Abstract
Neuromedin B (NMB) and its receptor regulate labor onset by mediating inflammatory factors; however the underlying mechanisms remain poorly understood. The present study is aimed to investigate the mechanisms of NMB-induced cyclo-oxygenase 2 (COX-2) expression and interleukin (IL)-6 generation in human primary myometrial cells. The results indicated that NMB could increase phosphorylation of nuclear factor κB (NF-κB) transcription factor p65 (p65) and Jun proto-oncogene, activator protein 1 (AP-1) transcription factor subunit (c-Jun), and in turn, markedly up-regulated the expression levels of COX-2 and IL-6. This up-regulation was significantly attenuated by knockdown of p65 or c-Jun, and enhanced by overexpression of p65 or c-Jun. Furthermore, we identified a potential interaction between p65 and c-Jun following NMB stimulation. In addition, a significant positive correlation was observed between the amount of phosphorylated p65 and the levels of COX-2 and IL-6, and between the amount of phosphorylated c-Jun and COX-2 and IL-6 levels. These data suggested that NMB-induced COX-2 and IL-6 expression were mediated via p65 and c-Jun activation.
Keywords: AP-1/c-Jun, COX-2, human primary myometrial cells, IL-6, neuromedin B, NF-κB/p65
Introduction
Preterm birth (PTB), defined as birth before 37 weeks, is the leading cause of perinatal morbidity and mortality worldwide [1]. It is estimated that over 41000 preterm infants are born each day across the world [1]. Preterm neonates are at a high risk of long-term disabilities, including mental retardation, cerebral palsy, lung disease, and hearing loss [2]. However, the precise mechanism controlling labor onset in humans remains unclear.
The key approach to women with suspected preterm labor continues to be the inhibition of uterine contractions. This focus on contraction inhibition has led to the development of agents affecting myometrial contractility [3]. Tocolysis is currently considered as an important strategy for PTB because it can inhibit uterine contractility to prolong pregnancy. There are a variety of tocolytic agents in current use, which act on the uterus in varying ways. The most common are calcium channel blockers, betamimetics, magnesium sulfate, cyclo-oxygenase (COX) inhibitors, oxytocin agonists, and nitric oxide. However, their lack of long-term efficacy, and maternal and fetal side effects, make them clinically unsatisfactory [4]. Thus, investigation of novel targets associated with uterine contraction might provide new insights into the prevention and treatment of PTB. Through our initial cDNA microarray screening, we found that the mRNA encoding neuromedin B (NMB) receptor (NMBR), but not the oxytocin receptor (OTR) or β2-adrenergic receptor, was differentially expressed in the human uterine myometrium during spontaneous or oxytocin-induced labor [5]. The levels of Nmbr mRNA and protein peaks at parturition and decreases sharply after delivery in mouse myometrial cells [6,7]. The NMBR agonist, NMB, selectively binds to NMBR to mediate many biological effects, such as contraction of uterine smooth muscle [7], as well as urogenital and gastrointestinal smooth muscles [8]. Maternal exposure to the NMB shortened the gestational age of mice [7]. All the above suggested that NMBR is likely to be an ideal candidate target in PTB. However, the specific mechanisms of NMB/NMBR in the regulation of labor onset remain to be determined.
The transcription factor nuclear factor κB (NF-κB) is known to play a fundamental role in a number of physiological processes. In resting cells, NF-κB is sequestered in the cytoplasm through direct interaction with a member of the IκB family of inhibitor proteins such as IκBα. Various stimuli could lead to the activation of the IKK complex which contains two IκB kinases, IKKα and IKKβ. Phosphorylation of IκBα by the IKK complex leads to its polyubiquitination and subsequent degradation. The liberated NF-κB dimer then translocates to the nucleus where it recognizes and binds specific DNA sequences termed as κB sites [9]. WIP1 phosphatases, a member of the Ser/Thr PP2C family, could suppress phosphorylation of p65 resulting in its inactivation [10]. Accumulating evidence demonstrated that NF-κB transcription factor p65 (p65) takes an active part in labor onset by regulating a variety of cytokines, including interleukin (IL)-6, type-2 COX enzyme (COX-2), IL-8, IL-1β, matrix metalloproteinase (MMP)-9 and tumor necrosis factor α (TNF-α) [11–18]. Our previous study found that NMB could activate p65 and induce the expression of IL-6 to control the free [Ca2+]i in mice myometrial cells [19]; but a higher level of inhibition of [Ca2+]i was detected in response to Nmbr-specific knockdown than p65-specific knockdown [19]. Therefore, we speculated that other pathways that might be involved in the Nmbr-mediated increase in [Ca2+]i in addition to the p65/IL-6 pathway. Beyond NF-κB/p65, activator protein 1 (AP-1) is another key mediator associated with inflammation-induced PTB [14]. AP-1 is a transcription factor comprising members of the Fos and Jun families [20]. Moreover, c-Jun (Jun proto-oncogene, AP-1 transcription factor subunit), the most widely investigated protein of AP-1, is involved in the expression of various inflammatory genes by binding to their transcription factor binding sites [21]. c-Jun binds at the proximal IL6 promoter to promote IL-6 generation in breast cancer cells [22]. The promoter of the human PTGS2 (COX-2) gene contains several transcription factor binding sites, including AP-1 and NF-κB [23]. AP-1 can directly bind to PTGS2 gene promoter to increase its expression in several cell types, including chondrosarcoma and tracheal smooth muscle cells [24,25], as well as human primary amnion cells [26]. Meanwhile, IL-6 and COX-2 expression was suppressed by AP-1 inactivation [27,28]. This evidence indicated that AP-1, in addition to p65, might be important to regulate the expression of IL-6 and COX-2 induced by NMB. Some studies have demonstrated a potential interaction between NF-κB and AP-1. The physical association of the leucine zipper domain of c-Jun and c-Fos with the Rel homology domain of the p65 subunit of NF-κB has been described, and this association enhances the transactivation of NF-κB-regulated genes [29]. In addition, Jun D co-operates with p65 to activate the proximal NF-κB site of the CCND1 (cyclin D1) promoter [30]. However, a functional co-operation between NF-κB and AP-1 proteins in NMB-induced myometrial gene expression has never been investigated.
Taken together, these findings prompted us to investigate whether both c-Jun and p65 were involved in the regulation of COX-2 and IL-6 expression by NMB in human primary myometrial cells.
Materials and methods
Human subjects
Fifteen uterine smooth muscle specimens were collected from 15 pregnant women (singleton pregnancy, no complications, no premature rupture, or signs of infection) admitted to the Obstetrical Department of Xiangya Hospital of Central South University from August 2015 to May 2016. The average age of the pregnant women was 29.3 ± 3.6 years (25–34 years). The mean gestational time was 39+6 weeks (38+4 to 40+3 weeks). Planned cesarean delivery was carried out at terms in all 15 women because of pelvic stenosis, breech presentation, or certain other social-related factors.
