Abstract
Spasticity is one of the major complications after stroke. Botulinum toxin type A (BoNT-A) injection is commonly used to manage focal spasticity. However, it is uncertain whether BoNT-A can improve activities of daily living function of paretic arm. The recovery of functions of the affected arm is also the aim of robotic upper limb (UL) therapy. The motorized exoskeleton assists the patient in a large 3D work environment by promoting movement for the UL (shoulder, elbow, wrist, hand). The combination of the BoNT-A injection and the robotic therapy might enhance functional recovery after stroke. We reported the case of a chronic stroke patient in which the injection of BoNT-A was combined with multi-joint exoskeleton training. The patient showed improvement in the motor control of the UL, supporting the feasibility of this approach.
Keywords: stroke, hemiplegia, botulinum toxins type A, exoskeleton device, upper extremity
INTRODUCTION
Focal spasticity is one of the major complications after stroke. Spasticity primarily affects the upper limb (UL) [15]; it contributes to disabilities that negatively impact functional recovery. In addition to functional limitations, spasticity, when inappropriately treated, may lead to reduced quality of life, increased pain and joint contractures [4]. Botulinum toxin type A (BoNT-A) injection is commonly used to manage focal spasticity of UL in adult stroke patients. Neurotoxin inhibits local neuromuscular cholinergic transmission, binding to motor nerve terminal pre-synaptic receptors, inhibits the secretion of acetylcholine causing the transitory paralysis of the muscle [5]. Motor UL recovery is greater if BoNT-A injection is followed by rehabilitation with respect to an injected without rehabilitation [1]. Parallel, in order to increase recovery of UL, in the past decades, the robotic therapy often focuses on increased strength and joint movement reduction of spasticity. Robotic exoskeleton can be assist the paretic UL in a large 1D, 2D or 3D environment by promoting movement [12]. Recent researches supported the usefulness of combined approach of BoNT-A injection and robot both for the recovery of lower limb and gait function and for the recovery of UL function in chronic stroke [3, 6]. We reported the effect of the first case of combined BoNT-A injection and multi-joint and 3D exoskeleton therapy on functional UL recovery in chronic stroke.
CASE REPORT
A 55-year-old right-handed Caucasian women presented an ischemic stroke in the area of the middle cerebral artery treated with thrombolytic therapy and then thrombectomy (Magnetic Resonance Imaging acquisition; Fig. 1) on 20 July 2016. The subject clinically presented a moderate hemiparesis of left side with UL spasticity [modified Ashworth scale (MAS): 0.66 ± 0.57] and sensorimotor deficit [Fugl-Meyer Assessment (FMA) UE 16].
Figure 1.
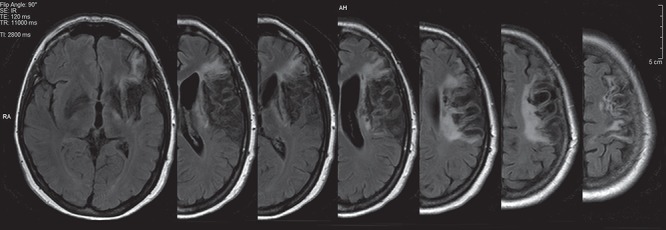
MRI acquisition. Axial views of T1-weighted magnetic resonance imaging before treatment.
She had the following comorbidity: aphasia without cognitive deficits (Mini-Mental State Examination >23), patent foramen ovale, interatrial septum aneurysm, fracture of the right clavicle in 2017, former smoker 15 cigarettes/die.
In order to treat focal spasticity of the right UL, has been performed, an ultrasound-guided BoNT-A (Xeomin®) injection. Ultrasonic imaging has been performed with a linear array transducer (MyLabTM 25 Gold Esoade). A total of 100 units (U) of BoNT-A have been infiltrated (Flexor Carpi Radialis, 20U; Flexor digitorum superficialis, 30U; Flexor digitorum profundus, 50U).
Immediately after the infiltration, the patient was subjected to 10 sessions of multi-joint and tridimensional exoskeleton robot-assisted therapy (40 mins; 5 times; 2 weeks) using Armeo® Power II. Every single session included exercises for the range of motion (ROM) of shoulder, elbow, wrist and hand coordination. The training characteristics (difficulty level, duration, visual detail) were set in conformity of residual ability of the patient. Selected exercises consisted in single-joint movements in a single axis (1D) or combined movements of single joint in two (2D) or three axes (3D), selective exercises for the opening and closing hand (GRASP) and multi-joint exercises in 1D, 2D or 3D movements (COORDINATION).
