Key Points
Question
Is fibrinogen concentrate noninferior to cryoprecipitate for treatment of bleeding related to acquired hypofibrinogenemia in cardiac surgery?
Findings
In this randomized trial of 735 adult patients who underwent cardiac surgery and developed clinically significant bleeding and hypofibrinogenemia post cardiopulmonary bypass, the mean number of blood components transfused within 24 hours post bypass was 16.3 units in the fibrinogen concentrate group and 17.0 units in the cryoprecipitate group; ratio of the mean number of units transfused was 0.96, which met the prespecified noninferiority margin ratio of less than 1.2.
Meaning
For management of cardiac surgery–associated bleeding related to acquired hypofibrinogenemia, fibrinogen concentrate may be considered for fibrinogen replacement.
Abstract
Importance
Excessive bleeding is a common complication of cardiac surgery. An important cause of bleeding is acquired hypofibrinogenemia (fibrinogen level <1.5-2.0 g/L), for which guidelines recommend fibrinogen replacement with cryoprecipitate or fibrinogen concentrate. The 2 products have important differences, but comparative clinical data are lacking.
Objective
To determine if fibrinogen concentrate is noninferior to cryoprecipitate for treatment of bleeding related to hypofibrinogenemia after cardiac surgery.
Design, Setting, and Participants
Randomized clinical trial at 11 Canadian hospitals enrolling adult patients experiencing clinically significant bleeding and hypofibrinogenemia after cardiac surgery (from February 10, 2017, to November 1, 2018). Final 28-day follow-up visit was completed on November 28, 2018.
Interventions
Fibrinogen concentrate (4 g; n = 415) or cryoprecipitate (10 units; n = 412) for each ordered dose within 24 hours after cardiopulmonary bypass.
Main Outcomes and Measures
Primary outcome was blood components (red blood cells, platelets, plasma) administered during 24 hours post bypass. A 2-sample, 1-sided test for the ratio of the mean number of units was conducted to evaluate noninferiority (threshold for noninferiority ratio, <1.2).
Results
Of 827 randomized patients, 735 (372 fibrinogen concentrate, 363 cryoprecipitate) were treated and included in the primary analysis (median age, 64 [interquartile range, 53-72] years; 30% women; 72% underwent complex operations; 95% moderate to severe bleeding; and pretreatment fibrinogen level, 1.6 [interquartile range, 1.3-1.9] g/L). The trial met the a priori stopping criterion for noninferiority at the interim analysis after 827 of planned 1200 patients were randomized. Mean 24-hour postbypass allogeneic transfusions were 16.3 (95% CI, 14.9 to 17.8) units in the fibrinogen concentrate group and 17.0 (95% CI, 15.6 to 18.6) units in the cryoprecipitate group (ratio, 0.96 [1-sided 97.5% CI, −∞ to 1.09; P < .001 for noninferiority] [2-sided 95% CI, 0.84 to 1.09; P = .50 for superiority]). Thromboembolic events occurred in 26 patients (7.0%) in the fibrinogen concentrate group and 35 patients (9.6%) in the cryoprecipitate group.
Conclusions and Relevance
In patients undergoing cardiac surgery who develop clinically significant bleeding and hypofibrinogenemia after cardiopulmonary bypass, fibrinogen concentrate is noninferior to cryoprecipitate with regard to number of blood components transfused in a 24-hour period post bypass. Use of fibrinogen concentrate may be considered for management of bleeding in patients with acquired hypofibrinogenemia in cardiac surgery.
Trial Registration
ClinicalTrials.gov Identifier: NCT03037424
This noninferiority trial compares the effects of fibrinogen concentrate vs cryoprecipitate on the number of red blood cell (RBC), platelet, and plasma units transfused 24 hours after cardiopulmonary bypass in adult patients with clinically significant bleeding and hypofibrinogenemia after cardiac surgery.
