Abstract
Objective:
To examine associations between circulating levels of the bone-derived protein osteocalcin (OC) and type 2 diabetes (T2D) risk in Latino children and adults.
Methods:
Serum OC was measured in 136 children and 531 adults who had the following T2D risk factors assessed, body mass index (BMI), Hemoglobin A1c (HbA1c), fasting and 2-hour glucose during an oral glucose tolerance test.
Results:
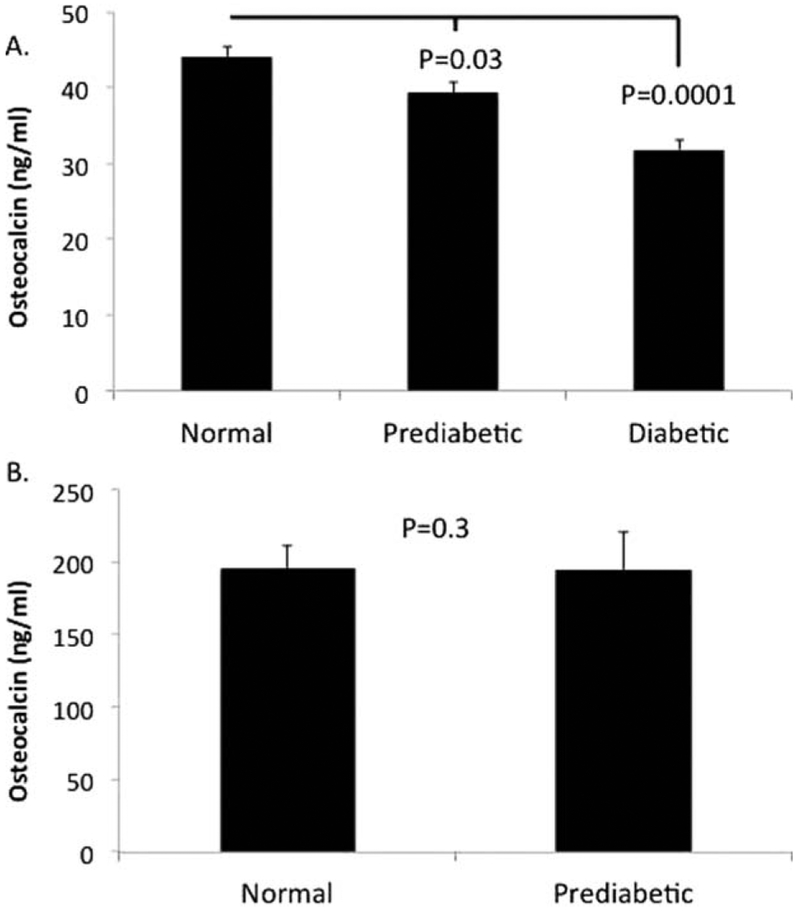
OC was significantly higher in children than adults (209.0 ± 12.1 vs. 41.0 ± 0.9 ng/ml, (p<0.000l). In adults, OC was inversely associated (all p<0.001) with BMI (r=−0.2), HbA1c (r=−0.2), fasting glucose (r=−0.16), and 2-hour glucose (r=−0.21), while there were no significant associations in children. There was a stepwise decrease in OC with increasing dysglycemia in adults, normoglycemic (44.1 ± 1.3 ng/ml), prediabetic (39.3 ± 1.3 ng/ml), and T2D (31.8 ± 1.2 ng/ml), (p<0.000l), whereas there were no differences between normal and prediabetic youth (195.7 ± 16.1 vs. 194.7 ± 25.8 ng/ml, p=0.3).
Conclusions:
OC was inversely associated with T2D risk in Latino adults; however, this pattern was not observed in children.
INTRODUCTION
Evidence has identified bone as an important regulatory tissue for energy metabolism, thereby modifying type 2 diabetes (T2D) risk (Confavreux et al., 2009). More specifically, both cross-sectional and prospective studies suggest a protective effect of the bone-derived protein osteocalcin (OC), on T2D risk in adults (Diaz-Lopez et al., 2013). In contrast, less is known about the association between OC and T2D risk in children with the majority of pediatric studies focused on non-Hispanic whites (Polgreen et al., 2012; Reinehr et al., 2010; Pollock et al., 2011; Prats-Puig et al., 2010; Boucher-Berry et al., 2012). Given the disproportionate rates of obesity, insulin resistance, and T2D in Latino youth, there is a need to better understand the relationship between OC and T2D risk in this population and how it may differ from adults. Therefore, the purpose of this study was to examine the associations between OC and T2D risk across the life course in a Latino population.
