Abstract
Salivary duct carcinoma (SDC) is a rare and aggressive malignancy with a high mortality and poor response to treatment in the advanced setting. Human epidermal growth factor 2 (HER2) can be amplified in a fraction of SDC. We describe the case of HER2+ metastatic SDC of the submandibular gland in a young pregnant woman treated by multimodal treatment (chemotherapy, radiotherapy and targeted therapy). During pregnancy, a 27-year-old woman developed SDC of the left submandibular gland with lung and bone metastases. Given the HER2 overexpression, she was treated with trastuzumab, paclitaxel and cisplatin. Since the tumor had arisen during pregnancy, triptorelin was administered after delivery. A complete remission was observed, and after eight cycles of chemotherapy, radiotherapy was started in association with trastuzumab and triptorelin. A prolonged disease control and complete visceral remission were observed. Multimodal therapy based on patient’s tumor characteristics showed good clinical efficacy in the treatment of metastatic SDC.
Keywords: salivary duct carcinoma, HER2-positive disease, brain metastases, multimodality treatment
INTRODUCTION
Salivary duct carcinoma (SDC) is an uncommon neoplasm of a ductal cell origin, which accounts for 3–9% of all malignant salivary gland tumors [1]. It occurs most commonly in the parotid gland in men aged > 50 years [1]. Interestingly, SDC shares significant morphologic and immunophenotypic overlap with ductal carcinoma of the breast, including human epidermal growth factor 2 (HER2) expression in up to 88% of cases [2, 3].
We describe here the case of HER2+ advanced ductal carcinoma of the submandibular gland in a young pregnant woman.
CASE REPORT
A 27-year-old Caucasian woman at the 28th week of gestation developed a mass on the left submandibular gland. A biopsy was performed and the pathology report revealed a high-grade carcinoma. At the 30th week, the child labor was induced and the newborn was found to be healthy. Computed tomography (CT) scan showed an extensive mass of the left submandibular gland along with synchronous bilateral pulmonary metastases. Magnetic resonance imaging (MRI) and CT scan of the head showed a mass infiltrating the submandibular gland, multiple regional lymph nodes and both cervical spine and lung metastases (Fig. 1).
Figure 1.
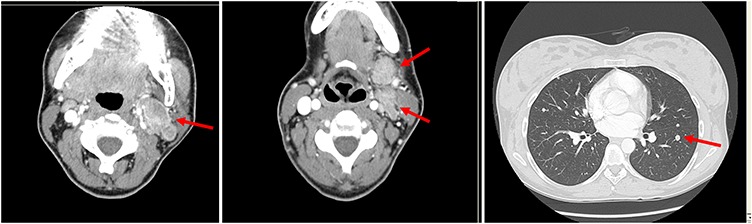
CT scan of the head with submandibulary gland tumor, multiple nodes and pulmonary metastases at diagnosis.
Positron emission tomography (PET) scan demonstrated fluorodeoxyglucose (FDG)-avidity in the left neck, in bilateral lungs and in the cervical spine (C1, C2, C3, C4, C6). The cancer was considered unresectable.
Pathology was reviewed at tertiary referral center for the treatment of head and neck cancers, and it revealed both intraductal and invasive components. Immunohistochemical analysis by chromogenic in situ hybridization showed strong positive (3+) HER2 expression. The tumor was also weakly androgen receptor (AR) positive (<20% of tumor cells at immunohistochemistry). Therefore, histopathological review concluded for HER2+ AR− SDC.
A palliative chemotherapy regimen with cisplatin (75 mg/m2; day 1; every 21 days), paclitaxel (80 mg/m2; days 1, 8 and 15) and trastuzumab (8 mg/kg dose-loading and 6 mg/kg maintenance treatment, every 21 days) was promptly initiated. Moreover, ovarian suppression with monthly triptorelin 3.75 mg was administered concomitantly to systemic therapy. The patient completed a total of eight cycles of chemotherapy. Compliance to treatment was excellent; the clinical and hematological tolerability was very good.
Radiological reassessments (CT, MRI and PET scans) obtained at the third and sixth courses of chemotherapy (December 2012) showed complete remission of the primary tumor as well as of all pulmonary and bone metastatic lesions (Fig. 2).
