Abstract
Purpose:
Novel paradigms have broadened our understanding of mechanisms through which complement mediates allograft inflammation/injury. Herein we review advances in the field and highlight therapeutic implications.
Recent Findings:
Pre-clinical and translational human trials have elucidated complement-dependent mechanisms of post-transplant ischemia-reperfusion (I/R) injury. Immune cell-derived, and intracellular, complement activation are newly linked to proinflammatory T cell immunity relevant to allograft rejection. Complement-induced immune regulation, including C5a ligation of C5a receptor 2 on T cells, C5a/C5a receptor 1 interactions on regulatory myeloid cells, and C1q binding to CD8+ T cells can inhibit proinflammatory T cells and/or prolong murine allograft survival. Pilot trials of complement inhibition to treat/prevent human I/R- or antibody-initiated allograft injury show promise.
Summary:
The complement system participates in allograft injury through multiple context- dependent mechanisms involving various components and receptors. These new insights along with development and implementation of individualized complement inhibitory strategies have potential to improve transplant outcomes.
Keywords: Complement, T cell Activation, Ischemia Reperfusion Injury, Allograft Inflammation, Antibody Mediated Rejection
INTRODUCTION
In the setting of transplantation, complement activation mediates immediate post-transplant ischemia-reperfusion (I/R) graft injury, anti-donor T cell alloimmunity, and functions as a key effector mechanism of antibody-initiated graft injury. New insights into complement’s broad immunomodulatory functions after transplantation has led to testing novel complement-directed therapeutics to limit early and late allograft injury and improve transplant outcomes.
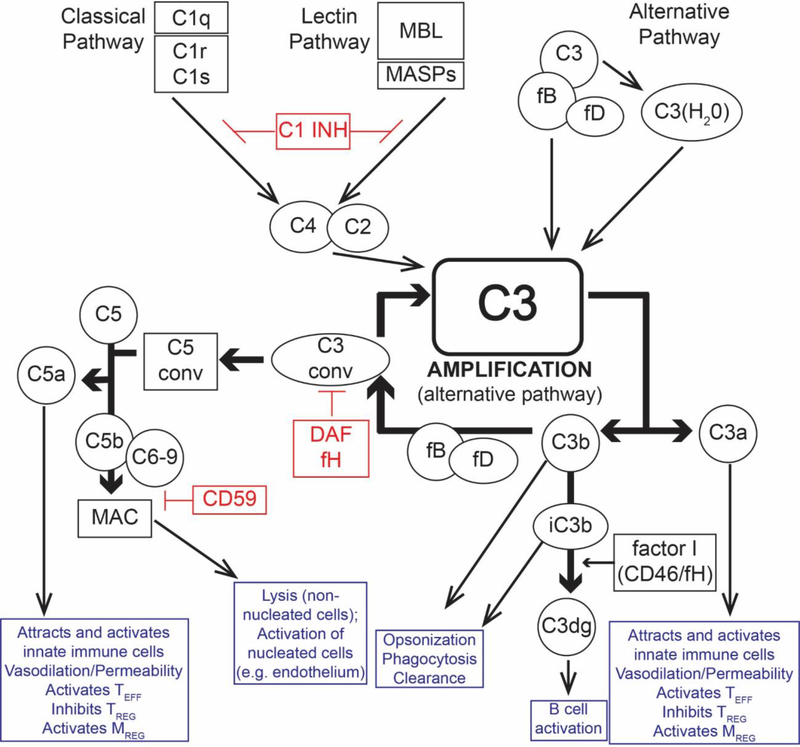
The complement system encompasses >30 proteins comprised of soluble and membrane bound zymogens, receptors, and regulators (Figure 1). While the liver produces the majority of the circulating (plasma) complement components, complement proteins are also produced locally by multiple other cells, including endothelial cells, parenchymal cells, and immune cells, including T cells and antigen presenting cells (APCs) (1, 2). Complement activation can be initiated via the classical, mannose binding lectin (MBL), and alternative pathways. The classical pathway relies on antibody (IgM or IgG) ligation of antigen with subsequent binding by the C1q molecule to the Fc region of the antibodies to yield bound C1qrs complexes that cleave C4, initiating the cascade (3). MBL, ficolins, and collectins are pattern recognition molecules for bacterial carbohydrate motifs that analogously initiate complement activation by binding to and activating mannose associated serine proteases (MASP). MBL can also recognize the Fc region of bound Ig molecules similar to C1q (4, 5). In the alternative pathway, complement component C3 spontaneously hydrolyzes and activates at low levels in the serum in a “tick over” mechanism that requires factor B (fB) and factor D (fD). The three pathways converge at the formation of C3 convertases, which cleave C3 into C3a (anaphylatoxin) and C3b (opsonin). Additional C3 convertases are then formed via the alternative pathway (in the absence of regulation, see below) resulting in amplification of the cascade. Subsequent formation of the C5 convertase results in cleavage of C5 into C5a (anaphylatoxin) and C5b, that latter of which binds with complement components C6-C8 and multiple C9 molecules to assemble the membrane attack complex (MAC). The MAC functions as a pore within cell membranes, promoting cytolysis in non-nucleated cells (e.g. bacteria or red blood cells) but inducing cellular activation rather than cell death in nucleated cells (e.g. endothelium) via non-canonical NFκB signaling (6).