Sample collection
The experimental protocols were approved by the Ethics Review Committee of Central South University. Myometrial tissues (1 cm × 1 cm × 1 cm) were obtained from the upper edge of the uterine incision during lower segment cesarean section after fetus delivery and before oxytocin injection. Myometrial tissues were isolated and digested and dissociated and myometrial cells were collected and cultured according to our previous publication [19]. Myometrial cells could be amplified by passage culture. Cells at passages 3–6 were used for the experiments. All participating women provided written consent.
Antibodies and reagents
Primary antibodies against total-p65, phospho-p65 (S536), total-c-Jun, phospho-c-Jun (Ser63/Ser73), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technologies (Danvers, MA, U.S.A.). Antibodies against NMBR weres purchased from Sigma–Aldrich (St. Louis, MO, U.S.A.). Antibodies against α-smooth muscle actin (α-SMA) were purchased from Abcam (Cambridge, U.K.). Horseradish peroxidase (HRP)–conjugated anti-rabbit or anti-mouse secondary antibodies were purchased from Cell Signaling Technologies (Danvers, MA, U.S.A.). An ECL-plus kit was purchased from Advansta (MenloPark, CA, U.S.A.). NMB was purchased from Bachem (Bubendorf, Switzerland). The human IL-6 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Enzo Life Sciences (New York, NY, U.S.A.). Polybrene was purchased from Invitrogen (San Diego, CA, U.S.A.).
Cell culture and treatment
Human primary myometrial cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml), and streptomycin (100 mg/ml) in 5% CO2 at 37°C in a humidified incubator. For cell treatment, 1 μM of NMB was added to the medium directly and incubated for various times (0.5, 1, 2, 4, or 6 h) before detection, while the same volume of medium was used as control.
Measurement of IL-6 generation
Myometrial cells were treated with 1 μM of NMB or vehicle for the indicated times. The culture supernatant was collected to detect IL-6 using an ELISA kit according to the manufacturer’s instructions.
qRT-PCR
Total RNA was isolated from untreated or NMB-treated cells using a Mini RNA Isolation II kit (Zymo Research, Irvine, CA, U.S.A.) according to the manufacturer’s instructions. CDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen). Real-time polymerase chain reaction was performed using iTaq SYBR Green Supermix (Bio-Rad, Hercules, CA, U.S.A.) on an ABI ViiA™ real-time PCR detection system (Thermo Scientific, Waltham, MA, U.S.A.). The value of 2–ΔΔCt was used to determine the fold difference between samples. The following primers were used for IL6: Forward: 5′-TACATCCTCGACGGCATCTC-3′, Reverse: 5′-GCCATCTTTGGAAGGTTCAG-3′, PTGS2: Forward: 5′-GGTTGCTGGTGGTAGGAATG-3′, Reverse: 5′-TAAAGCGTTTGCGGTACTCA-3′, ACTB (encoding β-actin as a reference): Forward: 5′-CTCCATCCTGGCCTCGCTGT-3′, Reverse: 5′-GCTGTCACCTTCACCGTTCC-3′.
Stable knockdown of p65 and c-Jun
Primary myometrial cells were infected with lentiviruses with shRNAs targeting p65 or c-Jun (Oligo-Engine, Seattle, WA, U.S.A.) and then selected with blasticidin (4 μg/ml; Invitrogen) for 2 weeks. The stable clones were pooled and used for further downstream experiments as indicated.
Stable overexpression of p65 and c-Jun
Primary myometrial cells were infected with lentiviruses containing cDNA sequence of p65 or c-Jun (Oligo-Engine, Seattle, WA, U.S.A.) and then selected with puromycin (2 μg/ml; Invitrogen) for 3 weeks to generate stable clones, which were pooled and used for further downstream experiments as indicated.
Western blotting
Protein samples were extracted from cells using radioimmunoprecipitation assay (RIPA) buffer [10 mM Tris/Cl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, and 140 mM NaCl]. Equivalent amounts of protein (30 μg of protein extract in each lane) were separated by 10% SDS/PAGE, transferred on to polyvinylidene fluoride (PVDF) membranes (Millipore Corporation, Bedford, MA, U.S.A.) and blocked with 5% non-fat milk/phosphate buffered saline-Tween 20 (PBST) solution for 1 h at room temperature. The membranes were then incubated with primary antibodies at 4°C overnight. The primary antibodies were detected using HRP–conjugated anti-rabbit or anti-mouse secondary antibodies and visualized using an ECL plus kit. GAPDH was used as the loading control. To normalize the Western blotting data, relative levels of phospho-p65, and phospho-c-Jun protein were normalized to the total p65 or c-Jun, respectively. Other non-phosphorylated proteins, like COX-2, were normalized to the internal control (GAPDH).
Immunocytochemical staining
Isolated myometrial cells (2 × 106 cells/dish) were cultured. Immunocytochemistry was used to examine the expression of NMBR in the surface and α-SMA in the cytoplasm. The cells were stained with anti-NMBR and anti-α-SMA polyclonal primary antibodies, followed by the appropriate HRP-linked secondary antibody. Cells were visualized under a microscope.
Immunofluorescence staining
Cells fixed with 4% paraformaldehyde (PFA) solution, and permeabilized with Triton-X-100 were incubated with an anti-p65 (mouse) and anti-c-Jun (rabbit) antibodies overnight, after incubation with Alexa Fluor 488–conjugated anti-mouse secondary antibody (Abcam) and Alexa Fluor 647 anti-rabbit secondary antibody, 2-(4-amidinophenyl)-1H-indole-6-carboxamidine (DAPI) was used for nuclear staining. The final sections were examined under a confocal laser scanning microscope.
Flow cytometry
Cells were suspended at 1 × 106 cells/ml, and 5 μl of Annexin V and propidium iodide staining solution were added to 200 μl of the cell suspension. After the cells were incubated at room temperature for 15 min in the dark, stained cells were assayed and quantified using a FACSort Flow Cytometer (Beckman Coulter, Brea, CA, U.S.A.). Cell debris was excluded from the analysis using an appropriate forward light scatter threshold setting. Compensation was used whenever necessary.
Statistical analysis
All quantitative data are expressed as mean ± standard deviation (SD) of at least three independent experiments. GraphPad Prism V software was used for statistical analyses. Differences between groups were compared using either Student’s t test or one-way analysis of variance (ANOVA) associated with Dunnett’s test. Correlation analysis between different indicators was carried out using Pearson correlation analysis. Statistical significance was defined as a P-value <0.05 (marked as *). Higher significance levels were set at P<0.01 (marked as **).
