include examining only a single stage of reward processing and not considering the psychometric properties (e.g., test-retest reliability, internal consistency) of these components.
Reward Processing in Certain vs. Uncertain Contexts
In order to obtain desirable outcomes in our daily lives, we are required to form predictions based on the cues that are available to us. In our complex environment these cues vary in degree of certainty, either fully predicting the receipt of a reward (i.e., certain) or providing only partial information (i.e., uncertain). Achieving a desired reward requires distinguishing between certain and uncertain cues, and forming predictions based on those cues facilitates adaptive responses and is a critical aspect of daily living. The distinction between certain and uncertain cues is critical, because mistaken certainty or uncertainty can lead to faulty predictions and contribute to poor daily functioning.
Extensive human and non-human animal research indicates that reward processing differs in several fundamental ways between certain versus uncertain contexts. Certainty is widely viewed as inherently desirable whereas uncertainty as inherently aversive and makes greater demands on cognitive control processes (Ladouceur, Gosselin, & Dugas, 2000; Luhmann, Chun, Yi, Lee, & Wang, 2008; Reuman, Jacoby, Fabricant, Herring, & Abramowitz, 2015). Furthermore, different brain networks are involved in reward processing when outcomes are certain versus uncertain. During certain contexts there is greater activity in the ventromedial prefrontal cortex, anterior cingulate cortex, and orbitofrontal cortex, whereas during uncertain contexts there is activity in a wider network including posterior parietal cortex, prefrontal cortex, and the striatum. Despite the importance of the certainty/uncertainty distinction for various cognitive processes (Esber & Haselgrove, 2011; Mushtaq, Bland, & Schaefer, 2011; Rushworth & Behrens, 2008), the longstanding scientific interest in uncertainty (Bertelson & Boons, 1960), and the relevance of uncertainty processing for psychopathology (e.g., anxiety and depressive disorders; Grupe & Nitschke, 2013; Hélie, Shamloo, Novak, & Foti, 2017; Lake & Labar, 2011; Zald & Treadway, 2017), no ERP studies have directly examined the impact of certain vs. uncertain reward related cues in schizophrenia.
Most of the reward-related ERPs shows a distinct pattern for certainty effects. For cue evaluation, cP300 is larger during trials when reward is possible than during trials when reward is not possible (Broyd et al., 2012; Novak et al., 2016; Novak & Foti, 2015; Vignapiano et al., 2016). However, cP300 does not appear to show a distinct pattern for certainty effects because studies of the relationship between uncertainty and cP300 are confounded by the impact of reward incentives. During feedback anticipation, SPN appears to index the degree of uncertainty, as it is larger for uncertain than for certain trials (Foti & Hajcak, 2012; Hughes et al., 2013), is greater for improbable than for probable outcomes (Catena et al., 2012), and increases in amplitude as outcomes become more uncertain (Fuentemilla et al., 2013; Megías et al., 2017). For feedback receipt, fRewP is larger for uncertain than for certain outcomes (Eppinger, Kray, Mock, & Mecklinger, 2008; Hewig et al., 2007; Holroyd, Krigolson, Baker, Lee, & Gibson, 2009; Wu & Zhou, 2009), and fP300 shows a similar pattern (Hajcak et al., 2005; Hajcak et al., 2007; Pfabigan et al., 2011; Wu & Zhou, 2009). Although certainty effects are well understood in studies of healthy controls, to our knowledge no study has examined ERPs associated with certainty effects across stages of reward processing in schizophrenia.
People with schizophrenia have difficulty making predictions based on environmental cues (Culbreth, Gold, Cools, & Barch, 2016; Gold, Waltz, Prentice, Morris, & Heerey, 2008; Strauss et al., 2014), and this difficulty may be due in part to an impairment in processing uncertain cues. For example, people with schizophrenia demonstrate cognitive control deficits (for review, see Barch, Culbreth, & Sheffield, 2018), and cognitive control is needed to resolve uncertainty (for review, see Mushtaq et al., 2011). These cognitive control deficits might also impair the maintenance of the value of a certain or uncertain cue (Strauss et al., 2014). Additionally, uncertainty relates to activity in dorsolateral prefrontal cortex and posterior parietal cortex (for meta-analysis, see White, Engen, Sørensen, Overgaard, & Shergill, 2014), and reduced activity in these brain regions during uncertain contexts has been observed in schizophrenia (Krug et al., 2014). These studies suggest that people with schizophrenia would show impairments in the processing of uncertainty, but it remains unclear whether the processing of certain cues is deficient or whether the ability to distinguish between certainty and uncertainty is impaired.
The Current Study
The primary goal of the present study was to determine whether patients with schizophrenia show an intact distinction between certain and uncertain conditions across three stages of reward processing. We used a novel cued reward paradigm that manipulated both certainty effects (certain and uncertain conditions) and valence effects (reward, loss, and neutral conditions). Regarding the primary research question of certainty effects, we predicted patients would show a diminished ability to distinguish between certain and uncertain conditions across the three stages of processing in light of the large cognitive control impairments seen in schizophrenia. The secondary aim was to determine whether patients show the expected valence-related differences in monetary outcomes: gain, loss, and breaking even (neither gain nor loss). For reward outcome we expected to replicate findings of an intact fRewP in schizophrenia, but the small number of studies for other ERP components did not support clear directional hypotheses.
Method
Participants
Study enrollment included 92 outpatients with schizophrenia and 74 healthy comparison participants (see Table 2). The research was approved by the Institutional Review Board at the VA Greater Los Angeles Healthcare System, and all participants provided written informed consent. These participants were part of a larger research project from which results for other experimental tasks have already been reported (Llerena, Wynn, Hajcak, Green, & Horan, 2016; Reddy, Waltz, Green, Wynn, & Horan, 2016). None of the ERP data presented in this manuscript have been reported elsewhere.
Table 2.
Summary of Demographics and Clinical Symptoms
| Characteristic | Controls | Patients | ||||
|---|---|---|---|---|---|---|
| n = 74 | n = 92 | |||||
| n | n | |||||
| Female/Male | 34/40 | 22/70 | X2 = 7.95, p < .01 | |||
| Mean | SD | Mean | SD | t | P | |
| Age (yrs) | 47.8 | 8.3 | 49.9 | 10.3 | −1.46 | .15 |
| Education (yrs.) | 14.6 | 1.8 | 13.1 | 1.8 | 5.03 | < .01 |
| Parental Education (yrs.)1 | 14.3 | 3.0 | 13.8 | 3.3 | 1.01 | .32 |
| Symptoms | ||||||
| PANSS - Negative Symptoms | - | - | 15.2 | 6.5 | ||
| PANSS - Positive Symptoms | - | - | 18.0 | 7.2 | ||
| PANSS - Disorganized Symptoms | - | - | 12.1 | 3.9 | ||
| PANSS - Excited | - | - | 5.6 | 2.4 | ||
| PANSS - Depression/Anxiety | - | - | 7.1 | 2.7 | ||
Note:
Information is missing for one control and three patients.
PANNS = Positive and Negative Syndrome Scale
The schizophrenia group, but not the controls, were reassessed on EEG four weeks later to examine test-retest reliability of ERP scores. These data are not directly relevant to the research question addressed in the current paper, but they are included in the supplementary material if the reader is interested in the test-retest reliability of these ERP scores.
Psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P; First, Spitzer, Gibbon, & Williams, 1996). Interviewers were trained to establish interrater reliability and obtained a minimum kappa of .75 for key psychotic and mood items and a minimum kappa of .85 for diagnostic accuracy. Training materials included a library of videotaped interviews developed by the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. Exclusion criteria for patients included substance dependence in the last six months or abuse in the last month, an identifiable neurological disorder, a current mood episode, loss of consciousness for more than one hour, and limited fluency in English. All patients were clinically stable as indicated by no hospitalizations within three months prior to study participation, no medication changes within six weeks prior to study participation, and no changes in housing status in the two months prior to study participation. With regard to medication status, 84% of participants were using atypical antipsychotics, 7% were using typical antipsychotics, 5% were using both atypical and typical antipsychotics, and 5% were not using antipsychotic medications.
Healthy controls were recruited though postings on websites. Exclusion criteria for controls included a neurological disorder, loss of consciousness for more than one hour, a psychotic disorder in a first-degree relative, and limited fluency in English. Healthy controls were also excluded for a history of psychotic disorder, bipolar disorder, recurrent depression, a lifetime history of substance dependence, or substance abuse in the last month as assessed by the SCID-I/P. The Structured Clinical Interview for DSM-IV Axis II Disorders (First, Gibbon, Spitzer, Williams, & Benjamin, 1996) was administered to healthy controls to assess for and exclude those with avoidant, paranoid, schizoid, or schizotypal personality disorders.