Figure 1:
Overview of the complement cascade and its diverse effects of on alloimmunity and inflammation. Complement activation can be initiated by the C1q-initiated classical pathway, the MASP-dependent mannose binding lectin (MBL) pathway, and spontaneous activation of the alternative pathway. All three pathways converge at the central amplification loop, which forms C3 convertases that cleave C3 to C3a and C3b, the latter initiating formation of the C5 convertase. Subsequent C5 cleavage yields C5a and C5b, ultimately forming the membrane attack complex (MAC, C5b-9), which activates nucleated cells (e.g. endothelium and parenchyma) and induces cytolysis in non-nucleated cells (e.g. red blood cells, bacteria). The anaphylatoxins C3a and C5a bind their respective receptors on immune and parenchymal cells to induce local inflammation, activate effector T cells, and TREG. C5a/C5aR1 paradoxically activates regulatory myeloid cells required for transplant tolerance in mice. The opsonin C3b and iC3b promote phagocytosis. iC3b also ligates complement receptor 2 (CD21) promoting B cell activation. Complement activation/amplification is restrained on self-cells by several membrane-bound and soluble regulatory proteins. Surface-expressed regulators include decay accelerating factor (DAF or CD55, accelerates the decay of cell-surface assembled C3 convertases), membrane cofactor protein (MCP or CD46, a co-factor for factor I that cleaves C3b to its inactive form, iC3b), and CD59 (protectin, inhibits formation of the MAC). Factor H is a soluble complement regulator that exhibits both decay accelerating and co-factor activity. C1 inhibitor (C1INH) inhibits C1qrs and MBL-MASP complexes, limiting classical pathway and MBL pathway activation, respectively. C1INH: C1 inhibitor; C3 conv: C3 convertase; C5 conv: C5 convertase; DAF: decay accelerating factor (CD55), fB: factor B; fD: factor D; fH: factor H; fI: factor I; MAC: membrane attack complex C5b-9; MBL: mannose-binding lectin; MASP: mannose-associated serine protease; TREG: Regulatory T cell
The anaphylatoxins C3a and C5a promote inflammation primarily by binding to their respective G-protein coupled receptors, C3aR1 and C5aR1. These receptors are expressed on a variety of cell types and mediate chemotaxis, cytokine release, APC activation, and T cell activation/expansion (1, 7).
Complement activation is tightly controlled by regulatory mechanisms at multiple steps throughout the cascade to prevent injury to self-cells while permitting activation on pathogens that do not express complement regulators (Figure 1) (3). C1-inhibitor (C1INH) is a soluble molecule that irreversibly binds C1r, C1s and MASPs to inactivate both the classical and MBL-initiated activation complexes. Decay accelerating factor (DAF; CD55) is a cell surface expressed protein that accelerates the decay of surface expressed C3 convertases, thereby limiting amplification of the complement cascade. Soluble factor H and surface-expressed membrane cofactor protein (MCP, CD46) are co-factors for factor I, which cleaves C3b into inactive C3b (iC3b) to irreversibly disable C3 convertases. Ubiquitously expressed carboxypeptidases rapidly inactivate the C3a and C5a anaphylatoxins, and surface expressed CD59 (protectin) prevents the formation of MACs.
COMPLEMENT AND ISCHEMIA-REPERFUSION INJURY
Obligate cold ischemic storage of deceased donor organs prior to transplantation causes hypoxia, ATP depletion, and mitochondrial damage within the graft. Subsequent reperfusion paradoxically exacerbates the damage by generating free oxygen radicals, a phenomenon known as I/R injury (8). Clinically, I/R injury manifests as delayed graft function (DGF) or primary graft dysfunction (PGD) in kidney and lung transplantation, respectively, and is associated with poor long term outcomes (9, 10).
Studies from multiple research groups have established complement’s critical role in post-transplant I/R injury (5, 11, 12). Neoantigens exposed on the surface of endothelial cells after I/R injury can be recognized by MBL (5), collectins (13), or naturally occurring IgM (11) to activate complement. Subsequent C3a and C5a ligation to their respective receptors on local endothelial and immune cells initiates inflammation and facilitates I/R injury (14, 15).
