Changes in temperature, caused by climate change, can alter the amount of power an animal’s muscle produces, which could in turn affect that animal’s ability to catch prey or escape predators. Some animals may cope with such changes, but other species could undergo local extinction as a result.
Keywords: Activity, force, locomotion, muscle, power, temperature
Abstract
Climate change can involve alteration in the local temperature that an animal is exposed to, which in turn may affect skeletal muscle temperature. The underlying effects of temperature on the mechanical performance of skeletal muscle can affect organismal performance in key activities, such as locomotion and fitness-related behaviours, including prey capture and predator avoidance. The contractile performance of skeletal muscle is optimized within a specific thermal range. An increased muscle temperature can initially cause substantial improvements in force production, faster rates of force generation, relaxation, shortening, and production of power output. However, if muscle temperature becomes too high, then maximal force production and power output can decrease. Any deleterious effects of temperature change on muscle mechanics could be exacerbated by other climatic changes, such as drought, altered water, or airflow regimes that affect the environment the animal needs to move through. Many species will change their location on a daily, or even seasonal basis, to modulate the temperature that they are exposed to, thereby improving the mechanical performance of their muscle. Some species undergo seasonal acclimation to optimize muscle mechanics to longer-term changes in temperature or undergo dormancy to avoid extreme climatic conditions. As local climate alters, species either cope with the change, adapt, avoid extreme climate, move, or undergo localized extinction events. Given that such outcomes will be determined by organismal performance within the thermal environment, the effects of climate change on muscle mechanics could have a major impact on the ability of a population to survive in a particular location.
Introduction
Global average temperatures are rising at a rate of about 0.2°C per decade; the rate of temperature change is much greater in some regions, such as up to three times higher in the Arctic (IPCC, 2018). Global climate change is also initiating more frequent and more intense extreme weather events, such as heavy rainfall and heat waves causing floods and drought, respectively (IPCC, 2018). These acute and chronic changes in the local environment and threats to animal habitats brought about through climate change have, and continue to, alter the behaviour, geographical range, and survival of many animal species (Perry et al., 2005; Chown et al., 2010; Evans et al., 2015; Beever et al., 2017).
Many previous studies focusing on the effects of global climate change on animals have considered the effects of altered temperature. Such studies have demonstrated that changes in temperature have been linked with shifts in geographical range of species, effectively causing local extinctions. For example, the movement of most species of fish in the North Sea to more Northern and/or deeper, therefore colder waters (Perry et al., 2005). There are many examples where explanation of such observed shifts, or prediction of likely future shifts, in species distribution is dependent on incorporating underlying temperature-induced physiological changes into the predictive models used (Chown et al., 2010, Somero, 2012; Evans et al., 2015). Some modelling studies have considered that rising temperatures could restrict the amount of time that individual animals can be active in their environment, thereby reducing the time available to partake in fitness-related behaviours (Evans et al., 2015). However, more attention needs to be given to the effects of temperature on the actual performance of animals while they are active to improve modelling of the effects of climate change on animal species survival and distribution.
Temperature affects the chemical and physical properties of animals and their environment, such as the rates of biochemical reactions within an animal and the density of fluids an animal moves through. Standard performance curves can be used to describe the change in performance with temperature, indicating the maximal performance, the optimal temperature for maximal performance (Topt), and the performance breadth, which is the range of temperature over which a specified level of performance can be attained (Fig. 1; Angilleta, 2009). For example, the reaction rates of metabolic enzymes are affected in a similar way to this standard curve with variation in Topt of a specific enzyme between species or populations (Hochachka and Somero, 2002). Such enzymes exhibit relatively rapid declines in performance at higher than optimal temperature with inactivation and eventual denaturation occurring. Therefore, both increases and decreases in environmental temperature due to climate change could result in reductions in performance. Figure 1.
Figure 1.
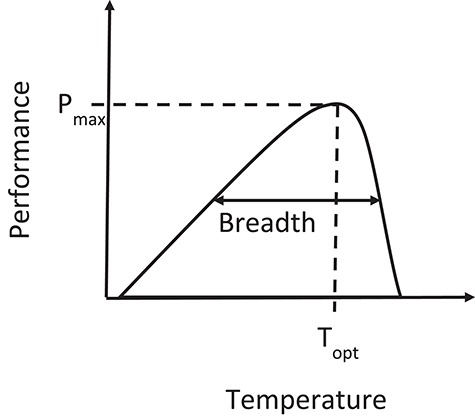
Theoretical performance curve showing the effect of temperature on performance. Topt is the optimum temperature to maximize performance; Pmax is the maximal performance; breadth is the performance breadth, which is the range of temperature over which performance is above a specified percentage of maximal performance.
Given that animal performance is influenced by the mechanical performance of skeletal muscle, this review will focus on the acute and chronic effects of temperature change on skeletal muscle mechanics and consider how such effects could influence animal behaviour and survival as a result of climate change. Skeletal muscle mechanics has been shown to constrain aspects of animal performance that are important in some behaviours, such as sprinting, as used during an escape response, and bite force, as would be used during some aggressive behaviours. For example, previous studies have found strong correlations between individual variation in skeletal muscle mechanics or activity of metabolic enzymes in muscle, as a proxy of muscle mechanics, and variation in maximal sprint performance within a lizard species (Johnson et al., 1993; Higham et al., 2011). Variation in isolated iliotibialis, a leg extensor, muscle power output between related lacertid lizard species has been found to be strongly correlated, r = 0.77, with variation in sprint performance (Van Hooydonck et al., 2014). A linkage has also been demonstrated between high performance in such muscle-powered activities and fitness, via longer-term survivorship or improved reproductive success (Miles, 2004; Lailvaux and Irschick, 2006; Husak et al., 2008). Therefore, this review will also consider, where possible, how any temperature-induced alterations in muscle mechanics may impact locomotor performance and behaviour. Determining the existence of linkages between changes in skeletal muscle mechanics and effects on locomotor performance and behaviour is key to understanding the extent to which muscle mechanics could constrain locomotion and behaviour in a changing climate. Where possible, this review will differentiate between temperature effects on endotherms and ectotherms. Endotherms use heat from metabolism to regulate their core body temperature, often within a narrow range of temperatures, whereas the body temperature of ectotherms is dependent on their external environment and can undergo large daily and seasonal changes (Angilletta, 2009).
Effects of acute temperature change on skeletal muscle mechanics
Changes in temperature can have profound effects on skeletal muscle mechanics. Studies, using skeletal muscle isolated from vertebrates, have generally demonstrated that up to an optimal, often relatively high, temperature, increased temperature causes greater force production, faster rates of force generation and relaxation, higher shortening speed, and enhanced power output (Bennett, 1984; Rall and Woledge, 1990; Syme, 2006; James, 2013). This general finding is consistent in both endotherms and ectotherms.
Muscle mechanics during short-term activity
When a neurone stimulates a muscle cell, calcium is released from the sarcoplasmic reticulum into the muscle cytoplasm, increasing calcium concentration to initiate a chain of events that allow myosin to interact more strongly with actin (Jones et al., 2004). Muscle force is produced via the interaction between myosin and actin, with myosin binding to actin to form cross-bridges that undergo a conformational change to produce force. These cross-bridges can be in low or high force–producing states (Ranatunga, 2018). When the neural stimulus ends, calcium is taken back into the sarcoplasmic reticulum, while parvalbumin also binds calcium in the cytoplasm and shuttles it to the sarcoplasmic reticulum, thereby reducing the concentration of calcium in the muscle cell; a reduction in calcium concentration decreases the number of interactions between myosin and actin, lowering muscle force to a ‘resting’ level (Berchtold et al., 2000). Therefore, greater calcium concentration in the cytoplasm causes higher numbers of cross-bridges to form and greater force production, whereas more rapid calcium release into the cytoplasm leads to faster force generation and more rapid calcium uptake from the cytoplasm causes faster muscle relaxation.