Experimental Task
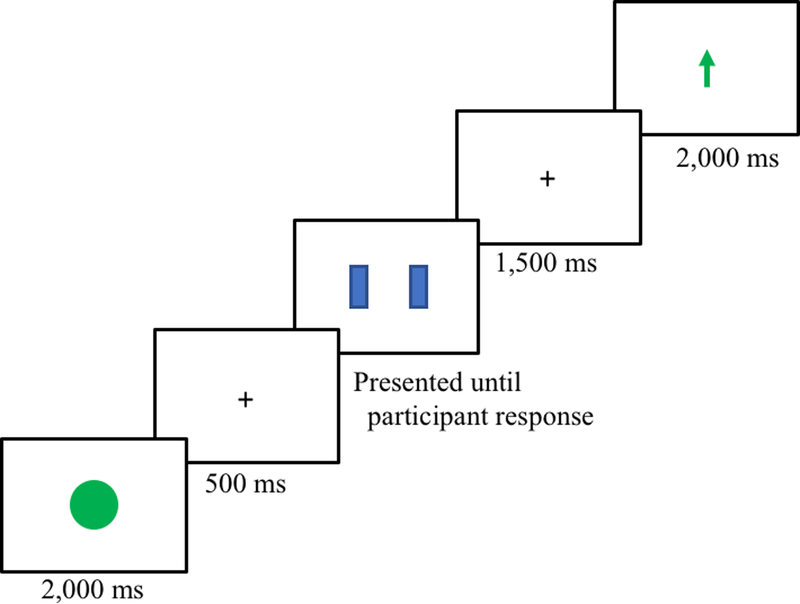
Participants completed a cued reward guessing task in order to examine three different stages of processing: cue evaluation, feedback anticipation, and feedback receipt. A schematic of an example trial can be seen in Figure 1. Each trial began with a 2,000 ms cue, which specified five possible outcomes for a particular trial: a complete green circle indicated a 100% chance of winning money, a half green and half white circle indicated a 50% chance of winning money and a 50% chance of breaking even (i.e., neither winning nor losing money), a whole white circle indicated a 100% chance of breaking even, a half red and half white circle indicated a 50% chance of losing money and a 50% chance of breaking even, and a whole red circle indicated a 100% chance of losing money. A fixation cross was then presented for 500 ms. The fixation cross was then replaced by two doors, and the participant selected one of the doors by pressing a left or right mouse button. Once the participant selected a door, the doors were removed from the screen and followed by a 1,500 ms fixation cross. Following the offset of the fixation cross, feedback was presented for 2,000 ms. The feedback stimulus consisted of one of three different stimuli: a green upward arrow indicated that the participant had won $0.50, a white “0” indicated that the participant had broken even, and a red downward arrow indicated that the participant had lost $0.25. These values were chosen to equate the subjective value of gains and losses (Tversky & Kahneman, 1992). Participants completed 140 trials (20 certain gain, 20 certain loss, 20 certain even, 40 uncertain gain, and 40 uncertain loss). Participants broke even on 50% of uncertain gain trials and on 50% of uncertain loss trials. The order of the trial type and feedback was random. Prior to beginning the task, participants were told that they would be given their cumulative winnings. Because the number of win/loss/even trials was fixed, cumulative earnings amounted to $10 for all participants.
Figure 1.
An example schematic of a certain gain trial.
Electrophysiological Data Recording and Reduction
EEG data recording and reduction procedures were identical for Session 1 and Session 2. Continuous EEG was recorded using an ActiveTwo BioSemi amplifier (BioSemi, Amsterdam, Netherlands). EEG signals were pre-amplified at the electrode with a gain of one and were digitized at a sampling rate of 1,024 Hz with a 24-bit analog-to-digital converter (least significant bit: 31.25 nV). EEG was filtered online using a low-pass, fifth-order sinc filter with a half-power cut-off of 204.8 Hz. EEG was recorded from 64 active scalp electrodes placed based on the 10/20 system using a custom cap (Cortech Solutions, Wilmington, North Carolina, USA). Two additional scalp electrodes were placed on the left and right mastoids. Electrooculogram was recorded from four additional sensors placed above and below the left eye and near the outer canthi. Electrodes were referenced online to a common mode sense electrode that formed a monopolar channel.
Data were subsequently filtered offline using ERPLab v6.1.4 (Lopez-Calderon & Luck, 2014). Cue- and feedback-related EEG data (i.e., cP300, fRewP, fP300) were digitally filtered using a sixth-order IIR Butterworth filter with half-amplitude cutoffs at .05 and 20 Hz. Feedback preceding activity (i.e., SPN) was digitally filtered using the same Butterworth filter but with half-amplitude cutoffs of .01 and 5 Hz. EEG data were algebraically rereferenced to averaged mastoids and epoched based on specifications reported below. Ocular artifact (i.e., eye blinks and saccadic eye movement) were then removed from the segmented waveforms using independent components analysis (ICA) implemented in the ERP PCA Toolkit v2.65 (Dien, 2010a). Specifically, any ICA components that correlated at .9 or above with the scalp topography of a blink template and at .8 or above with the scalp topography of vertical and horizontal saccade templates were removed from the data. Following ocular artifact correction, trials that contained more than a 100 μV step within 100 ms intervals or a voltage difference of 300 μV through the duration of the epoch were rejected.
Following artifact correction and rejection, individual-subject ERPs were analyzed, and EEG sites for analysis were chosen based on grand average waveforms and prior studies of cP300 (e.g., Novak & Foti, 2015), SPN (e.g., Brunia, van Boxtel, et al., 2011), fRewP (e.g., Foti, Weinberg, Dien, & Hajcak, 2011), and fP300 (e.g., Kujawa, Smith, Luhmann, & Hajcak, 2013). In order to reduce the biasing effects of background EEG noise on ERP measurements, all ERPs were scored using a mean amplitude approach (Clayson, Baldwin, & Larson, 2013; Luck, 2014).
Following data extraction of ERP measurements, the internal consistency of scores was assessed to ensure that ERP scores met appropriate standards (Clayson & Miller, 2017b; Hajcak, Meyer, & Kotov, 2017; Infantolino, Luking, Sauder, Curtin, & Hajcak, 2018). To this end, participants were excluded if their data did not have an adequate number of trials in a given ERP average to obtain reliability standards. The number of trials needed to achieve a reliability threshold of .70 was calculated. This threshold of .70 was deemed acceptable based on published guidelines for ERP score reliability for paradigms that are in the early stages of development (Clayson & Miller, 2017b), such as the one used in the current study. In addition to internal consistency, test-retest reliability of ERP scores was examined in people with schizophrenia, and these analyses are reported in the supplementary material.
Generalizability theory was used to calculate dependability, which is a measure of internal consistency analogous to Cronbach’s alpha from classical test theory (see Baldwin, Larson, & Clayson, 2015; Brennan, 2001; Shavelson & Webb, 1991). ERP score reliability was examined using the ERP Reliability Analysis Toolbox v 0.4.7 (Clayson & Miller, 2017a). See supplementary material for additional information about reliability analyses. Supplementary Tables 1–4 summarize the number of trials needed to obtain a dependability point estimate of .70 for each ERP component and event type for certainty and valence effects. For the primary analyses of interest (i.e., examination of certainty effects), the data for all participants met the necessary trial cutoffs for each ERP, except for SPN (one control and one patient were excluded), and the overall internal consistency of the data was high (controls: .83 < ϕs < .98; patients: .87 < ϕs < .98).
For secondary analyses (i.e., examination of valence effects), the paradigm did not include enough trials to achieve adequate dependability for all valence effects during certain and uncertain conditions. Thus, trial cutoffs for the event types that were within the number of trials presented during the paradigm were used for determining exclusion criteria. These exclusion criteria resulted in up to one control and four patients be excluded from any given analysis of valence effects. Internal consistency estimates appeared lower for valence effects during certain conditions (controls: .47 < ϕs < .83; patients: .63 < ϕs < .76) than during uncertain conditions (controls: .79 < ϕs < .90; patients: .77 < ϕs < .92). Given the low internal consistency estimates for some valence effects for certain conditions, results should be interpreted with caution.