Collectin-11 is a C-type lectin and pattern recognition molecule that contains a binding motif for the MBL-associated proteases (MASP)-1, 2, and 3 and has a high avidity for L-fucose. Unlike MBL, which is primarily produced in the liver, collectin-11 is produced by a variety of cell types (including renal epithelial cells). Farrar et al hypothesized that collectin-11 could serve as a local initiator of inflammation and complement activation (13). In a model of native kidney I/R injury, collectin-11 expression rapidly increased after reperfusion and co-localized with deposition of the complement split product C3d in the renal cortex. Specifically, collectin-11 only deposited in the areas expressing L-fucose (proximal tubule), despite being broadly expressed throughout the collecting system (ex: distal tubules). Kidneys from Collec11−/− animals subjected to I/R showed nominal C3d deposition, diminished infiltration by immune cells (neutrophils and macrophages), and preserved renal function as measured by blood urea nitrogen (BUN) levels. In vitro studies using wild type (WT) or Collec11−/− renal tubular epithelial cells co-cultured with WT or MASP-2 deficient serum revealed that both collectin-11 and MASP-2 were required to induce C3d deposition.
To understand if local kidney-produced or systemic collectin-11 mediated I/R injury, the investigators used a transplant model wherein WT and Collec11−/− kidney allografts were subjected to cold ischemia (25mins) and transplanted into Collec11−/− or WT recipients, respectively. Collectin-11 deficiency in the allograft, but not the recipient, limited post-transplant graft injury at 24 hours and 7 days, and was associated with lower BUN at day 7. The findings identified local kidney produced collectin-11 binding of I/R-induced L-fucose as an important mediator of MASP-2 dependent complement activation, inflammation, and graft injury.
In separate work, Chun et al studied the effect of complement activation in a murine heterotopic cardiac transplant model of I/R injury (12). Whereas fully allogeneic heart allografts transplanted without prolonged cold ischemia into WT recipients and treated with cytotoxic T-lymphocyte associated protein 4 (CTLA4)-Ig survived > 60 days, the same allogeneic hearts subjected to 8 hour cold ischemic storage (CIS) were rejected with a mean survival time (MST) of 37 days despite CTLA4-Ig. Remarkably, allografts subjected to 8 h CIS but transplanted into recipients deficient in C3 survived >60 days (same kinetics as WT hearts without prolonged CIS), implicating recipient-derived complement activation as crucial for IR-induced, costimulatory blockade resistant, rejection in this model. Mechanistic studies showed that prolonged allograft CIS led to increases in early post-transplant serum levels of TNFα and IL1β, and amplified graft infiltration by T cells at 48 hours post-transplant. These early inflammatory changes were associated with augmented numbers of alloreactive IFNγ-producing T cells at 21 days post-transplant. Recipient C3 deficiency blunted both the early inflammatory and late adaptive immune changes induced by prolonged CIS.
Allografts subjected to 8 hour CIS and transplanted into mannose-binding lectin deficient recipients survived for >60d, implicating the MBL pathway. Peri-transplant (day 0 and 1 only) pharmacologic blockade of the MBL pathway using C1INH, which blocks MASP-dependent MBL pathway complement activation, in conjunction with CTLA4-Ig, prolonged the MST of WT hearts subjected to 8 hour CIS to >60 days and blunted the development of anti-donor immunity at 21 days post-transplant. Together, this work and the collectin-11 paper described above link MBL-pathway complement activation (Figure 1) to post-transplant I/R injury in two different pre-clinical organ transplant models.
Translational work by Jordan and colleagues showed that C1INH administration can potentially limit DGF and improve kidney function in human recipients of deceased donor kidneys (16). In a prospective, double blind, placebo controlled, single center trial, seventy dialysis-dependent end stage renal patients receiving deceased donor kidney transplants at risk for DGF (e.g. extended criteria donors, kidney donor profile index score ≥85, donation after cardiac death) were randomized to receive C1INH (50U/kg) or placebo day 0 and 1 post-transplant. While the authors did not observe differences in the primary outcome (number of patients requiring dialysis or the average number of dialysis sessions required between the two groups at 1 week post-transplant), 5 subjects in the placebo arm but none in the C1INH arm remained dialysis-dependent 15–30 days post-transplant. At one year post-transplant C1INH treatment was associated with higher estimated glomerular filtration rates. The investigators did not observe differences in adverse outcomes between the two groups (including infectious or thrombotic complications), highlighting the known safety profile of this therapy that is FDA-approved for the treatment of C1INH deficiency.
