Abstract
Fin whales in the Gulf of California constitute a resident population genetically isolated from the rest of the North Pacific Ocean. Its small population size and the scarce information available about its dynamics in a semi-enclosed sea underline the importance of conducting studies about its reproduction. Given the monsoonal regime that dominates the oceanographic habitat of this region, we hypothesized seasonality in the population’s reproductive activity. To test this, we validated and assayed testosterone and progesterone from blubber biopsies of free-ranging individuals. Lactating females exhibited low progesterone concentrations, whereas a group of females of unknown reproductive stage, but with extremely high progesterone concentrations, showed strong evidence of separation and were considered to be likely ovulating or pregnant. A seasonal model of testosterone concentrations showed a high peak during the late summer. This trend was supported by the first documentation of courtship events and by the recording of a female with high progesterone concentration during summer and re-sighted with a calf 1 year later. Therefore, the breeding in this resident population would be seasonal, as it is in migratory baleen whales, but occurring during the summer/autumn, which is the least productive season in the Gulf of California. Our study represents an important input to assist in future management policies of this protected population.
Keywords: Blubber, fin whale, progesterone, seasonal reproduction, testosterone
Fin whales in the Gulf of California constitute a resident population. To investigate its reproductive cycle, we measured the sex steroid hormones in their blubber. The temporal trend of testosterone, patterns in progesterone values, and courtship events suggest a seasonal cycle in which reproduction occurs during the least productive season.
Introduction
Understanding the reproductive behavior of protected species is important for the development of effective conservation policies and management tools. It is especially crucial for small populations occupying restricted areas, such as islands, mountain lakes, or semi-enclosed seas, which make them more vulnerable to environmental changes and anthropogenic disturbances (Willi et al., 2006). Although most baleen whales are known to regulate their breeding and feeding according to seasonal migrations between low and high latitude grounds (Mackintosh, 1965; Corkeron and Connor, 1999), the fin whale (Balaenoptera physalus) does not conform to this general pattern everywhere. The year-round occurrence of groups of this species, especially in mid-latitudes, has been reported (Silva et al., 2013; Scales et al., 2017), and the existence of small non-migratory populations is widely accepted (Fujino, 1960; Bérubé et al., 1998).
Genetic evidence shows that the fin whale population in the Gulf of California (Fig. 1) is unique and reproductively isolated (Bérubé et al., 2002). A recent integrated study based on aerial surveys, genetic markers, and photographic capture/recapture produced a population abundance estimate of around 300 individuals [95% credible interval (CI) = 150–420] (Pardo et al., 2016). Currently, there is no evidence that these animals move outside the Gulf of California, due to which the population is considered non-migratory. This resident strategy may suggest that the gulf fulfills the population’s requirements year-round, despite a strong monsoonal regime (Adams and Comrie, 2003) that favors high biological production during winter/spring, but more oligotrophic conditions during summer/autumn (Álvarez-Borrego and Lara-Lara, 1991). Although the habitat used by fin whales in the Gulf of California is not well known, it is possible that they aggregate during summer in some areas that remain productive due to upwelling or mixing, triggered by strong tidal regimes or other specific morphological features, capable of sustaining a rich macrofaunal community throughout the year (Brusca et al., 2005).
Figure 1.
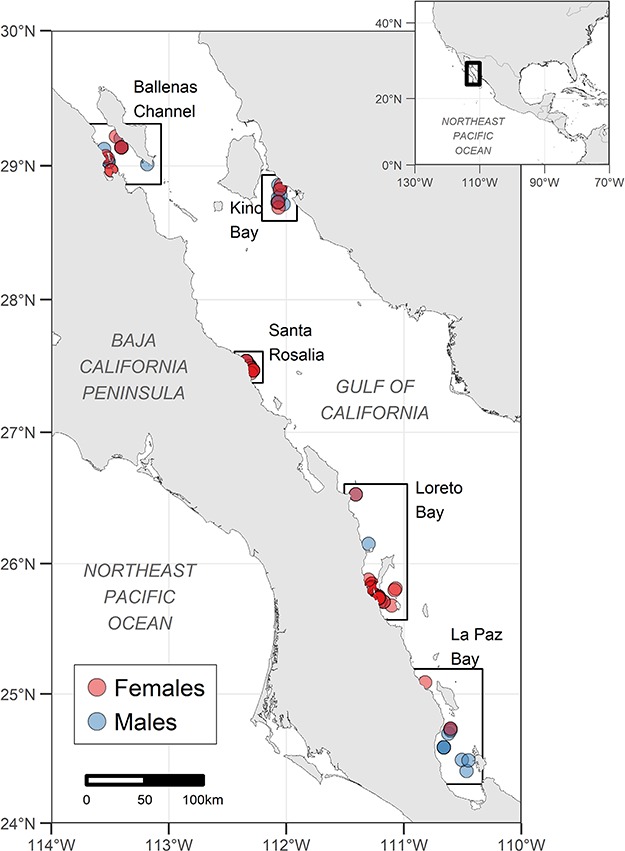
The Gulf of California in the Northeast Pacific Ocean. Colored dots show the geographic distribution of blubber biopsy samples from female (red) and male (blue) fin whales, used in this study. Each squared polygon was labeled according to the closest, most recognized geographical feature.
In non-migratory mysticete populations, the primary driving forces on the reproductive strategies are still uncertain. In the Mediterranean Sea, the occurrence of fin whale newborns year-round, with a peak between September and January, suggests a breeding season not well defined for that resident population (Notarbartolo di Sciara et al., 2003). Similarly, year-round calving and ovulation frequency on the inshore Bryde’s whales (Balaenoptera edeni) off South Africa proved that this resident population is an aseasonal breeder (Best, 2001). In contrast, the resident humpback whale (Megaptera novaeangliae) population in the Arabian Sea showed seasonal reproductive habits lasting from January to May (Mikhalev, 1997).
Given the strong restraints of cetacean studies in acquiring the basic knowledge of the physiological mechanisms driving reproduction, most of the current information comes from anatomical examination of gonads in commercially harvested, stranded, or by-catch animals (Lockyer, 1984), as well as from experiments in captivity (Daoquan et al., 2006; Bergfelt et al., 2011). In free-ranging cetaceans, most of the information about the life history parameters has been acquired through data collected from photo-identified animals (Agler et al., 1993). Nevertheless, this fieldwork approach in areas not easily accessible may result in fragmentary information on individual sighting histories and their reproductive state. In this context, the measurement of hormones has become an important complementary tool for generating progressive and significant contributions to knowledge on baleen whale physiology (Hunt et al., 2013).
There is strong evidence that mating behavior is regulated by changes in sex steroid hormone concentrations (Nelson et al., 1990). Within these, progesterone and testosterone have been analyzed in different wildlife species (Schwarzenberger, 2007). Progesterone is mainly produced during the luteal phase of the estrous cycle and during pregnancy, in order to maintain quiescence of the uterus until the fetus is mature (Graham and Clarke, 1997), whereas testosterone concentrations respond to various factors, such as sexual maturation, competition, female estrous, and stress (Dixson and Anderson, 2004; Hansen, 2009). In cetaceans, steroid hormone concentrations have been quantified in serum, urine, saliva, milk, feces, ocular secretion, baleen, earplug, muscle, and blow (Yoshioka et al., 1994; Amaral, 2010; Trumble et al., 2013; Hunt et al., 2017). Due to their lipophilic character (Prins et al., 1996), these hormones have been detected also in blubber of several odontocetes and in humpback, bowhead (Balaena mysticetus), and minke whales (Balaenoptera acutorostrata) (Mansour et al., 2002; Amaral, 2010; Kellar et al., 2014; Cates et al., 2019). The time lag between the production of sex steroids and their deposition in blubber and whether this tissue is representative of the current reproductive status of individuals are still uncertain. In the bowhead whale, there is a strong positive relationship between serum and blubber progesterone levels only when pregnant females are included (Kellar et al., 2014). This means that even if blubber does not represent a target tissue or a vehicle of progesterone (Graham and Clarke, 1997), the magnitude and duration of its production during gestation are such that it is possible to detect its changes in blubber. Similarly, testosterone concentration in male blubber has produced useful data on seasonal reproductive patterns in humpback whales (Vu et al., 2015) and short-beaked common dolphins (Delphinus delphis) (Kellar et al., 2009).