Cue-related activity.
Stimulus-locked epochs were extracted from 200 ms prior to presentation of the cue to 800 ms following cue presentation. The first 200 ms of the epoch served as a prestimulus baseline. Cue-related activity corresponding to cP300 was extracted as the average activity from 400 to 650 ms following cue presentation at Pz.
Feedback-preceding activity.
Stimulus-locked epochs were extracted from 200 ms prior to the offset of the doors to 1,500 ms following the offset of the doors. The first 200 ms of the epoch served as a prestimulus baseline. Feedback-preceding activity corresponding to SPN was extracted as the average activity from 1,000 to 1,500 ms following the offset of the doors. Activity was averaged over six right-frontal sites (FC4, FC6, C4, C6, F4, F6), because SPN is lateralized over the right-hemisphere (for review, see Brunia, van Boxtel, et al., 2011).
Feedback-related activity.
Stimulus-locked epochs were extracted from 200 ms prior to presentation of the feedback stimulus to 800 ms following feedback presentation. The first 200 ms of the epoch served as a prestimulus baseline. Feedback-related activity corresponding to fRewP was extracted as the average activity from 225 to 300 ms following feedback presentation at FCz and Fz. Feedback-related activity corresponding to fP300 was extracted as the average activity from 325 to 500 ms following feedback presentation at Cz.
Certainty effects appeared to broadly impact ERP activity within the fP300 time window (approximately 200 to 800 ms following the presentation of feedback). The robust fP300 response led to concerns of overlap between fP300 and fRewP. We conducted a temporospatial principal components analysis (PCA) on feedback-related ERP activity, but we were not able to isolate fRewP from fP300 (see supplementary material). Given that fP300 exhibited a robust response and we were unable to isolate fRewP from fP300, we chose to focus solely on feedback-related activity indexed by fP300 throughout the remainder of the manuscript. fRewP findings are presented in the supplementary material.
Clinical Ratings
Symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS; Kay, Opler, & Lindenmayer, 1989). The PANSS is a 30-item structured interview that assessed five symptom dimensions: positive (observed internal consistency: Cronbach’s α = .82), negative (α = .84), disorganized (α = .70), excited (α = .72), and anxiety/depression (α = .61).
Data Analysis
The primary focus was on group differences for certainty processing for cue evaluation, feedback anticipation, and feedback receipt. Separate 2-Group (controls, patients) × 2-Certainty Effect (certain trials, uncertain trials) repeated measures analyses of variance (ANOVAs) were conducted for cP300, SPN, and fP300.
Follow-up secondary analyses were then conducted to examine valence effects within certain and within uncertain conditions. Valence effects within certain and uncertain outcomes were examined separately due to the asymmetry in outcomes across effects. On the one hand, uncertain gain trials could result in gain or breaking even outcomes, but not a loss outcome. On the other hand, uncertain loss trials could result in loss or breaking even outcomes, but not a gain outcome. Hence, the asymmetry in potential outcomes precludes the certainty effects and valence effects from being examined using one ANOVA for each ERP. For cP300 and SPN, separate 2-Group × 3-Valence Effect within Certain Outcomes (certain gain cues, certain loss cues, certain even cues) and 2-Group × 2-Valence Effect within Uncertain Outcomes (uncertain gain cues, uncertain loss cues) ANOVAs were conducted. For fP300, separate 2-Group × 3-Valence Effect within Certain Outcomes and 2-Group × 4-Valence Effect within Uncertain Outcomes (uncertain gain cues, gain outcomes; uncertain gain cues, even outcomes; uncertain loss cues, loss outcomes; uncertain loss cues, even outcomes) were conducted. For all ANOVAS, partial-eta2 was reported as a measurement of effect size, and a Huynh-Feldt epsilon adjustment was applied to correct for possible violations of sphericity for factors with more than two levels. Significant effects were followed up with independent samples or paired samples t tests. Cohen’s d was reported as a measurement of effect size for t tests.
Results
Sample Characteristics
With regard to demographic characteristics (see Table 2 for summary information and statistical analyses), there was a larger proportion of women in the control group than in the patient group. Controls had higher personal, but not parental, levels of education compared with patients.
Cue-Related Activity
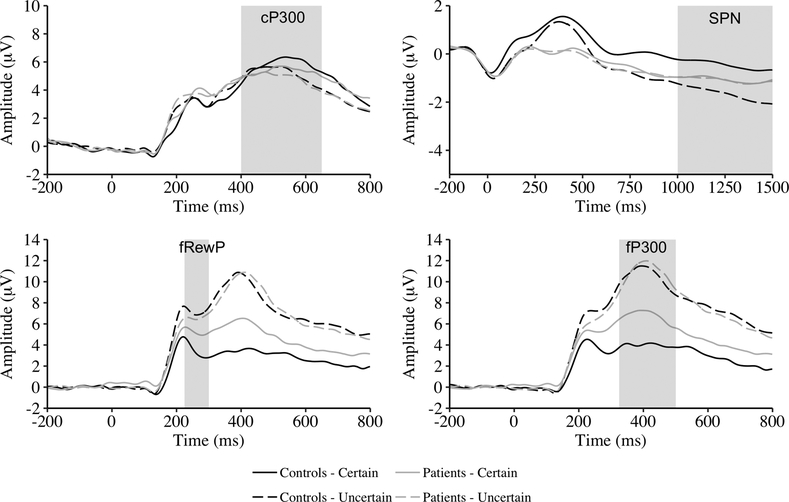
Summary information for cP300 scores are shown in Tables 3 and 4. Grand average waveforms for cP300 are shown in Figures 2 and 3, and voltage maps are shown in supplementary Figure 1. Main effects and interactions for each ANOVA on cP300 amplitude are shown in Table 5 and interpreted below.
Table 3.
Summary of ERP Amplitudes (μV) for Certain and Uncertain Trials for Controls (n = 73) and Patients (n = 89)
| Component | Group | Certain | Uncertain |
|---|---|---|---|
| M (SD) | M (SD) | ||
| cP300 | Controls | 5.8 (4.5) | 5.2 (4.1) |
| Patients | 5.2 (3.9) | 4.8 (3.6) | |
| SPN | Controls | −.1 (0.8) | −.4 (0.8) |
| Patients | −.2 (0.9) | −.2 (1.0) | |
| fP300 | Controls | 4.1 (3.7) | 10.6 (6.8) |
| Patients | 6.8 (4.8) | 10.9 (7.0) |
Note. cP300 = cue P300; SPN = stimulus-preceding negativity; fP300 = feedback P300
Table 4.
Summary of cP300 and SPN Amplitudes (μV) as a Function of Certain/Uncertain Outcome and Group
| Component | Group | Certain Gain | Certain Loss | Certain Even | Uncertain Gain | Uncertain Loss | |
|---|---|---|---|---|---|---|---|
| n | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||
| cP300 | Controls | 72 | 6.4 (5.1) | 6.2 (4.5) | 4.7 (5.1) | 5.2 (4.3) | 5.2 (4.4) |
| Patients | 85 | 6.2 (4.3) | 5.4 (4.1) | 4.4 (4.3) | 4.9 (4.0) | 4.8 (3.6) | |
| SPN | Controls | 71 | −.1 (10) | −.1 (10) | −1 (0.9) | −.4 (0.9) | −.4 (0.9) |
| Patients | 85 | −.2 (1.0) | −.3 (1.0) | −.2 (0.9) | −.3 (1.0) | −.3 (1.0) |
Note. cP300 = cue P300; SPN = stimulus-preceding negativity
Figure 2.
Grand average waveforms for cue-related activity (i.e., cue P300 [cP300]), feedback-preceding activity (i.e., stimulus-preceding negativity [SPN]) and feedback receipt (i.e., feedback P300 [fP300]) for certain and uncertain trials as a function of group. Note different amplitude scales.
Figure 3.