C1INH therapy also improved lung function in a small cohort of lung transplant patients with PGD (10). Sequential patients undergoing lung transplantation with diminished PaO2/FiO2 (an early marker of PGD) were treated with C1INH and compared against an untreated cohort that developed PGD within 72 hours of transplant. Therapeutic C1INH (after evidence of graft dysfunction), rapidly corrected blood oxygenation and was associated with diminished time on mechanical ventilation. Together, these two trials provide evidence that the mechanisms described in the pre-clinical models are relevant in human transplantation and support testing MBL-pathway complement inhibitors in patients receiving allografts at risk for I/R injury.
In contrast to the encouraging effects of C1INH, a small 5 center prospective, randomized trial testing anti-C5 monoclonal antibody, eculizumab, in kidney transplantation showed no effect in preventing DGF (17) (35.9% vs 41.7%, eculizumab vs placebo, p=0.398), suggesting that prevention of DGF may require proximal complement inhibition (at or prior to the C3 convertase step).
In another approach, investigators treated donor lungs with a nebulized C3a receptor (C3aR1) antagonist to ameliorate brain death-associated I/R injury in a murine lung transplant model (18). Lungs harvested from donor mice after induced brain death were compared to induced brain death donors treated with nebulized C3aR1-antagonist. All allografts were subjected to 18 hours of cold storage prior to transplantation. C3aR1-antagonist treatment diminished post-transplant I/R injury score at 6 hours post-implantation, blunted neutrophil and macrophage graft infiltration, and reduced inflammatory cytokine (e.g. TNFα, IL1Bβ) intragraft gene expression. These effects were associated with improved acute rejection scores at 5 days post-transplant in the C3aR1-antagonist treated animals compared to non-treated induced brain death allografts. The findings support the need to further develop and test C3aR1 antagonists for use in human transplant recipients.
Using a distinct therapeutic strategy, investigators developed a lipid-tailed C3 convertase inhibitor that can be perfused into donor organs and inserts into the endothelial cell membranes within the allograft. Pre-clinical studies showed it could prevent kidney I/R injury in rodents (19) and improved early islet allograft inflammation in a humanized mouse model (20). Studies testing efficacy of the analogous human reagent Mirococept (APT070) to prevent DGF in human recipients of deceased donor kidneys are ongoing (21).
COMPLEMENT AND T CELL- MEDIATED REJECTION
Immune cell-derived complement regulates T cell immune responses through local production of C3a/C5a and subsequent autocrine and paracrine C3a/C3aR1 and C5a/C5aR1 signaling (1, 7, 22) on T cells and antigen presenting cells (APCs). On T cells, C3a/C3aR1 and C5a/C5aR1 ligations transmit proliferative and survival signals to amplify effector T cell expansion and differentiation. Absence of DAF [lifts restraint on production of C3a and C5a (23)] augments T cell immunity and accelerates murine cardiac allograft rejection (24) while anti-C5 (25), C5aR1 antagonism (26), or absence of C3aR1 signaling (27) reduces T cell priming and improves allograft survival in mice (Figure 1).
Simultaneous to activating effector T cell responses, immune cell-derived C3a/C3aR1 and C5a/C5aR1 ligations on APCs induces upregulation of costimulatory molecules and cytokines (28) to further amplify the effector T cell response. In 2017, Sheen et al used murine systems to show that Toll-like receptor (TLR)-induced dendritic cell (DC) activation, a process crucial for pathogenic immune responses directed at transplanted organs, requires immune cell production of complement and subsequent C3a/C3aR1 and C5a/C5aR1 ligations on the APC (29). DCs harvested from WT mice stimulated with the TLR 9 agonist CpG DNA expressed higher surface levels of activation markers (CD80/CD86 and MHC II), produced more inflammatory cytokines (including TNFα, IL1β), and amplified allogeneic CD4+ T cell proliferation ex vivo. Conversely, DCs from identically stimulated mice deficient in C3aR1 and C5aR1 showed no increase in activation markers or inflammatory cytokines and showed a diminished ability to induce allogeneic T cell proliferation. Using complement C3/C5-deficient bone marrow chimeras the group showed that the effects were mediated by immune cell-derived complement. Following cardiac transplantation, CpG administration augmented the allospecific IFNγ CD8+ T cell responses and induced accelerated graft rejection. In contrast, in the absence of recipient immune cell expression of C3aR1/C5aR1, CpG had no effect (prolonged graft survival and sustained low frequencies of donor reactive T cells). Analogous processes apply to human T cell/APC interactions; C3a and C5a are locally produced during in vitro cultures of human T cells and allogeneic dendritic cells, resulting in alloreactive T cell activation and expansion (1, 30).