Introduction
Excessive bleeding necessitating blood component transfusion is common after cardiac surgery and is associated with increased risk of morbidity and mortality and associated costs.1 The pathophysiology of cardiac surgery–related bleeding is often multifactorial but includes acquired hypofibrinogenemia (fibrinogen level <1.5-2.0 g/L), which is associated with excessive bleeding in children2 and adults.3 In cases of excessive bleeding and acquired hypofibrinogenemia, guidelines recommend treatment with either cryoprecipitate or fibrinogen concentrate.4,5
Although both products are plasma-derived, they have distinct features. Cryoprecipitate is a nonpurified product; hence, in addition to fibrinogen it contains fibronectin and platelet microparticles, as well as coagulation factors VIII, XIII, and von Willebrand factor.6 Its fibrinogen content varies widely (from 3-30 g/L per unit); in most cases it must be maintained and shipped in a frozen state and then thawed and pooled (typically 5-10 unit pools) before administration; and it has a limited shelf life after thawing (<4-6 hours), increasing wastage.7,8 Fibrinogen concentrates are pathogen-reduced and purified; have standardized fibrinogen content (20 g/L); are lyophilized, allowing for easy storage, reconstitution, and administration; and have longer shelf life after reconstitution (up to 24 hours), which reduces wastage. There is divergent practice regarding the preferred product for fibrinogen replacement.9 Cryoprecipitate remains the therapy of choice in many areas (including North America). In many European countries, however, fibrinogen concentrates have replaced cryoprecipitate, even though very few studies have directly compared these products.10,11
In this multicenter randomized clinical trial in patients undergoing cardiac surgery and requiring fibrinogen replacement because of clinically significant bleeding related to acquired hypofibrinogenemia, the objective was to determine if fibrinogen concentrate was noninferior to cryoprecipitate as measured by the amount of allogeneic blood components (red cells, platelets, and plasma) administered.
Methods
Trial Oversight
This was an investigator-initiated, multicenter, randomized clinical trial conducted at 11 centers in Canada. The trial design and methodology have been previously published12; the study protocol and statistical analysis plan are available in Supplement 1, and aspects of the trial design and methodology are included in the eMethods in Supplement 2. The trial coordinating center was the Department of Anesthesia and Pain Management Clinical Trials Unit at the University Health Network (Toronto, Ontario, Canada). The trial was overseen by an independent data and safety monitoring committee, which reviewed adverse events after every 100 patients and reviewed a preplanned interim analysis performed after approximately 600 evaluable patients were enrolled. Study monitors independently reviewed all primary outcomes and adverse events. The trial was performed in accordance with the principles of the Declaration of Helsinki and applicable regulatory requirements. Research ethics board approval was obtained at each site before trial initiation, including approval of a delayed written consent process. Consent was delayed until after treatment (in accordance with the Canadian Tri-Council Policy Statements on the ethical conduct of research involving humans),13 in part because it is not possible to reliably predict before surgery which patients will require fibrinogen replacement for bleeding control during or after surgery, at which time there is a need for rapid access to therapy.
Patients
Adult patients undergoing cardiac surgery with cardiopulmonary bypass for whom fibrinogen replacement was ordered in response to clinically significant postbypass bleeding deemed related to acquired hypofibrinogenemia (fibrinogen plasma level <2.0 g/L by the Clauss method or FIBTEM [fibrin-based thromboelastometry test extrinsically activated with tissue factor and containing the platelet inhibitor cytochalasin D]–derived clot amplitude at 10 minutes <10 mm by thromboelastometry) were eligible for enrollment.14,15 In line with clinical practice, patients with bleeding were also eligible if hypofibrinogenemia was suspected but plasma fibrinogen levels were unavailable at the time of decision-making.