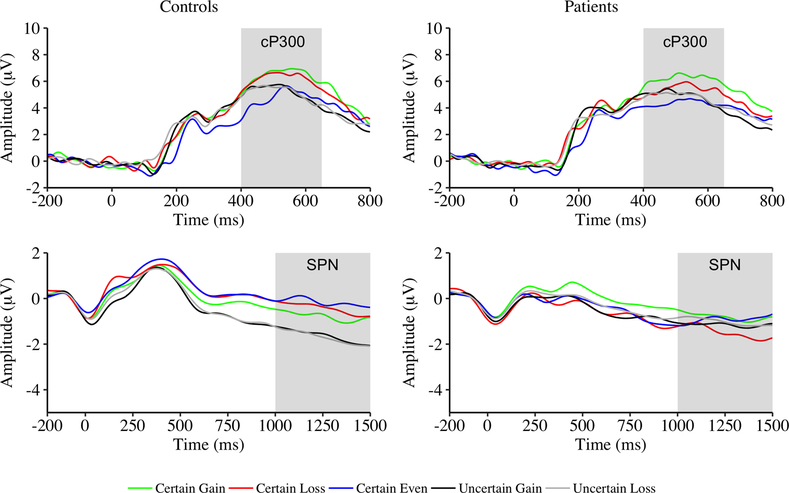
Grand average waveforms for cue-related activity (i.e., cue P300 [cP300]) and feedback-preceding activity (i.e., stimulus-preceding negativity [SPN]) for certain and uncertain trials as a function of valence effect and group. Note different amplitude scales.
Table 5.
Analyses of Variance (ANOVAs) for cue P300 (cP300) and Stimulus Preceding Negativity (SPN) Amplitudes
| ERP | ANOVA | Effect | |
|---|---|---|---|
| cP300 | Group × Certainty Effect | Group | F(1, 160) = 0.69, p = .41, = .004 CI[.00, .05] |
| Certainty | F(1, 160) = 13.12, p < .001, = .08 CI[.02, .16] | ||
| Interaction | F(1, 160) = 0.57, p = .45, = .004 CI[.00, .04] | ||
| Group × Valence Effect within Certain Condition | Group | F(1, 155) = 0.46, p = .50, = .003 CI[.00, .04] | |
| Certain Outcome | F(2, 310) = 31.24, p < .001, = .17 CI[.10, .24] | ||
| Interaction | F(2, 310) = 0.79, p = .46, = .005 CI[.00, .03] | ||
| Group × Valence Effect within Uncertain Condition | Group | F(1, 155) = 0.27, p = .61, = .002 CI[.00, .04] | |
| Uncertain Outcome | F(1, 155) = 0.03, p = .87, < .001 CI[.00, .02] | ||
| Interaction | F(1, 155) = 0.002, p = .97, < .001 CI[.00, .004] | ||
| SPN | Group × Certainty Effect | Group | F(1, 158) < 0.01, p = .96, < .001 CI[.00, .009] |
| Certainty | F(1, 158) = 15.74, p < .001, = .09 CI[.02, .18] | ||
| Interaction | F(1, 158) = 12.65, p < .001, = .07 CI[.01, .16] | ||
| Group × Valence Effect within Certain Condition | Group | F(1, 154) = 1.58, p = .21, = .01 CI[.00, .06] | |
| Certain Outcome | F(2, 308) = 1.14, p = .32, = .01 CI[.00, .03] | ||
| Interaction | F(2, 308) = 0.33, p = .72, = .002 CI[.00, .02] | ||
| Group × Valence Effect within Uncertain Condition | Group | F(1, 154) = 0.70, p = .41, = .005 CI[.00, .05] | |
| Uncertain Outcome | F(1, 154) = 0.12, p = .73, = .001 CI[.00, .03] | ||
| Interaction | F(1, 154) = 0.43, p = .52, = .003 CI[.00, .04] |
Note. Significant main effects and interactions (ps < .05) are bolded for ease of identification. Significant analyses are interpreted in the cP300 and SPN sections of the Results. CI = 95% confidence interval for .
cP300.
Certainty Effect.
The Group × Certainty ANOVA on cP300 showed a main effect of certainty. cP300 amplitude was larger for certain trials than for uncertain trials. The main effect of group and Group × Certainty interaction were not significant.
Valence Effect within Certain Trials.
The Group × Valence Effect ANOVA yielded a main effect of valence within certain outcomes. cP300 was larger for certain gain trials than for both certain loss trials and certain even trials, t(156) = 2.37, p = .02, d = .19; t(156) = 7.59, p < .001, d = .61, respectively. cP300 was also larger for certain loss trials than for certain even trials, t(156) = 5.41, p < .001, d = .43. The main effect of group and Group × Valence Effect interaction were not significant.
Valence Effect within Uncertain Trials.
A Group × Valence Effect ANOVA on cP300 amplitudes within uncertain outcomes did not yield any significant main effects or a significant interaction.
Feedback-Preceding Activity
Summary information for SPN amplitudes are shown in Tables 3 and 4. Grand average waveforms are shown in Figures 2 and 3, and voltage maps are shown in supplementary Figure 2. Main effects and interactions for each ANOVA on SPN amplitude are shown in Table 5 and interpreted below.
SPN.
Certainty Effect.
The Group × Certainty ANOVA yielded a main effect of certainty with larger SPN for uncertain trials than for certain trials. The main effect of group was not significant. The Group × Certainty interaction was significant. Only controls showed larger SPN for uncertain than for certain trials, t(71) = −4.59, p < .001, d = .51. None of the remaining comparisons were significant (|ts| < 1.3, ps > .20, ds < .21).
Valence Effect within Certain and Uncertain Trials.
Neither the Group × Valence ANOVA within certain outcomes nor the Group × Valence ANOVA within uncertain outcomes yielded any significant main effects or interactions.
Feedback Receipt Activity
Summary information for fP300 amplitudes are shown in Tables 3 and 6. Grand average waveforms are shown in Figures 2 and 4. Main effects and interactions for each ANOVA on fP300 amplitude are shown in Table 7 and interpreted below.
Table 6.
Summary of fP300 Amplitudes (μV) as a Function of Certain/Uncertain Outcome and Group
| Component | Group | Certain Gain | Certain Loss | Certain Even | Uncertain Gain, Gain | Uncertain Gain, Even | Uncertain Loss, Loss | Uncertain Loss, Even | |
|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | ||
| fP300 | Controls | 72 | 5.0 (4.5) | 4.5 (4.2) | 2.4 (3.6) | 13.0 (7.9) | 9.1 (7.2) | 10.9 (7.3) | 9.2 (6.6) |
| Patients | 89 | 8.1 (5.6) | 7.3 (5.4) | 4.9 (4.8) | 13.0 (8.1) | 9.6 (6.5) | 10.9 (7.5) | 10.1 (7.4) |
Note. fP300 = feedback P300
Figure 4.
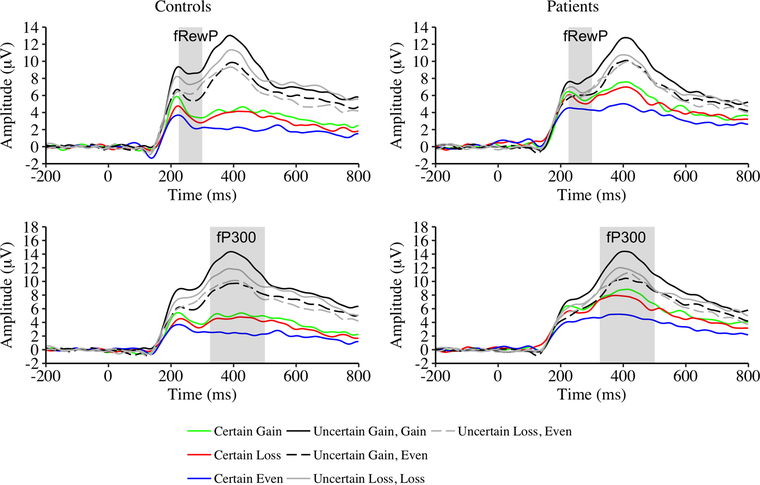
Grand average waveforms for feedback receipt (i.e., feedback P300 [fP300]) for certain and uncertain trials as a function of valence effect and group.
Table 7.
Analyses of Variance (ANOVAs) for feedback P300 (fP300) Amplitudes
| ERP | ANOVA | Effect | |
|---|---|---|---|
| fP300 | Group × Certainty Effect | Group | F(1, 160) = 3.59, p = .06, = .02 CI[.00, .08] |
| Certainty | F(1, 160) = 119.21, p < .001, = .43 CI[.31, .52] | ||
| Interaction | F(1, 160) = 5.85, p = .02, = .04 CI[.001, .11] | ||
| Group × Valence Effect within Certain Condition | Group | F(1, 159) = 16.47, p < .001, = 09 CI[.03, .19] | |
| Certain Outcome | F(2, 318) = 58.19, p < .001, = .27 CI[.19, .34] | ||
| Interaction | F(2, 318) = 0.48, p = .62, = .003 CI[.00, .02] | ||
| Group × Valence Effect within Uncertain Condition | Group | F(1, 159) = 0.11, p = .74, = .001 CI[.00, .03] | |
| Uncertain Outcome | F(3, 477) = 56.35, p < .001, = .26 CI[.19, .32] | ||
| Interaction | F(3, 477) = 0.92, p = .43, = .006 CI[.00, .02] |
Note. Significant main effects and interactions (ps < .05) are bolded for ease of identification. Significant analyses are interpreted in the fP300 section of the Results. CI = 95% confidence interval for .
fP300.