RESEARCH DESIGN AND METHODS
Participants
Data and samples used for the present investigation were derived from 667 self-identified Latino youth and adults (ages 7–85 years old, 20.4% children, and 79.6% adults), who participated in the Arizona Insulin Resistance Registry. Details of the Arizona Insulin Resistance Registry are presented elsewhere (Shaibi et al., 2013). Briefly, the overall purpose of the project was to establish infrastructure to: 1) facilitate clinical research opportunities for the Latino community of Phoenix, AZ and 2) support transdisciplinary research collaborations among biomedical researchers in the area. Participants consented to a comprehensive cardiometabolic screening visit as well as banking of samples, including serum, to support future research. This study was approved by the Institutional Review Board of the Arizona State University and all participants provided written informed consent (parental consent and child assent for those <18 years of age) prior to study procedures.
Measures
The following descriptive and clinical characteristics were extracted from the database: age, sex, height, weight, body mass index (BMI), and BMI percentile for children. T2D risk was assessed from data collected during a fasting blood draw [hemoglobin Ale (HbA1c) and glucose] and, in participants ≥12 years of age, a standard 75 gram oral glucose tolerance test (OGTT) was administered with 2-hour glucose collected. Glycemic status (normal, prediabetes, and T2D) was determined according to the American Diabetes Association classification (American Diabetes Association, 2014). Fasting serum total OC levels were measured by sandwich immunometric assay using antihuman osteocalcin antibody (Meso-Scale Discoveries, Gaithersburg, Md). Intraassay CV=5.7% and 5.8% at 12.9 and 32.5 ng/mL, and interassay CV=21.1% and 19.3% at 13.4 and 35.9 ng/mL.
Analysis
Pearson correlation analysis was used to examine associations between OC and BMI, (BMI percentile for youth), HbA1c, fasting glucose and 2-hour post-challenge glucose. Differences in mean OC levels by glycemic status (healthy, prediabetes and T2D) were analyzed by analysis of variance in adults and by independent sample t-test in youth (no cases of T2D were detected in youth). All analyses were conducted separately in youth and adults using SPSS V20.0 with significance set at p<0.05.
RESULTS
Demographic characteristics of study participants by age are presented in Table 1. Correlations between OC and T2D risk factors are presented in Table 2. There was a significant inverse association between OC and all T2D risk factors in adults while none of the associations were significant in children. Similarly there was a significant stepwise decrease in mean OC levels with increasing dys-glycemia in adults (Figure 1A), whereas there were no differences between normal and prediabetic youth (Figure 1B). OC levels were considerably higher in youth compared to adults (209.0 ±12.1 vs. 41.0 ±0.9 ng/ml, p<0.0001). Therefore, we compared OC by decade of life and found that OC levels were highest in the first and second decade (253 ± 20.4 and 205 ± 13.1 ng/ml, respectively) and by the third decade decreased (to 39.0 ± 0.8 ng/ml) and remained relatively stable throughout adulthood. In addition to these age-associated differences, OC levels were significantly higher in males than females, in both youth (male: 265.9 ± 18.6 vs. female: 156.0 ± 12.3 ng/ml, p<0.001) and adults (male: 48.1 ± 1.6 vs. female: 37.2 ± 1.0 ng/ml, p<0.00l). Further, OC levels were higher in US-born compared to foreign-born adults (48.1 ±3.6 vs. 39.9 ± 0.8 ng/ml, p=0.03) and youth (227.4 ± 17.1 vs. 181.8 ± 15.6 ng/ml, p=0.06).
TABLE 1.
Descriptive Characteristics by Age Group
| Adults (≥ 18) | Children (<18) | |
|---|---|---|
| Age (years) | 36.3 ± 0.5 | 14.0 ± 0.2 |
| Sex (M/F) | 192/339 | 68/68 |
| Country of Origin (US/Foreign)a | 69/427 | 68/46 |
| Height (cm) | 163.6 ± 0.4 | 159.8 ± 1.2 |
| Weight (kg) | 80.8 ± 0.9 | 63.0 ± 2.0 |
| BMI (kg/m2)/BMI percentile (%) | 30.2 ± 0.3 | 73.2 ± 2.6 |
Collected in a subset of the cohort
TABLE 2.