‘The exoskeleton, used for the therapy, is composed by an orthosis for the UL with six degrees of freedom: three for the shoulder, one for the elbow flexion, one for the forearm supination, and one for the wrist flexion. Each joint is powered by a motor and equipped with 2D sensors. The device can support the patient’s UL weight, providing a feeling of fluctuation. The interface used for the exergame, is designed to simulate UL gestures and provide a simple virtual environment’ [10].
The effects has been monitored through the MAS, the Fugl-Meyer assessment scale for Upper Extremity (FMAUE), the National Institute of Health Stroke scale (NIHSS), the Barthel Index (BI) and the Medical Research Council before (T0) and after treatment (T1).
Descriptive analysis has been used to describe the data; the score change was calculated using Effectiveness (EFC) formula:
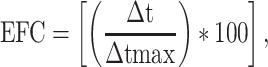 |
where Δt is the differences between the post-treatment time point (T1) and the baseline (T0) and  tmax are the differences between maximum score and baseline score (T0) [14]. For MAS and MRC scales were performed an average of the score recorded in the movements of wrist, forearm, elbow and shoulder. The ROM was recorded with exoskeleton in the three axes of space.
tmax are the differences between maximum score and baseline score (T0) [14]. For MAS and MRC scales were performed an average of the score recorded in the movements of wrist, forearm, elbow and shoulder. The ROM was recorded with exoskeleton in the three axes of space.
After combined treatment a reduction in muscle tone has been recorded (MAS; T0 = 6.6±0.57, T1 = 3.3±0.57; EFC = 6.81%). An improvement in strength measured by MRC (T0 = 2±1.69, T1 = 2.75±1.9, EFC = 25%) and functional movements (FMA; T0 = 16, T1 = 17, EFC = 2%) and signs and symptoms due to stroke (NIHSS; T0 = 3, T1 = 0, EFC = 7.69%) has been recorded. No effect was recorded in the performance of daily life activities (BI). Increase in ROM has been recorded (T0 = 200 399 cm3, T1=288 931 cm3; Fig. 2; complete data in Table 1).
Figure 2.
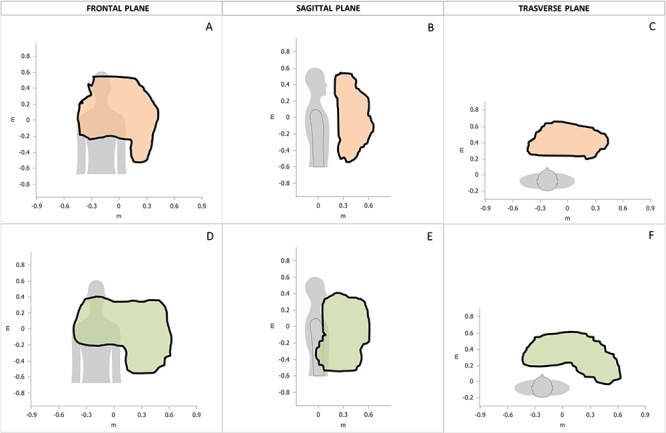
Area and total volume drawn with passive mobilization. Area and total volume drawn with passive mobilization of the UL up to the articular limit in the three planes of the space recorded with Armeo® Power and support by a blinded therapist in three axes of space and analyzed with Armeo®Control Software.
Table 1.