Certainty Effect.
The Group × Certainty ANOVA on fP300 amplitude yielded a main effect of certainty with larger fP300 for uncertain than for certain trials. The main effect of group was not significant. The ANOVA also yielded a significant interaction. Controls and patients showed larger fP300 for uncertain trials than for certain trials, t(72) = −9.21, p < .001, d = 1.08; t(88) = −6.21, p < .001, d = .66, respectively. Patients showed larger fP300 for certain trials than controls, t(159) = −4.06, p < .001, d = .64, and group differences were not observed for uncertain trials, t(159) = −.34, p = .74, d = .05.
Valence Effect within Certain Trials.
A Group × Valence ANOVA on fP300 amplitude indicated a main effect of group with patients showing larger fP300 than controls. The main effect of valence was also significant. fP300 was larger for certain gain than for both certain loss and certain even trials, t(160) = 2.37, p = .02, d = .19; t(160) = 10.02, p < .001, d = .79, respectively. fP300 for certain loss trials was larger than for certain even trials, t(160) = −8.37, p < .001, d = .66. The Group × Valence interaction was not significant.
Valence Effect within Uncertain Trials.
The main effect of valence was significant, and the follow-up t tests are shown in Supplementary Table 5 and summarized here. fP300 was largest when the participant won than when the participant broke even or when it was possible to lose money (regardless of monetary outcome; ts > 6.8, ps < .001, ds > .54). When it was possible to lose money, fP300 was larger when the participant lost money than when the participant broke even (regardless of whether it was possible to win or lose money; ts > 4.0, ps < .001, ds > .31). fP300 amplitudes were similar when the participant broke even and it was possible to win or lose money, t(160) = −1.10, p = .27, d = .09. The main effect of group and Group × Valence interaction were not significant.
Discussion
The primary aim of the study was to determine whether people with schizophrenia demonstrate intact processing across three stages of reward processing under certain vs. uncertain conditions (i.e., certainty effects). To our knowledge this is the first study to examine certainty effects across multiple stages of reward processing in either healthy or schizophrenia samples within a single paradigm. Controls demonstrated the expected certainty effects (see Table 1), showing clear ERP differentiation between certain vs. uncertain conditions across all three stages. Although initial cue evaluation (i.e., cP300) was intact in the schizophrenia group, they showed an atypical pattern of reduced differentiation between certain vs. uncertain conditions during subsequent stages. During feedback anticipation (i.e., SPN), the schizophrenia group showed no distinction between certain and uncertain conditions. During feedback receipt (i.e., fP300), they showed a significant, but diminished, distinction between conditions. During feedback receipt, the schizophrenia group’s ERPs were similar to controls for the uncertain context but were larger than controls for the certain context. In other words, the schizophrenia group appeared to process certain events more similar to uncertain events than controls did. These findings show, for the first time across multiple stages of reward processing, that the fundamental distinction between certain and uncertain reward processing is altered in schizophrenia.
Certainty Effects Across Reward-Processing Stages
Our multi-stage paradigm enabled us to identify how abnormal certainty effects temporally unfold in schizophrenia. During the initial cue evaluation stage, both groups showed a similarly clear cP300 differentiation between the certain versus uncertain conditions. While this finding might appear inconsistent with prior studies showing impaired processing in uncertain conditions in schizophrenia, the other studies used tasks that involved relatively complex learning and risky/implicit decision making processes (e.g., Albrecht, Waltz, Frank, & Gold, 2016; Cheng, Tang, Li, Lau, & Lee, 2012; Fond et al., 2013; Reddy et al., 2013). An important feature of the present paradigm is that participants were informed via explicit cues about the upcoming probability (100% vs. 50%) of reward, punishment or breaking even outcomes, without the added cognitive processing demands like those in prior studies. Thus, the schizophrenia group was able to properly encode the basic distinction between certain and uncertain contexts in response to explicit and simple cues in our paradigm that removed cognitive challenges in understanding for participants.
Despite intact initial encoding, the schizophrenia group showed disturbed certainty effects in the subsequent stages. During feedback anticipation, SPN amplitudes did not differentiate between certain and uncertain conditions in the schizophrenia group. This is consistent with neuroimaging and self-report studies showing impaired anticipation of reward or pleasure in this disorder (Buck & Lysaker, 2013; Li et al., 2015; Radua et al., 2015; Subramaniam et al., 2015). It has been proposed that impairments in reward anticipation might be due to deficits in either cue responsivity or cognitive control (e.g., Culbreth, Moran, & Barch, 2017). Our findings suggest deficits in cognitive control rather than cue responsivity, because cP300 amplitudes were intact in the schizophrenia group. Hence, cognitive control is more likely to contribute to anticipatory disturbances in schizophrenia.
During feedback receipt, the schizophrenia group continued to show a diminished certainty effect. This reflected exaggerated fP300 responses in the certain condition but normal fP300 in the uncertain condition. The schizophrenia group’s tendency to process certain events more similar to uncertain events than controls did is maladaptive in light of the widely held view that certainty is inherently desirable, whereas uncertainty is inherently aversive and makes greater demands on cognitive control processes (Ladouceur et al., 2000; Luhmann et al., 2008; Reuman et al., 2015). Furthermore, intolerance of uncertainty (e.g., Grupe & Nitschke, 2013), an aversion of uncertainty that reflects a belief that a negative event will likely occur, is commonly observed in other forms of psychopathology, particularly internalizing disorders such as anxiety disorders and depression (Carleton, 2014; Grupe & Nitschke, 2013; Lake & Labar, 2011; McEvoy & Mahoney, 2012; Tanovic, Gee, & Joormann, 2018). The schizophrenia group, in contrast, showed an atypical response to feedback receipt that could reflect a belief that certainty is improbable. Speculatively, a fundamental disturbance in the ability to distinguish feedback during certain versus uncertain contexts could impact reality testing, and thereby contribute to the onset or maintenance of delusional beliefs or hallucinatory experiences. A bias toward processing feedback as though it is uncertain would also be expected to adversely impact a variety of cognitive control and motivational processes required for adaptive functioning (Esber & Haselgrove, 2011; Mushtaq et al., 2011; Rushworth & Behrens, 2008).
Valence Effects Across Reward-Processing Stages
There were no group differences in valence effects for any stage of reward processing during either certain or uncertain conditions. This finding converges with a few studies showing normal valence-related activity in schizophrenia for cP300 during cue evaluation (Vignapiano et al., 2016) and for fP300 (Houthoofd et al., 2013) during feedback receipt. These findings are also broadly consistent with ERP studies of the late positive potential that showed intact valence-related effects for responses to emotional images in schizophrenia (e.g., Horan, Foti, Hajcak, Wynn, & Green, 2012; Horan, Wynn, Kring, Simons, & Green, 2010). However, nonsignificant group differences for valence effects should be interpreted in the context of lower internal consistency for valence effects than for certainty effects, particularly for valence types within certain trials. In this novel paradigm there were fewer trials for each valence type, and future work should include more trials per valence type to ensure adequate internal consistency.
Limitations and Conclusion
The following limitations should be considered. First, individuals with schizophrenia were mostly male, and sex differences have been observed for some of the present ERPs, such as SPN (Greimel et al., 2018). However, follow-up analyses indicated that none of the main effects of sex or interactions with sex were significant , but these nonsignificant effects might be due to low statistical power for examining sex as a factor. It is also possible that a history of substance abuse/dependence or major depressive episodes contributed to the present findings in the patient sample (see Supplementary Table 15; Baskin-Sommers & Foti, 2015). Follow-up analyses separately added history of substance abuse/dependence and history of major depressive episodes to the ERP ANOVAs, and these ANOVAs did not yield any significant main effects or interactions associated with either additional factor . Second, individuals with schizophrenia were also chronically ill, and it is unclear whether present findings would generalize to individuals in the early course of the illness. Third, individuals with schizophrenia were receiving antipsychotic medications and other types of medication at clinically determined dosages, which might have impacted ERP findings. For example, a study of P300 to performance-related feedback indicated that P300 amplitude in schizophrenia was comparable to healthy controls only after individuals with schizophrenia were treated with antipsychotic medications (Houthoofd et al., 2013). Additional research is needed in determine the impact of medication treatment on ERPs associated with reward processing. Fourth, it is possible that people with schizophrenia were unable to distinguish between certain and uncertain contexts, because they failed to understand or remember the meaning of the cues. Although we did not directly test whether patients understood the meaning of each cue, group differences were not observed in cP300 amplitudes for certainty effects or valence effects, which suggests that initial cue evaluation was intact.