Blood bank technologists screened and randomized eligible patients if they had none of the following exclusion criteria: receipt of fibrinogen concentrate or cryoprecipitate within 24 hours before surgery; history of severe allergic reaction to fibrinogen concentrate or cryoprecipitate; refusal of blood components for religious or other reasons; plasma fibrinogen level greater than 3.0 g/L within 30 minutes of treatment order (to avoid increasing levels above the upper limit of normal [4.0 g/L]); and known pregnancy.
Trial Procedures
Participants were randomly assigned (1:1) to study groups using a pseudorandom number generator (PROC PLAN procedure in SAS) in randomly permuted blocks of 4, stratified by center. Allocation was blinded; randomization schedule was kept at the blood banks in sequentially numbered opaque sealed envelopes (prepared by Ergomed GmbH), which were opened when the order for fibrinogen replacement was received. Patients in the fibrinogen concentrate group received 4 g of fibrinogen concentrate (Fibryga; Octapharma AG), to be infused over approximately 10 minutes, and those in the cryoprecipitate group received 10 units of cryoprecipitate, to be infused according to local practice. The dosages for fibrinogen concentrate16,17,18 and cryoprecipitate19,20 were consistent with recently published trials and guidelines. Cryoprecipitate dose in Canada is standardized at 10 units per dose,21 and although the amount of fibrinogen in each unit of cryoprecipitate is variable, on average each 10-unit pool contains approximately 4 g of fibrinogen (Canadian Blood Services data; Dana Devine, PhD, Canadian Blood Services, email communication, September 2019).
Patients were to only receive the allocated product for 24 hours after cardiopulmonary bypass, after which only cryoprecipitate was used for fibrinogen replacement. It was not feasible to blind clinicians involved in product administration, but clinicians not involved in product administration, patients, data collectors, and outcome assessors were blinded. To maintain blinding, chart labels for both products stated “Fibrinogen Study Product 4 grams.” Data on race were collected for descriptive purposes as reported by patients based on predefined categories.
There were no other alterations to patient care. Each hospital had an established protocol for bleeding management that was not altered for the purposes of this study. Tranexamic acid use was routine at all centers. Administration of blood components and hemostatic agents (other than cryoprecipitate and fibrinogen concentrate), use of cell salvage, and viscoelastic testing were based on local hospital protocols.
Outcomes
The primary efficacy outcome was cumulative allogeneic blood component units (red blood cells, platelets, and plasma) administered for 24 hours after termination of cardiopulmonary bypass. Since 2 types of platelets are issued in Canada (75% standard buffy-coat pools from 4 allogeneic donors and 25% apheresis units from a single allogeneic donor), both types were counted as a 4-unit transfusion. Secondary outcomes included individual blood component units administered for 24 hours after termination of cardiopulmonary bypass, all transfusions from beginning of surgery to postoperative day 7, bleeding severity (according to the universal definition of perioperative bleeding [UDPB])22 during the 24 hours after cardiopulmonary bypass, and pretreatment and posttreatment fibrinogen levels. The UDPB components “delay in chest closure” and “cryoprecipitate or fibrinogen concentrate administration” were not used. Postoperative follow-up was 28 days. Adverse events were classified using the Medical Dictionary for Regulatory Activities (version 21.1) system of nomenclature. Adverse events that started or worsened on or after the first dose of the investigational product were classified as treatment-emergent. Data on duration of mechanical ventilation, intensive care unit stay, and hospitalization after surgery were also collected.
Statistical Analysis
Sample size was based on demonstrating noninferiority of fibrinogen concentrate relative to cryoprecipitate with respect to the primary efficacy outcome. Determination of noninferiority was based on a 1-sided type I error probability of .025 and a noninferiority margin of 20% for the ratio of mean number of units transfused per group. A 20% margin was deemed by an expert panel to be a plausible trade-off in efficacy in exchange for the potential advantages of fibrinogen concentrate relative to those of cryoprecipitate. An empirical distribution function was calculated based on a mean of 16 units and a standard deviation of 14 units (derived from a recent study on bleeding management in cardiac surgery)23 and was used to estimate power by performing 10 000 simulations for each of the possible sample sizes. Calculations showed that 1200 patients would provide an empirical power greater than 90% (assuming a 10% drop-out rate and equal mean units transfused per group).