Results
| T0 | T1 | tmax | EFC (%) | ||
|---|---|---|---|---|---|
| FMA | 16 | 17 | 66 | 2 | |
| NIHSS† | 3 | 0 | 42 | 7.69 | |
| MRCa | 2 ± 1.7 | 2.75 ± 1.9 | 5 | 25 | |
| BI | 81 | 81 | 100 | — | |
| MAS†,a | 0.6 ± 0.6 | 0.3 ± 0.6 | 5 | 6.81 | |
| ROM | |||||
| SHOULDER | Abduction† | −66.7° | −77.3° | −80/0° | 79,69 |
| Adduction | 18.9° | 23.3° | −0/40° | 20,85 | |
| Extention† | 43.9° | 42.7° | 0/45° | 109,09 | |
| Flexion | 116.9° | 116.8° | 45/120° | −3,22 | |
| Int. Rot.† | 6.2° | 1.9° | 0/45° | 69,35 | |
| Ext. Rot. | 83.6° | 86.1° | 45/90° | 39,06 | |
| ELBOW | Extention | 100° | 100° | 0/100° | — |
| Flexion† | 6.9° | 1.8° | 100/0° | 5,47 | |
| FOREARM | Pronation† | −55.1° | −64.2° | −65/0° | 91,91 |
| Supination | 59.1° | 64.1° | 0/65° | 84,74 | |
| HAND | Opening† | −4.2° | −4.5° | −6/0° | 16,66 |
| Closing | 36.7° | 44.0° | 0/45° | 87,95 | |
†A decreased score is indicative of a better outcome.
Mean score ± standard deviation of multi-joint evaluation for the UL.
Clinical differences after and before protocol. The degrees of passive joint ROM for UL have been recorded with robotic exoskeleton with the support of a blinded physical therapist. tmax, maximal score scale; EFC, effectiveness; FMA, Fugl-Meyer assessment scale for Upper Extremity; NIHSS, National Institute of Health Stroke scale; MRC, Medical Research Council scale for muscle strength; BI, Barthel Index; MAS: Modified Ashwort scale.
DISCUSSION
As it is a single case, the results have only a conceptual value; notwithstanding, the immediate use of multi-joint exoskeleton training after BoNT-A injection did not imply any adverse effect and a good compliance. We recorded a UL spasticity reduction (MAS +6.81%), followed by a motor improvement (MRC +25%) like as previous robot-assisted studies (both single joint and multi-joint) [3, 12, 13]. Although the BoNT-A injection may weaken the strength of the spastic elbow, wrist and finger flexors, it allows improvements to the release of the hand grip and reaching tasks ability. The combination with robot-assisted training can induce a plastic reorganization at the muscular afferents, spinal motor neurons, interneuron system and beyond and facilitates neural plasticity and motor relearning through goal-oriented training program [8]. The robotics device allows to train patients in an intensive and task-oriented and top-down therapy way, increasing patients’ compliance and motivation. The cognitive top-down stimulation is allowed by means the introduction of visual feedback performed through exergaming [9]. In addition, by using computer-assisted devices for regaining UL function, the robot can easily apply new constraints, in order to optimize the required movement pattern. Therefore, the complexity of a motor task to be learned can be controlled for more precisely with robotics than in conventional treatment approaches leading to neuroplasticity-dependent functional modifications [2, 8].
The potential of our case report is in comparing the obtained results with those of other studies, in order to design a specific RCT with multi-joints robotic therapy and BoNT-A injection. First of all, Gandolfi et al. [6] tested a group of patients who received robot-assisted UL training and BoNT-A injection comparing it with a control group who received conventional therapy combined with BoNT-A injection. So, their research question was to test if robotic training may enhance the efficacy of BoNT-A. Clinically, they reported significant UL spasticity reduction in both groups, without reporting significant between-group differences. Only quantitative analysis of muscle strength and activity showed significant differences in favor of robotic treatment. Gandolfi et al. used an end-effector device working in a bi-dimensional space with video feedback (45 mins × 2 sessions × 5 weeks) [6]. Their sample size was based on the data of MAS score reported in Pennati et al. [11]. Both the studies involved chronic patients, but Pennati performed 10 sessions, 60 mins each, 2–3 times per week. The main difference with the study of Pennati et al. is the choice of the control condition: the latter authors enrolled a group receiving the robotic treatment without BoNT-A injection [11]. According to previous studies, our treatment also lasted 10 sessions, 5 per week and lasting 40 mins each. In terms of primary outcome, Gandolfi et al. found an improvement in MAS score of 0.25 in the robotic group (versus 1.25 in conventional group; whereas Pennati et al. found 0.86 versus 0.67). A sample size calculation performed using the Gandolfi’s data (SD = 3, α-level = 5%, power = 80%) highlighted the need of 248 patients, suggesting a very complicated study. However, we used an exoskeleton and not an end effector, and we hypothesized that it may increase the effects of robotic training. In fact, the improvement in MAS score in our study was of 3.3 points. Combining these data and that of the control group of Gandolfi et al., we found the sample size of 34 patients per group, defining a more feasible study (using the Pennati’s data, the resulting sample size was even smaller, but probably less reliable) [6, 11]. Analyzing the secondary outcome, the FMA, we just found a slight improvement, in line with the results of Gandolfi and Pennati who both found higher improvements in their control groups than in their experimental group [6, 11]. This case report supports the idea of the feasibility of this combined approach and can be interesting for designing an Randomized Controlled Trial in which an exoskeleton can be combined with BoNT-A injection for the recovery of UL in chronic stroke.