The present paradigm was designed to examine the impact of certainty effects and valence effects across three reward processing stages. However, the manipulation of both certainty and valence limited the interpretation of some ERP effects. For example, cP300 differentiation between certain and uncertain outcomes was impacted by valence effects within certain trials, such that certain gain and certain loss trials were related to larger cP300 than certain even trials, but cP300 did not significantly differentiate between valence effects with uncertain trials. During feedback receipt, we were unable to examine fRewP activity due to the robust, overlapping fP300 response elicited by uncertainty (see supplementary material). Future research might consider whether certainty effects can be fully disentangled from valence effects during each stage of reward processing using alternative paradigms.
Despite these limitations, the present study highlights a fundamental impairment in distinguishing between certainty and uncertainty in people with schizophrenia. Distinguishing between certainty and uncertainty is a vital process, because predicting the future requires consideration of the probabilities of potential outcomes. The prediction of rewards based on information in the environment is essential for independent living. Although valence effects have received considerable attention in schizophrenia, future research should also consider certainty effects in schizophrenia.
Supplementary Material
Acknowledgements
This work was supported by a VA Merit (5I01CX000593 to W.P.H.). Writing of this manuscript was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. M.F.G. has been a consultant to AbbVie, ACADIA, DSP, and Takeda, is on the scientific advisory board of Luc, and has received unrelated research funds from FORUM. All the other authors do not have any conflict of interest.
Footnotes
This study was approved by the Institutional Review Board at the VA Greater Los Angeles Healthcare System (PCC# 2012–081111).
References
- Albrecht MA, Waltz JA, Frank MJ, & Gold JM (2016). Probability and magnitude evaluation in schizophrenia. Schizophrenia Research: Cognition, 5, 41–46. doi: 10.1016/j.scog.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasi A (1997). Psychological testing (6th ed.). New York: Macmillan. [Google Scholar]
- Baldwin SA, Larson MJ, & Clayson PE (2015). The dependability of electrophysiological measurements of performance monitoring in a clinical sample: A generalizability and decision analysis of the ERN and Pe. Psychophysiology, 52, 790–800. doi: 10.1111/psyp.12401 [DOI] [PubMed] [Google Scholar]
- Barch DM, Culbreth A, & Sheffield J (2018). Systems level modeling of cognitive control in psychiatric disorders: A focus on schizophrenia In Murray JD (Ed.), Computational Psychiatry (pp. 145–173): Academic Press. [Google Scholar]
- Barch DM, & Dowd EC (2010). Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophrenia Bulletin, 36, 919–934. doi: 10.1093/schbul/sbq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, & Luking K (2016). Mechanisms underlying motivational deficits in psychopathology: Similarities and differences in depression and schizophrenia In Simpson EH & Balsam PD (Eds.), Behavioral Neuroscience of Motivation (Vol. 27, pp. 411–449). Switzerland: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98, 227–239. doi: 10.1016/j.ijpsycho.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Bertelson P, & Boons JP (1960). Time uncertainty and choice reaction time. Nature, 187, 531–532. [DOI] [PubMed] [Google Scholar]
- Brennan RL (2001). Generalizability theory: Statistics for social science and public policy. New York, NY: Springer-Verlag. [Google Scholar]
- Broyd SJ, Richards HJ, Helps SK, Chronaki G, Bamford S, & Sonuga-Barke EJS (2012). An electrophysiological monetary incentive delay (e-MID) task: A way to decompose the different components of neural response to positive and negative monetary reinforcement. Journal of Neuroscience Methods, 209, 40–49. doi: 10.1016/j.jneumeth.2012.05.015 [DOI] [PubMed] [Google Scholar]
- Brunia CHM, Hackley SA, van Boxtel GJM, Kotani Y, & Ohgami Y (2011). Waiting to perceive: Reward or punishment? Clinical Neurophysiology, 122, 858–868. doi: 10.1016/j.clinph.2010.12.039 [DOI] [PubMed] [Google Scholar]
- Brunia CHM, van Boxtel GJM, & Böcker KBE (2011). Negative slow waves as indices of anticipation : The Bereitschaftspotential, the contingent negative variation, and the stimulus-preceding negativity In Kappenman ES & Luck SJ (Eds.), The Oxford handbook of event-related potential components (pp. 189–207). New York, NY: Oxford University Press. [Google Scholar]
- Buck B, & Lysaker PH (2013). Consummatory and anticipatory anhedonia in schizophrenia: Stability, and associations with emotional distress and social function over six months. Psychiatry Research, 205, 30–35. doi: 10.1016/j.psychres.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Carleton RN (2014). The intolerance of uncertainty construct in the context of anxiety disorders: Theoretical and practical perspectives. Expert Review of Neurotherapeutics, 12, 937–947. doi: 10.1586/ern.12.82 [DOI] [PubMed] [Google Scholar]
- Carpenter B, Gelman A, Hoffman M, Lee D, Goodrich B, Betancourt M, … Riddell A (2017). Stan: A probabilistic programming language. Journal of Statistical Software, 76. doi: 10.18637/jss.v076.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catell RB (1966). The scree test for the number of factors. Multivariate Behavioral Research, 1, 245–276. [DOI] [PubMed] [Google Scholar]
- Catena A, Perales JC, Megías A, Cándido A, Jara E, & Maldonado A (2012). The brain network of expectancy and uncertainty processing. PloS one, 7, e40252–40211. doi: 10.1371/journal.pone.0040252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RCK, Wang Y, Huang J, Shi Y, Wang Y, Hong X, … Kring AM (2010). Anticipatory and consummatory components of the experience of pleasure in schizophrenia: Cross-cultural validation and extension. Psychiatry Research, 175, 181–183. doi: 10.1016/j.psychres.2009.01.020 [DOI] [PubMed] [Google Scholar]
- Cheng GLF, Tang JCY, Li FWS, Lau EYY, & Lee TMC (2012). Schizophrenia and risk-taking: Impaired reward but preserved punishment processing. Schizophrenia Research, 136, 122–127. doi: 10.1016/j.schres.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Cicchetti DV (2001). The precision of reliability and validity estimates re-visited: Distinguishing between clinical and statistical significance of sample size requirements. Journal of Clinical and Experimental Neuropsychology, 23, 695–700. doi: 10.1076/jcen.23.5.695.1249 [DOI] [PubMed] [Google Scholar]
- Clawson A, Clayson PE, Keith CM, Catron C, & Larson MJ (2017). Conflict and performance monitoring throughout the lifespan: An event-related potential (ERP) and temporospatial component analysis. Biological Psychology, 124, 87–99. doi: 10.1016/j.biopsycho.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Clawson A, Clayson PE, & Larson MJ (2013). Cognitive control adjustments and conflict adaptation in major depressive disorder. Psychophysiology, 50, 711–721. doi: 10.1111/psyp.12066 [DOI] [PubMed] [Google Scholar]
- Clayson PE, Baldwin SA, & Larson MJ (2013). How does noise affect amplitude and latency measurement of event-related potentials (ERPs)? A methodological critique and simulation study. Psychophysiology, 50, 174–186. doi: 10.1111/psyp.12001 [DOI] [PubMed] [Google Scholar]
- Clayson PE, & Miller GA (2017a). ERP Reliability Analysis (ERA) Toolbox: An open-source toolbox for analyzing the reliability of event-related potentials. International Journal of Psychophysiology, 111, 68–79. doi: 10.1016/j.ijpsycho.2016.10.012 [DOI] [PubMed] [Google Scholar]
- Clayson PE, & Miller GA (2017b). Psychometric considerations in the measurement of event-related brain potentials: Guidelines for measurement and reporting. International Journal of Psychophysiology, 111, 57–67. doi: 10.1016/j.ijpsycho.2016.09.005 [DOI] [PubMed] [Google Scholar]
- Culbreth AJ, Gold JM, Cools R, & Barch DM (2016). Impaired activation in cognitive control regions predicts reversal learning in schizophrenia. Schizophrenia Bulletin, 42, 484–493. doi: 10.1093/schbul/sbv075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth AJ, Moran EK, & Barch DM (2017). Effort-cost decision-making in psychosis and depression: Could a similar behavioral deficit arise from disparate psychological and neural mechanisms? Psychological Medicine, 38, 1–16. doi: 10.1017/S0033291717002525 [DOI] [PubMed] [Google Scholar]
- Dien J (2006). Progressing towards a consensus on PCA of ERPs. Clinical neurophysiology: Official journal of the International Federation of Clinical Neurophysiology, 117, 699–702; 10.1016/j.clinph.2005.09.029 [DOI] [PubMed] [Google Scholar]