Earlier studies of captured fin whales from the Atlantic and Pacific migratory populations showed that both females and males reach sexual maturity between 3 and 15 years old (Rice, 1963; Lockyer, 1972; Aguilar et al., 1988). The mating season duration varies among populations, and conception probably occurs during winter (Mizroch et al., 1984). Usually, females give birth to a single calf after a gestation period of about 11 months (Lockyer, 1984) and nurse them for 7–11 months before weaning (Mizroch et al., 1984). Sex steroid hormones have been studied in the serum of fin whales caught in the North Atlantic during the summers of 1981–89 (Kjeld et al., 1992). Based on concentrations found, it was possible to determine progesterone concentrations in pregnant females, as well as a temporal change in testosterone in males.
Although fin whales occur throughout the entire Gulf of California, there are some important gathering regions that have been identified (Pardo et al., 2015; Jiménez López et al., 2019) such as the Ballenas Channel, Kino Bay, Santa Rosalia, Loreto Bay, and La Paz Bay (Fig. 1). Nevertheless, the seasonality of such aggregations, as well as the reproductive behavior of the population, remains poorly understood. During 3 years of monthly surveys (1983–86) in the Ballenas Channel (Fig. 1), only 1% of sightings included mother/calf pairs (Tershy et al., 1990). Calves can be seen year-round throughout the Gulf, similar to what has been observed for the resident population of the Mediterranean Sea (Notarbartolo di Sciara et al., 2003). Despite that, the lack of information about the body length of calves observed has prevented the verification of seasonality in calving or mating events within the Gulf of California.
Given the strong seasonality of the oceanographic environment in the Gulf of California (Álvarez-Borrego and Lara-Lara, 1991), and considering the high energetic cost of reproduction for baleen whales (Lockyer and Brown, 1981), we hypothesized that this resident fin whale population has a seasonal reproductive cycle, which would be detectable from sexual hormone concentrations in blubber biopsies. Our objectives were as follows: (i) to quantify testosterone and progesterone concentrations in the blubber of fin whale individuals and relate them to their sighting histories, and (ii) to verify the existence of seasonal variations in the species’ testosterone concentrations. This is the first study based on reproductive hormones of free-ranging fin whales and constitutes a relevant advance in understanding this population’s dynamics in the Gulf of California.
Materials and methods
Sample collection
Samples and sighting data were collected under permits issued by “Secretaría de Medio Ambiente y Recursos Naturales” (SGPA/DGVS 08021/06, 00506/08, 09760/08, 01110/15, 00255/16, and 00987/17, issued to D. Gendron, and SGPA/DGVS//036624/17 and 002162/18, issued to M.A. Pardo; CITES export permits: MX 88860) and complied with the criteria of the Institutional Animal Care and Use Committee of the University of Alaska. We collected 84 skin/blubber biopsies from 34 females (four with more than one sample) and 44 males (one with two samples) of fin whales, spanning 2007–09 (n = 33) and 2015–17 (n = 51) in the following five main aggregation regions in the Gulf of California: La Paz Bay, Loreto Bay, Santa Rosalia, Kino Bay, and Ballenas Channel (Fig. 1). All biopsies were collected in accordance with the standard protocol (Costa-Urrutia et al., 2013), using modified crossbow arrows, shot into the animal’s mid-lateral region, and stored in liquid nitrogen before being processed.
Whale identification
The sex of each sampled animal was determined using skin (Bérubé and Palsbøll, 1996). Fin whales were identified individually using photographs of the dorsal fin profile and presence of permanent scars (Agler et al., 1990). Adult females recorded with a calf were assumed to be lactating (n = 3), whereas the rest were considered as in an unknown reproductive stage. All males were considered as animals of unknown reproductive stage.
Hormone extraction and quantification
Hormone extractions from blubber were carried out using the methods described by Mansour et al. (2002) with modifications. Approximately 0.06–0.15 g of blubber sample was crushed manually with 500 μL of ethanol. After centrifugation (2500 rpm for 15 min; Beckman Coulter GS-6R Centrifuge), the supernatant was collected, and the pellet was re-suspended in 500 μL of ethanol. Once dried, 2 mL of a 4:1 mixture of acetone and ethanol were added to the samples. Then, samples were mixed with a vortex (1 min; VWR Vortex-Genie 2), centrifuged (2500 rpm for 15 min), and evaporated. The same procedure was done first with 1 mL of ether and then with 2 mL of a 1:1 mixture of hexane and acetonitrile, twice. The final extracts were then frozen at −20°C until analyzed. Prior to the final analyses, the samples were re-dissolved in methanol. Progesterone for females and testosterone for males were quantified using commercially available enzyme immunoassay kits from Arbor Assays (catalogue #K025-H1; #K032-H1). Manufacturer cross-reactivity of progesterone assay with other steroids was as follow: 172% 3ß-hydroxy-progesterone, 188% 3a-hydroxy-progesterone, 2.7% 11ß-hydroxy-progesterone, 147% 11a-hydroxy-progesterone, 7.0% 5a-dihydroprogesterone, 5.9% pregnenolone, and <0.1% corticosterone and androstenedione. As for testosterone assay, the cross-reactivity was 100% with testosterone and 56.8% 5a-dihydrotestosterone, 0.27% androstendione, 0.04% androsterone and Dehydroepiandrosterone (DHEA), 0.03% cholesterol, 0.02% 17ß-estradiol, and <0.02% progesterone, pregnenolone, hydrocortisone, cholic acid, and cholic derivatives. All samples were processed and quantified in duplicate and were rerun if the coefficient of variation (CV) was higher than 10%. The maximum assay range allowed was between 50 and 3200 pg mL−1. If the concentration exceeded this range, the sample was rerun at varying dilutions. To validate the assays, we employed parallelism and accuracy tests in a pooled sample (derived by combining equal volumes of all rinse samples), done by randomly combining 20 samples for testosterone and 18 for progesterone. Parallelism test was based on the simultaneous run of pooled samples with the standard curve and the determination of parallelism between these two curves. The accuracy test determined the level at which the measured hormone concentration matched the concentration of the standard hormone added to the sample pool. This test allows the detection of the potential interference from other metabolites in the samples and is expressed as a linear regression of the measured hormone as a function to the standard. A slope much higher or much lower than one represents an over- or under-estimation of the hormone, respectively.