The primary analysis set comprised all randomized patients who had undergone cardiac surgery with cardiopulmonary bypass, received at least 1 (partial or complete) dose of either treatment, and for whom informed consent was obtained. The per-protocol analysis excluded patients who did not undergo cardiac surgery with cardiopulmonary bypass, received the incorrect treatment, received less than 80% of the planned dose, or received the first treatment more than 24 hours after cardiopulmonary bypass. The prespecified subgroups for analysis were elective vs nonelective surgery, simple vs complex surgery, and a subgroup excluding patients who were in critical state before surgery (blinded adjudication based on predefined conditions such as acute aortic dissection or cardiac arrest immediately before surgery). Post hoc subgroups analyzed were patients undergoing nonelective surgery and not in critical state before surgery, patients who had a fibrinogen measurement before product administration, patients with confirmed hypofibrinogenemia (with Clauss method) before product administration, and patients with at least moderate blood loss according to the UDPB.22
A 2-sample, 1-sided test of the null hypothesis that the treatment group fibrinogen concentrate to cryoprecipitate ratio of the mean number of allogenic blood components was 1.2 or greater (ie, 20% noninferiority margin) was used to evaluate noninferiority using Poisson regression modeling for count data. Noninferiority would be demonstrated if the upper limit of the model-derived 1-sided 97.5% CI was less than 1.2. If noninferiority was demonstrated, an analogous test for superiority was to be performed, testing the hypothesis that the treatment group ratio of the mean number of allogenic blood components was 1.0 or greater against the alternative that it was less than 1.0, at a 1-sided type I error level of α = .025. No α adjustment was made because of the hierarchical ordering of the tests. This analysis was repeated for the secondary allogeneic blood component transfusion outcomes.
There was 1 prespecified interim analysis after approximately 50% of the planned 1200 evaluable patients were enrolled, which used an adjusted type I error rate of .00258 according to the O’Brien-Fleming method24 for a noninferiority stop. The trial was also to be stopped for futility if the predictive power for the test of noninferiority was less than 0.25.
Secondary outcomes were examined using descriptive statistics, the Wilcoxon rank-sum test, point estimates and risk ratios with 2-sided 95% CIs, and the Hodges-Lehmann estimator of median differences with 95% CIs, where appropriate. Kaplan-Meier estimates for time-to-death distribution were calculated and graphically presented. Comparisons were prespecified as superiority analyses with α = .05.
Several post hoc analyses were performed: (1) noninferiority was assessed for the outcome of cumulative allogeneic blood component units administered from investigational product administration to 24 hours after cardiopulmonary bypass; (2) treatment × site interaction was accounted for by including site in the model for the primary outcome as a fixed-effect variable (as 3 categories based on number of patients enrolled: <40; 40-99; >99); and (3) the relationship of critical state before surgery was explored by adjusting for it in the comparisons for the primary outcome and mortality. The results of secondary outcomes and post hoc analyses should be interpreted as exploratory, as no correction for type I error was made.