Building upon these findings, unique studies by Arbore and colleagues showed a distinctive role for complement in human T cell immunity by demonstrating that the intracellular production of complement cleavage product C5a is crucially involved in human T cell activation, in part via activation of the inflammasome and production of IL1β(31). Human CD4+ T cells express CD46, which binds C3b and functions as a co-factor for factor I cleavage of C3b into iC3b (see Figure 1, note that there is no murine homologue for CD46, although Crry has some analogous function). When the investigators activated human CD4+ T cells with anti-CD3 plus anti-CD46, the T cells produced intracellular C5a and released C5a into culture supernatants. Subsequent autocrine C5aR1 signaling induced reactive oxygen species-dependent upregulation of NLRP3 and IL1β gene transcripts, and enhanced IFNγ production. The in vivo corollary of in vitro antibody-induced CD46 activation of human T cells remains unclear, but untested implications include the possibility that T cell-expressed CD46 ligation with APC-surface bound C3b could have similar effects. Despite lacking a CD46 homolog, murine CD4+ T-cells also relied on intrinsic NLRP3 mediated IL1β production for optimal IFNγ production. Thus, together with previous data showing that immune cell produced C5a/C5aR1 signaling induces T cell proliferation and survival (32), the findings expand mechanisms through which immune cell derived complement impacts T cell responses across species.
In contrast to complement-dependent promotion of effector T cell responses, immune cell-derived, locally produced C3a/C5a inhibits the generation and stability of regulatory T cells (TREG) in mice and humans (7, 22, 33), an effect that further augments antigen specific effector T cell responses. Conversely, TREG-dependent allograft survival/tolerance is facilitated by blocking C3aR1/C5aR1 signaling (7).
The inhibitory effects of C3aR1/C5aR1 signaling on TREG generation are modulated by T cell-expressed C5aR2 that binds C5a but lacks the intracellular signaling domain to initiate G-protein coupled receptor signaling (34). Verghese and colleagues showed that absence of C5aR2 limits, while transgenic overexpression of C5aR2 enhances, in vivo TREG generation (35). While C5aR2 has been shown by others to interact with β-arrestin and/or inhibit ERK1/2 signaling in other cell types (36–38), mechanistic studies in the transplant system supported the conclusion that T cell-expressed C5aR2 limits C5aR1-initiated signals by acting as a decoy receptor and binding locally produced C5a (34, 39). Using murine TREG-dependent cardiac transplant survival models, the investigators further showed that absence of recipient C5aR2 deficiency accelerated graft rejection while C5aR2 overexpression prolonged graft survival. As C5aR2 dependent TREG induction applies to human T cells (1), the data suggest that strategies aimed at inducing T cell C5aR2 expression could positively impact transplant outcomes, an approach that is yet to be tested.
Though complement activation is traditionally considered proinflammatory, studies from the tumor immunology literature described an immunosuppressive role for complement signaling that activates myeloid derived suppressor cells (MDSCs) or regulatory myeloid cells (40, 41). Regulatory myeloid cells, which inhibit effector T cell responses and augment TREG in vivo, are known to be required for costimulatory blockade induced heart transplant survival in mice (42). Using novel myeloid-cell specific C5aR1 conditional knockout mice, Llaudo et al showed C5a/C5aR1 signaling on myeloid cells is a key mediator of regulatory myeloid cell induction/function post-transplant. Absence of C5aR1 specifically on myeloid cells (and not granulocytes) increased anti-donor T cell immunity, decreased TREG, and accelerated graft rejection despite costimulatory blockade (43).
In separate studies addressing a distinct effect of complement on T cell responses, Ling and colleagues showed that C1q modulates CD8+ T cell responses by controlling cellular metabolism (44). Metabolic reprogramming is a necessary step for T cell activation as naïve and memory T cells depend on oxidative phosphorylation while effector cells utilize glycolysis to fulfill their energy needs (45, 46). To address why C1q deficiency, but not C3 deficiency, is associated with systemic lupus erythematosus (SLE), Ling et al studied a graft versus host disease model of SLE. They showed that C1q-deficient recipients of WT spleen cells developed worse renal pathology, with increased numbers of germinal center B cells, T follicular helper cells, and lupus autoantibodies than identically treated WT or C3-deficient mice. C1q-deficient CD8+ T cells exhibited greater Granzyme B secretion, increased BLIMP-1 expression (promotes cytotoxic T cell functions), and higher cell turnover than WT cells. C1q deficiency specifically affected the CD8+ T cell memory precursor effector cell (MPEC) population (defined as CD44+CD62L+) and metabolic assays revealed that MPECs from C1q−/− mice had reduced spare respiratory capacity (a measure of oxidative phosphorylation) and diminished mitochondrial mass. Mitochondrial mass was restored in C1q-deficient CD8+ T cells incubated with C1q in vitro, suggesting a T cell extrinsic effect. Mechanistically, cell surface-expressed p32/C1qR, a mitochondrial protein, was endocytosed after binding the globular head of the C1q molecule and co-localized to the mitochondria. The findings present a novel paradigm of C1q-mediated CD8+ T cell regulation via metabolic reprogramming.