Isolated skeletal muscle mechanics has often been determined using isometric studies, whereby the muscle is activated while kept at an overall constant length (Josephson, 1993; Caiozzo, 2002). The maximal isometric force that a skeletal muscle can produce increases as temperature rises (Bennett, 1984; Rall and Woledge, 1990; Syme, 2006; James, 2013). However, at higher temperatures, this change in force is usually relatively low, and above the optimal temperature, a gradual reduction in force occurs (Fig. 2). For example, a comparison of maximum isometric force produced by single fibres from species of ectothermic fish from the Antarctic, North Sea, and Central Africa demonstrated that the muscle of each fish produced maximal force at temperatures around those that occurred in their natural environment, with decreases in force at lower and higher temperatures, such that each species outperformed the other species when in its own physiological temperature range (Altringham and Johnston, 1986). Skeletal muscle in endotherms has also been found to show high thermal sensitivity of isometric force production outside of physiological temperature ranges, regardless of whether the muscle is from the body core, where temperature is relatively constant, or the periphery of the body, such as found in diaphragm (core) and soleus (peripheral) muscle isolated from laboratory mice, Mus musculus (James et al., 2015), or in muscle from different regions of endothermic fish (Altringham and Block, 1997; Bernal et al., 2005; Donley et al., 2012). As temperature rises, there is no change in the number of cross-bridges that form (myosin heads attached to actin binding sites) within skeletal muscle, but there is an increase in the proportion of cross-bridges that are in a high force producing state, thereby enhancing muscle force generation (Bershitsky and Tsaturyan, 2002; Decostre et al., 2005; Colombini et al., 2008; Ranatunga, 2018). Figure 2.
Figure 2.
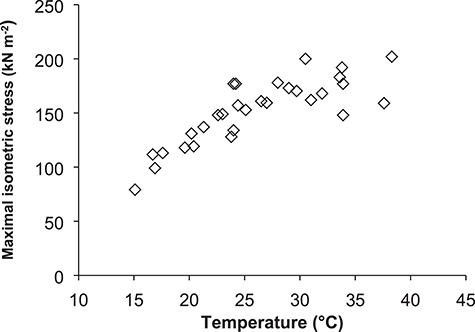
Effect of temperature on the maximal isometric stress (force normalized to muscle cross-sectional area) generated by isolated mouse diaphragm muscle. Each data point represents the maximum stress generated by one muscle at that temperature. Eight muscles were subjected to four different temperatures each. Temperature was randomized for each muscle. Based on data presented in James et al. (2015).
During isometric actions, temperature does not just affect the maximal amount of force produced. Rates of force generation and relaxation, during isometric activities, increase as temperature rises (Ranatunga, 1982; John-Alder et al., 1988; Swoap et al., 1993; Altringham and Block, 1997; De Ruiter and De Haan, 2000; Wilson et al., 2000; Herrel et al., 2007; James et al., 2015). Data from both ectothermic and endothermic species indicate that the rate of change decreases as the optimal temperature for maximal mechanical performance of muscle is approached. Changes in rates of force generation and relaxation could have important influences on whole animal performance; in some species, they may influence the stride frequency that an animal can attain (Johnson et al., 1993; see discussion below on temperature effects on work loops). Increased temperature raises myofibrillar ATPase activity and the rate at which parvalbumin binds calcium, thereby increasing rates of isometric force generation and relaxation, respectively (Barany, 1967; Stein et al., 1982; Hou et al., 1992). For example, as temperature increased from 5°C to 35°C, there was a high correlation (r = 0.99) between change in rate of myofibrillar ATPase activity and rate of force generation in skinned psoas muscle fibres from rabbit, Oryctolagus cuniculus (Brenner and Eisenberg, 1986).
Isolated muscle power output has traditionally been assessed during force–velocity experiments, whereby muscle is activated to produce force while at constant length and then shortened at a constant velocity or at a constant force (Josephson, 1993; Caiozzo, 2002). Such controlled shortening actions are repeated numerous times on a muscle preparation to determine the relationship between force output and shortening velocity in that muscle. Power output is calculated as force generated multiplied by shortening velocity. During such force–velocity experiments, performance of skeletal muscle generally improves as temperature rises, with increases in estimates of maximum shortening velocity and power output (Hill, 1938; Ranatunga, 1982; Marsh and Bennett, 1985; Johnston and Gleeson, 1987; Coughlin et al., 1996; Ranatunga, 1998). Again, the rate of change decreases as temperature approaches that normally used during locomotion. Notably, Olberding and Deban (2017) demonstrated, during force–velocity experiments, that frog, Osteopilus septentrionalis, plantaris muscle work output and shortening velocity exhibited much lower thermal sensitivity during low force, as would be used during routine movement or postural control, than high force contractions, such as would be used during maximal activities.
Work loop experiments were developed to allow closer in vitro simulation of the type of muscle actions that occur in vivo during power-producing activities (Josephson, 1993; Caiozzo, 2002). The force generated by the muscle is plotted against the length change to produce a work loop, the area of which represents the work done during a length change cycle (Fig. 3). The power generated by the muscle can be determined as the sum of work done divided by the time taken to do that work. As temperature rises up to normal body temperature, there is an increase in work loop power output, in muscle isolated from amphibians, fish, mammals, and reptiles (Johnson and Johnston, 1991; Swoap et al., 1993; Altringham and Block, 1997; Rome et al., 1999; Donley et al., 2007; Herrel et al., 2007; Seebacher and James, 2008; James et al., 2012; Seebacher et al., 2014; James et al., 2015). As temperature rises, work loop power output can be enhanced by a combination of increasing the maximal shortening velocity of the muscle such that maximal power output is achieved at a higher cycle frequency and by increasing the area of the work loop via the following: (i) reducing the time taken to generate force and to relax; (ii) enhancing the muscle's ability to produce force while shortening; and (iii) a reduction in the passive resistance to lengthening (Fig. 3; James, 2013). Thermal sensitivity of muscle power output varies between species with, in general, higher thermal sensitivity in skeletal muscle from endotherms. Importantly, power output determined by work loops also indicates that increased temperature above, or below, that normally experienced can result in a decrease in power output, such that the optimal temperature for skeletal muscle power output generally seems to be about the normal active body temperature. Figure 3.
Figure 3.
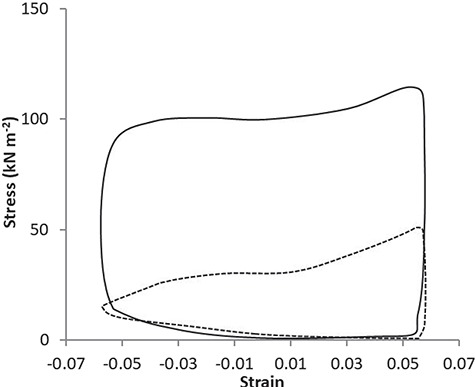
Typical effects of temperature on work loop shape. Mouse soleus work loop shapes at maximal power output at 15.3°C (broken line) and 37.4°C (solid line) in the same muscle preparation. Maximal power output was produced at a length change cycle frequency of 1 Hz at 15.3°C and 5 Hz at 37.4°C. Force was normalized to muscle cross-sectional area to calculate muscle stress and muscle length change was normalized to muscle length to calculate strain. Based on data presented in James et al. (2015). These work loop shapes demonstrate that at the higher temperature, there was more rapid force generation, greater maximal force, improved maintenance of force during shortening (likely to be at least partly due to an increased maximal shortening velocity, thereby altering the force–velocity relationship), and more rapid force relaxation.