Variables with missing values are noted where applicable and patients with missing values were excluded from the relevant analyses. All analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
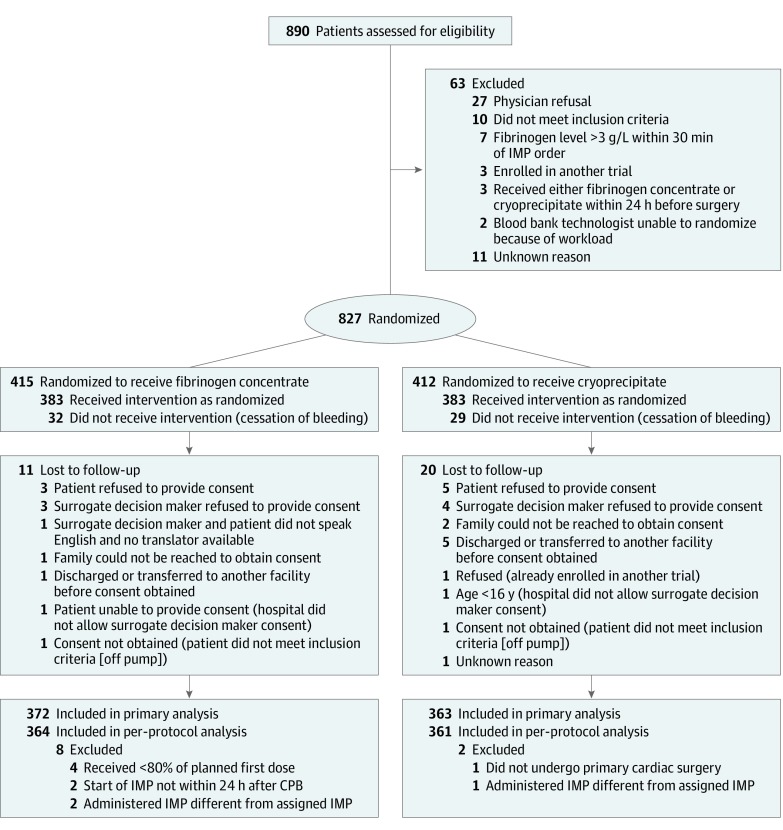
Patient recruitment continued from February 10, 2017, to November 1, 2018, with the final follow-up visit on November 28, 2018. The trial was stopped based on the recommendation of the independent data and safety monitoring committee for meeting the criterion for noninferiority at the interim analysis. Of the 15 412 patients who underwent cardiac surgery at the 11 participating sites, 827 (5.3%) were screened and randomized (Figure 1). Sixty-one randomized patients were not treated because of cessation of bleeding. Informed consent could not be obtained for 31 treated patients, leaving 735 patients in the primary analysis set (n = 372 for fibrinogen concentrate, n = 363 for cryoprecipitate). After excluding 10 patients with major protocol deviations, 725 patients were included in the per-protocol analysis (Figure 1). No patients in the primary analysis set had missing transfusion or adverse event information; thus, they were all included in the relevant analyses.
Figure 1. Patient Flow in the FIBRES Study of the Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery.
CPB indicates cardiopulmonary bypass; IMP, investigational medicinal product.
Baseline demographics and surgical characteristics were well balanced except for critical state before surgery (Table 1). Patients received a median of 4 (interquartile range [IQR], 4-4) g of fibrinogen concentrate and 10 (IQR, 10-10) units of cryoprecipitate (Table 2; eTable 1 in Supplement 2).
Table 1. Characteristics of the Study Population at Baseline.