- Dien J (2010a). The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods, 187, 138–145. doi: 10.1016/j.jneumeth.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Dien J (2010b). Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology, 47, 170–183. doi: 10.1111/j.1469-8986.2009.00885.x [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, & Mangun GR (2007). Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Human Brain Mapping, 28, 742–763. doi: 10.1002/hbm.20304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Tucker DM, Potts G, & Hartry-Speiser A (1997). Localization of auditory evoked potentials related to selective intermodal attention. Journal of Cognitive Neuroscience, 9, 799–823. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mock B, & Mecklinger A (2008). Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia, 46, 521–539. doi: 10.1016/j.neuropsychologia.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Esber GR, & Haselgrove M (2011). Reconciling the influence of predictiveness and uncertainty on stimulus salience: A model of attention in associative learning. Proceedings of the Royal Society B: Biological Sciences, 278, 2553–2561. doi: 10.1098/rspb.2011.0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, & Benjamin J (1996). Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York, NY: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams J (1996). Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Verison 2.0). New York State Psychiatric Institute, New York: Biometrics Research Department. [Google Scholar]
- Fond G, Bayard S, Capdevielle D, Del-Monte J, Mimoun N, Macgregor A, … Raffard S (2013). A further evaluation of decision-making under risk and under ambiguity in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience, 263, 249–257. doi: 10.1007/s00406-012-0330-y [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2012). Genetic variation in dopamine moderates neural response during reward anticipation and delivery: Evidence from event-related potentials. Psychophysiology, 49, 617–626. doi: 10.1111/j.1469-8986.2011.01343.x [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, & Hajcak G (2011). Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping, 32, 2207–2216. doi: 10.1002/hbm.21182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L, Cucurell D, Marco-Pallares J, Guitart-Masip M, Morís J, & Rodríguez-Fornells A (2013). Electrophysiological correlates of anticipating improbable but desired events. NeuroImage, 78, 135–144. doi: 10.1016/j.neuroimage.2013.03.062 [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, & Green MF (2007). Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93, 253–260. doi: 10.1016/j.schres.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer JE, Kelley NJ, Pornpattananangkul N, Mittal VA, & Nusslock R (2018). Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. International Journal of Psychophysiology, 132, 184–202. doi: 10.1016/j.ijpsycho.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, & Heerey EA (2008). Reward processing in schizophrenia: A deficit in the representation of value. Schizophrenia Bulletin, 34, 835–847. doi: 10.1093/schbul/sbn068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Barch DM, & Gold JM (2015). Effort-based decision making: A novel approach for assessing motivation in schizophrenia. Schizophrenia Bulletin, 41, 1035–1044. doi: 10.1093/schbul/sbv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, & Lee J (2015). Social cognition in schizophrenia. Nature Reviews Neuroscience, 16, 620–631. doi: 10.1038/nrn4005 [DOI] [PubMed] [Google Scholar]
- Green MF, Horan WP, Lee J, McCleery A, Reddy LF, & Wynn JK (2018). At issue: Social disconnection in schizophrenia and the general Community. Schizophrenia Bulletin, 44, 242–249. doi: 10.1093/schbul/sbx082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E, Bakos S, Landes I, Töllner T, Bartling J, Kohls G, & Schulte-Körne G (2018). Sex differences in the neural underpinnings of social and monetary incentive processing during adolescence. Cognitive, Affective, & Behavioral Neuroscience, 18, 296–312. doi: 10.3758/s13415-018-0570-z [DOI] [PubMed] [Google Scholar]
- Grupe DW, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14, 488–501. doi: 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, & Simons RF (2005). Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology, 42, 161–170. doi: 10.1111/j.1469-8986.2005.00278.x [DOI] [PubMed] [Google Scholar]
- Hajcak G, Meyer A, & Kotov R (2017). Psychometrics and the neuroscience of individual differences: Internal consistency limits between-subjects effects. Journal of Abnormal Psychology, 126, 823–834. doi: 10.1037/abn0000274 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, & Simons RF (2007). It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology, 44, 905–912. doi: 10.1111/j.1469-8986.2007.00567.x [DOI] [PubMed] [Google Scholar]
- Hélie S, Shamloo F, Novak K, & Foti D (2017). The roles of valuation and reward processing in cognitive function and psychiatric disorders. Annals of the New York Academy of Sciences, 1395, 33–48. doi: 10.1111/nyas.13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstadter GC (1964). Principles of psychological measurement. New York: Appleton-Century Crofts. [Google Scholar]
- Hewig J, Trippe R, Hecht H, Coles MGH, Holroyd CB, & Miltner WHR (2007). Decision-making in Blackjack: An electrophysiological analysis. Cerebral Cortex, 17, 865–877. doi: 10.1093/cercor/bhk040 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Krigolson OE, Baker R, Lee S, & Gibson J (2009). When is an error not a prediction error? An electrophysiological investigation. Cognitive, Affective, and Behavioral Neuroscience, 9, 59–70. [DOI] [PubMed] [Google Scholar]
- Horan WP, & Blanchard JJ (2003). Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophrenia Research, 65, 125–137. doi: 10.1016/S0920-9964(02)00410-3 [DOI] [PubMed] [Google Scholar]
- Horan WP, Foti D, Hajcak G, Wynn JK, & Green MF (2012). Intact motivated attention in schizophrenia: Evidence from event-related potentials. Schizophrenia Research, 135, 95–99. doi: 10.1016/j.schres.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Kring AM, Simons RF, & Green MF (2010). Electrophysiological correlates of emotional responding in schizophrenia. Journal of Abnormal Psychology, 119, 18–30. doi: 10.1037/a0017510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL (1965). A rationale and test for the number of factors in factor analysis. Psychometrika, 30, 179–185. [DOI] [PubMed] [Google Scholar]
- Houthoofd S, Morrens M, Sabbe B, Schrijvers D, Vandendriessche F, Hulstijn W, & de Bruijn ERA (2013). Trait and state aspects of internal and external performance monitoring in schizophrenia. International Journal of Psychophysiology, 87, 42–51. doi: 10.1016/j.ijpsycho.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Hughes G, Mathan S, & Yeung N (2013). EEG indices of reward motivation and target detectability in a rapid visual detection task. NeuroImage, 64, 590–600. doi: 10.1016/j.neuroimage.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Infantolino ZP, Luking KR, Sauder CL, Curtin JJ, & Hajcak G (2018). Robust is not necessarily reliable: From within-subjects fMRI contrasts to between-subjects comparisons. NeuroImage, 173, 146–152. doi: 10.1016/j.neuroimage.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, & Morosini PL (2008). The new approach: Psychosocial functioning as a necessary outcome criterion for therapeutic success in schizophrenia. Current Opinion in Psychiatry, 21, 630–639. doi: 10.1097/YCO.0b013e328314e144 [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, & Lindenmayer J-P (1989). The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. The British Journal of Psychiatry, 155, 59–65. [PubMed] [Google Scholar]
- Krug A, Cabanis M, Pyka M, Pauly K, Kellermann T, Walter H, … Kircher T (2014). Attenuated prefrontal activation during decision-making under uncertainty in schizophrenia: A multi-center fMRI study. Schizophrenia Research, 152, 176–183. doi: 10.1016/j.schres.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Kujawa A, Smith E, Luhmann C, & Hajcak G (2013). The feedback negativity reflects favorable compared to nonfavorable outcomes based on global, not local, alternatives. Psychophysiology, 50, 134–138. doi: 10.1111/psyp.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur R, Gosselin P, & Dugas MJ (2000). Experimental manipulation of intolerance of uncertainty: A study of a theoretical model of worry. Behaviour Research and Therapy, 38, 933–941. [DOI] [PubMed] [Google Scholar]