In endotherms, there is evidence that some discrepancies in thermal sensitivity between skeletal muscles may relate to differences in location of each muscle in the body that result in variation in the range of temperature experienced. Skeletal muscle from the core of the body is maintained at a more narrow range of temperatures and has been found to have greater thermal sensitivity than muscle from the periphery in fish exhibiting regional endothermy (Altringham and Block, 1997; Bernal et al., 2005; Donley et al., 2007, 2012) with comparably little difference found in mouse (James et al., 2015). Work loop power output showed higher thermal sensitivity in muscle isolated from the deep endothermic core of yellow fin tuna, Thunnus albacares, than from the more superficial region of skeletal muscle in this fish (Altringham and Block, 1997). A comparison between bat, Carollia perspicillata, wing muscle (extensor carpi radialis longus) and mouse, M. musculus, limb muscle (extensor digitorum longus) demonstrated that isometric force generation and relaxation times and maximal shortening velocity had lower thermal sensitivity, below core body temperature, in the bat wing muscle, which is likely to be subjected to much higher temperature ranges during flight than would occur in mouse limb muscle (Rummel et al., 2018). Therefore, it seems that muscles subjected to a more narrow range of temperatures, as would be expected in core body muscles in endotherms, can become specialized to produce higher mechanical performance over a narrow thermal range, while having higher thermal sensitivity, as predicted by theory of a generalist–specialist continuum (Angilleta, 2009; Angilletta et al., 2010). However, such differences may be dependent on species and the mechanical property measured.
Muscle mechanics during sustained activity
In some fitness-related animal behaviours, high performance cannot be attributed to an ability to produce bursts of high muscle force or power but is instead due to an ability to sustain muscle activity over a series of actions. Prolonged muscle and locomotor performance is affected by fatigue. Skeletal muscle fatigue is defined as a reversible progressive reduction in contractile function (Allen et al., 2008).
While other mechanical variables show a typical trend of a temperature-induced improvement in performance, this is not consistently shown in the literature considering sustained muscle activity. Work by James et al. (2012) reported that the endurance of iliotibialis muscle isolated from Xenopus tropicalis, measured via a change in maximal work loop power over a protocol of repeated activations, increased with temperature (Fig. 4). A temperature-induced improvement in fatigue resistance has been reported in previous work using both protocols of repeated isometric tetani and cycles of active shortening (Roots et al., 2009). Conversely, there is conflicting evidence that increased temperature has limited effects on fatigue resistance (Place et al., 2009, Nocella et al., 2013) or may even cause fatigue to occur more quickly (Segal et al., 1986). Figure 4.
Figure 4.
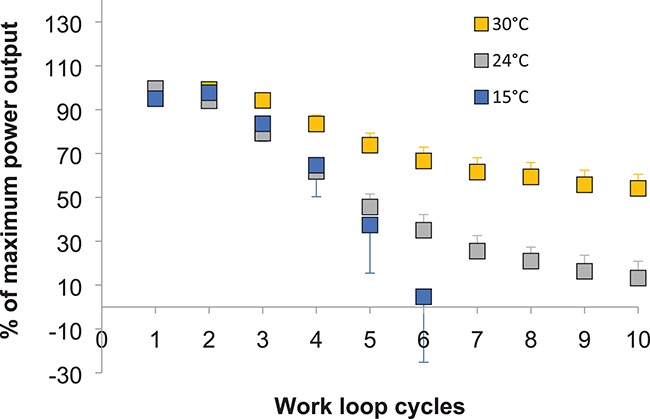
Fatigue resistance of power production improved as test temperature increased during a series of work loops in X. tropicalis iliotibialis muscle. Orange, grey, and blue symbols represent 30°C, 24°C, and 15°C data, respectively, for individuals housed at 24°C. Data represented as mean ± sem, n = 8, plotted as a percentage of the maximum power output produced by each individual. Based on data presented in James et al. (2012).
Ambiguity in findings make it difficult to make comparisons between studies, and such disparity is likely to arise, at least in part, from methodological discrepancies, including whether the test temperature was close to normal body temperature, relevance of the fatigue protocol with respect to real-world function, and the complexity around aetiology of fatigue. As an example, previous work defines fatigue by an arbitrary decline in performance relative to a pre-fatigue maximal (Roots et al., 2009) or over an arbitrary number of predefined activations (ranging from 10 to 105 activations) (James et al., 2012, Nocella et al., 2013, Place et al., 2009) and is either measured during maximal or sub-maximal activation, force, or power-generating conditions. Fatigue occurs as a two-phase decline, whereby the initial rapid decline in muscle function may take longer and be attenuated at higher temperatures, but an increased temperature may cause performance to decline more rapidly in the latter phase where the loss of force occurs more slowly (Roots et al., 2009, Nocella et al., 2013). These distinct phases are not always considered in previous work and may be apparent without a temperature-induced change in the end point of fatigue (Nocella et al., 2013).
Interestingly, the previously outlined temperature-induced increase in fatigue resistance reported by James et al. (2012) did not translate to whole-organismal endurance in the same species, X. tropicalis, where jump performance was decreased either side of an optimal temperature (Herrel and Bonneaud, 2012). This may indicate that exertion capacity may be limited by factors external to the muscular contractile elements (Herrel and Bonneaud, 2012) or that the chosen fatigue protocol may not relate closely enough to the in vivo locomotor demands of the whole organism. Studies using relatively long duration fatigue protocols may not accurately represent movements involved in feeding and burst locomotion, which are performed using single or small numbers of muscle activations (Olberding and Deban, 2017, Wilson et al., 2002). Burst performance, which is the result of a small number of high-force muscle activations, has been documented to be important for performance (Segre et al., 2015, Bennett, 1994) and survival (Watkins, 1996), so a reduction in phase one of the fatigue response with increasing temperature may be beneficial to some species in more burst like, shorter, periods of prolonged muscular activity.
The aetiology of fatigue is complex, is species and muscle specific, and is also temperature sensitive (Allen et al., 2008, Fitts, 2008, James et al., 2012, Debold et al., 2016). For example, skeletal muscle fatigue has, at least in part, been attributed to accumulation of inorganic phosphate and hydrogen ions, which have been shown to directly inhibit interactions between myosin and actin, during the cross-bridge cycle, and to indirectly cause lowered myofibrillar Ca2+ sensitivity, such that less force is produced at a specific calcium concentration, and there is a reduction in sarcoplasmic reticulum Ca2+ release (Allen et al., 2008, Fitts, 2008; Debold et al., 2016). There is evidence to indicate that increasing temperature may reduce the effects of high concentrations of inorganic phosphate and hydrogen ions on muscle force production (Coupland et al., 2001, Debold et al., 2016), which would support evidence showing that temperature can, at least in some muscles, improve fatigue resistance.
A further important aspect of fatigue is the mechanical efficiency of muscle activity, which has also been demonstrated to be temperature sensitive. Data by Edwards et al. (1972) indicated that increasing temperature reduced the mechanical efficiency of isometric contractions in human quadriceps muscle in situ, such that higher temperatures elicited greater energy cost and more rapid fatigue while undertaking the same activity. However, it has been more recently demonstrated that temperature effects on mechanical efficacy can be related to contractile velocity and, more specifically, a rightward shift in the efficiency–velocity relationship, whereby low-velocity muscle action may be less efficient at higher temperatures due to an increased speed of cross-bridge cycling, and at higher temperature, mechanical efficacy increases (Ferguson et al., 2002).
Despite the outlined challenges with summarizing the current literature, it is likely that the effect of temperature on prolonged muscle activity is species specific. A disparity in subsequent locomotor performance between predator and prey as a result of changing temperature may affect the survival and abundance of a particular species. For example, Allan et al. (2015) found that exposure to an elevated temperature influenced predator–prey interactions of two common reef fish. The piscivorous dottyback (Pseudochromis fuscus) demonstrated increased attack speed, while the planktivorous damselfish (Pomacentrus wardi) had decreased escape speeds and distances leading to increased predation rates. Other work has demonstrated that temperature effects on predator–prey relationships can, in some instances, also favour prey (Abrahams et al., 2007).