Data analysis
The first step was to estimate the probability distributions of both hormones to obtain their ranges and central tendencies. A preliminary diagnosis of the progesterone’s frequency distribution (see Supplementary Text 1) showed a possible bimodal pattern in the logarithmic scale, with frequent extreme high values. Therefore, we performed a Bayesian mixture model of normal distributions on the progesterone observations (Marin et al., 2005; Dahl, 2006), which allowed us to evaluate if there was evidence for such bimodal structure and to estimate the probability that the progesterone values come from each of the clusters detected by the model. For the testosterone, whose frequency distribution did not show apparent bimodal structure (see Supplementary Text 1), we made a simple Bayesian estimation of the mean (Gelman et al., 2013). Both models were based on logarithmic likelihoods of the hormone concentrations.
In order to address the potential seasonality of fin whale reproductive cycle in the Gulf of California, we stated the testosterone concentrations as a sinusoidal function of the day of the year, whose parameter probabilities were estimated through a Bayesian regression analysis (Gelman et al., 2013). A log-normal likelihood was stated for the testosterone observations, with uninformative priors. Given the limited amount of observations throughout the year cycle, we decided to complete the yearly series by replicating the observations made during winter as the first part of a hypothetical second year. This was based on the idea that it is not possible that very high values of testosterone maintain indefinitely and, because the predominantly low concentrations observed during the first part of the year suggest that it was a transition period.
All Bayesian analyses were performed within the Just Another Gibbs Sampler (JAGS) language (Plummer, 2003) to sample from the posterior distributions of the stochastic parameters through a Markov Chain Monte Carlo procedure. We ran 1 million iterations in three independent chains, keeping one of every 20 iterations to avoid autocorrelation of chain states, and discarding an adaptation (i.e. burn-in) phase of the first 10% of iterations per chain. The final sample size for each posterior distribution was of 135 000 iterations. All data processing, analyses, and graphical representations were performed in R (R Development Core Team, 2019). The algebraic details of the models and the JAGS codes are provided in Supplementary Text 1.
Results
Assay validation
Progesterone and testosterone assays were successfully validated. Serial dilutions of pooled extracts showed parallelism with the standard curves of progesterone and testosterone (see Supplementary Fig. 1). The slopes of the regressions between added and measured masses were close to a hypothetical 1:1 ratio, which suggests that the method was highly accurate in measuring the hormone concentrations from the samples. The slope was 0.83 for progesterone (95% CI = 0.78–0.87) and 0.83 for testosterone (95% CI = 0.76–0.9). Additionally, the high Bayesian R2 (BR2) suggests strong linearity between control and blubber extracts, which indicates that the assay was measuring primarily the same antigen ins both groups. The BR2 reached 0.998 in progesterone (95% CI = 0.994–0.999) and 0.997 in testosterone (95% CI = 0.985–0.997) (see Supplementary Fig. 2). The recovery efficiency was 111% (CV = 21.73%) for progesterone and 91.71% (CV = 23.91%) for testosterone.
Progesterone and testosterone concentrations
The results of the mixture model of normal distributions strongly supported a bimodal structure in the progesterone concentrations. The first normal distribution of the mixture corresponded to the lowest values, with a median of 1.56 ng g−1 (95% CI = 0.97–2.76), whereas the distribution of the higher values had a median of 27.12 ng g−1 (95% CI = 8.55–64.78) (Table 1), with a slightly broader posterior probability (Fig. 2). The overlap between the curves was barely noticeable, limited to the tails of the distributions, and beyond the extreme limits of the 95% credible intervals of both clusters (Fig. 2). This separation was confirmed by the probabilities of cluster source for the range of the data (Fig. 2), which showed that the overlap only occurs at intermediate values, with probabilities less than 0.1. At that point, the probability that lower values come from cluster B is virtually 0 and vice versa. The results show that 61% (95% CI = 34–82) of the females were likely to come from cluster A (lower values) and 39% (95% CI = 18–66) from cluster B (higher values) (Fig. 2; see Supplementary Text 1). The testosterone exhibited a median of 0.88 ng g−1 (95% CI = 0.59–1.32) (Table 1; see Supplementary Table 1).
Table 1.
Posterior distribution summary of progesterone and testosterone means (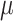 , ng g−1) in blubber of fin whales from the Gulf of California.
, ng g−1) in blubber of fin whales from the Gulf of California.
| Mean | SD | 2.5% | 25.0% | 50.0% | 75.0% | 97.5% |
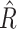
|
N eff (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone | ||||||||||
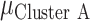
|
1.58 | 1.30 | 0.97 | 1.33 | 1.56 | 1.84 | 2.76 | 1.00 | 92.86 | |
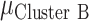
|
26.05 | 1.66 | 8.55 | 19.43 | 27.12 | 36.24 | 64.78 | 1.00 | 100 | |
| Testosterone | ||||||||||
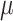
|
0.88 | 1.23 | 0.59 | 0.77 | 0.88 | 1.01 | 1.32 | 1.00 | 65 | |
A Gelman–Rubin convergence statistic (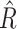 ) close to one indicates good convergence of chains. The Neff is the effective sample size as the percentage of the total iterations retained from all chains
) close to one indicates good convergence of chains. The Neff is the effective sample size as the percentage of the total iterations retained from all chains
Figure 2.
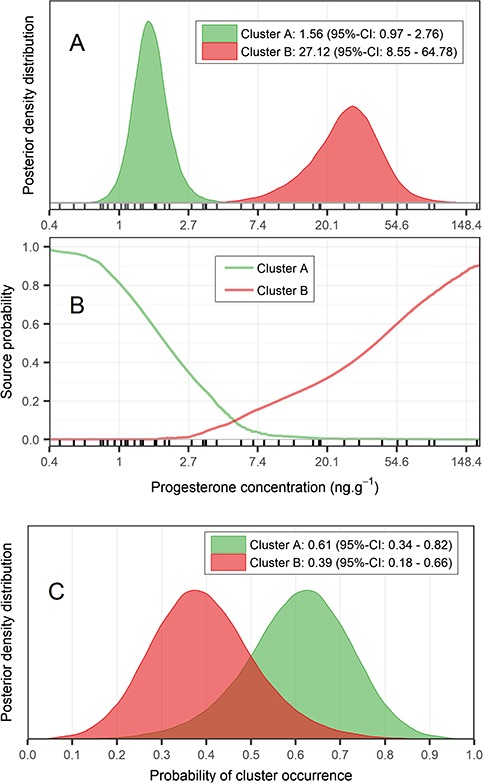
Panel A. Posterior probability distributions of progesterone concentrations (logarithmic scale) for the two clusters estimated by the Bayesian mixture model. Panel B. Posterior probability that any value within the observed range of concentrations comes from either Cluster A or Cluster B. Panel C. Posterior probability of cluster occurrence for each cluster.