Association between Osteocalcin and type 2 diabetes risk factors in children and adults.
| Variable | Adults (≥18yo) | Children (<18yo) |
|---|---|---|
| BMI/BMI % | r= −0.20, p<0.001 | r= −0.03, p=0.8 |
| Fasting Glucose | r= −0.16, p<0.001 | r= 0.12, p=0.2 |
| 2-Hour Glucose | r= −0.21, p<0.001 | r= 0.12, p=0.2 |
| HbA1c | r= −0.20, p<0.001 | r= 0.04, p=0.7 |
Fig. 1.
Osteocalcin levels and dysglycemia in adults (A) and youth (B).
CONCLUSIONS
The results of this study indicate that lower OC levels are associated with increasing T2D risk factors and worsening glycemic profile in adults. However, this pattern was not observed in children. It is hypothesized that the protective effect of OC on T2D is due to a function of OC’s direct effect on the β-cell to stimulate insulin secretion as well as an indirect effect on adipocytes to stimulate secretion of adiponectin, a known insulin sensitizer (Patti et al., 2013). A balance between insulin secretion and insulin sensitivity is critical for maintaining glucose homeostasis. In the presence of a healthy β-cell, insulin resistance can be compensated for by increased insulin secretion. Therefore, the stimulatory effects of OC on β-cell function may be critical in populations that are susceptible to insulin resistance, such as Latinos.
In contrast, the lack of a protective effect in youth may be a function of physiologic elevations in OC secondary to an increase in bone turnover during childhood (Bayer, 2014). Despite the high OC levels in our pediatric cohort, there were no significant associations with T2D risk factors (BMI, HbA1c, fasting and 2-hour blood glucose) and OC levels did not differ between healthy and prediabetic youth. These results are in contrast to those of Pollock et al (2011), who found that prepubertal Caucasian and African-American children with prediabetes exhibited lower total OC levels compared to normoglycemic peers. Differences between the present findings and those of Pollock et al. may be driven by developmental differences in the populations studied. Pollock et al (2011) studied only prepubertal youth (mean age 9.2 ± O.lyears) whereas those in the present investigation were likely mid to late puberty (mean age of 14.9 ± 0.4 years). Therefore, we suspect that factors during pubertal growth may modulate the relationship between OC and T2D risk. For example, it is plausible that bone-specific insulin resistance, as recently described by Wei J et al.(2014) in mouse models, could attenuate the protective effect of OC on diabetes. The phenomenon of pubertal insulin resistance is well-described in the literature but is usually presented in relation to skeletal muscle insulin resistance. Another possible reason for the lack of a protective effect of OC on diabetes risk during development could be a classic endocrine negative feedback loop between elevated OC and insulin secretion whereby beyond a certain point, increasing levels of OC may decrease insulin secretion. The idea of a negative feedback loop in relationship to OC and T2D risk in youth was recently put forth by Rochefort et al. (2011), who found that 6-months of exercise training led to increases in OC as well as fasting insulin despite reductions in adiponectin. The authors postulated that above a certain threshold, the negative feedback may subsequently reduce β-cell function (Rochefort et al., 2011). Given that the present study was cross-sectional and included youth who were at various stages of pubertal development, this hypothesis needs to be tested further.
The novelty of this paper is that it focuses on a Latino population that is disproportionately impacted by insulin resistance and T2D in both children (Pettitt et al., 2014) and adults (Cowie et al., 2006). Moreover, there is evidence that some of the pathophysiologic processes contributing to diabetes risk among Latinos may be unique to this ethnic group (Lê et al., 2011). Our data suggest that country of origin may also influence osteocalcin levels but despite evidence that acculturation may be associated with T2D risk in Latinos, we did not find differential T2D rates by country of origin on this cohort (data not shown). In addition to focusing on Latinos, the inclusion of pediatric and adults participants in the same study is novel. Very little research has taken a life course approach to diabetes and given that up to 50% of Latino youth may develop T2D in their lifetimes (Narayan et al., 2003), it is critical to examine T2D risk factors across the lifespan. A limitation of this study is the lack of information regarding menstrual cycle status or timing in relation to data collection as OC levels can vary throughout the cycle in women. To our knowledge, this is the first study to examine the relationship between OC and T2D risk factors in children and adults in any population and our preliminary results suggest that the relationship between OC and T2D risk varies by age. Future studies that evaluate prospective trajectories in OC levels in relation to T2D risk are warranted.