Can the effects of acute temperature change on skeletal muscle mechanics constrain locomotion and behaviour?
A key question as to whether changes in skeletal muscle performance are important to survival of individuals or species is whether there is a direct link between variation in the mechanical performance of skeletal muscle, changes in locomotor performance, and subsequent alterations in behaviour. Modelling conducted by Seebacher et al. (2015) demonstrated that individual variation in the mechanical performance of isolated skeletal muscle could partially explain observed differences in the overall swimming performance between those individual zebrafish, which in turn explained the time a fish was active in a novel environment and in turn an individual’s boldness in approaching a novel object. Thus, simplistically, longer, lower-weight fishes that had muscle that produced higher force at a quicker rate were more likely to have higher sustained and sprint swimming performance, such that they were more likely to spend more time active and to cover a greater distance in the behavioural test in the novel environment and were in turn more likely to show higher boldness in approaching a novel object (Seebacher et al., 2015). These findings suggest that variation in the mechanical performance of skeletal muscle can explain differences observed between individuals in locomotor performance and, in turn, behaviour. Thus, could effects of temperature on muscle mechanics and locomotion alter behaviour?
One of the best examples of the effects of acute temperature on locomotor performance and behaviour is the fight or flight response observed in some lizard species. At higher temperatures, such lizards will tend to flee from perceived predation risk. However, as environmental, and hence body, temperature decreases, it becomes increasingly likely that an individual will act aggressively and may attempt to bite the predator rather than run away (Hertz et al., 1982; Crowley and Pietruszka, 1983; Mautz et al., 1992). Sprint performance in lizards has high thermal sensitivity, with maximal and near maximal performance occurring over a narrow range of relatively high temperatures (Bennett, 1980; Huey and Kingsolver, 1989; Swoap et al., 1993; Herrel et al., 2007). In contrast, the maximal bite force produced by Trapelus pallida, a species of agamid lizard that exhibits this temperature-related shift between fight and flight behaviour, is relatively independent of temperature (Herrel et al., 2007). Herrel et al, (2007) demonstrated that the thermal sensitivity of the mechanical performance of muscle could explain the effects of temperature on bite force and running performance measured in this species. Isolated jaw muscle from T. pallida exhibited almost no change in maximal isometric force production between test temperatures of 20°C and 40°C, whereas caudofemoralis, a large muscle that retracts the femur, reduced in maximal isometric force by 20% between 35°C and 20°C (Herrel et al., 2007). However, while isometric force production is important for jaw muscles during biting, the caudofemoralis muscle is likely to act primarily to produce power during sprinting. The maximal power produced by isolated caudofemoralis muscle during work loops decreased by more than 40% between 35°C and 20°C (Herrel et al., 2007); other previous work on the desert iguana, Dipsosaurus dorsalis, has also found that isolated limb muscle power output, determined by work loops, has a high thermal sensitivity (Swoap et al., 1993). Overall, previous findings indicate that at least in these species studied, the acute thermal effects on the mechanical performance of skeletal muscle cause large changes in sprint performance but not bite force, due to the type of muscle activity being undertaken, as well as the differing intrinsic properties of the muscles involved. These thermal effects on sprint performance drive the observed shift from escape behaviour to aggressive behaviour at lower temperature in such lizard species. However, in some species of lizard, such as the Jamaican Grey anole Anolis lineatopus, individuals start their escape response from predators sooner at lower temperatures, rather than using any form of defensive behaviour, presumably as a different strategy to account for lower mechanical performance of limb muscle at reduced temperatures (Rand, 1964).
Michelangeli et al. (2018) demonstrated that behaviour of individuals of delicate skink, Lampropholis delicate, was linked to thermal sensitivity of performance such that a distinct ‘hot’ group of individuals had higher preferred body temperature, lower breadth of selected body temperature, higher optimal performance temperature, and lower thermal performance breadth than the ‘cold’ group; the hot group, when at a common temperature, spent more time active, spent more time exploring and less time hiding when in a novel environment, and had faster maximal sprint speeds than the cold group. These findings suggest that thermal physiology can constrain behaviour in predictable ways, affecting the way that individuals use niches within a habitat.
While the published literature is relatively sparse, it seems likely that colder or hotter environments will affect the mechanical performance of skeletal muscle and could in turn influence aspects of animal performance and behaviour, but further work is needed to better elucidate such relationships. What is clear is that some species are able to respond to changes in environmental temperature by undergoing acclimation of physiological processes or spending time in a ‘dormant’ state.
Chronic responses to temperature—acclimation and dormancy
As environmental temperature alters over time, individuals may be able to continue to be active and survive at that temperature, if their thermal sensitivity is sufficiently low, or may undertake a physiological response, such as acclimation or dormancy, or may be forced to move to an environment with a more favourable temperature.
Thermal acclimation
Acclimation responses occur in many species that are subjected to relatively large, seasonal changes in temperature and involve physiological changes to improve aspects of performance at the seasonal temperature. In many, but not all, cases acclimation responses can be beneficial, enabling changes in the thermal sensitivity of physiological processes to effect seasonal compensation in performance, such as to improve the mechanical performance of skeletal muscle and consequently locomotor performance at relatively low temperatures (Johnston and Temple, 2002; Wilson and Franklin, 2002; Seebacher, 2005; Angilletta, 2009). For example, muscle power output increased with temperature in caudofemoralis (the main muscle to power swimming) isolated from saltwater crocodiles, Crocodylus porosus, regardless of acclimation temperature (Seebacher and James, 2008; Fig. 5). However, cold-acclimated (20°C) individuals had a higher rate of caudofemoralis force generation and relaxation than those that were warm acclimated (30°C), enabling higher work loop power output in cold-acclimated muscle when tested at either acclimation temperature (Seebacher and James, 2008). In this study, the thermal sensitivity of myofibrillar ATPase activity of caudofemoralis muscle decreased with cold acclimation, providing at least some explanation for the results obtained (Seebacher and James, 2008). Glanville and Seebacher (2006) found, in much smaller individuals of the same species, that cold acclimation (20°C) enabled individuals to achieve higher maximum sustained swimming performance (UCrit) than warm-acclimated (30°C) individuals when tested at the cold acclimation temperature, the reverse being true at 30°C. Therefore, in the saltwater crocodile, the cold acclimation of skeletal muscle helps explain the compensation in swimming performance seen at lower temperatures in cold-acclimated animals. In another example, acclimation of red (slower, more aerobic) muscle mechanics and swimming performance was compared between rainbow smelt, Osmerus mordax, and rainbow trout, Oncorhynchus mykiss, that tend to encounter relatively higher and lower ranges of temperature, respectively, over a year (Shuman and Coughlin, 2018). In both species, red muscle isolated from cold-acclimated individuals had faster rates of isometric force generation and relaxation than warm-acclimated individuals at the two test temperatures used, representing relatively cold and mid-range environmental temperatures, although this effect was greater in smelt (Shuman and Coughlin, 2018). In work loop studies, there was no significant effect of acclimation group on maximum skeletal muscle power output (Shuman and Coughlin, 2018). In both species, cold-acclimated fish showed higher maximum steady swimming speed, than warm-acclimated fish, at the mid-range test temperature, the difference between acclimation groups being much greater in smelt, the species that are subjected to a higher annual range in temperature (Shuman and Coughlin, 2018). Figure 5.
Figure 5.