Together, these data linking complement to effector and regulatory T cell immunity highlight the importance of context, cell type, and the specific complement component in understanding the effects of the complement cascade. These concepts are essential for proper interpretation of trials testing complement inhibition for prevention and/or treatment of allograft injury.
COMPLEMENT AND ANTIBODY-MEDIATED TRANSPLANT INJURY
In addition to the recent recognition that complement influences T cell immunity, studies over the past 20 years have revealed that complement influences B cell immune responses. The complement receptor 1 (CR1, CD35) and complement receptor 2 (CR2, CD21) recognize the opsonins C3b and iC3b, respectively, the latter lowering the threshold for B cell activation and promoting antigen retention by follicular dendritic cells (47, 48) (Figure 1). Confirming relevance of a link between complement and antibody production in transplantation, C3-deficient mice failed to produce high-affinity IgG responses against major histocompatibility antigens in skin grafts (49).
Complement activation is also an important effector mechanism of antibody-mediated transplant injury in murine models (50) and human transplant recipients (51, 52). Donor specific alloantibodies activate complement via the classical pathway, ultimately inducing graft inflammation and injury through C3a/C5a production and MAC formation (51). While MAC formation mediates lysis in non-nucleated cells, terminal complement activation on donor human endothelial cells induces non-canonical NF-κB signaling, initiating a pro-inflammatory gene program that facilitates recruitment of alloreactive T cells required for the development of allograft injury (6).
Building upon these fundamental observations, clinicians have studied the efficacy of eculizumab, an anti-human C5, to prevent and/or treat antibody-mediated rejection (ABMR) in transplant recipients. Eculizumab plus plasma exchange reduced the incidence of ABMR in 26 sensitized kidney transplant recipients compared to a control group (53), but showed mixed results when treating ABMR in other small cohort trials (54, 55). Cases of ABMR resistant to eculizumab (53) suggest that complement components proximal to the C5 convertase can contribute to antibody-initiated pathology. Pilot studies testing blockade of upstream classical and MBL pathway activation with C1INH for treatment (56) or prevention (57) of ABMR showed safety and promise, though larger scale studies are needed. Given the known complement-independent (e.g. Fc-receptor-mediated, among others) effector mechanisms (51) of antibody-initiated injury it is likely that complete abrogation of the complement cascade will only be partially efficacious in treating ABMR and may predispose to infectious complications.
CONCLUSION
Experimental data accumulated over the past decade have uncovered previously unidentified mechanisms through which complement mediates post-transplant allograft injury. Preclinical studies showing a role for MBL-initiated complement activation as a crucial mechanism underlying I/R injury (5, 11–13) is being effectively translated into clinical care (10, 16). Although larger validation trials are required, the findings demonstrate that mechanisms deciphered from rodent work can be exploited to rapidly and positively impact human transplant outcomes.
Among the unexpected observations uncovered in the past decade is the paradigm shifting work linking immune cell-derived C3a/C5a to T cell and APC responses (with some differences) in mice and humans (3, 31, 32). Newly uncovered mechanisms are that complement initiated signaling through various receptors can exert these effects by modulating cell intrinsic innate immune components [TLR signaling in DCs (29), inflammasome activation in T cells (31)]. Despite clear proinflammatory effects of complement in transplantation, complement-initiated signals are also required for regulatory myeloid cell function (C5a/C5aR1-dependent, required for murine transplant survival) and can inhibit CD8+ T cell responses (C1q-dependent) (43, 44). Data from pre-clinical and early human trials have also revealed multiple complement dependent mechanisms that participate in antibody mediated allograft injury (16, 17, 53–57). Together with the pharmaceutical industry’s growing pipeline of complement inhibitors (58), these new fundamental discoveries, and the associated understanding of the complexity through which complement components and their receptors impact immune responses, support the need to study the effects of targeting complement as a therapeutic strategy to improve transplant outcomes.
Funding/Acknowledgments:
The work was supported by NIH grants R01 AI071185 and AI132405 awarded to PSH and K08 AI135101 to NC
Footnotes
Disclosures: The authors have no relevant conflicts of interest to disclose
Human and Animal Rights:
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1. •.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant. 2013;13(10):2530–9.Study showing complement regulation of T cell immunity is operant in human cells.
- 2.Cravedi P, Heeger PS. Complement as a multifaceted modulator of kidney transplant injury. J Clin Invest. 2014;124(6):2348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathern DR, Heeger PS. Molecules Great and Small: The Complement System. Clin J Am Soc Nephrol. 2015;10(9):1636–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold JN, Dwek RA, Rudd PM, Sim RB. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett. 2006;106(2):103–10. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177(7):4727–34. [DOI] [PubMed] [Google Scholar]
- 6. •.Jane-Wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, et al. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-κB signaling in endothelial cells. Circulation. 2013;128(23):2504–16.Complement-induced activation of allograft endothelial cells via nuclear factor-κB signaling.