Results
Primary cell culture and identification
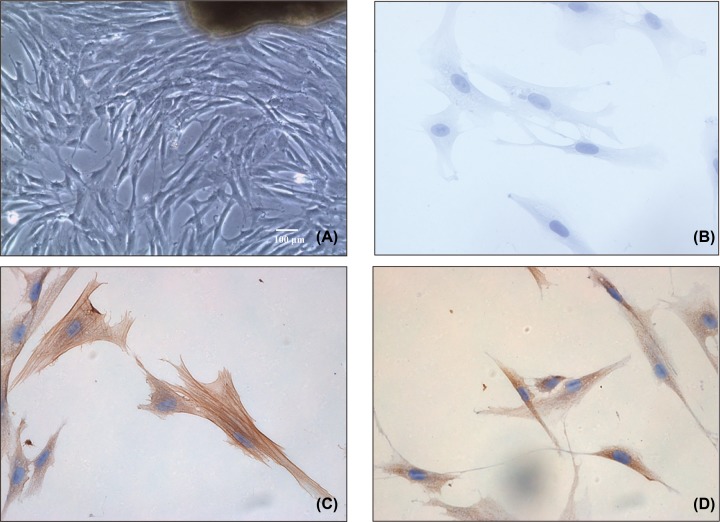
Cultured primary myometrial cells were long and fusiform in shape. Cell cloning was performed after 48 h of culture, the medium was first changed on day 5, and fusion was apparent in some clones 1 week later. The majority of cultured cells were observed to be long and fusiform or polygonal in shape under inverted microscopy (Figure 1A). Immunocytochemistry results showed α-SMA was expressed in abundance in the cytoplasm (Figure 1C) and NMBR was present in the membrane of the cultured myometrial cells (Figure 1D), compared with the negative control (Figure 1B). Taken together, these results showed that the cultured myometrial cells isolated from patients’ term myometrium demonstrated abundant levels of α-SMA and NMBR proteins.
Figure 1. The cultured primary myometrial cells from term myometrium and their verification.
(A) Cultured primary myometrial cells were observed as long and fusiform in shape under inverted microscopy. At least five fields were counted. (B) The negative control for immunocytochemistry. At least five fields were counted. (C) The positive expression of α-SMA, as assessed by immunocytochemistry. At least five fields were counted. (D) The positive expression of NMBR, as assessed by immunocytochemistry. Original magnification ×40 (A) and ×400 (B–D). At least five fields were counted.
NMB up-regulates the expression of p-p65, p-c-Jun, IL-6, and COX-2 in primary myometrial cells
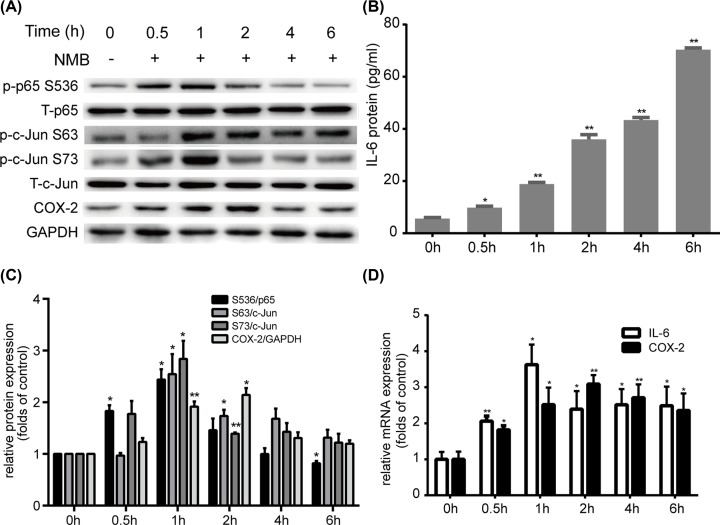
Our previous study established the ideal dose of NMB in our system as 1 μM [19]. Therefore, we treated myometrial cells with 1 μM NMB for 0, 0.5, 1, 2, 4, or 6 h and then detected the expression of p65, c-Jun, IL-6, and COX-2 at the mRNA and protein levels. Our results showed that NMB treatment led to rapid phosphorylation of p65 and c-Jun without affecting their total levels (Figure 2A,C). Phosphorylation of p65 on Ser536, c-Jun on Ser63 and Ser73 were induced within 0.5 h of NMB treatment and reached a peak at 1 h (Figure 2A,C). IL6 mRNA expression (Figure 2D) peaked at 1 h, and the IL-6 protein level (Figure 2B) increased dramatically following NMB treatment. COX-2 mRNA and protein levels (Figure 2A,C,D) peaked at 2 h. These findings demonstrated that NMB could significantly increase the levels of p-p65, p-c-Jun, IL-6, and COX-2 in primary myometrial cells.
Figure 2. The effect of NMB on p65, c-Jun, COX-2 and IL-6 expression in cultured primary myometrial cells.
Cells were treated with 1 μM NMB for 0, 0.5, 1, 2, 4, and 6 h, and the protein levels were analyzed using Western blotting or ELISA, and the mRNA levels were analyzed by qRT-PCR. (A) Expression of total p65, p-p65 (Ser536), total c-Jun, p-c-Jun (Ser63, Ser73) and COX-2 was analyzed using Western blotting with GAPDH as a control standard. (B) IL-6 in the supernatants was quantified by ELISA. (C) The relative level of the COX-2 protein was normalized to that of the internal control (GAPDH). The relative levels of phospho-p65, and phospho-c-Jun protein were normalized to total p65 or c-Jun, respectively. (D) IL6 and PTGS2 mRNA levels were analyzed by qRT-PCR. Blots are representative. Data are expressed as mean ± SD of three independent experiments (n=3). *P<0.05; **P<0.01, against the control group.