Reproductive seasonality
The seasonal model of testosterone concentrations showed a well-defined predicted high peak during late summer (August) and lower concentrations during late winter (February/March) (Fig. 3, panel A). The probability of positive amplitude in the sinusoidal curve was 100%, indicating high certainty on the curve trend. The variability explained by the model was relatively low (BR2 = 0.29; 95% CI = 0.13–0.42), but expected, since our model could not take into account inter-individual variability, and there was lack of observations during parts of the cycle. The information associated with the sightings of biopsied fin whales allowed us to detect some cases that suggested a seasonal pattern in the reproductive cycle of the population and confirmed the results of the seasonal model of testosterone concentrations. The female C003 was observed during summer (August 2015) in the Ballenas Channel (Fig. 1) with the highest progesterone concentration of all females biopsied (173.36 ng g−1). The same female was observed twice the next summer (July 2016) in Loreto Bay accompanied by a calf, presumably lactating, with a low progesterone concentration in blubber (3.51 ng g−1). Another female (C021) was observed during summer (August 2015) in the Ballenas Channel with a calf (a biopsy could not be collected). Almost 6 months later, during winter (February 2016), it was re-sighted and biopsied in Kino Bay without the calf, exhibiting a low progesterone concentration (0.76 ng g−1), which suggests that weaning probably occurred before winter. Finally, in the Ballenas Channel, our team witnessed two courtship events during summer, never described before for this species. On 12 August 2015, an individual was followed by three other fin whales at an anomalous highly sustained speed. The individuals of the group were seen at least twice side-lunging and obstructing each other’s path. Fortunately, we were able to collect biopsies from the four animals, and genetic analysis confirmed that the leading animal was a female exhibiting a medium progesterone concentration of 18.26 ng g−1 (cluster B; Fig. 2), whereas the other three animals were males with high testosterone concentrations (7.86, 6.32, and 3.58 ng g−1), above the highest limit of the 95% CI of the testosterone posterior probability. Finally, on 17 September 2018, we observed another courtship event between one female and two males (sex genetically confirmed), in the same area. As with the previous event, males were chasing the female, breaching alternately, and obstructing each other’s path (Fig. 4a–c; see Supplementary Video 1). On numerous occasions, both males turned on their longitudinal axes while surfacing, showing one of their flippers and half of the fluke (Fig. 4d and e).
Figure 3.
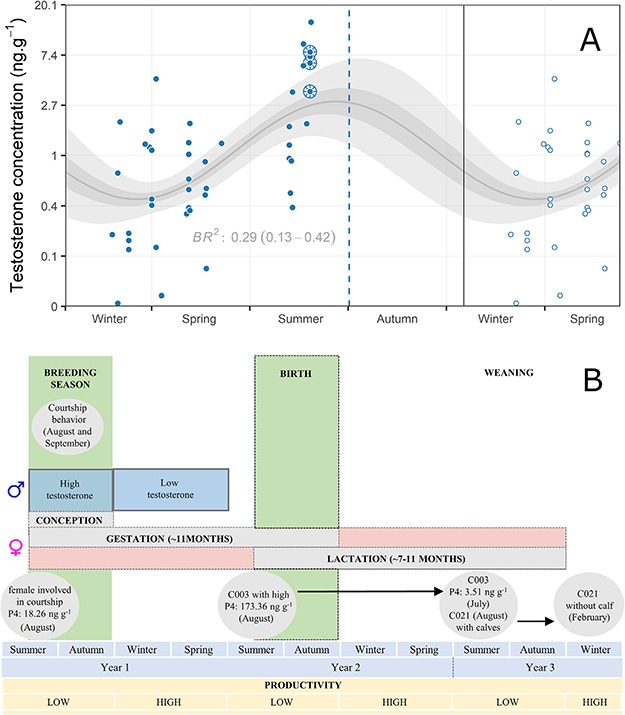
Panel A. Mean seasonal trend (gray sinusoidal curve) of testosterone concentrations in fin whale blubber biopsies in the Gulf of California, spanning 2007–09 and 2015–17. Shaded areas represent the 75% (dark) and 95% (light) credible intervals of the prediction. The mean BR2 with its 95% credible interval are shown. The Y-axis is in logarithmic scale. Color-filled dots represent the original observations and the empty dots represent the repetitions to complete the yearly series (see Materials and Methods). Observations surrounded by sliced circles represent animals involved in the first courtship event (2015). The vertical blue dashed line shows the day of the second courtship (2018; one female followed by two competing males, of unknown hormone concentrations). Panel B. Schematic reproductive cycle of the resident fin whale population of the Gulf of California, based on the seasonal hormone analysis of this study, the sighting histories of pregnant, lactating females and the female involved in the courtship event [with concentration of progesterone (P4)], weaning, and courtship events observed (gray circles). Black dashed lines represent a period of uncertainty during which each event is proposed.
Figure 4.
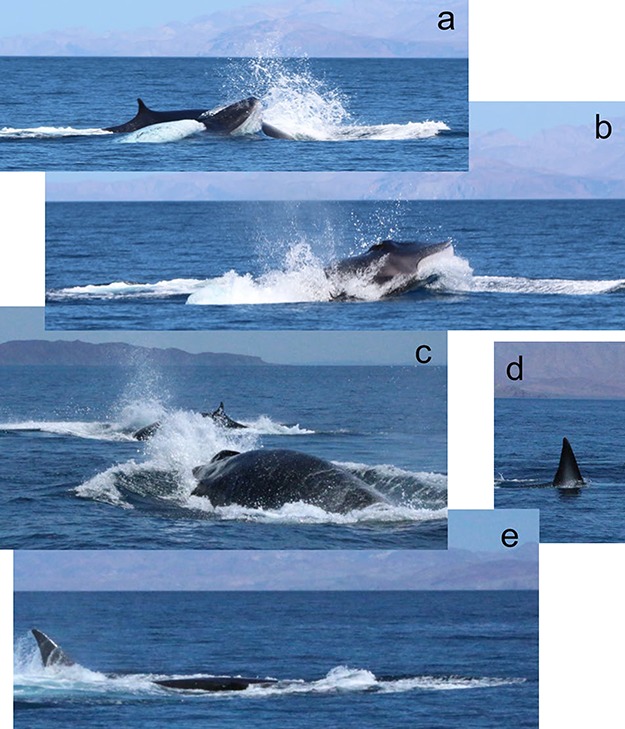
Two fin whale males during a presumed courtship display for a female (not shown) that always leaded their main path. They made fast and strong breaching displays very close to each other (a). Many times, the entire head was visible above the surface (b). They often irrupted each other’s path (c), and rolled on their longitudinal axes, showing half of the fluke (d and e) and a flipper out of the water. Photographs by G. Busquets-Vass (all rights granted).
Discussion
To our knowledge, this is the first study quantifying progesterone and testosterone in free-ranging fin whales. The use of blubber, through non-lethal sampling techniques, allows the assessment of reproductive status at the population level, rather than only of hunted or sick individuals. Nevertheless, steroid hormones pathway in blubber remains unexplored. Due to their lipophilic properties, these hormones enter from capillaries into the fat cells via diffusion (Deslypere et al., 1985), but there is no information about their accumulation or enzyme action on them once in the blubber. Neither testosterone (Kellar et al., 2009) nor progesterone (Kellar et al., 2006) are significantly different through the blubber in several species of delphinids, but in beluga whales (Delphinapterus leucas), cortisol concentration increased with blubber depth, with highest concentrations in the inner layer (Trana et al., 2015). Despite the valuable information provided by these studies, they were carried out on odontocetes and there is still a lack of knowledge about mysticetes. Since blubber is a complex matrix characterized by different metabolic activity along its thickness, fatty acids, contaminant concentrations, and adipocyte size have been found to vary (Ackman et al., 1975; Iverson, 2009; Waugh et al., 2013). Thus, due to the greater blubber thickness of large whales, a different concentration of steroid hormones through the tissue can be expected. For this reason, to obtain comparable results in steroid analyses, it may be preferable to use the same biopsy subsample layer. Furthermore, as a result of these differences among species and the lack of knowledge of some physiological mechanism, it is crucial to validate the technique for measuring any hormone for each new species considered. Although knowledge of fin whale physiology is still limited, our validation was successfully carried out (see Supplementary Fig. 1; Fig. 2), and its results strongly support the use of blubber biopsies of fin whales to investigate reproductive aspects of the population and to detect long-term changes.