- 7.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210(2):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorsuch WB, Chrysanthou E, Schwaeble WJ, Stahl GL. The complement system in ischemia-reperfusion injuries. Immunobiology. 2012;217(11):1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponticelli C Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014;29(6):1134–40. [DOI] [PubMed] [Google Scholar]
- 10.Sommer W, Tudorache I, Kuhn C, Avsar M, Salman J, Ius F, et al. C1-esterase-inhibitor for primary graft dysfunction in lung transplantation. Transplantation. 2014;97(11):1185–91. [DOI] [PubMed] [Google Scholar]
- 11. •.Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, et al. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015;131(13):1171–80.Description of preformed antibodies as pathogenic mediators of transplant associated ischemia reperfusion injury.
- 12. •.Chun N, Fairchild RL, Li Y, Liu J, Zhang M, Baldwin WM, et al. Complement Dependence of Murine Costimulatory Blockade-Resistant Cellular Cardiac Allograft Rejection. Am J Transplant. 2017;17(11):2810–9.Shows recipient mannose-binding lectin pathway initiated recipient complement activation as a mediator of cardiac allograft ischemia reperfusion injury and late graft loss.
- 13. •.Farrar CA, Tran D, Li K, Wu W, Peng Q, Schwaeble W, et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J Clin Invest. 2016;126(5):1911–25.Identification of allograft-derived collectin-11 as an initiator of pathogenic complement activation and renal allograft ischemia reperfusion injury.
- 14.Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, et al. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23(9):1474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalli PN, Zhou W, Sacks S, Medof ME, Heeger PS. Locally produced and activated complement as a mediator of alloreactive T cells. Front Biosci (Schol Ed). 2009;1:117–24. [DOI] [PubMed] [Google Scholar]
- 16. •.Jordan SC, Choi J, Aubert O, Haas M, Loupy A, Huang E, et al. A phase I/II, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Am J Transplant. 2018.Translational human trial showing promise of C1-inhibitor therapy for improving outcomes in kidney transplant recipients of organs at risk for delayed graft function.
- 17.Heeger P, Akalin E, Baweja M, Bloom R, Florman S, Haydel B, et al. Lack of Efficacy of Eculizumab for Prevention of Delayed Graft Function (DGF) in Deceased Donor Kidney Transplant Recipients. Am J Transplant. 2018;18(S4). [DOI] [PubMed] [Google Scholar]
- 18.Cheng Q, Patel K, Lei B, Rucker L, Allen DP, Zhu P, et al. Donor pretreatment with nebulized complement C3a receptor antagonist mitigates brain-death induced immunological injury post-lung transplant. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel H, Smith RA, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol. 2006;17(4):1102–11. [DOI] [PubMed] [Google Scholar]
- 20.Xiao F, Ma L, Zhao M, Smith RA, Huang G, Jones PM, et al. APT070 (mirococept), a membrane-localizing C3 convertase inhibitor, attenuates early human islet allograft damage in vitro and in vivo in a humanized mouse model. Br J Pharmacol. 2016;173(3):575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassimatis T, Qasem A, Douiri A, Ryan EG, Rebollo-Mesa I, Nichols LL, et al. A double-blind randomised controlled investigation into the efficacy of Mirococept (APT070) for preventing ischaemia reperfusion injury in the kidney allograft (EMPIRIKAL): study protocol for a randomised controlled trial. Trials. 2017;18(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J Immunol. 2013;190(12):5921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. [DOI] [PubMed] [Google Scholar]
- 24.Pavlov V, Raedler H, Yuan S, Leisman S, Kwan WH, Lalli PN, et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol. 2008;181(7):4580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raedler H, Vieyra MB, Leisman S, Lakhani P, Kwan W, Yang M, et al. Anti-complement component C5 mAb synergizes with CTLA4Ig to inhibit alloreactive T cells and prolong cardiac allograft survival in mice. Am J Transplant. 2011;11(7):1397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gueler F, Rong S, Gwinner W, Mengel M, Brocker V, Schon S, et al. Complement 5a receptor inhibition improves renal allograft survival. J Am Soc Nephrol. 2008;19(12):2302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz J, Mathern D, Heeger P. C3a Receptor Regulates the CD8 T-Cell Alloresponse via Intrinsic and Extrinsic Mechanisms Am J Transplant. 2018;18(S4). [Google Scholar]
- 28.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheen JH, Strainic MG, Liu J, Zhang W, Yi Z, Medof ME, et al. TLR-Induced Murine Dendritic Cell (DC) Activation Requires DC-Intrinsic Complement. J Immunol. 2017;199(1):278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, Fazekasova H, Wang N, Sagoo P, Peng Q, Khamri W, et al. Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol. 2011;48(9–10):1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. ••.Arbore G, West EE, Spolski R, Robertson AAB, Klos A, Rheinheimer C, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4⁺ T cells. Science. 2016;352(6292):aad1210.Novel mechanism describing requisite complement-induced, T cell-intrinsic, inflammasome activation for development of Th1 immunity in human CD4+ T cells.