ACKNOWLEDGMENTS
This work was supported by the Mayo Clinic Center for Individualized Medicine and the Mayo/ASU Center for Metabolic and Vascular Biology. Data management support was provided by Grant UL1-RR-4150 from the Mayo Clinic to use Research Electronic Data Capture (RED-Cap). The authors are grateful to the children and their families who participated in this study. The authors thank Veronica Vital and the staff of the Clinical Research Unit for their help with enrolling and testing participants and Angela Dalenberg for her assistance with measuring osteocalcin.
Contract grant sponsor: Mayo Clinic; Contract grant number: UL1-RR-024150
LITERATURE CITED
- American Diabetes Association. 2014. Diagnosis and classification of diabetes mellitus. Diabetes Care 37 Suppl 1(January):S81–90. [DOI] [PubMed] [Google Scholar]
- Bayer M. 2014. Reference values of osteocalcin and procollagen type I N-propeptide plasma levels in a healthy Central European population aged 0–18 years. Osteoporos Int 25(2):729–36. [DOI] [PubMed] [Google Scholar]
- Boucher-Berry C, Speiser PW, Carey DE, Shelov SP, Accacha S, Fennoy I, Rapaport R, Espinal Y, Rosenbaum M. 2012. Vitamin D, osteocalcin, and risk for adiposity as comorbidities in middle school children. J Bone Miner Res 27(2):283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux CB, Levine RL, Karsenty G. 2009. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 310(1–2):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW, 2006. Prevalence of diabetes and impaired fasting glucose in adults in the U. S. Diabetes Care 29(6): 1263–1268. [DOI] [PubMed] [Google Scholar]
- Díaz-López A, Bulló M, Juanola-Falgarona M, Martínez-González MA, Estruch R, Covas MI, Arós F, Salas-Salvadó J. 2013. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study. J Clin Endocrinol Metab 98(11):4524–31. [DOI] [PubMed] [Google Scholar]
- Garanty-Bogacka B1, Syrenicz M, Rać M, Krupa B, Czaja-Bulsa G, Walczak M, Sowińska-Przepiera E, Syrenicz A. 2013. Association between serum osteocalcin, adiposity and metabolic risk in obese children and adolescents. Endokrynol Pol (5):346–52. [DOI] [PubMed] [Google Scholar]
- Lê KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M, Hu HH, Nayak KS, Goran MI. 2011. Ethnic Differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care 34: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003; 290(14): 1884–1890 [DOI] [PubMed] [Google Scholar]
- Patti A, Gennari L, Merlotti D, Dotta F and Nuti R. 2013. Endocrine actions of osteocalcin. Int J Endocrinol 2013:846480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM, Liese AD, Linder B, Mayer-Davis EJ, Pihoker C, Saydah SH, Standiford DA, Hamman RF; SEARCH for Diabetes in Youth Study Group. 2014. Prevalence of diabetes in U. S. youth in 2009: The SEARCH for diabetes in youth study. Diabetes Care 37(February): 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgreen L, Jacobs D, Nathan B. 2012. Association of osteocalcin with obesity, insulin resistance, and cardiovascular risk factors in young adults. Obesity(Silver Spring) 20(11, November 2012):2194–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, Bassali RW, Davis CL. 2011. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J Clin Endocrinol Metab 96(7):E1092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats-Puig A, Mas-Parareda M, Riera-Pérez E, González-Forcadell D, Mier C, Mallol-Guisset M, Díaz M, Bassols J, de Zegher F, Ibáñez L, López-Bermejo A. 2010. Carboxylation of osteocalcin affects its association with metabolic parameters in healthy children. Diabetes Care 33(3):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, and Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between bsteocalcin, leptin and insulin resistance in obese children before and after weight loss. 2010. Int J Obes (Lond) 34(5):852–858. [DOI] [PubMed] [Google Scholar]
- Rochefort GY, Rocher E, Aveline PC, Garnero P, Bab I, Chappard C, Jaffré C, Benhamou CL. 2011. Osteocalcin-insulin relationship in obese children: a role for the skeleton in energy metabolism. Clin Endocrinol (Oxf) 75(2):265–70. [DOI] [PubMed] [Google Scholar]
- Shaibi GQ, Coletta DK, Vital V, Mandarino LJ. 2013. The Design And Conduct Of A community-based registry and biorepository: A focus on cardi-ometabolic health in Latinos. Clin Transi Sci 6(6):429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner WS, Karsenty G. 2014. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest 124(4): 1781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]