The lack of long sighting histories for most of the individuals sampled prevented us from determining hormone concentrations according to age classes. The highest progesterone concentration observed in this study (in summer) belonged to a female for which pregnancy was confirmed by its re-sighting a year later accompanied with a calf. This constitutes the first quantification of blubber progesterone in a pregnant fin whale. The wide range of progesterone values observed in this study (Fig. 2; see Supplementary Table 1) is in agreement with the high variability found in North Atlantic fin whale serum (Kjeld et al., 1992) and in blubber of other cetaceans (Mansour et al., 2002; Pérez et al., 2011).
The presence of a group of females with high progesterone concentrations (cluster B, Fig. 2) may be explained by the luteal phase of the ovarian cycle or by the gestation. In this sense, the progesterone concentration of the female involved in the first courtship (2015) would reinforce the existence of this sexually active group of females (cluster B) in our mixture model. The intermedium progesterone concentration, close to the lower limit of the cluster B, could indicate that this female was in estrous (ovulating period) or recently conceived from a previous mating event during the same season. Nevertheless, it is still unknown which phase of the ovarian cycle may be detected through progesterone in large whales. Kjeld (1992), based on progesterone values in serum, identified the following three groups of females in the North Atlantic fin whale: (i) with low progesterone values (young sexually immature females); (ii) with intermediate values; and (iii) with high values (pregnant females). Due to the similar length and age with the first group, the author suggests that the second group represents mainly females that have recently maturated and in which an ovulation or a recent conception cannot be discarded. By contrast, blubber is considered a matrix that represents only long-term changes (Kellar et al., 2014). Thus, considering that progesterone levels fall after ovulation if conception does not occur, it is likely that blubber reflects only pregnancy, which would explain the clearly distinction of two groups in our study, in accordance with previous studies on the same tissue (Mansour et al., 2002; Kellar et al., 2006) and in discordance with the serum results of Kjeld (1992).
The wide range of values in cluster B also may be linked to a high variability in the timing of conception during the breeding season, or simply to its lower sample size. In various mammals, progesterone serum levels gradually rise through the gestation (Bedford et al., 1972; Boyd et al., 1993). Nevertheless, the progesterone trend and whether its concentration in blubber reflects different gestation states are still unclear in cetaceans. Bottlenose dolphins (Tursiops truncatus) and killer whales (Orcinus orca) showed higher progesterone concentrations in serum during early and mid-pregnancy compared to late pregnancy (Katsumata et al., 2006; Bergfelt et al., 2011). In blubber of three species of dolphins, no difference was found in progesterone concentrations with respect to fetal length (Kellar et al., 2006). In mysticetes, however, blubber is highly variable in thickness and composition, and no studies have investigated progesterone throughout the entire gestational period.
Even if the physiological processes that drive progesterone production in cetaceans are not clear, we hypothesized, based on studies in captive odontocetes (Bergfelt et al., 2011), a rapid decrease of the hormone immediately before and during parturition. This was confirmed by the low progesterone concentrations found in lactating females in this study. Furthermore, since fin whales show long calving intervals of at least 2 years (Mizroch et al., 1984), we assumed that progesterone production, in resting females, was similar to that of lactating females, according to the pattern reported previously in blue whale (Balaenoptera musculus) feces (Valenzuela et al., 2018) and blubber (Atkinson et al., 2019). These animals would be represented by the cluster A (Fig. 2) identified in this study.
The high testosterone concentrations we observed in late summer coincide with the pattern found in the serum of North Atlantic fin whales, whose testosterone increase was more than 4-fold during late summer (August–September) compared to the concentrations of the early summer (June) (Kjeld et al., 1992). Nevertheless, that migratory population showed this rise in the summer feeding grounds, several months before the reproductive activity that takes place in winter (December–January). According to several studies, this increment in testosterone observed in migratory individuals, while they are still in their feeding areas at high latitudes, could suggest a physiological preparation before reaching the reproductive areas (Kjeld et al., 2004; Vu et al., 2015). In our study, little data prior to summer (May–June) prevented the detection of such a preparation stage. Nevertheless, the observation of two courtship displays in August and September may discard the scenario of a long preparatory phase in the males of the Gulf of California population and rather suggests the start of the mating season during the late summer. Since the mating season generally occurs over several months (Evans, 1987), it is possible that testosterone concentrations keep increasing during the autumn. Due to the lack of samples in autumn, and the unknown time lag in detection of these hormones in the blubber, we could not determine the precise range of the breeding season. Nevertheless, low values of testosterone in winter (Fig. 3, panel A) suggest the termination of mating occurs before this season.
Based on the evidence found in our study and what has been described about the gestation and lactation durations in other fin whale populations (Lockyer, 1984; Mizroch et al., 1984), we propose a hypothetical reproductive cycle for the resident fin whale population in the Gulf of California (Fig. 3, panel B). Mating activity would occur during the late summer/autumn, part of gestation and lactation would take place around winter/spring, and weaning would follow during summer/autumn, depending on when conception occurs.
In marine and terrestrial mammals, a seasonal reproductive cycle implies a limited period for giving birth, usually as an adaptation to changes in the environment (McGuire et al., 2010). In migratory whale species, the calving season seems to be driven partially by the necessity to avoid thermal stress for the newborns, giving birth in warm, low-latitude waters (Gaskin, 1982). Comparing to high latitudes, the sea surface temperature in the Gulf of California in winter is relatively warm (20.37 ± 1.92) (Escalante et al., 2013). In fact, during this season, the Gulf and surroundings are used as an important calving area by several migratory whale species, such as the gray whale (Rice et al., 1982), the humpback whale (Urbán and Aguayo, 1987), and the blue whale (Gendron and Ugalde De La Cruz, 2012). This led us to discard temperature stress as a driving factor of the seasonal reproductive cycle for the fin whale in the Gulf of California.
Seasonal reproductive strategies have also been observed among animals that live in more stable temperature conditions (Clutton-Brock and Harvey, 1978). In these cases, food availability may be an important limiting factor for any biological activity related to reproduction. As reported for the resident fin whales of the Mediterranean Sea (Relini et al., 1992; Canese et al., 2006), those in the Gulf of California are observed feeding all year (Ladrón de Guevara et al., 2008). However, their main prey in the gulf is the euphausiid Nyctiphanes simplex (Tershy, 1992), which is most abundant in winter/spring and decreases in summer (Brinton and Townsend, 1980), when they shift their diet to small pelagic fish (Tershy, 1992; Gendron et al., 2001). Thus, although this population does not fast as other migratory populations do, resources may not be enough year-round (in terms of prey abundance or nutritional quality) to support the strong energetic demands of pregnant and lactating females, for which the energetic cost can double that of the gestation and fetal development period (Lockyer, 1981). In most migratory populations of mysticetes, females fast or feed infrequently during lactation, often showing poor body condition (Aguilar and Borrell, 1990), and relying on the energy reserves stored during the feeding season. Nevertheless, in the Gulf of California, most females with calves appear in good body condition (D. Gendron, unpublished data), suggesting that they do not fast during lactation. Based on our results, it is likely that early- or mid-lactation, when calves are completely dependent on the mother’s milk (Oftedal, 1997), would start slightly before the most productive period of the year, that is the winter/spring (Álvarez-Borrego and Lara-Lara, 1991). Thus, the lactation period for the female C003 probably began in autumn, and therefore the weaning would have taken place during the following summer/autumn after we observed her with a calf. This is supported by the sighting of the female C021 with her calf in late summer (August), and its posterior re-sighting 6 months later during winter (February) without the calf (Fig. 3, panel B). This is analogous to the pattern of migratory rorquals, whose calves are weaned just before or immediately after the female’s arrival to the feeding grounds (Oftedal, 1997). All these observations support the hypothesis that prey availability or quality could be crucial in the regulation of the reproductive cycle of the resident fin whale population of the Gulf of California.