P65 and c-Jun play a vital role in NMB-induced COX-2 and IL-6 expression in primary myometrial cells
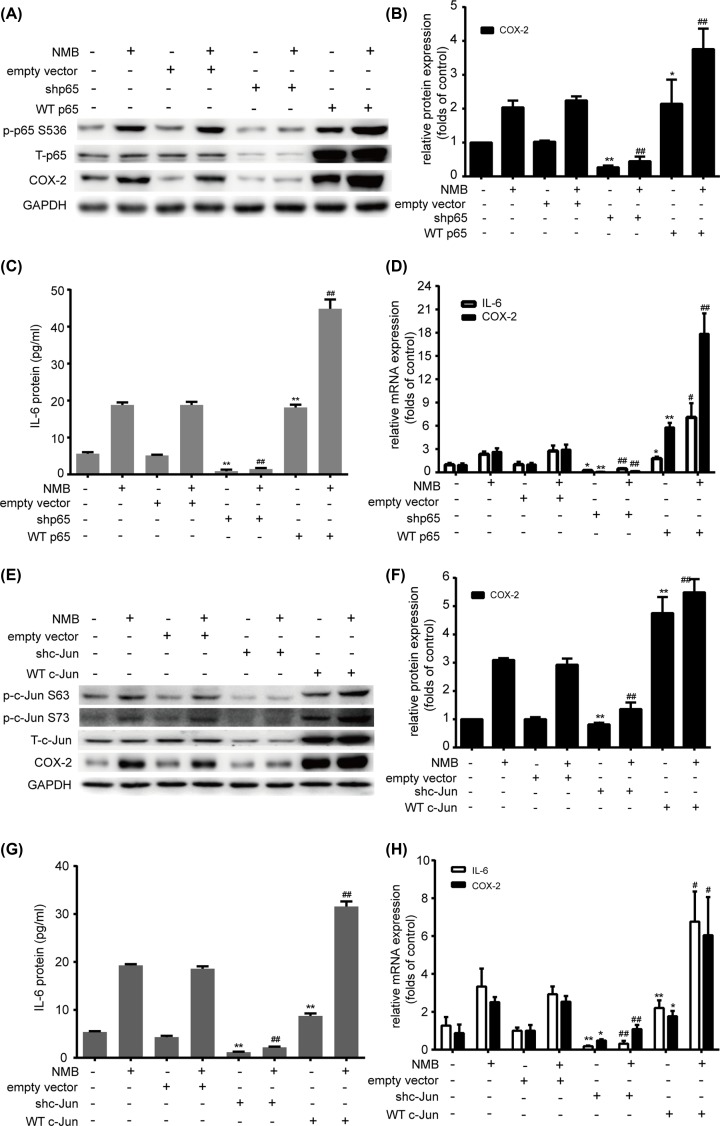
To further determine whether p65 and c-Jun regulate COX-2 and IL-6 directly in myometrial cells, we depleted or overexpressed p65 or c-Jun. Efficacy of the knockdown or overexpression was verified by Western blotting analysis and qRT-PCR (Supplementary Figure S1). The results showed that p65 knockdown led to decreased COX-2 and IL-6 mRNA and protein levels with or without NMB treatment, compared with the empty vector group (Figure 3A–D). However, p65 overexpression resulted in increased COX-2 and IL-6 mRNA and protein levels, with or without NMB treatment, compared with the empty vector group (Figure 3A–D). Next, c-Jun was deleted or overexpressed to evaluate its function. Similarly, we found that c-Jun deletion evidently suppressed COX-2 and IL-6 mRNA and protein up-regulation with or without NMB treatment, compared with the empty vector group (Figure 3E–H), while c-Jun overexpression increased COX-2 and IL-6 mRNA and protein levels with or without NMB treatment, compared with the empty vector group (Figure 3E–H). Collectively, these results indicated the essential roles of p65 and c-Jun in NMB-induced expression of COX-2 and IL-6 in myometrial cells.
Figure 3. Induction of COX-2 and IL-6 by NMB is mediated via p65 and c-Jun.
(A–D) Primary myometrial cells were infected with lentiviruses with WTp65 overexpresssion or knockdown or the empty vector, and then treated with 1 μM NMB for 1 h. P-p65 (S536), p65, and COX-2 (A) levels were probed by Western blotting with GAPDH as an internal standard. The relative abundance of PTGS2 was quantified (B). The release of IL-6 was measured by ELISA (C). IL6 and COX-2 mRNA induction was determined using qRT-PCR (D). (E–H) Primary myometrial cells were infected with lentiviruses with WT c-Jun overexpresssion or knockdown or the empty vector, and then treated with 1 μM NMB for 1 h. P-c-Jun (S63 and S73), c-Jun, and COX-2 (E) levels were analyzed using Western blotting with GAPDH as an internal standard. The relative abundance of COX-2 was quantified (F). The release of IL-6 was measured (G). IL6 and PTGS2 mRNA expression levels were determined using qRT-PCR (H). Blots are representative. Data are expressed as mean ± SD of three independent experiments (n=3). *P<0.05; **P<0.01, against the empty vector group; #P<0.05; ##P<0.01, against the empty vector + NMB group.
Possible interaction between p65 and c-Jun in NMB-induced COX-2 and IL-6 expression in primary myometrial cells
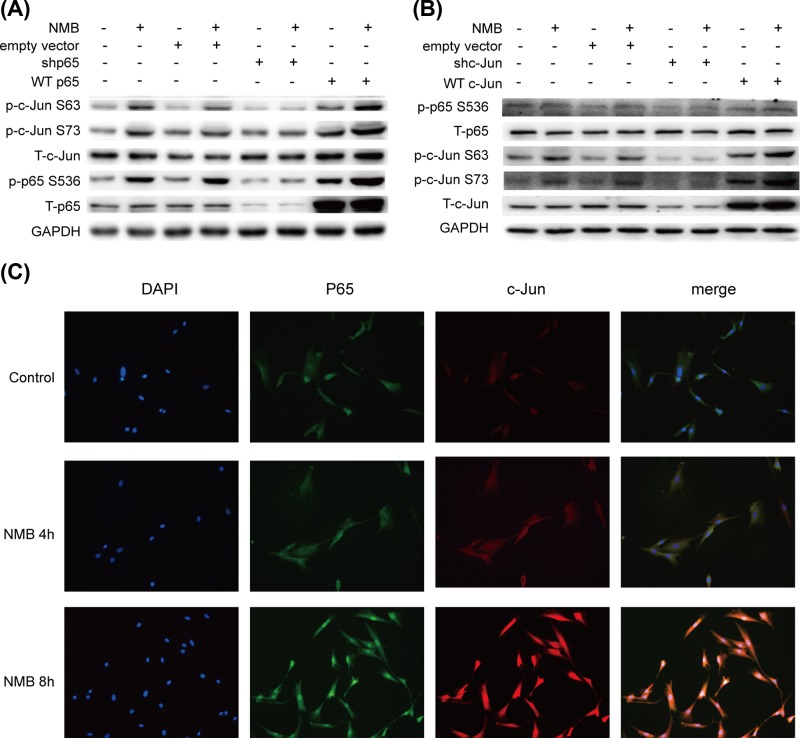
To our surprise, we found that level of phosphorylated c-Jun in the p65 knockdown group treated with or without NMB was significantly lower than that in the empty vector group (Figure 4A). While the level of phosphorylated c-Jun in the p65 overexpression group treated with or without NMB was significantly higher than that in the empty vector group (Figure 4A). Similarly, the level of phosphorylated p65 in the c-Jun knockdown group treated with or without NMB was significantly lower than that in the empty vector group (Figure 4B), and the level of phosphorylated p65 in the c-Jun overexpression group treated with or without NMB was significantly higher than that in the empty vector group (Figure 4B). Besides, we explored the cellular distribution of p65 and c-Jun using immunofluorescence. Both p65 and c-Jun translocated from the cytoplasm to the nucleus consecutively after NMB stimulation, and co-localization of p65 and c-Jun in the nucleus was observed following NMB treatment (Figure 4C), strongly suggesting an association between p65 and c-Jun.