| Characteristic | No. (%) | |
|---|---|---|
| Fibrinogen Concentrate (n = 372) | Cryoprecipitate (n = 363) | |
| Age, median (IQR), y | 65 (54-72) | 64 (53-72) |
| Sex | ||
| Women | 113 (30.4) | 105 (28.9) |
| Men | 259 (69.6) | 258 (71.1) |
| Race | ||
| White | 287 (77.2) | 273 (75.2) |
| Asian | 51(13.7) | 58 (16.0) |
| Black | 3 (0.81) | 6 (1.6) |
| Aboriginal | 5 (1.3) | 2 (0.55) |
| Othera | 26 (7.0) | 24 (6.6) |
| Body mass index, median (IQR)b | 22.2 (19.6-25.4) | 22.8 (19.6-25.6) |
| NYHA classc | ||
| I (least severe) | 104 (27.9) | 112 (30.8) |
| II | 110 (29.6) | 108 (29.8) |
| III | 110 (29.6) | 103 (28.4) |
| IV (most severe) | 48 (12.9) | 40 (11.0) |
| Myocardial infarction, d | ||
| No. | 367 | 358 |
| None | 294 (80.1) | 299 (83.5) |
| 0-90 | 39 (10.6) | 30 (8.4) |
| >90 | 34 (9.3) | 29 (8.1) |
| Ejection fraction, % | ||
| No. | 357 | 347 |
| >50 | 256 (71.7) | 263 (75.8) |
| 31-50 | 51 (14.3) | 57 (16.4) |
| 21-30 | 28 (7.8) | 12 (3.5) |
| <21 | 22 (6.2) | 15 (4.3) |
| Pulmonary pressure, mm Hg | ||
| No. | 308 | 303 |
| ≤30 | 225 (73.1) | 215 (71.0) |
| 31-55 | 58 (18.8) | 68 (22.4) |
| >55 | 25 (8.1) | 20 (6.6) |
| Hypertension | 234 (62.9) | 240 (66.1) |
| Dyslipidemia | 185 (49.7) | 185 (51.0) |
| Congestive heart failure | 113 (30.4) | 91 (25.1) |
| Atrial fibrillation | 81 (21.8) | 80 (22.0) |
| Diabetes mellitus | 80 (21.5) | 74 (20.4) |
| Chronic lung disease | 53 (14.3) | 37 (10.2) |
| Stroke/TIA | 46 (12.4) | 49 (13.5) |
| Peripheral vascular disease | 37 (10.0) | 34 (9.4) |
| Active endocarditis | 19 (5.1) | 18 (5.0) |
| CCS class IV angina | 15 (4.0) | 13 (3.6) |
| Dialysis (preoperative) | 9 (2.4) | 14 (3.9) |
| Intra-aortic balloon pump | 10 (2.7) | 3 (0.8) |
| VAD/ECMO | 9 (2.4) | 9 (2.5) |
| Critical state before surgeryd | 63 (16.9) | 38 (10.5) |
| Preoperative laboratory values, median (IQR) | ||
| Creatinine, μmol/L | 88 (72-107) [n = 359] |
86 (72-106) [n = 352] |
| Hemoglobin, g/dL | 13.4 (11.6-14.6) [n = 366] |
13.5 (11.7-14.8) [n = 356] |
| Platelet count, ×103/μL | 189 (154-235) [n = 365] |
185 (152-230) [n = 356] |
| International normalized ratio | 1.02 (0.98-1.20) [n = 335] |
1.03 (0.98-1.15) [n = 334] |
| Surgical factors | ||
| Nonelective surgery | 141 (37.9) | 128 (35.3) |
| Complex surgerye | 267 (71.8) | 260 (71.6) |
| Procedure (% of procedures)f | 653 (100.0) | 612 (100.0) |
| Aortic valve procedure | 165 (25.3) | 146 (23.9) |
| Surgery on aorta | 161 (24.7) | 177 (28.9) |
| CABG surgery | 153 (23.4) | 146 (23.9) |
| Mitral valve procedure | 68 (10.4) | 70 (11.4) |
| Tricuspid valve procedure | 31 (4.7) | 35 (5.7) |
| ASD/VSD repair | 20 (3.1) | 8 (1.3) |
| Heart transplant | 18 (2.8) | 10 (1.6) |
| Complex congenital | 11 (1.7) | 11 (1.8) |
| Otherg | 26 (4.0) | 9 (1.5) |
| Cardiopulmonary bypass duration, median (IQR), min | 143 (102-209) | 134 (99-200) |
Abbreviations: ASD, atrial septal defect; CABG, coronary artery bypass graft; CCS, Canadian Cardiovascular Society; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; NYHA, New York Heart Association; TIA, transient ischemic attack; VAD, ventricular assist device; VSD, ventricular septal defect.
Other races marked as unknown or not applicable.
Calculated as weight in kilograms divided by height in meters squared.