- 32.Sheen JH, Heeger PS. Effects of complement activation on allograft injury. Curr Opin Organ Transplant. 2015;20(4):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14(2):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, et al. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42(31):9406–15. [DOI] [PubMed] [Google Scholar]
- 35.A Verghese D, Demir M, Chun N, Fribourg M, Cravedi P, Llaudo I, et al. T Cell Expression of C5a Receptor 2 Augments Murine Regulatory T Cell (T. J Immunol. 2018;200(6):2186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croker DE, Monk PN, Halai R, Kaeslin G, Schofield Z, Wu MC, et al. Discovery of functionally selective C5aR2 ligands: novel modulators of C5a signalling. Immunol Cell Biol. 2016;94(8):787–95. [DOI] [PubMed] [Google Scholar]
- 37.Karsten CM, Wiese AV, Mey F, Figge J, Woodruff TM, Reuter T, et al. Monitoring C5aR2 Expression Using a Floxed tdTomato-C5aR2 Knock-In Mouse. J Immunol. 2017;199(9):3234–48. [DOI] [PubMed] [Google Scholar]
- 38.Pundir P, MacDonald CA, Kulka M. The Novel Receptor C5aR2 Is Required for C5a-Mediated Human Mast Cell Adhesion, Migration, and Proinflammatory Mediator Production. J Immunol. 2015;195(6):2774–87. [DOI] [PubMed] [Google Scholar]
- 39.Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, et al. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem. 2005;280(48):39677–80. [DOI] [PubMed] [Google Scholar]
- 40.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ajona D, Ortiz-Espinosa S, Moreno H, Lozano T, Pajares MJ, Agorreta J, et al. A Combined PD-1/C5a Blockade Synergistically Protects against Lung Cancer Growth and Metastasis. Cancer Discov. 2017;7(7):694–703. [DOI] [PubMed] [Google Scholar]
- 42.Ochando J, Conde P, Bronte V. Monocyte-Derived Suppressor Cells in Transplantation. Curr Transplant Rep. 2015;2(2):176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llaudo I, Fribourg M, Edward Medof M, Conde P, Ochando J, Heeger PS. C5aR1 regulates migration of suppressive myeloid cells required for costimulatory blockade-induced murine allograft survival. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. ••.Ling GS, Crawford G, Buang N, Bartok I, Tian K, Thielens NM, et al. C1q restrains autoimmunity and viral infection by regulating CD8. Science. 2018;360(6388):558–63.New paradigm of T cell regulation by C1q-mediated metabolic reprogramming.
- 45.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160(11):5273–9. [PubMed] [Google Scholar]
- 48.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–50. [DOI] [PubMed] [Google Scholar]
- 49.Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation. 2001;72(7):1310–8. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Arp J, Liu W, Faas SJ, Jiang J, Gies DR, et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179(7):4451–63. [DOI] [PubMed] [Google Scholar]
- 51.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8(11):670–8. [DOI] [PubMed] [Google Scholar]
- 53.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11(11):2405–13. [DOI] [PubMed] [Google Scholar]
- 54.Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9(1):231–5. [DOI] [PubMed] [Google Scholar]
- 55.Burbach M, Suberbielle C, Brochériou I, Ridel C, Mesnard L, Dahan K, et al. Report of the inefficacy of eculizumab in two cases of severe antibody-mediated rejection of renal grafts. Transplantation. 2014;98(10):1056–9. [DOI] [PubMed] [Google Scholar]
- 56.Montgomery RA, Orandi BJ, Racusen L, Jackson AM, Garonzik-Wang JM, Shah T, et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody-Mediated Rejection Following Kidney Transplantation: Results of a Randomized Double-Blind Placebo-Controlled Pilot Study. Am J Transplant. 2016;16(12):3468–78. [DOI] [PubMed] [Google Scholar]
- 57. •.Vo AA, Zeevi A, Choi J, Cisneros K, Toyoda M, Kahwaji J, et al. A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients. Transplantation. 2015;99(2):299–308.Pilot clinical study showing safety and potential efficacy of C1INH therapy for prevention of antibody-mediated rejection in high risk transplant recipients.
- 58.Thurman JM, Le Quintrec M. Targeting the complement cascade: novel treatments coming down the pike. Kidney Int. 2016;90(4):746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]