Figure 4. Potential interaction between NMB-induced expression of p65 and c-Jun.
(A) Primary myometrial cells were infected with lentiviruses with WTp65 overexpresssion or knockdown or the empty vector, and then treated with 1 μM NMB for 1 h. Levels of p65, c-Jun, p-p65 (S536), p-c-Jun (S63, S73) was analyzed by Western blotting with GAPDH as an internal standard. (B) Primary myometrial cells were infected with lentiviruses with WTc-Jun overexpresssion or knockdown or the empty vector, and then treated with 1 μM NMB for 1 h. Levels of p65, c-Jun, p-p65 (S536), p-c-Jun (S63, S73) were analyzed by Western blotting with GAPDH as an internal standard. Blots are representative of three independent experiments. (C) The cellular distribution of p65 (green, Alexa488) and c-Jun protein (red, Alexa647) at 0, 4, and 8 h after NMB treatment was visualized using confocal microscopy. Cell nuclei were stained with DAPI (blue). The images are representative of three independent experiments.
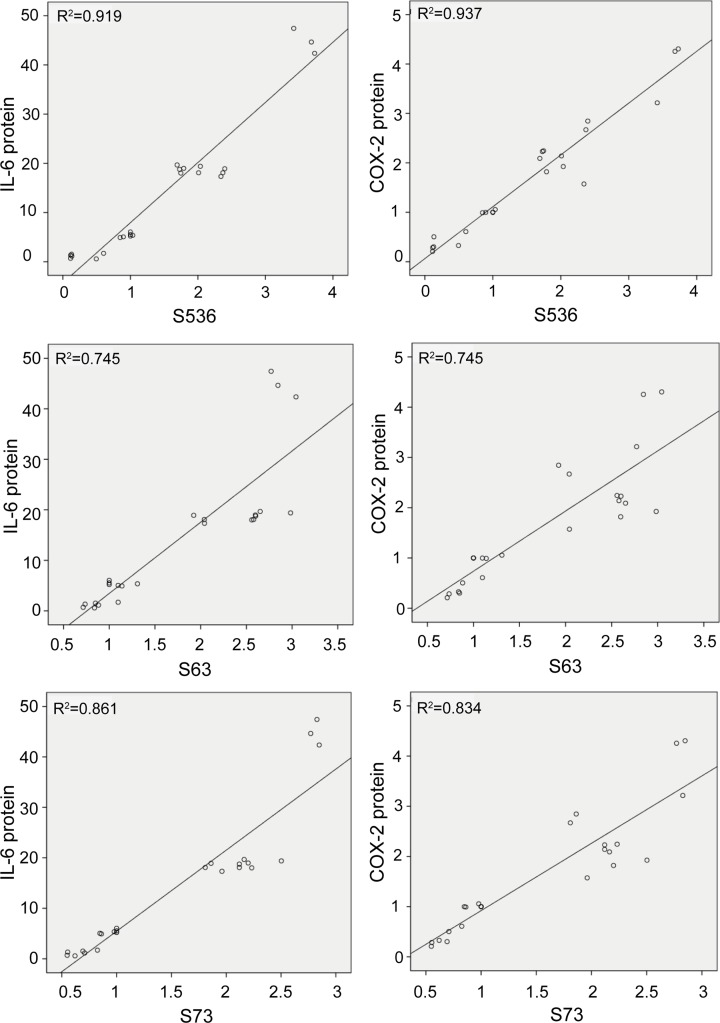
Correlations between NMB-induced phosphorylation of p65 or c-Jun and COX-2 or IL-6 protein levels in primary myometrial cells
As shown in Figure 5, positive correlations were detected between the level of phosphorylated p65 and the level of IL-6, and between the level of phosphorylated p65 and the level of COX-2 when induced by NMB (r = 0.959, P<0.05, and r = 0.968, P<0.05, respectively). Positive correlations were also observed between the level of phosphorylated c-Jun S63 and the level of IL-6 (r = 0.863, P<0.05), between the level of phosphorylated c-Jun S63 and the level of COX-2 (r = 0.863, P<0.05), between the level of phosphorylated c-Jun S73 and the level of IL-6 (r = 0.928, P<0.05), and between the level of phosphorylated c-Jun S73 and the level of COX-2 (r = 0.913, P<0.05) induced by NMB.
Figure 5. Correlations among NMB-induced expression of p-p65, p-c-Jun, COX-2, and IL-6 in primary myometrial cells.
Positive correlation was determined for p-p65 (S536) and IL-6 (r = 0.959, P<0.05), for p-p65 (S536) and COX-2 (r = 0.968, P<0.05), for p-c-Jun S63 and IL-6 (r = 0.863, P<0.05), for p-c-Jun S63 and COX-2 (r = 0.863, P<0.05), for p-c-Jun S73 and IL-6 (r = 0.928`, P<0.05), and for p-c-Jun S73 and COX-2 (r = 0.913, P<0.05).
Discussion
Many genes and associated pathways might contribute to preterm and term labor initiation [31,32]. In our initial cDNA microarray experiment, uterus fundus myometrial tissues were obtained from women undergoing cesarean section deliveries at term before the onset of labor and in labor. NMBR, but not the OTR or β2-adrenergic receptor, was differentially expressed [5]. The reason for this difference could be that the specimens were obtained from different parts of the uterus. OTR gene expression occurs in the cervix and lower uterine segment, with less pronounced changes in the uterine fundus [33]. The upper and lower regions of the human uterus function differently. The upper segment has a more contractile phenotype, contracting to push down and initiate delivery of the baby, while the lower segment maintains a more relaxed phenotype, promoting delivery of the baby through the lower segment of the uterus and cervix [34]. Therefore, we believe that NMBR will be a strong candidate target for tocolytics. However, the role of NMB/NMBR in regulating labor onset and the potential molecular mechanism are not fully defined.
Primary myometrial cell culture provides a suitable model to investigate the mechanism of NMB in labor initiation. The present study was conducted using cultures of primary myometrial cells isolated from women who chose planned cesarean section in our hospital. Immunochemical staining techniques demonstrated strong expression of α-SMA, thus confirming the myogenic origin of the cultured cells. The observed strong expression of NMBR in primary myometrial cells is consistent with our previous study [7].