Reproduction responds to a complex interaction between hypothalamic–pituitary–gonad axis and environmental factors (Nelson et al., 1990). A short reproductive period in a restricted area makes this population more vulnerable to environmental changes and anthropogenic disturbances. In fact, changes in survival rate of the calves (Lockyer, 1984), prey availability (Lockyer, 1986), or fertility (Hohn et al., 2007) (e.g. for exposure to contaminants) may negatively affect the reproductive rate of a population and more so if it does not breed continually during the year. This also highlights the importance of specific measures that should be taken into account in the conservation plan of this population.
Conclusions
Progesterone and testosterone assays were validated for the first time in free-ranging fin whales. Our study provides preliminary baseline parameters for reproductive hormones in fin whale blubber that, in the future, will allow the monitoring of their variations. In particular, the separation of two clusters in progesterone concentration lays the basis for the future determination of different reproductive categories of females. Furthermore, contrary to what has been observed in other resident mysticete populations, here we report a seasonal reproductive cycle for the fin whale population of the Gulf of California, which provides a useful tool for estimating other reproductive parameters for its management. In order to get a more precise insight into its reproductive dynamics, future studies need to incorporate photogrammetry analyses to account for different stages of individual development at varying hormone concentrations, to increase the time series monitoring to detect more details of the seasonal cycle, and to estimate possible inter-annual anomalies from it.
Supplementary Material
Acknowledgements
We sincerely thank the students and volunteers of the Cetacean Ecology Laboratory at CICIMAR-IPN and the Marine Macroecology Laboratory at CICESE, who were involved in fieldwork sample collection, and processing. We are grateful to G. Busquets-Vass for providing copyright of courtship photos (Fig. 4). We would also like to thank F. Favoretto, J. Gómez-Gutiérrez, C.J. Hernández-Camacho, and J. Del-Angel for their valuable comments on the manuscript.
E.C. and D.G. conceived the study. E.C., D.G., M.A.P., and H.P.P. were involved in the sample collection. E.C., S.A., and K. M performed the hormone analysis and L.E.P. performed genetic analysis. M.A.P. carried out the statistical analyses. E.C., D.G., and M.A.P wrote the original draft. All authors reviewed the manuscript.
Funding
This work was supported by Instituto Politécnico Nacional (SIP 2007–09 and 2015–17; PI: D.G.), Comisión Nacional de Áreas Naturales Protegidas—Secretaría de Medio Ambiente y Recursos Naturales (PROCER/DGOR/06/2015, PROCER/CCER/DEPC/05/2016; PI: M.A.P.), and Fondo Mexicano para la Conservación de la Naturaleza (Project No. M.1701-021; PI: M.A.P.). E.C. was funded by CONACYT (Ph.D. Scholarship No.704818) and Instituto Politécnico Nacional (Scholarship “Programa Institucional de Formación de Investigadores”). Hormone analysis was partially funded by University of Alaska, Society for Marine Mammalogy (Small Grants in Aid of Research), Sociedad Mexicana de Mastozoología Marina, and Cetacean Society International.
Conflict of interest statement
The authors declare no competing financial and non-financial interests.
References
- Ackman RG, Hingley JH, Eaton CA, Sipos JC, Mitchell ED (1975) Blubber fat deposition in mysticeti whales. Can J Zool 53: 1332–1339. [DOI] [PubMed] [Google Scholar]
- Adams DK, Comrie AC (2003) The north American monsoon. Am Meteorol Soc 23: 1453–1464. [Google Scholar]
- Agler BA, Beard JA, Bowman RS, Corbett HD, Frohock SW, Hawvermale MP, Katona SK, Sadove SS, Seipt IE (1990) Fin whale (Balaenoptera physalus) photographic identification: methodology and preliminary results from the western North Atlantic. Reports Int Whal Comm 12: 349–356. [Google Scholar]
- Agler BA, Schooley RL, Frohock SE, Katona SK, Seipt IE (1993) Reproduction of photographically identified fin whales, Balaenoptera physalus, from the Gulf of Maine. J Mammal 74: 577–587. [Google Scholar]
- Aguilar A, Borrell A (1990) Patterns of lipid content and stratification in the blubber of fin whales (Balaenoptera physalus). J Mammal 71: 544–554. [Google Scholar]
- Aguilar A, Olmos M, Lockyer CH (1988) Sexual maturity in fin whales (Balaenoptera physalus) caught off Spain. Rep Int Whal Comm 38: 317–322. [Google Scholar]
- Álvarez-Borrego S, Lara-Lara JR (1991) The physical environment and primary productivity of the Gulf of California. Gulf Penins Prov Californias 47: 555–567. [Google Scholar]
- Amaral RS. (2010) Use of alternative matrices to monitor steroid hormones in aquatic mammals: a review. Aquat Mamm 36: 162–171. [Google Scholar]
- Atkinson S, Gendron D, Branch TA, Mashburn KL, Melica V, Enriquez-Paredes L, Brownell RL Jr (2019) Pregnancy rate and biomarker validation from the blubber of eastern North Pacific blue whales. Mar Mammal Sci 1–23. [Google Scholar]
- Bedford CA, Challis JR, Harrison FA, Heap RB (1972) The role of oestrogens and progesterone in the onset of parturition in various species. J Reprod Fertil Suppl 16: Suppl-16. [PubMed] [Google Scholar]
- Bergfelt DR, Steinetz BG, Lasano S, West KL, Campbell M, Adams GP (2011) Relaxin and progesterone during pregnancy and the post-partum period in association with live and stillborn calves in bottlenose dolphins (Tursiops truncatus). Gen Comp Endocrinol 170: 650–656. [DOI] [PubMed] [Google Scholar]
- Bérubé M, Aguilar A, Dendanto D, Larsen F, Notarbartolo Di Sciara G, Sears R, Sigurjónsson J, Urban RJ, Palsbøll PJ (1998) Population genetic structure of North Atlantic, Mediterranean Sea and Sea of Cortez fin whales, Balaenoptera physalus (Linnaeus 1758): analysis of mitochondrial and nuclear loci. Mol Ecol 7: 585–599. [DOI] [PubMed] [Google Scholar]
- Bérubé M, Palsbøll P (1996) Identification of sex in cetaceans by multiplexing with three ZFX and ZFY specific primers. Mol Ecol 5: 283–287. [DOI] [PubMed] [Google Scholar]
- Bérubé M, Urbán-Ramírez J, Dizon AE, Brownell RL, Palsbøll PJ (2002) Genetic identification of a small and highly isolated population of fin whales (Balaenoptera physalus) in the Sea of Cortez, México. Conserv Genet 3: 183–190. [Google Scholar]
- Best PB. (2001) Distribution and population separation of Bryde’s whale Balaenoptera edeni off southern Africa. Mar Ecol Prog Ser 220: 277–289. [Google Scholar]
- Boyd IL, Lockyer C, Marsh HD (1993) Reproduction in marine mammals In Reynolds JE, Rommel SA, eds, Biology of Marine Mammals. Smithson Inst Press, Washington, DC, pp. 218–286. [Google Scholar]