Figure 2.
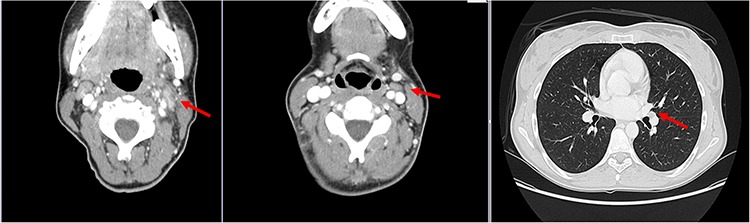
At CT scan, evidence of complete remission of submandibulary gland tumor, multiple nodes and pulmonary metastases.
After multidisciplinary discussion, the patient was then given intensity-modulated radiotherapy (IMRT) using megavoltage equipment (6 MV linear accelerator), using a simultaneous integrated boost approach delivering in 30 fractions 60 Gy to the primary site and positive lymph nodes, 50 Gy on microscopic volume and 45 Gy on cervical vertebrae bones. During IMRT, trastuzumab was continued (weekly regimen). After the conclusion of IMRT, maintenance treatment of trastuzumab was given.
The treatment was protracted for 9 months; at this point, she unfortunately developed a voluminous expansive brain lesion in spite of a persistent complete response at all other secondary lesion sites. The patient received a local multimodality treatment at Istituto Nazionale Tumori Regina Elena. She underwent left frontal craniotomy with complete surgical removal of the central nervous system metastasis confirmed by postoperative MRI. The patient refused adjuvant stereotactic radiotherapy. At a subsequent follow-up MRI, on April 2015, a brain relapse was detected. At this time, the patient underwent stereotactic therapy, delivering 32 Gy in 19 fractions.
In the meantime, the treatment of triptorelin was continued uninterruptedly until the date of death of the patient due to brain and liver progression, occurred 36 months after the diagnosis, while the other sites (lung and bones) remained stable.
DISCUSSION
SDC is a malignant salivary neoplasm with a poor prognosis. Due to its rarity and highly aggressive behavior, all cases of SDC should be discussed within a multidisciplinary team in order to provide a tailored approach.
Chemotherapy as a single treatment modality is currently considered for disseminated disease.
In the present case, given the young age of the patient, we decided to administer upfront treatment with platinum-based chemotherapy [4].
It is widely accepted that SDC shows remarkable morphologic and biologic resemblance to high-grade mammary ductal carcinoma [5]. Given the marked HER2 expression shown by the tumor, we decided to treat the patient with trastuzumab, currently indicated for HER2-amplified breast cancer [6]. Moreover, the efficacy of trastuzumab concomitant to taxane-containing regimens had already been demonstrated in breast cancer patients [7]. This prompted us to prescribe a combination of cisplatin, paclitaxel and trastuzumab; this combination led to the disappearance of all visible disease. Based on this excellent result, treatment with trastuzumab was continued.
In breast cancer, endocrine therapy is used in hormone receptor-positive disease. In this setting, gonadotropin-releasing hormone agonists (GnRH) inhibit cancer proliferation and may confer a survival benefit in premenopausal patients [8]. Even though our case did not overexpress hormone receptors (estrogen and progesterone), we decided to associate triptorelin in combination with anti-HER2-based systemic therapy. The decision to administer triptorelin was taken upon the fact that, in spite of the weak AR expression of our case (<20%), the manipulation of GnRH-R signaling by analogues such as triptorelin has an important role in AR+ SDC, as revealed by phase II studies [9]. This is even more appropriate if we consider a hormone-sensitive adenocarcinoma appearing during pregnancy, when hormonal changes are dramatically accentuated.
Last, the patient received radiotherapy, in line with the observation [10] that patients with a partial or complete clinical response to chemotherapy may be benefit from further cytoreduction.
Safety and efficacy of high-dose radiotherapy combined with hormonal treatment were observed.
At the time of encephalic progression, aggressive local therapy might be considered in patient with oligoprogressive disease. Resection and stereotactic radiotherapy are both reasonable options to pursue local control with minimal morbidity.