NYHA functional classification: I = no limitation of physical activity and no symptoms; II = slight limitation of physical activity (ordinary physical activity results in fatigue, palpitation, dyspnea); III = marked limitation of physical activity (less than ordinary activity causes fatigue, palpitation, or dyspnea); IV = unable to carry on any physical activity without discomfort (symptoms of heart failure at rest).
Determined by blinded adjudication for patients who underwent emergency surgery deemed to be in a critical state, based on criteria outlined in Supplement 2.
Procedures other than CABG surgery only, single valve only, or repair of atrial septal defect only.
Totals exceed 100% because some patients underwent more than 1 procedure.
Examples of other procedures included aortic root enlargement, left ventricular aneurysmectomy, and left atrial appendage resection.
Table 2. Details of Intervention, Perioperative Laboratory Values, Fibrinogen Levels, and Bleeding.
| Variable | Median (IQR) | |
|---|---|---|
| Fibrinogen Concentrate (n = 372) | Cryoprecipitate (n = 363) | |
| Dosage of investigational product | ||
| Mean (SD) | 4.8 (2.1) g | 12.9 (8.5) units |
| Median (IQR) | 4 (4-4) g | 10 (10-10) units |
| Doses of investigational product, No. (%) | ||
| 1 | 309 (83.1) | 296 (81.5) |
| 2 | 49 (13.2) | 49 (13.5) |
| 3 | 10 (2.7) | 10 (2.8) |
| ≥4 | 4 (1.1) | 8 (2.2) |
| Time from start of surgery to order of first dose, h | 5.8 (4.5-7.3) | 5.8 (4.6-7.1) |
| Time from order of first dose to administration, min | 45 (32-62) | 47 (37-60) |
| Time from end of CPB to administration of first dose, min | 97 (52-159) | 104 (59-160) |
| Plasma fibrinogen level, g/L | ||
| Pretransfusion | 1.6 (1.3-1.9) [n = 352] | 1.6 (1.3-1.9) [n = 346] |
| Posttransfusion | 2.5 (2.1-2.9) [n = 324] | 2.3 (2.0-2.6) [n = 296] |
| Change from pretransfusion | 0.90 (0.6-1.2) [n = 306] | 0.70 (0.5-1.0) [n = 280] |
| Blood components transfused before randomization, mean (SD), units | 4.3 (6.1) | 4.5 (6.0) |
| Cell salvage blood, mL | 373 (0-918) | 400 (0-876) |
| Nadir hematocrit during cardiopulmonary bypass, % | 26 (23-30) | 25 (23-30) |
| Hemoglobin, g/dL | ||
| Intraoperative post-CPB | 9.1 (8.3-9.9) [n = 348] | 8.9 (8.2-9.7) [n = 342] |
| Day of surgery (last recorded value) | 9.6 (8.6-10.9) [n = 365] | 9.5 (8.7-10.7) [n = 356] |
| Postoperative day 1 (last recorded value) | 9.0 (8.3-9.9) [n = 367] | 9.0 (8.3-10.0) [n = 361] |
| Platelet count, ×103/μL | ||
| Intraoperative post-CPB | 107 (84-136) [n = 280] | 103 (81-129) [n = 278] |
| Day of surgery (last recorded value) | 132 (107-165) [n = 362] | 133 (107-163) [n = 353] |
| Postoperative day 1 (last recorded value) | 117 (91-145) [n = 366] | 115 (92-143) [n = 359] |
| International normalized ratio | ||
| Intraoperative post-CPB | 1.6 (1.4-1.8) [n = 295] | 1.6 (1.5-1.8) [n = 277] |
| Day of surgery (last recorded value) | 1.3 (1.2-1.4) [n = 355] | 1.3 (1.2-1.4) [n = 340] |
| Postoperative day 1 (last recorded value) | 1.2 (1.1-1.4) [n = 342] | 1.2 (1.1-1.3) [n = 335] |
| Bleeding categories according to modified UDPB classification, No. (%)a | ||
| ≥2 (moderate to massive) | 349 (93.8) | 347 (95.6) |
| <2 (insignificant to mild) | 23 (6.2) | 16 (4.4) |
| 24-h chest tube drainage, mL | 800 (500-1350) | 830 (540-1350) |
| Reexploration, No. (%) | 53 (14.2) | 44 (12.1) |
| Prothrombin complex concentrate, No. (%) | 110 (29.6) | 98 (27.0) |
| Recombinant factor VII, No. (%) | 35 (9.4) | 28 (7.7) |
Abbreviations: CPB, cardiopulmonary bypass; IQR, interquartile range; UDPB, universal definition of perioperative bleeding.