- Brinton E, Townsend AW (1980) Euphausiids in the Gulf of California—the 1957 cruises. Calif Coop Ocean Fish Investig Reports 21: 211–236. [Google Scholar]
- Brusca RC, Findley LT, Hastings PA, Hendrickx ME, Torre-Cosío J, Van Der Heiden AM (2005) Macrofaunal diversity in the Gulf of California. Biodiversity Ecosyst Conserv North Mex. [Google Scholar]
- Canese S, Cardinali A, Fortuna CM, Giusti M, Lauriano G, Salvati E, Greco S (2006) The first identified winter feeding ground of fin whales (Balaenoptera physalus) in the Mediterranean Sea. J Mar Biol Assoc United Kingdom 86: 903–907. [Google Scholar]
- Cates KA, Atkinson S, Gabriele CM, Pack AA, Straley JM, Yin S (2019) Testosterone trends within and across seasons in male humpback whales (Megaptera novaeangliae) from Hawaii and Alaska. Gen Comp Endocrinol. . doi: 10.1016/j.ygcen.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T, Harvey PH (1978) Mammals, resources and reproductive strategies. Nature 273: 191–195. [DOI] [PubMed] [Google Scholar]
- Corkeron PJ, Connor RC (1999) Why do baleen whales migrate? Mar Mammal Sci 15: 1228–1245. [Google Scholar]
- Costa-Urrutia P, Sanvito S, Victoria-Cota N, Enríquez-Paredes L, Gendron D (2013) Fine-scale population structure of blue whale wintering aggregations in the Gulf of California. PLoS One 8: e58315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl DB. (2006) Model-based clustering for expression data via a Dirichlet process mixture model. In Kim-Anh Do, Peter Müller, Marina Vannucci (Eds.), Bayesian Inference Gene Expression and Proteomics, Cambridge University Press, 4: 201–218. [Google Scholar]
- Daoquan C, Yujiang H, Qingzhong Z, Ding W (2006) Reproductive seasonality and maturity of male Neophocaena phocaenoides asiaeorientalis in captivity: a case study based on the hormone evidence. Mar Freshw Behav Physiol 39: 163–173. [Google Scholar]
- Deslypere JP, Verdonck L, Vermeulen A (1985) Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab 61: 564–570. [DOI] [PubMed] [Google Scholar]
- Dixson AF, Anderson MJ (2004) Sexual behavior, reproductive physiology and sperm competition in male mammals. Physiol Behav 83: 361–371. [DOI] [PubMed] [Google Scholar]
- Escalante F, Valdez-Holguín JE, Álvarez-Borrego S, Lara-Lara JR (2013) Variación temporal y espacial de temperatura superficial del mar, clorofila α y productividad primaria en el Golfo de California. Ciencias Mar 39: 203–215. [Google Scholar]
- Evans PGH. (1987) The Natural History of Whale and Dolphins. Christopher Helm, London. [Google Scholar]
- Fujino K. (1960) Immunogenetic and marking approaches to identifying subpopulations of the North Pacific whales. Sci Rep Whales Res Inst 15: 84–142. [Google Scholar]
- Gaskin DE. (1982) The Ecology of Whales and Dolphins. Ltd. Heinemann Educational Books, London, UK. [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB (2013) Bayesian Data Analysis. Chapman and Hall/CRC. [Google Scholar]
- Gendron D, Aguíñiga S, Carriquiry JD (2001) d 15 and d 13 C in skin biopsy samples: a note on their applicability for examining the relative trophic level in three rorqual species. J Cetacean Res Manag 3: 41–44. [Google Scholar]
- Gendron D, Ugalde De La Cruz A (2012) A new classification method to simplify blue whale photo-identification technique. J Cetacean Res Manag 12: 79–84. [Google Scholar]
- Graham DJ, Clarke CL (1997) Physiological action of progesterone in target tissues. Endocr Rev 18: 502–519. [DOI] [PubMed] [Google Scholar]
- Hansen PJ. (2009) Effects of heat stress on mammalian reproduction. Philos Trans R Soc B Biol Sci 364: 3341–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn A, Ewing R, Zaias J (2007) Reproduction in relation to conservation and commercial exploitation. Reprod Biol Phylogeny Cetacea 7: 371–389. [Google Scholar]
- Hunt KE, Lysiak NS, Robbins J, Moore MJ, Seton RE, Torres L, Buck CL (2017) Multiple steroid and thyroid hormones detected in baleen from eight whale species. Conserv Physiol 5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KE, et al. (2013) Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv Physiol 1: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson SJ. (2009) Blubber. In: Encyclopedia of Marine Mammals (Second Edition), William F. Perrin, Bernd Würsig and J.G.M. Thewissen (Eds.), Academic Press: 115–120. [Google Scholar]
- Jiménez López ME, Palacios DM, Jaramillo Legorreta A, Urbán JR, Mate BR (2019) Fin whale movements in the Gulf of California, Mexico, from satellite telemetry. PLoS One 14. doi: e0209324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata E, Jaroenporn S, Katsumata H, Konno S, Maeda Y, Watanabe G, Taya K (2006) Body temperature and circulating progesterone levels before and after parturition in killer whales (Orcinus orca). J Reprod Dev 52: 65–71. [DOI] [PubMed] [Google Scholar]
- Kellar NM, Keliher J, Trego ML, Catelani KN, Hanns C, George JCC, Rosa C (2014) Variation of bowhead whale progesterone concentrations across demographic groups and sample matrices. Endanger Species Res 22: 61–72. [Google Scholar]
- Kellar NM, Trego ML, Marks CI, Chivers SJ, Danil K, Archer FI (2009) Blubber testosterone: a potential marker of male reproductive status in short-beaked common dolphins. Mar Mammal Sci 25: 507–522. [Google Scholar]
- Kellar NM, Trego ML, Marks CI, Dizon AE (2006) Determining pregnancy from blubber in three species of delphinids. Mar Mammal Sci 22: 1–16. [Google Scholar]
- Kjeld JM, Alfredsson Á, Ólafsson Ö, Tryland M, Christensen I, Stuen S, Árnason A (2004) Changes in blood testosterone and progesterone concentrations of the North Atlantic minke whale (Balaenoptera acutorostrata) during the feeding season. Can J Fish Aquat Sci 61: 230–237. [Google Scholar]
- Kjeld JM, Sigurjonsson J, Arnason A (1992) Sex hormone concentrations in blood serum from the North Atlantic fin whale (Balaenoptera physalus). J Endocrinol 134: 405–413. [DOI] [PubMed] [Google Scholar]
- Ladrón de Guevara P, Lavaniegos BE, Heckel G (2008) Fin whales (Balaenoptera physalus) foraging on daytime surface swarms of the euphausiid Nyctiphanes simplex in Ballenas Channel, Gulf of California, Mexico. J Mammal 89: 559–566. [Google Scholar]
- Lockyer C. (1972) The age at sexual maturity of the southern fin whale (Balaenoptera physalus) using annual layer counts in the ear plug. ICES J Mar Sci 34: 276–294. [Google Scholar]
- Lockyer C. (1981) Growth and energy budgets of large baleen whales from the southern hemisphere. Food Agric Organ 3: 379–487. [Google Scholar]