Earlier studies reported increased concentrations of certain cytokines, most notably IL-6 in the serum and amniotic fluid of patients with preterm labor [35,36]. And the presence of IL-6 in the cervico-vaginal zone could predict preterm delivery [37]. Our previous study showed that NMB induced labor onset in mice associated with increased IL6 mRNA expression [7]. In addition, IL-6 expression increased via the rela/p65 pathway in cultured mouse primary myometrial cells in vitro in response to NMB treatment [19]. This study confirmed that NMB could also significantly increase the expression of IL-6 at the mRNA and protein levels in human primary myometrial cells.
Prostaglandins (PGs) are believed to be involved in uterine contractions, cervical ripening and fetal membrane rupture during parturition [38]. COX-2 is the rate-limiting enzyme involved in PG synthesis. It increases in amnion cells and human myometrium in both term and preterm labor [11,18]. To the best of our knowledge, this is the first study to report that treatment of human primary myometrial cells with NMB could result in dramatic increase in COX-2 mRNA and protein levels. Therefore, COX-2, together with IL-6, is also likely to play a pivotal role in NMB-induced labor onset.
We next explored how NMB induced COX-2 and IL-6 expression in human primary myometrial cells. Through a combination of analyses, we demonstrated that activation of both p65 and c-Jun signaling are decisive factors in COX-2 and IL-6 up-regulation following NMB stimulation. In support of our observation, the PTGS2 gene promoter has NF-κB and AP-1 binding sites, and activated p65 and c-Jun could induce PTGS2 expression in several cell types, including human cervical cancer cells and human rheumatoid arthritis synovial fibroblasts [39,40]. In lipopolysaccharide (LPS)-induced inflammation, both NF-κB and AP-1 are activated and thus induce the expression of IL-6 and COX-2 [27,28]. Similarly, in our correlation analysis, we found that levels of phosphorylated p65 and c-Jun correlated positively with COX-2 and IL-6 expression. Therefore, the p65 and c-Jun pathways are hypothesized to be involved in NMB-induced up-regulation of COX-2 and IL-6. Besides, signal transducer and activator of transcription 3 (STAT3) were reported to bind to the PTGS2 promoter region after cortisol stimulation in human amnion fibroblasts [41]. Whether other transcription factors like STAT3 mediate NMB-induced COX-2 induction in human myometrial cells require further investigation. Notably, as p65 or c-Jun knockdown led to cultured primary myometrial cells apoptosis (Supplementary Figure S2), we cannot rule out the possibility that this apoptosis could have a potential impact on the subsequent following experiments. NF-κB is a family of transcription factors that play critical roles in inflammation, immunity, cell proliferation, differentiation, and survival. Many miRNAs are transcribed by NF-κB and participate in the negative feedback loops to prevent sustained or excessive activation of NF-κB pathway [42]. Thus, as a key in physiological process, NF-κB not only activates inflammatory proteins like IL-6 but activates long non-coding RNA and other process in repair and cancer.
Evidence indicates that AP-1 can also exert a transactivating function independent of its binding to AP-1 sites via protein–protein interactions with other transcriptional regulators, including NF-κB and β-catenin [29,43]. A physical association of the basic leucine zipper regions of Jun/Fos with the Rel homology domain of the p65 NF-κB subunit resulting in enhanced transactivation of NF-κB-regulated genes has been previously demonstrated [29,30]. Interestingly, our current work predicted potential interaction between p65 and c-Jun in association with NMB action. This notion is supported by three lines of experimental evidence: (i) Either p65 or c-Jun knockdown could suppress downstream COX-2 and IL-6 expression in NMB treated cells. (ii) Knockdown of p65 could attenuate NMB-induced phosphorylation of c-Jun, and overexpression of p65 could increase the level of phosphorylated c-Jun induced by NMB. C-Jun knockdown or overexpression could affect NMB-induced phosphorylation of p65. (iii) High p65 co-occurrence with c-Jun was observed in the nuclei of NMB-treated myometrial cells. Thus, we believe that a similar mechanism contributes to PTGS2 or IL6 gene transactivation via the proximal NF-κB element of the promoter.
Our studies have important clinical implications. (1) In our pilot study, we established for the first time that NMBR is dramatically up-regulated during labor onset and sharply decreased after delivery, which indicates a close association with labor onset. (2) NMBR induced NF-κB and AP-1 activation. Thus, NMBR might up-regulate NF-κB, AP-1-responsive proinflammatory cytokines, chemokines, and MMPs in myometrial cells. NMBR may synergize with these mediators and induce labor onset. Therefore, targeting NMBR or developing NMBR antagonists might reduce the inflammation cascade and suppress uterine contraction, thus preventing preterm labor.
However, our study has some limitations. First, because of time and money limits, the sample size (n=3) for each dataset is relatively small, which is a weakness of our study. Second, although we demonstrated that NMBR activation could improve COX-2 and IL-6 expression by up-regulating p65 and c-Jun expression in vitro, we did not investigate whether such a phenomenon was replicated in vivo. Third, we established the ideal concentration of NMB as 1 μM in cell culture conditions based on our pilot study, which might not be consistent with physiological NMB concentrations during gestation and at parturition. Fourth, we reported that transcription factors p65 and c-Jun are involved in NMB-induced inflammatory cascade, but we cannot rule out the role of other transcription factors, such as STAT3 and Sp1, which are considered as typical factors involved in the inflammatory signaling pathway. Further studies are required to help fully understand the in vivo and in vitro involvement of the NMB/NMBR signaling pathway to provide robust evidence for NMBR as a target in anti-PTB therapy.
In summary, our study showed that in primary myometrial cells, NMB-induced COX-2 expression and IL-6 secretion were mediated through p65 and c-Jun activation. These results provide new insights into the mechanisms of NMB induced expression of COX-2 and generation of IL-6, with an aim of providing more evidence to explore NMBR as a novel therapeutic target.
Supplementary Material
Acknowledgments
We thank Dr. Fei Luo (The Second Xiangya Hospital of Cental South University, Changsha, Hunan, China) for the manuscript revision.