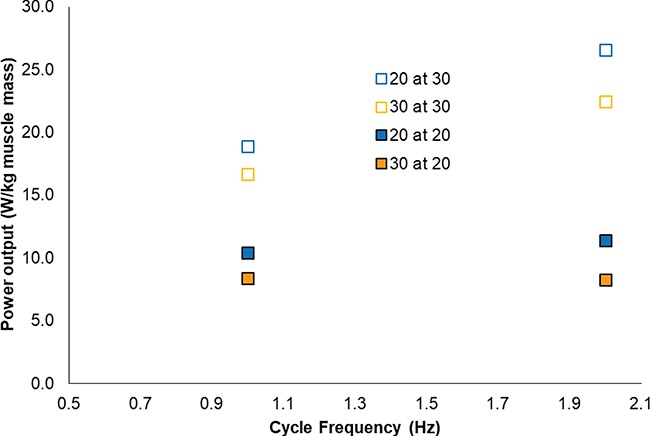
Power output increased with temperature and was greater in cold-acclimated animals (20°C, blue symbols) than warm-acclimated animals (30°C, orange symbols), regardless of test temperature (open symbols at 30°C, closed symbols at 20°C) or frequency of length change cycles, in caudofemoralis muscle isolated from saltwater crocodile. Data represents mean ± sem, n = 8. Based on data presented in Seebacher and James (2008).
Usually, acclimation occurs when the seasonal change in temperature is predictable and large when compared to daily variation in temperature. In contrast, species that live in environments with relatively high daily variation in temperature may have lower thermal sensitivity enabling them to perform reasonably well over a comparatively broad range of temperatures (Angilletta, 2009). For example, amphibian tadpoles, which often live in relatively small volumes of water that can be subjected to high daily thermal variability, on the whole seem unable to acclimate, instead having comparably low thermal sensitivity such that locomotor performance is near maximal across a broad range of temperatures (Niehaus et al., 2011). In contrast, Wilson and Franklin (1999) demonstrated that one species of tadpole, the striped marsh frog, Limnodynastes peronei, when acclimated to 10°C achieved about 50% greater maximum swimming velocity and acceleration, at a relatively low temperature of 10°C, in comparison to those acclimated to 24°C. When acclimation period at 10°C was increased from 6 weeks to 8 months, there was also a marked reduction in swimming performance at 24°C (Wilson and Franklin, 1999). Niehaus et al. (2011) tested whether daily thermal variation around a mean acclimation temperature caused differences in thermal sensitivity of performance in striped marsh frog. They found that swimming performance and skeletal muscle lactate dehydrogenase (a marker enzyme for anaerobic metabolic capacity in skeletal muscle) activity showed the same thermal sensitivity across a temperature range of 14–34°C regardless of the acclimation treatment, demonstrating that large daily thermal variation does not affect acclimation response in some species (Niehaus et al., 2011).
Some studies on thermal acclimation indicate that the capacity to acclimate to temperature change varies between species and can vary between populations within a species, possibly determined by the long-term variability in temperature that a population or species is subjected to (Johnston and Temple, 2002; Angilletta, 2009; Seebacher et al., 2012; Le Roy et al., 2017). Phenotypic plasticity in response to temperature seems to be more common in ectotherms living in large bodies of water as daily changes in temperature are buffered by the water volume unlike any large, often gradual, seasonal changes in temperature (Johnston and Temple, 2002). Species with the capacity to acclimate in response to temperature change have, in some studies, been shown to alter the mechanical properties of skeletal muscle via mechanisms, such as changes in myofibrillar ATPase activity, differential expression of contractile protein isoforms, or changes in calcium handling (Johnston and Temple, 2002; Syme 2006). Such species that are able to acclimate may be better equipped to deal with, at least, small long-term changes in environmental temperature that can occur as a result of climate change. However, it is unclear whether these species can deal well with an overall more variable climate, particularly as such variability could mask the cue to acclimate.
Dormancy
Avoidance of extreme temperature and drought can be achieved via undergoing periods of dramatically reduced metabolism, often referred to as ‘dormancy.’ The effects of periods of dormancy on the mechanical performance of skeletal muscle and locomotion have not been extensively studied. Periods of dormancy to avoid drought and extreme temperatures are termed aestivation and can extend up to many months or years (Pinder et al., 1992).
Aestivation for 3 months did not affect burst swimming performance of the green-striped burrowing frog, Cyclorana alboguttata (Hudson and Franklin, 2002). In addition, 9 months of aestivation in the green-striped burrowing frog did not cause any significant changes in the maximal power output or fatigue resistance of isolated iliofibularis or sartorius muscles during work loop studies (Symonds et al., 2007), despite significant reduction in the rate of tetanus force relaxation in iliofibularis muscle from aestivated individuals. Green-striped burrowing frogs are able to maintain skeletal muscle mass and cross-sectional area over these durations of dormancy (Symonds et al., 2007; Mantle et al., 2009), at least partially via reduced metabolism and increased expression of anti-apoptotic genes (Reilly et al., 2013), enabling absolute muscle mechanical performance to be maintained ready for emergence from aestivation.
While climate change is more likely to cause regions to be on average warmer than colder, extreme weather events are likely to be more common and some of the overall findings from studies on hibernation may provide useful insight into dormancy in general. Up to 4 months of hibernation, while submerged in water, in the common frog, Rana temporaria, had no effect on maximal isometric force, force–velocity relationships from isovelocity experiments, or work loop power output in sartorius muscle (West et al., 2006). In the 13-lined ground squirrel, Ictidomys tridecemlineatus, 3 months of hibernation did not cause a change in the maximal power output of soleus muscle (Fig. 6) but did reduce fatigue resistance during work loop studies (James et al., 2013). Previous studies on ground squirrels have indicated little or no change in skeletal muscle fibre type but a decrease in skeletal muscle mitochondrial respiration rates, which would help explain the mechanical properties observed (James et al., 2013). Many other hibernating mammals, such as bears, bats, and various rodents, undergo limited muscle atrophy, with no change in muscle fibre type or small shifts toward more oxidative fibres and little or no changes in the mechanical properties of skeletal muscle (Cotton, 2016). Figure 6.
Figure 6.
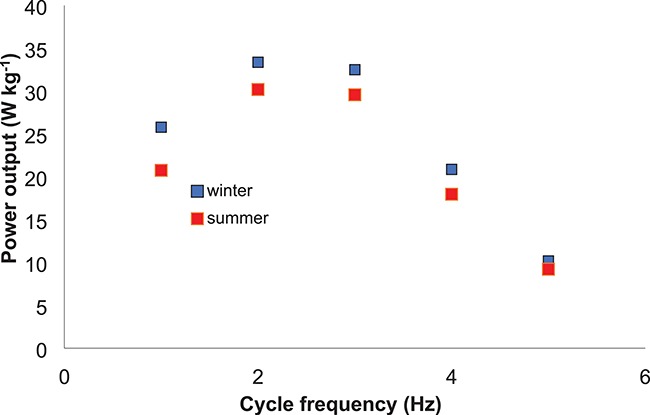
There was no significant difference in the soleus muscle power output–cycle frequency relationship between muscle removed from 13-lined ground squirrel in the summer (red) compared with that removed from those that had undergone 3 months of torpor in winter (blue). Data represent mean ± sem. Based on data presented in James et al. (2013).
These studies on hibernation reinforce the findings on aestivation to demonstrate that some species are able to spend substantial periods of time in a dormant state to avoid unfavourable climatic conditions without appreciable effects on subsequent mechanical performance of skeletal muscle, which is particularly important as post-dormancy such species tend to focus on fitness-related behaviours, such as finding and securing a mate. It is possible that further climate change could extend the periods of dormancy required. However, the possible duration of dormancy is limited, not least by the supply of stored energy required to maintain survival (Storey and Storey, 2007).