Limitations
Being a combined protocol, it is not possible to clearly interpret which of the two interventions the increase is attributable to. This observation should be considered only as an anecdotal episode.
ACKNOWLEDGEMENTS
The authors thanks for the subject participation.
Conflict of Interest statement
None declared.
Funding
This work was not supported by any grant or other financial support.
Ethical approval
The authors confirm that the current study has been conducted in an ethical and responsible manner and is in full compliance with all relevant codes of experimentation and legislation.
Consent
The patient received and signed the informed consent.
References
- 1. Devier D, Harnar J, Lopez L, Brashear A, Graham G. Rehabilitation plus OnabotulinumtoxinA improves motor function over OnabotulinumtoxinA alone in post-stroke upper limb spasticity: a single-blind, randomized trial. Toxins (Basel) 2017;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol 2004;3:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erbil D, Tugba G, Murat TH, Melike A, Merve A, Cagla K, et al. Effects of robot-assisted gait training in chronic stroke patients treated by botulinum toxin-a: a pivotal study. Physiother Res Int 2018;23: e1718. [DOI] [PubMed] [Google Scholar]
- 4. Francisco GE, McGuire JR. Poststroke spasticity management. Stroke 2012;43:3132–6. [DOI] [PubMed] [Google Scholar]
- 5. Frevert J. Xeomin: an innovative new botulinum toxin type A. Eur J Neurol 2009;16:11–3. [DOI] [PubMed] [Google Scholar]
- 6. Gandolfi M, Valè N, Dimitrova EK, Mazzoleni S, Battini E, Filippetti M, et al. Effectiveness of robot assisted upper limb training on spasticity, function andmuscle activity in chronic stroke patients treated with Botulinum toxin: a randomized single-blinded controlled trial. Front Neurol 2019;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaji R. Direct central action of intramuscularly injected botulinum toxin: is it harmful or beneficial? J Physiol 2013;591:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair 2008;22:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morone G, Spitoni GF, De Bartolo D, Ghanbari Ghooshchy S, Di Iulio F, Paolucci S, et al. Rehabilitative devices for a top-down approach. Expert Rev Med Devices 2019;16:187–95. [DOI] [PubMed] [Google Scholar]
- 10. Palermo E, Hayes DR, Russo EF, Calabrò RS, Pacilli A, Filoni S. Translational effects of robot-mediated therapy in subacute stroke patients: an experimental evaluation of upper limb motor recovery. PeerJ 2018;6: e5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pennati GV, Da Re C, Messineo I, Bonaiuti D. How could robotic training and botolinum toxin be combined in chronic post stroke upper limb spasticity? A pilot study. Eur J Phys Rehabil Med 2015;51:381–7. [PubMed] [Google Scholar]
- 12. Poli P, Morone G, Rosati G, Masiero S. Robotic technologies and rehabilitation: new tools for stroke patients’ therapy. Biomed Res Int 2013;2013: 153872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saita K, Morishita T, Hyakutake K, Fukuda H, Shiota E, Sankai Y, et al. Combined therapy using botulinum toxin A and single-joint hybrid assistive limb for upper-limb disability due to spastic hemiplegia. J Neurol Sci 2017;373:182–7. [DOI] [PubMed] [Google Scholar]
- 14. Vanclay F. Functional outcome measures in stroke rehabilitation. Stroke 1991;22:105–8. [DOI] [PubMed] [Google Scholar]
- 15. Wissel J, Schelosky LD, Scott J, Christe W, Faiss JH, Mueller J. Early development of spasticity following stroke: a prospective, observational trial. J Neurol 2010;257:1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