Abbreviations
- AP-1
activator protein 1
- cDNA
complementary DNA
- COX-2
cyclo-oxygenase 2
- c-Jun
Jun proto-oncogene, AP-1 transcription factor subunit
- EGTA
ethylene glycol-bis (beta-aminoethyl ether) - N, N, N’, N’-tetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HRP
horseradish peroxidase
- IKK
IκB kinase
- IL
interleukin
- IκB
I kappa B
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor κB
- NMB
Neuromedin B
- NMBR
NMB receptor
- OTR
oxytocin receptor
- PG
prostaglandin
- PP2C
Protein Phosphatase 2C
- PTB
preterm birth
- qRT-PCR
Real-time Quantitative Reverse Transcription PCR
- STAT3
signal transducer and activator of transcription 3
- WIP1
The wild-type p53-induced Phosphatase 1
- α-SMA
α-smooth muscle actin
Author Contribution
Weishe Zhang, Jingfei Chen and Texuan Zhu conceived the presented idea. Jingfei Chen and Texuan Zhu performed the experiment. Yanhua Zhao completed the analytical methods. Jingfei Chen and Jiejie Zhang wrote the manuscript. Weishe Zhang supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81270719, 81571516].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Platt M.J. (2014) Outcomes in preterm infants. Public Health 128, 399–403 10.1016/j.puhe.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 2.Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E. et al. (2015) Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385, 430–440 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 3.Simhan H.N.and Caritis S.N. (2007) Prevention of preterm delivery. N. Engl. J. Med. 357, 477–487 10.1056/NEJMra050435 [DOI] [PubMed] [Google Scholar]
- 4.Hosli I., Sperschneider C., Drack G., Zimmermann R., Surbek D.and Irion O. (2014) Tocolysis for preterm labor: expert opinion. Arch. Gynecol. Obstet. 289, 903–909 10.1007/s00404-013-3137-9 [DOI] [PubMed] [Google Scholar]
- 5.Zhang W.S., Liang Q.H., Xie Q.S., Wu Z.D.and Wu X.H. (2007) Scanning of drug targets related to uterus contraction from the uterine smooth muscles by cDNA microarray. Zhong Nan Da Xue Xue Bao Yi Xue Ban 32, 579–583 [PubMed] [Google Scholar]
- 6.Zhang W., Xie Q., Wu Z., Wu X.and Liang Q. (2009) Expression of NMBR in myometrium in pregnant mice at different gestational ages and its relation with parturition. Zhong Nan Da Xue Xue Bao Yi Xue Ban 34, 531–536 [PubMed] [Google Scholar]
- 7.Zhang W.S., Xie Q.S., Wu X.H.and Liang Q.H. (2011) Neuromedin B and its receptor induce labor onset and are associated with the RELA (NFKB P65)/IL6 pathway in pregnant mice. Biol. Reprod. 84, 113–117 10.1095/biolreprod.110.085746 [DOI] [PubMed] [Google Scholar]
- 8.Bedard T., Mountney C., Kent P., Anisman H.and Merali Z. (2007) Role of gastrin-releasing peptide and neuromedin B in anxiety and fear-related behavior. Behav. Brain Res. 179, 133–140 10.1016/j.bbr.2007.01.021 [DOI] [PubMed] [Google Scholar]
- 9.Christian F., Smith E.L.and Carmody R.J. (2016) The regulation of NF-kappaB subunits by phosphorylation. Cells 5, 10.3390/cells5010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chew J., Biswas S., Shreeram S., Humaidi M., Wong E.T., Dhillion M.K. et al. (2009) WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat. Cell Biol. 11, 659–666 10.1038/ncb1873 [DOI] [PubMed] [Google Scholar]
- 11.Allport V.C., Pieber D., Slater D.M., Newton R., White J.O.and Bennett P.R. (2001) Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol. Hum. Reprod. 7, 581–586 10.1093/molehr/7.6.581 [DOI] [PubMed] [Google Scholar]
- 12.Lindstrom T.M.and Bennett P.R. (2005) The role of nuclear factor kappa B in human labour. Reproduction 130, 569–581 10.1530/rep.1.00197 [DOI] [PubMed] [Google Scholar]
- 13.Lim S., MacIntyre D.A., Lee Y.S., Khanjani S., Terzidou V., Teoh T.G. et al. (2012) Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes. PLoS ONE 7, e34707 10.1371/journal.pone.0034707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacIntyre D.A., Lee Y.S., Migale R., Herbert B.R., Waddington S.N., Peebles D. et al. (2014) Activator protein 1 is a key terminal mediator of inflammation-induced preterm labor in mice. FASEB J. 28, 2358–2368 10.1096/fj.13-247783 [DOI] [PubMed] [Google Scholar]
- 15.Mitchell J.A.and Lye S.J. (2002) Differential expression of activator protein-1 transcription factors in pregnant rat myometrium. Biol. Reprod. 67, 240–246 10.1095/biolreprod67.1.240 [DOI] [PubMed] [Google Scholar]
- 16.Khanjani S., Terzidou V., Johnson M.R.and Bennett P.R. (2012) NFkappaB and AP-1 drive human myometrial IL8 expression. Mediators Inflamm. 2012, 504952 10.1155/2012/504952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terzidou V., Lee Y., Lindstrom T., Johnson M., Thornton S.and Bennett P.R. (2006) Regulation of the human oxytocin receptor by nuclear factor-kappaB and CCAAT/enhancer-binding protein-beta. J. Clin. Endocrinol. Metab. 91, 2317–2326 10.1210/jc.2005-2649 [DOI] [PubMed] [Google Scholar]
- 18.Choi S.J., Oh S., Kim J.H.and Roh C.R. (2007) Changes of nuclear factor kappa B (NF-kappaB), cyclooxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9) in human myometrium before and during term labor. Eur. J. Obstet. Gynecol. Reprod. Biol. 132, 182–188 10.1016/j.ejogrb.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 19.Zhang W.S., Fei K.L., Wu M.T., Wu X.H.and Liang Q.H. (2012) Neuromedin B and its receptor influence the activity of myometrial primary cells in vitro through regulation of Il6 expression via the Rela/p65 pathway in mice. Biol. Reprod. 86, 1–7 10.1095/biolreprod.111.095984 [DOI] [PubMed] [Google Scholar]