Conclusions and future directions
The present review has demonstrated that changes in temperature can have profound effects on the mechanical performance of skeletal muscle, in turn affecting locomotor performance, behaviour, and potential survival. In some species acclimation, dormancy or other behavioural responses could reduce or remove potential effects of climate change on performance; there are limits to the efficacy of such approaches to dealing with climate change and many species are unable to acclimate or undergo dormancy. It is clear that the effects of temperature on muscle physiology differ between species and in some cases between populations within a species, which adds further complexity to modelling potential effects of climate change on fitness and species distribution. For example, the capacity of mosquitofish (Gambusia holbrooki) to acclimate critical sustained swimming performance and skeletal muscle metabolic enzyme activities has been found to vary between populations, even between populations from the same lineage (Seebacher et al., 2012). However, some species from relatively stable thermal environments, such as the Antarctic fish Pagothenia borchgrevinki, seem unable to acclimate burst locomotor performance in response to environmental temperature change (Wilson et al., 2001), although they are able to improve critical sustained swimming performance at higher temperatures via acclimation (Seebacher et al., 2005). While thermal acclimation incurs energetic costs, it can in some cases enable performance to be improved across a season and may facilitate some species to better buffer against thermal effects of climate change. In contrast, other species may also cope well with thermal effects of climate change by having relatively low thermal sensitivity, which although this can entail a relatively lower performance, it would be maintained over a wider temperature range.
As discussed, individuals may respond to climate change by rapidly altering behaviours or timing of behaviours, such as mating, locomotion, food acquisition, habitat use, and predator avoidance (Beever et al., 2017). For example, in response to higher temperatures, some animals use microhabitats that provide lower temperatures, as has been observed in fish, mammals, and reptiles. However, the success of such an approach depends on availability of, and competition for, such refuges and the ability of such animals to have sufficient time outside the refuge to undertake key fitness-related behaviours (Beever et al., 2017).
While many journal papers considering effects of temperature on skeletal muscle and locomotor performance set such studies against a background of climate change, there are not enough data to indicate whether increases or decreases in environmental temperature, of the magnitude likely in climate change scenarios, will cause meaningful effects on skeletal muscle mechanics, locomotor performance, and behaviour in the species studied as few studies have estimated the likely remaining capacity of a population to buffer against such changes. Responses of a species to climate change would ideally also need to be considered in terms of their wider ecosystem to account for climate change effects on such aspects as predator–prey interactions (Gabriel et al., 2005).
Further work needs to be done to better simulate the effects of prolonged locomotor activity in isolated muscle preparations to clarify the effects of temperature on sustained mechanical performance of skeletal muscle. As most previous studies have focused on thermal effects on one level of organization in a species removed from its environment, it is difficult to fully comprehend how important the broader effects of climate change on muscle mechanics will be in influencing local extinction rates of species. Therefore, additional work is needed to better understand the effects of temperature-induced changes on the mechanical performance of skeletal muscle on fitness-related whole animal performance, behaviour, and in turn the ability of a species to maintain its geographical range. Further work is also needed to investigate whether different climate change–related variables interact in their effects on skeletal muscle and whole animal performance, e.g. changes in ocean pH, habitat fragmentation, dehydration (more likely during periods of drought), and effects of pollutants (such as plastics, etc).
References
- Abrahams MV, Mangel M, Hedges K (2007) Predator–prey interactions and changing environments: who benefits. Philos Trans R Soc Lond B Biol Sci 362: 2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BJ, Domenici P, Munday PL, McCormick ML (2015) Feeling the heat: the effect of acute temperature changes on predator-prey interactions in coral reef fish. Conserv Physiol 3: cov011. doi: 10.1093/conphys/cov011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332. [DOI] [PubMed] [Google Scholar]
- Altringham JD, Block BA (1997) Why do tuna maintain elevated slow muscle temperatures? Power output of muscle isolated from endothermic and ectothermic fish. J Exp Biol 200: 2617–2627. [DOI] [PubMed] [Google Scholar]
- Altringham JD, Johnston IA (1986) Evolutionary adaptation to temperature in fish muscle cross bridge mechanisms: tension and ATP turnover. J Comp Physiol B 156: 819–821. [Google Scholar]
- Angilletta MJ., Jr (2009) Thermal Adaptation. Oxford University Press, Oxford [Google Scholar]
- Angilletta MJ Jr, Cooper BS, Schuler MS, Boyles JG (2010) The evolution of thermal physiology in endotherms. Front Biosci E2: 861–881. [DOI] [PubMed] [Google Scholar]
- Barany M. (1967) ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol 50: 197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beever EA, Hall LE, Varner J, Loosen AE, Dunham JB, Gahl MK, Smith FA, Lawler JJ (2017) Behavioral flexibility as a mechanism for coping with climate change. Front Ecol Environ 15: 299–308. [Google Scholar]
- Bennett AF. (1980) The thermal dependence of lizard behaviour. Anim Behav 28: 752–762. [Google Scholar]
- Bennett AF. (1984) Thermal dependence of muscle function. Am J Physiol 247: R217–R229. [DOI] [PubMed] [Google Scholar]
- Bennett AF. (1994) Exercise performance of reptiles. Adv Vet Sci Comp Med 38B: 113–138. [PubMed] [Google Scholar]
- Berchtold MW, Brinkmeier H, Müntener M (2000) Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80: 1215–1265. [DOI] [PubMed] [Google Scholar]
- Bernal D, Donley JM, Shadwick RE, Syme DA (2005) Mammal-like muscles power swimming in a cold-water shark. Nature 437: 1349–1352. [DOI] [PubMed] [Google Scholar]
- Bershitsky SY, Tsaturyan AK (2002) The elementary force generation process probed by temperature and length perturbations in muscle fibres from rabbit. J Physiol 540: 971–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B, Eisenberg E (1986) Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Nat Acad Sci U S A 83: 3542–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiozzo VJ. (2002) Plasticity of skeletal muscle phenotype: mechanical consequences. Muscle Nerve 26: 740–768. [DOI] [PubMed] [Google Scholar]
- Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ Jr, Stenseth NC, Pertoldi C (2010) Adapting to climate change: a perspective from evolutionary physiology. Clim Res 43: 3–15. [Google Scholar]
- Colombini B, Nocella M, Benelli G, Cecchi G, Bagni MA (2008) Effect of temperature on cross-bridge properties in intact frog muscle fibers. Am J Physiol 294: C1113–C1117. [DOI] [PubMed] [Google Scholar]
- Cotton CJ. (2016) Skeletal muscle mass and composition during mammalian hibernation. J Exp Biol 219: 226–234. doi: 10.1242/jeb.125401. [DOI] [PubMed] [Google Scholar]
- Coughlin DJ, Zhang G, Rome LC (1996) Contraction dynamics and power production of pink muscle of the scup (Stenotomus chrysops). J Exp Biol 199: 2703–2712. [DOI] [PubMed] [Google Scholar]