Although of anecdotal nature, the present case supports consideration for aggressive multimodal therapeutic approaches in selected patients affected by metastatic SDC. In particular, the combination of traditional treatment modalities (chemotherapy) with further different approaches (targeted therapy, hormone therapy and radiotherapy) has determined a complete response and has improved the quality of life of the patient. Complete remission allowed local analgesia and the absence of cranial nerve deficiencies for the duration of her illness.
To this end, selection of the initial therapy should be guided by both immunohistochemical and androgen receptor expression of the tumor and by patient performance status. For patients responding to systemic treatment, consolidation with radiotherapy seems justified. Interestingly, the disease remained controlled at the treated sites (lung and neck), while progressed at brain and liver. Even though brain is usually seen as a ‘sanctuary’ for drugs [11], it was also demonstrated that the blood–brain barrier can be altered by brain metastases, especially from HER-positive cancers [12]. In this setting, the ability to cross the blood–brain barrier was described for both bicalutamide [13] and trastuzumab [14].
Acknowledgements
L.L. has disclosed funding (to her institution) for clinical studies and research from AstraZeneca, Boehringer Ingelheim, Eisai, Merck Serono, MSD, Novartis and Roche, has received compensation for service as a consultant/advisor and/or for lectures from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Debiopharm, Eisai, Merck Serono, MSD, Novartis, Roche and Sobi and has received travel coverage for meetings from Bayer, Bristol-Myers Squibb, Debiopharm, Merck Serono, MSD and Sobi.
Conflict of interest statement
C.B., S.C., G.S. and A.F. state no conflict of interest.
Ethical approval and consent
Not applicable.
Guarantor
Cristiana Bergamini, MD.
REFERENCES
- 1. Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Loning T. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer 2005;103:2526–33. [DOI] [PubMed] [Google Scholar]
- 2. Colmenero Ruiz C, Patron Romero M, Martin PM. Salivary duct carcinoma: a report of nine cases. J Oral Maxillofac Surg 1993;51:641–6. [DOI] [PubMed] [Google Scholar]
- 3. Williams MD, Roberts D, Blumenschein GR Jr, Temam S, Kies MS, Rosenthal DI, et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol 2007;31:1645–52. [DOI] [PubMed] [Google Scholar]
- 4. Debaere D, Vander Poorten V, Nuyts S, Hauben E, Schoenaers J, Schöffski P, et al. Cyclophosphamide, doxorubicin, and cisplatin in advanced salivary gland cancer. B-ENT 2011;7:1–6. [PubMed] [Google Scholar]
- 5. Simpson RH. Salivary duct carcinoma: new developments--morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol 2013;7:S48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schramm A, De Gregorio N, Widschwendter P, Fink V, Huober J. Targeted therapies in HER2-positive breast cancer - a systematic review. Breast Care 2015;10:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamberg P, Bos MM, Braun HJ, Stouthard JM, Deijk GA, Erdkamp FL, et al. Randomized phase II study comparing efficacy and safety of combination-therapy trastuzumab and docetaxel vs. sequential therapy of trastuzumab followed by docetaxel alone at progression as first-line chemotherapy in patients with HER2+ metastatic breast cancer: HERTAX trial. Clin Breast Cancer 2011;11:103–13. [DOI] [PubMed] [Google Scholar]
- 8. De Iuliis F, Lanza R, Scarpa S. Are we ready to change clinical practice after the 'soft and text' results? Future Oncol 2015;11:2857–60. [DOI] [PubMed] [Google Scholar]
- 9. Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol 2018;29:979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma J, Liu Y, Yang X, Zhang CP, Zhang ZY, Zhong LP. Induction chemotherapy in patients with resectable head and neck squamous cell carcinoma: a meta-analysis. World J Surg Oncol 2013;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res 2007;13:1656–62. [DOI] [PubMed] [Google Scholar]
- 12. Yonemori K, Tsuta K, Ono M, Shimizu C, Hirakawa A, Hasegawa T, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010;116:302–8. [DOI] [PubMed] [Google Scholar]
- 13. Cockshott ID. Bicalutamide: clinical pharmacokinetics and metabolism. Clin Pharmacokinet 2004;43:855–78. [DOI] [PubMed] [Google Scholar]
- 14. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 2007;18:23–8. [DOI] [PubMed] [Google Scholar]