- Lockyer C. (1984) Review of baleen whale (Mysticeti) reproduction and implications for management. Rep Int Whal Comm 6: 27–50. [Google Scholar]
- Lockyer C. (1986) Body fat condition in Northeast Atlantic fin whales, Balaenoptera physalus, and its relationship with reproduction and food resource. Can J Fish Aquat Sci 43: 142–147. [Google Scholar]
- Lockyer C, Brown SG (1981) The migration of whales In Animal Migration. Cambridge University Press, New York, NY, pp. 105–137. [Google Scholar]
- Mackintosh NA. (1965) The Stocks of Whales. Fishing News (Books). Ltd. London, London. [Google Scholar]
- Mansour AAH, Mkay DW, Lien J, Orr JC, Banoub JH, ØIen N, Stenson G (2002) Determination of pregnancy status from blubber samples in minke whales (Balaenoptera acutorostrata). Mar Mammal Sci 18: 112–120. [Google Scholar]
- Marin JM, Kerrie M, Christian PR (2005) Bayesian modelling and inference on mixtures of distributions. Handb Stat 25: 459–507. [Google Scholar]
- McGuire N, Calisi R, Benteley G, Breed M, Moore J (2010) Seasonality: hormones and behavior. Encycl Anim Behav 3: 108–118. [Google Scholar]
- Mikhalev YA. (1997) Humpback whales Megaptera novaeangliae in the Arabian Sea. Mar Ecol Prog Ser 149: 13–21. [Google Scholar]
- Mizroch SA, Rice DW, Breiwick JM (1984) The fin whale, Balaenoptera physalus. Mar Fish Rev 46: 20–24. [Google Scholar]
- Nelson RJ, Badura LL, Goldman BD (1990) Mechanisms of seasonal cycles of behavior. Annu Rev Psychol 41: 81–108. [DOI] [PubMed] [Google Scholar]
- Notarbartolo di Sciara G, Zanardelli M, Jahoda M, Panigada S, Airoldi S (2003) The fin whale Balaenoptera physalus (L. 1758) in the Mediterranean Sea. Mamm Rev 33: 105–150. [Google Scholar]
- Oftedal OT. (1997) Lactation in whales and dolphins: evidence of divergence between baleen- and toothed-species. J Mammary Gland Biol Neoplasia 2: 205–230. [DOI] [PubMed] [Google Scholar]
- Pardo MA, Gendron D, Urbán-Ramirez J, Enríquez-Paredes L, Pérez-Puig H, Rosales Nanduca H, Viloria Gómora L, Heckel G, Carone E, Jimenez E (2015) Evaluación de la abundancia, tendencia poblacional y distribución espacial del rorcual común en el Golfo de California. In Programa de Conservación de Especies En Riesgo (PROCER).
- Pardo MA, Gendron D, Urbán-Ramirez J, Enríquez-Paredes L, Pérez-Puig H, Rosales Nanduca H, Viloria Gómora L, Heckel G, Carone E, Jimenez E (2016) Determinación del estado y dinámica poblacional del rorcual común en el Golfo de California. In Programa de Conservación de Especies En Riesgo (PROCER).
- Pérez S, García-López Á, De Stephanis R, Giménez J, García-Tiscar S, Verborgh P, Mancera JM, Martínez-Rodriguez G (2011) Use of blubber levels of progesterone to determine pregnancy in free-ranging live cetaceans. Mar Biol 158: 1677–1680. [Google Scholar]
- Plummer M. (2003) A program for analysis of Bayesian graphical models using Gibbs sampling In Proceedings of the 3rd International Workshop on Distributed Statistical Computing, pp. 20–22.
- Prins JB, O’Rahilly S, Chatterjee VK (1996) Steroid hormones and adipose tissue. Eur J Clin Invest 26: 259–261. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2019) R: a language and environment for statistical computing. R Found Stat Comput Vienna, Austria. doi: 10.1007/978-3-540-74686-7. [DOI]
- Relini G, Relini LO, Cima C, Fasciana C, Fiorentino F, Palandri G, Relini M, Tartaglia MP, Zamboni G, Torchia A (1992) Macroplancton, Meganyctiphanes norvegica, and fin whales, Balaenoptera physalus along some transects in the Liguria Sea. Eur Res Cetaceans 6: 134–137. [Google Scholar]
- Rice DW. (1963) Progress report on biological studies of the larger Cetacea in the waters off California. Nor Hvalfangst-Tidende 52: 181–187. [Google Scholar]
- Rice DW, Wolman AA, Withrow DE (1982) Distribution and numbers of gray whales on their Baja California winter grounds. In Int. Whal. Commn. Sci. Comm. Doc., SC/34/PS12 pp 1–22.
- Scales KL, Schorr GS, Hazen EL, Bograd SJ, Miller PI, Andrews RD, Zerbini AN, Falcone EA (2017) Should I stay or should I go? Modelling year-round habitat suitability and drivers of residency for fin whales in the California Current. Divers Distrib 23: 1204–1215. [Google Scholar]
- Schwarzenberger F. (2007) The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species. Int Zoo Yearb 41: 52–74. [Google Scholar]
- Silva MA, Prieto R, Jonsen I, Baumgartner MF, Santos RS (2013) North Atlantic blue and fin whales suspend their spring migration to forage in middle latitudes: building up energy reserves for the journey? PLoS One 8. doi: 10.1371/journal.pone.0076507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tershy BR. (1992) Body size, diet, habitat use, and social behavior of Balaenoptera whales in the Gulf of California. J Mammal 73: 477–486. [Google Scholar]
- Tershy BR, Breese D, Strong CS (1990) Abundance, seasonal distribution and population composition of balaenopterid whales in the canal de Ballenas, gulf of California, Mexico. Rep Int Whal Comm Spec Issue 12: 369–375. [Google Scholar]
- Trana MR, Roth JD, Tomy GT, Anderson WG, Ferguson SH (2015) Influence of sample degradation and tissue depth on blubber cortisol in beluga whales. J Exp Mar Bio Ecol 462: 8–13. [Google Scholar]
- Trumble SJ, Robinson EM, Berman-Kowalewski M, Potter CW, Usenko S (2013) Blue whale earplug reveals lifetime contaminant exposure and hormone profiles. Proc Natl Acad Sci 110: 16922–16926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán J, Aguayo L (1987) Spatial and seasonal distribution of the humpback whale, Megaptera novaeangliae, in the Mexican Pacific. Mar Mammal Sci 3: 333–344. [Google Scholar]
- Valenzuela M, Atkinson S, Mashburn K, Gendron D (2018) Fecal steroid hormones reveal by reproductive state in female blue whales from the Gulf of California, Mexico. Gen Comp Endocrinol 261: 127–135. [DOI] [PubMed] [Google Scholar]
- Vu ET, Clark C, Catelani K, Kellar NM, Calambokidis J (2015) Seasonal blubber testosterone concentrations of male humpback whales (Megaptera novaeangliae). Mar Mammal Sci 31: 1258–1264. [Google Scholar]
- Waugh CA, Nichols PD, Schlabach M, Noad M, Nash SB (2013) Vertical distribution of lipids, fatty acids and organochlorine contaminants in the blubber of southern hemisphere humpback whales (Megaptera novaeangliae). Mar Environ Res 94: 24–31. [DOI] [PubMed] [Google Scholar]
- Willi Y, Van Buskirk J, Hoffmann AA (2006) Limits to the adaptive potential of small populations. Annu Rev Ecol Evol Syst 37: 433–458. [Google Scholar]
- Yoshioka M, Okumura T, Aida K, Fujise Y (1994) A proposed technique for quantifying muscle progesterone content in minke whales (Balaenoptera acutorostrata). Can J Zool 72: 368–370. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