- Coupland ME, Puchert E, Ranatunga KW (2001) Temperature dependence of active tension in mammalian (rabbit psoas) muscle fibres: effect of inorganic phosphate. J Physiol 536: 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SR, Pietruszka RD (1983) Aggressiveness and vocalization in the leopard lizard (Gambelia wislizennii): the influence of temperature. Anim Behav 31: 1055–1060. [Google Scholar]
- De Ruiter CJ, De Haan A (2000) Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflugers Arch 440: 163–170. [DOI] [PubMed] [Google Scholar]
- Debold EP, Fitts RH, Sundberg CW, Nosek TM (2016) Muscle fatigue from the perspective of a single crossbridge. Med Sci Sports Exerc 48: 2270–2280. [DOI] [PubMed] [Google Scholar]
- Decostre V, Bianco P, Lombardi V, Piazzesi G (2005) Effect of temperature on the working stroke of muscle myosin. Proc Natl Acad Sci U S A 102: 13927–13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley JM, Sepulveda CA, Aalbers SA, McGillivray DG, Syme DA, Bernal D (2012) Effects of temperature on power output and contraction kinetics in the locomotor muscle of the regionally endothermic common thresher shark (Alopias vulpinus). Fish Physiol Biochem 38: 1507–1519. [DOI] [PubMed] [Google Scholar]
- Donley JM, Shadwick RE, Sepulveda CA, Syme DA (2007) Thermal dependence of contractile properties of the aerobic locomotor muscle in the leopard shark and shortfin mako shark. J Exp Biol 210: 1194–1203. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Harris RC, Hultman E, Kaijser L, Koh D, Nordesjo LO (1972) Effect of temperature on muscle energy metabolism and endurance during successive isometric contractions, sustained to fatigue, of the quadriceps muscle in man. J Physiol 220: 335–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TG, Diamond SE, Kelly MW (2015) Mechanistic species distribution modelling as a link between physiology and conservation. Conserv Physiol 3: cov056. doi: 10.1093/conphys/cov056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RA, Ball D, Sargeant AJ (2002) Effect of muscle temperature on rate of oxygen uptake during exercise in humans at different contraction frequencies. J Exp Biol 205: 981–987. [DOI] [PubMed] [Google Scholar]
- Fitts RH. (2008) The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104: 551–558. [DOI] [PubMed] [Google Scholar]
- Gabriel W, Luttbeg B, Sih A, Tollrian R (2005) Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am Nat 166: 339–353. [DOI] [PubMed] [Google Scholar]
- Glanville EJ, Seebacher F (2006) Compensation for environmental change by complementary shifts of thermal sensitivity and thermoregulatory behaviour in an ectotherm. J Exp Biol 209: 4869–4877. [DOI] [PubMed] [Google Scholar]
- Herrel A, Bonneaud C (2012) Temperature dependence of locomotor performance in the tropical clawed frog, Xenopus tropicalis. J Exp Biol 215: 2465–2470. [DOI] [PubMed] [Google Scholar]
- Herrel A, James RS, Van Damme R (2007) Fight versus flight: physiological basis for temperature dependent behavioral shifts in lizards. J Exp Biol 210: 1762–1767. [DOI] [PubMed] [Google Scholar]
- Hertz PE, Huey RB, Nevo E (1982) Fight versus flight: body temperature influences defensive responses of lizards. Anim Behav 30: 676–679. [Google Scholar]
- Higham TE, Korchari PG, McBrayer LD (2011) How muscles define maximum running performance in lizards: an analysis using swing- and stance-phase muscles. J Exp Biol 214: 1685–1691. [DOI] [PubMed] [Google Scholar]
- Hill AV. (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc B 126: 136–195. [Google Scholar]
- Hochachka PW, Somero GN (2002) Biochemical Adaptation. Oxford University Press, Oxford. [Google Scholar]
- Hou TT, Johnson JD, Rall JA (1992) Effect of temperature on relaxation rate and Ca2+, Mg2+ dissociation rates from parvalbumin of frog muscle fibres. J Physiol 449: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson NJ, Franklin CE (2002) Effect of aestivation on muscle characteristics and locomotor performance in the green-striped burrowing frog, Cyclorana alboguttata. J Comp Physiol B 172: 177–182. [DOI] [PubMed] [Google Scholar]
- Huey RB, Kingsolver JG (1989) Evolution of thermal sensitivity of ectotherm performance. Trends Ecol Evol 4: 131–135. [DOI] [PubMed] [Google Scholar]
- Husak JF, Fox SF, Van Den Bussche RA (2008) Faster male lizards are better defenders not sneakers. Anim Behav 75: 1725–1730. [Google Scholar]
- IPCC (2018) Summary for policymakers In V Masson-Delmotte, P Zhai, HO Pörtner, D Roberts, J Skea, PR Shukla, A Pirani, W Moufouma-Okia, C Péan, R Pidcock, S Connors, JBR Matthews, Y Chen, X Zhou, MI Gomis, E Lonnoy, T Maycock, M Tignor, T Waterfield, eds, Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. World Meteorological Organization, Geneva, Switzerland, 32 pp. [Google Scholar]
- James RS. (2013) A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J Comp Physiol B 183: 723–733. [DOI] [PubMed] [Google Scholar]
- James RS, Staples J, Brown JCL, Tessier S, Storey KB (2013) The effects of hibernation on the contractile and biochemical properties of skeletal muscles in the thirteen-lined ground squirrel, Ictidomys tridecemlineatus. J Exp Biol 216: 2587–2594. [DOI] [PubMed] [Google Scholar]
- James RS, Tallis J, Angilletta M (2015) Regional thermal specialisation in a mammal: temperature affects power output of core muscle more than that of peripheral muscle in adult mice (Mus musculus). J Comp Physiol B 185: 135–142. [DOI] [PubMed] [Google Scholar]
- James RS, Tallis J, Herrel A, Bonneaud C (2012) Warmer is better: thermal sensitivity of both maximal and sustained power output in the iliotibialis muscle isolated from adult Xenopus tropicalis. J Exp Biol 215: 552–558. [DOI] [PubMed] [Google Scholar]
- John-Alder HB, Barnhart MC, Bennett AF (1988) Thermal sensitivity of swimming performance and muscle contraction in northern and southern populations of tree frogs (Hyla crucifer). J Exp Biol 142: 357–372. [Google Scholar]
- Johnson TP, Johnston IA (1991) Power output of fish muscle fibres performing oscillatory work: effects of acute and seasonal temperature change. J Exp Biol 157: 409–423. [Google Scholar]
- Johnson TP, Swoap SJ, Bennett AF, Josephson RK (1993) Body size, muscle power output and limitations on burst locomotor performance in the lizard Dipsosaurus dorsalis. J Exp Biol 174: 199–213. [Google Scholar]
- Johnston IA, Gleeson TT (1987) Effect of temperature on contractile properties of skinned muscle fibres from three toad species. Am J Physiol 252: R371–R375. [DOI] [PubMed] [Google Scholar]
- Johnston IA, Temple GK (2002) Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. J Exp Biol 205: 2305–2322. [DOI] [PubMed] [Google Scholar]
- Jones D, Round J, de Haan A (2004) Skeletal Muscle from Molecules to Movement. Churchill Livingstone, London. [Google Scholar]
- Josephson RK. (1993) Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol 55: 527–546. [DOI] [PubMed] [Google Scholar]
- Lailvaux SP, Irschick DJ (2006) A functional perspective on sexual selection: insights and future prospects. Anim Behav 72: 263–273. [Google Scholar]
- Le Roy A, Loughland I, Seebacher F (2017) Differential effects of developmental thermal plasticity across three generations of guppies (Poecilia reticulata): canalization and anticipatory matching. Sci Rep 7: 4313. doi: 10.1038/s41598-017-03300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle BL, Hudson NJ, Harper GS, Cramp RL, Franklin CE (2009) Skeletal muscle atrophy occurs slowly and selectively during prolonged aestivation in Cyclorana alboguttata (Günther 1867). J Exp Biol 212: 3664–3672. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Bennett AF (1985) Thermal dependence of isotonic contractile properties of skeletal muscle and sprint performance of the lizard Dipsosaurus dorsalis. J Comp Physiol B 155: 541–551. [DOI] [PubMed] [Google Scholar]
- Mautz WJ, Daniels CB, Bennett AF (1992) Thermal dependence of locomotion and aggression in a xantusiid lizard. Herpetologica 48: 271–279. [Google Scholar]