- Lake JI, & Labar KS (2011). Unpredictability and uncertainty in anxiety: A new direction for emotional timing research. Frontiers in Integrative Neuroscience, 5, 55. doi: 10.3389/fnint.2011.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE, & Farrer TJ (2012). Performance monitoring and cognitive control in individuals with mild traumatic brain injury. Journal of the International Neuropsychological Society, 18, 323–333. doi: 10.1017/S1355617711001779 [DOI] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE, Keith CM, Hunt IJ, Hedges DW, Nielsen BL, & Call VRA (2016). Cognitive control adjustments in healthy older and younger adults: Conflict adaptation, the error-related negativity (ERN), and evidence of generalized decline with age. Biological Psychology, 115, 50–63. doi: 10.1016/j.biopsycho.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Li Z, Lui SSY, Geng F-L, Li Y, Li WX, Wang C-Y, … Chan RCK (2015). Experiential pleasure deficits in different stages of schizophrenia. Schizophrenia Research, 166, 98–103. doi: 10.1016/j.schres.2015.05.041 [DOI] [PubMed] [Google Scholar]
- Llerena K, Wynn JK, Hajcak G, Green MF, & Horan WP (2016). Patterns and reliability of EEG during error monitoring for internal versus external feedback in schizophrenia. International Journal of Psychophysiology, 105, 39–46. doi: 10.1016/j.ijpsycho.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. doi: 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2014). An introduction to the event-related potential technique (2nd ed.). Cambridge, MA: The MIT Press. [Google Scholar]
- Luhmann CC, Chun MM, Yi DJ, Lee D, & Wang XJ (2008). Neural dissociation of delay and uncertainty in intertemporal choice. Journal of Neuroscience, 28, 14459–14466. doi: 10.1523/JNEUROSCI.5058-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, McCleery A, Moore MM, Wynn JK, Green MF, & Horan WP (2018). ERP indices of performance monitoring and feedback processing in psychosis: A meta-analysis. International Journal of Psychophysiology, 132, 365–378. doi: 10.1016/j.ijpsycho.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy PM, & Mahoney AEJ (2012). To be sure, to be sure: Intolerance of uncertainty mediates symptoms of various anxiety disorders and depression. Behavior Therapy, 43, 533–545. doi: 10.1016/j.beth.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Megías A, Navas JF, Perandrés-Gómez A, Maldonado A, Catena A, & Perales JC (2017). Electroencephalographic evidence of abnormal anticipatory uncertainty processing in gambling Disorder patients. Journal of Gambling Studies, 1–18. doi: 10.1007/s10899-017-9693-3 [DOI] [PubMed] [Google Scholar]
- Morey RD, Hoekstra R, Rouder JN, Lee MD, & Wagenmakers E-J (2016). The fallacy of placing confidence in confidence intervals. Psychonomic Bulletin & Review, 23, 103–123. doi: 10.3758/s13423-015-0947-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq F, Bland AR, & Schaefer A (2011). Uncertainty and cognitive control. Frontiers in Psychology, 2, 249. doi: 10.3389/fpsyg.2011.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak BK, Novak KD, Lynam DR, & Foti D (2016). Individual differences in the time course of reward processing: Stage-specific links with depression and impulsivity. Biological Psychology, 119, 79–90. doi: 10.1016/j.biopsycho.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Novak KD, & Foti D (2015). Teasing apart the anticipatory and consummatory processing of monetary incentives: An event-related potential study of reward dynamics. Psychophysiology, 52, 1470–1482. doi: 10.1111/psyp.12504 [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Cockburn J, & Pauli WM (2017). Learning, reward, and decision making. Annual Review of Psychology, 68, 73–100. doi: 10.1146/annurev-psych-010416-044216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan DM, Alexopoulos J, Bauer H, & Sailer U (2011). Manipulation of feedback expectancy and valence induces negative and positive reward prediction error signals manifest in event-related brain potentials. Psychophysiology, 48, 656–664. doi: 10.1111/j.1469-8986.2010.01136.x [DOI] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52, 449–459. doi: 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, & Fusar-Poli P (2015). Ventral striatal activation during reward processing in psychosis: A neurofunctional meta-analysis. JAMA psychiatry (Chicago, Ill.), 72, 1243–1251. doi: 10.1001/jamapsychiatry.2015.2196 [DOI] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Gold JM, Marder SR, … Green MF (2017). Understanding the association between negative symptoms and performance on effort-based decision-making tasks: The importance of defeatist performance beliefs. Schizophrenia Bulletin, 44, 1217–1226. doi: 10.1093/schbul/sbx156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ, & Green MF (2013). Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology, 39, 456–463. doi: 10.1038/npp.2013.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Waltz JA, Green MF, Wynn JK, & Horan WP (2016). Probabilistic reversal learning in schizophrenia: Stability of deficits and potential causal mechanisms. Schizophrenia Bulletin, 42, 942–951. doi: 10.1093/schbul/sbv226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuman L, Jacoby RJ, Fabricant LE, Herring B, & Abramowitz JS (2015). Uncertainty as an anxiety cue at high and low levels of threat. Journal of Behavior Therapy and Experimental Psychiatry, 47, 111–119. doi: 10.1016/j.jbtep.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, & Behrens TEJ (2008). Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience, 11, 389–397. doi: 10.1038/nn2066 [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Murphy TI, Homan D, Chantziantoniou DK, & Khan S (2010). Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology, 47, 260–270. doi: 10.1111/j.1469-8986.2009.00942.x [DOI] [PubMed] [Google Scholar]
- Shavelson RJ, & Webb NM (1991). Generalizability theory: A primer. Thousand Oaks, CA: SAGE Publications, Inc. [Google Scholar]
- Stan Development Team. (2017). CmdStan: The command-line interface to Stan, version 2.14.0. Retrieved from http://mc-stan.org
- Strauss GP, Waltz JA, & Gold JM (2014). A review of reward processing and motivational impairment in schizophrenia. Schizophrenia Bulletin, 40, S107–S116. doi: 10.1093/schbul/sbt197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Hooker CI, Biagianti B, Fisher M, Nagarajan S, & Vinogradov S (2015). Neural signal during immediate reward anticipation in schizophrenia: Relationship to real-world motivation and function. NeuroImage: Clinical, 9, 153–163. doi: 10.1016/j.nicl.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanovic E, Gee DG, & Joormann J (2018). Intolerance of uncertainty: Neural and psychophysiological correlates of the perception of uncertainty as threatening. Clinical Psychology Review, 60, 87–99. doi: 10.1016/j.cpr.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Tversky A, & Kahneman D (1992). Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty, 5, 297–323. doi: 10.1007/bf00122574 [DOI] [Google Scholar]
- Ventura J, Subotnik KL, Gitlin MJ, Gretchen-Doorly D, Ered A, Villa KF, … Nuechterlein KH (2015). Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophrenia Research, 161, 407–413. doi: 10.1016/j.schres.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignapiano A, Mucci A, Ford J, Montefusco V, Plescia GM, Bucci P, & Galderisi S (2016). Reward anticipation and trait anhedonia: An electrophysiological investigation in subjects with schizophrenia. Clinical Neurophysiology, 127, 2149–2160. doi: 10.1016/j.clinph.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Wallis JD (2007). Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience, 30, 31–56. doi: 10.1146/annurev.neuro.30.051606.094334 [DOI] [PubMed] [Google Scholar]
- White TP, Engen NH, Sørensen S, Overgaard M, & Shergill SS (2014). Uncertainty and confidence from the triple-network perspective: Voxel-based meta-analyses. Brain and Cognition, 85, 191–200. doi: 10.1016/j.bandc.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Wu Y, & Zhou X (2009). The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research, 1286, 114–122. doi: 10.1016/j.brainres.2009.06.032 [DOI] [PubMed] [Google Scholar]
- Wynn JK, Horan WP, Kring AM, Simons RF, & Green MF (2010). Impaired anticipatory event-related potentials in schizophrenia. International Journal of Psychophysiology, 77, 141–149. doi: 10.1016/j.ijpsycho.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, & Treadway MT (2017). Reward processing, neuroeconomics, and psychopathology. Annual Review of Clinical Psychology, 13, 471–495. doi: 10.1146/annurev-clinpsy-032816-044957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.