- 20.Vesely P.W., Staber P.B., Hoefler G.and Kenner L. (2009) Translational regulation mechanisms of AP-1 proteins. Mutat. Res. 682, 7–12 10.1016/j.mrrev.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 21.Yang C.M., Lee I.T., Lin C.C., Wang C.H., Cherng W.J.and Hsiao L.D. (2013) c-Src-dependent MAPKs/AP-1 activation is involved in TNF-alpha-induced matrix metalloproteinase-9 expression in rat heart-derived H9c2 cells. Biochem. Pharmacol. 85, 1115–1123 10.1016/j.bcp.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 22.Ndlovu M.N., Van Lint C., Van Wesemael K., Callebert P., Chalbos D., Haegeman G. et al. (2009) Hyperactivated NF-{kappa}B and AP-1 transcription factors promote highly accessible chromatin and constitutive transcription across the interleukin-6 gene promoter in metastatic breast cancer cells. Mol. Cell. Biol. 29, 5488–5504 10.1128/MCB.01657-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosaka T., Miyata A., Ihara H., Hara S., Sugimoto T., Takeda O. et al. (1994) Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur. J. Biochem. 221, 889–897 10.1111/j.1432-1033.1994.tb18804.x [DOI] [PubMed] [Google Scholar]
- 24.Wu M.H., Chen L.M., Hsu H.H., Lin J.A., Lin Y.M., Tsai F.J. et al. (2013) Endothelin-1 enhances cell migration through COX-2 up-regulation in human chondrosarcoma. Biochim. Biophys. Acta 1830, 3355–3364 10.1016/j.bbagen.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 25.Hsu C.K., Lee I.T., Lin C.C., Hsiao L.D.and Yang C.M. (2015) Sphingosine-1-phosphate mediates COX-2 expression and PGE2 /IL-6 secretion via c-Src-dependent AP-1 activation. J. Cell. Physiol. 230, 702–715 10.1002/jcp.24795 [DOI] [PubMed] [Google Scholar]
- 26.Lee Y.S., Terzidou V., Lindstrom T., Johnson M.and Bennett P.R. (2005) The role of CCAAT/enhancer-binding protein beta in the transcriptional regulation of COX-2 in human amnion. Mol. Hum. Reprod. 11, 853–858 10.1093/molehr/gah194 [DOI] [PubMed] [Google Scholar]
- 27.Shin J.S., Hong Y., Lee H.H., Ryu B., Cho Y.W., Kim N.J. et al. (2015) Fulgidic acid isolated from the rhizomes of Cyperus rotundus suppresses LPS-induced iNOS, COX-2, TNF-alpha, and IL-6 expression by AP-1 inactivation in RAW264.7 macrophages. Biol. Pharm. Bull. 38, 1081–1086 10.1248/bpb.b15-00186 [DOI] [PubMed] [Google Scholar]
- 28.Lee H.J., Shin J.S., Lee W.S., Shim H.Y., Park J.M., Jang D.S. et al. (2016) Chikusetsusaponin IVa methyl ester isolated from the roots of Achyranthes japonica suppresses LPS-induced iNOS, TNF-alpha, IL-6, and IL-1beta expression by NF-kappaB and AP-1 inactivation. Biol. Pharm. Bull. 39, 657–664 10.1248/bpb.b15-00572 [DOI] [PubMed] [Google Scholar]
- 29.Stein B., Baldwin A.S. Jr, Ballard D.W., Greene W.C., Angel P.and Herrlich P. (1993) Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12, 3879–3891 10.1002/j.1460-2075.1993.tb06066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toualbi-Abed K., Daniel F., Guller M.C., Legrand A., Mauriz J.L., Mauviel A. et al. (2008) Jun D cooperates with p65 to activate the proximal kappaB site of the cyclin D1 promoter: role of PI3K/PDK-1. Carcinogenesis 29, 536–543 10.1093/carcin/bgm293 [DOI] [PubMed] [Google Scholar]
- 31.Irani R.A.and Foster S. (2015) Overview of the mechanisms of induction of labor. Semin. Perinatol. 39, 426–429 10.1053/j.semperi.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 32.Zhou X., Jiang Z., Zou Y., Yin Y., Zuo Q.and Sun L. (2015) Role of SOCS3 in the Jak/stat3 pathway in the human placenta: different mechanisms for preterm and term labor. Acta Obstet. Gynecol. Scand. 94, 1112–1117 10.1111/aogs.12708 [DOI] [PubMed] [Google Scholar]
- 33.Havelock J.C., Keller P., Muleba N., Mayhew B.A., Casey B.M., Rainey W.E. et al. (2005) Human myometrial gene expression before and during parturition. Biol. Reprod. 72, 707–719 10.1095/biolreprod.104.032979 [DOI] [PubMed] [Google Scholar]
- 34.Mosher A.A., Rainey K.J.and Bolstad S.S. (2013) Development and validation of primary human myometrial cell culture models to study pregnancy and labour. BMC Pregnancy Childbirth 13, 10.1186/1471-2393-13-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oz M., Polat B., Ozgu E., Seckin K.D., Tasin C.and Danisman N. (2015) Interleukin-6 and C-reactive protein levels in the amniotic fluid as indicators of preterm delivery in Turkish women. Clin. Exp. Obstet. Gynecol. 42, 801–804 [PubMed] [Google Scholar]
- 36.Greig P.C., Murtha A.P., Jimmerson C.J., Herbert W.N., Roitman-Johnson B.and Allen J. (1997) Maternal serum interleukin-6 during pregnancy and during term and preterm labor. Obstet. Gynecol. 90, 465–469 10.1016/S0029-7844(97)00294-9 [DOI] [PubMed] [Google Scholar]
- 37.Hadzi Lega M., Daneva Markova A., Stefanovic M.and Tanturovski M. (2015) Interleukin 6 and fetal fibronectin as a predictors of preterm delivery in symptomatic patients. Bosn. J. Basic Med. Sci. 15, 51–56 10.17305/bjbms.2015.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Challis J.R., Sloboda D.M., Alfaidy N., Lye S.J., Gibb W., Patel F.A. et al. (2002) Prostaglandins and mechanisms of preterm birth. Reproduction 124, 1–17 10.1530/rep.0.1240001 [DOI] [PubMed] [Google Scholar]
- 39.Kim S.H., Oh J.M., No J.H., Bang Y.J., Juhnn Y.S.and Song Y.S. (2009) Involvement of NF-kappaB and AP-1 in COX-2 upregulation by human papillomavirus 16 E5 oncoprotein. Carcinogenesis 30, 753–757 10.1093/carcin/bgp066 [DOI] [PubMed] [Google Scholar]
- 40.Yang C.M., Chen Y.W., Chi P.L., Lin C.C.and Hsiao L.D. (2017) Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-kappaB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 132, 77–91 10.1016/j.bcp.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 41.Wang W., Guo C., Zhu P., Lu J., Li W., Liu C. et al. (2015) Phosphorylation of STAT3 mediates the induction of cyclooxygenase-2 by cortisol in the human amnion at parturition. Sci. Signal. 8, ra106 10.1126/scisignal.aac6151 [DOI] [PubMed] [Google Scholar]
- 42.Liu B., Sun L., Liu Q., Gong C., Yao Y., Lv X. et al. (2015) A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 27, 370–381 10.1016/j.ccell.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 43.Toualbi K., Guller M.C., Mauriz J.L., Labalette C., Buendia M.A., Mauviel A. et al. (2007) Physical and functional cooperation between AP-1 and beta-catenin for the regulation of TCF-dependent genes. Oncogene 26, 3492–3502 10.1038/sj.onc.1210133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.