- Michelangeli M, Goulet C, Kang HS, Wong BBM, Chapple D (2018) Integrating thermal physiology within a syndrome: locomotion, personality and habitat selection in an ectotherm. Funct Ecol 32: 1–12. [Google Scholar]
- Miles DB. (2004) The race goes to the swift: fitness consequences of variation in sprint performance in juvenile lizards. Evol Ecol Res 6: 63–75. [Google Scholar]
- Niehaus AC, Wilson RS, Seebacher F, Franklin CE (2011) Striped marsh frog (Limnodynastes peronii) tadpoles do not acclimate metabolic performance to thermal variability. J Exp Biol 214: 1965–1970. [DOI] [PubMed] [Google Scholar]
- Nocella M, Cecchi G, Bagni MA, Colombini B (2013) Effect of temperature on crossbridge force changes during fatigue and recovery in intact mouse muscle fibers. PLoS One 8: e78918. 10.1371/journal.pone.0078918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olberding JP, Deban SM (2017) Effects of temperature and force requirements on muscle work and power output. J Exp Biol 220: 2017–2025. [DOI] [PubMed] [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308: 1912–1913. [DOI] [PubMed] [Google Scholar]
- Pinder AW, Storey KB, Ultsch GR (1992) Estivation and hibernation In Feder ME, Burggren WW, eds, Environmental Physiology of the Amphibians. University of Chicago Press, Chicago, pp. 251–274. [Google Scholar]
- Place N, Yamada T, Zhang SJ, Westerblad H, Bruton JD (2009) High temperature does not alter fatigability in intact mouse skeletal muscle fibres. J Physiol 587: 4717–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JA, Woledge RC (1990) Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol 259: R197–R203. [DOI] [PubMed] [Google Scholar]
- Ranatunga KW. (1982) Temperature dependence of shortening velocity and rate of isometric tension development in rat skeletal muscle. J Physiol 329: 465–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW. (1998) Temperature dependence of mechanical power output in mammalian (rat) skeletal muscle. Exp Physiol 83: 371–376. [DOI] [PubMed] [Google Scholar]
- Ranatunga KW. (2018) Temperature effects on force and actin-myosin interaction in muscle: a look back on some experimental findings. Int J Mol Sci 19: E1538. doi: 10.3390/ijms19051538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand AS. (1964) Inverse relationship between temperature and shyness in the lizard Anolis lineatopus. Ecology 45: 863–864. [Google Scholar]
- Reilly BD, Schlipalius DI, Cramp RL, Ebert PR, Franklin CE (2013) Frogs and estivation: transcriptional insights into metabolism and cell survival in a natural model of extended muscle disuse. Physiol Genomics 45: 377–388. [DOI] [PubMed] [Google Scholar]
- Rome LC, Swank D, Coughlin DJ (1999) The influence of temperature on power production during swimming II. Mechanics of red muscle fibres in vivo. J Exp Biol 202: 333–345. [DOI] [PubMed] [Google Scholar]
- Roots H, Ball G, Talbot-Ponsonby J, King M, McBeath K, Ranatunga KW (2009) Muscle fatigue examined at different temperatures in experiments on intact mammalian (rat) muscle fibers. J Appl Physiol 106: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel AD, Swartz SM, Marsh RL (2018) Low thermal dependence of the contractile properties of a wing muscle in the bat Carollia perspicillata. J Exp Biol 221: jeb180166. doi: 10.1242/jeb.180166. [DOI] [PubMed] [Google Scholar]
- Seebacher F. (2005) A review of thermoregulation and physiological performance in reptiles: what is the role of phenotypic flexibility. J Comp Physiol B 175: 453–461. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Davison W, Lowe CJ, Franklin CE (2005) A falsification of the thermal specialization paradigm: compensation for elevated temperatures in Antarctic fishes. Biol Lett 1 10.1098/rsbl.2004.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, Holmes S, Roosen NJ, Nouvian M, Wilson RS, Ward AJW (2012) Capacity for thermal acclimation differs between populations and phylogenetic lineages within a species. Funct Ecol 26: 1418–1428. [Google Scholar]
- Seebacher F, James RS (2008) Plasticity of muscle function in a thermoregulating ectotherm (Crocodylus porosus): biomechanics and metabolism. Am J Physiol 294: R1024–R1032. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Little AG, James RS (2015) Skeletal muscle contractile function predicts activity and behaviour in zebrafish. J Exp Biol 218: 3878–3884. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Tallis JA, James RS (2014) The cost of muscle power production: muscle oxygen consumption per unit work increases at low temperatures in Xenopus laevis Daudin. J Exp Biol 217: 1940–1945. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA, White TP (1986) Skeletal muscle fatigue in vitro is temperature dependent. J Appl Physiol 61: 660–665. [DOI] [PubMed] [Google Scholar]
- Segre PS, Dakin R, Zordan VB, Dickinson MH, Straw AD, Altshuler DL (2015) Burst muscle performance predicts the speed, acceleration, and turning performance of Anna’s hummingbirds. Elife 4: e11159. doi: 10.7554/eLife.11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman JL, Coughlin DJ (2018) Red muscle function and thermal acclimation to cold in rainbow smelt, Osmerus mordax, and rainbow trout, Oncorhynchus mykiss. J Exp Zool A 329: 547–556. [DOI] [PubMed] [Google Scholar]
- Somero GN. (2012) The physiology of global change: linking patterns to mechanisms. Annu Rev Mar Sci 4: 39–61. [DOI] [PubMed] [Google Scholar]
- Stein RB, Gordon T, Shriver J (1982) Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J 40: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB, Storey JM (2007) Tribute to P. L. Lutz: putting life on `pause’—molecular regulation of hypometabolism. J Exp Biol 210: 1700–1714. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Johnson TP, Josephson RK, Bennett AF (1993) Temperature, muscle power output, and limitations on burst locomotor performance of the lizard Dipsosaurus dorsalis. J Exp Biol 174: 185–197. [Google Scholar]
- Syme DA. (2006) Functional properties of skeletal muscle In Shadwick RE, Lauder GV, eds, Fish Physiology: Fish Biomechanics, Series Randall DJ, Farrell AP, eds Vol 23 Academic Press, Elsevier, pp. 179–224. [Google Scholar]
- Symonds BL, James RS, Franklin CE (2007) Getting the jump on skeletal muscle disuse atrophy: preservation of contractile performance in aestivating Cyclorana alboguttata (Günther, 1867). J Exp Biol 210: 825–835. [DOI] [PubMed] [Google Scholar]
- Vanhooydonck B, James RS, Tallis J, Aerts P, Tadic Z, Tolley KA, Measey GJ, Herrel A (2014) Is the whole more than the sum of its parts? Evolutionary trade-offs between burst and sustained locomotion in lacertid lizards. Proc R Soc B 281: 20132677. doi: 10.1098/rspb.2013.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TB. (1996) Predator-mediated selection on burst swimming performance in tadpoles of the Pacific tree frog, Pseudacris regilla. Physiol Biochem Zool 69: 154–167. [Google Scholar]
- West TG, Donohoe PH, Staples JF, Askew GN (2006) The role for skeletal muscle in the hypoxia-induced hypometabolic responses of submerged frogs. J Exp Biol 209: 1159–1168. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Franklin CE (1999) Thermal acclimation of locomotor performance in tadpoles of the frog Limnodynastes peronii. J Comp Physiol B 169: 445–451. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Franklin CE (2002) Testing the beneficial acclimation hypothesis. Trends Ecol Evol 17: 66–70. [Google Scholar]
- Wilson RS, Franklin CE, Davison B, Kraft P (2001) Stenotherms at sub-zero temperatures: thermal dependence of swimming performance in Antarctic fish. J Comp Physiol B 171: 263–269. [DOI] [PubMed] [Google Scholar]
- Wilson RS, James RS, Johnston IA (2000) Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog, Xenopus laevis. J Comp Physiol B 170: 117–124. [DOI] [PubMed] [Google Scholar]
- Wilson RS, James RS, Van Damme R (2002) Trade-offs between speed and endurance in the frog Xenopus laevis: a multi-level approach. J Exp Biol 205: 1145–1152. [DOI] [PubMed] [Google Scholar]