UDPB determinants used in this study include postoperative chest tube output; units of red blood cells, plasma, and platelets transfused; use of factor concentrates; and surgical reexploration (classification scheme included in the eAppendix in Supplement 2).22 For this study the following components of the UDPB score were not used: delay in chest closure and use of cryoprecipitate or fibrinogen concentrate. One intraoperative death was included in the severe or massive category in the fibrinogen concentrate group.
Transfusions
In the primary analysis set, mean allogeneic blood component units administered were 16.7 (SD, 16.4) units within 24 hours after termination of cardiopulmonary bypass and 22.4 (SD, 23.1) units from beginning of surgery to postoperative day 7. The mean 24-hour postbypass cumulative allogeneic blood component transfusions were 16.3 (95% CI, 14.9 to 17.8) units in the fibrinogen concentrate group and 17.0 (95% CI, 15.6 to 18.6) units in the cryoprecipitate group, and the mean ratio was 0.96 (1-sided 97.5% CI, −∞ to 1.09; P < .001 for nonferiority; 2-sided 95% CI, 0.84 to 1.09; P = .50 for superiority]) (Table 3 and Figure 2).
Table 3. Primary and Secondary Outcomes: Allogeneic Blood Component Transfusions.
| Population | Fibrinogen Concentrate | Cryoprecipitate | Mean Difference (95% CI) | Unadjusted Ratio of LS Means (1-Sided 97.5% CI) | Noninferiority P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Median (IQR) | LS Mean (95% CI) | No. | Median (IQR) | LS Mean (95% CI) | ||||
| Primary Outcome: Cumulative Allogeneic Blood Components Transfused Within 24 h After Cardiopulmonary Bypassa | |||||||||
| Primary analysis set | 372 | 12.0 (5.5 to 22.0) |
16.3 (14.9 to 17.8) |
363 | 14.0 (7.0 to 23.0) |
17.0 (15.6 to 18.6) |
−0.73 (−3.10 to 1.64) |
Unadjusted 0.96 (−∞ to 1.09) |
<.001 |
| Adjusted 0.91 (−∞ to 1.03)c |
<.001 | ||||||||
| Per-protocol setb | 364 | 12.0 (6.0 to 22.0) |
16.4 (15.0 to 18.0) |
361 | 14.0 (7.0 to 22.0) |
16.9 (15.5 to 18.5) |
−0.50 (−2.90 to 1.89) |
Unadjusted 0.97 (−∞ to 1.10) |
<.001 |
| Adjusted 0.92 (−∞ to 1.05)c |
<.001 | ||||||||
| Secondary Outcome: Red Blood Cell Transfusions Within 24 h After Cardiopulmonary Bypass | |||||||||
| Primary analysis set | 372 | 2.0 (0.0 to 5.0) |
3.3 (3.0 to 3.7) |
363 | 2.0 (1.0 to 5.0) |
3.3 (2.9 to 3.7) |
0.23 (−0.55 to 1.00) |
1.00 (0 to 1.18) |
.02 |
| Per-protocol set | 364 | 2.0 (0.0 to 5.0) |
3.3 (3.0 to 3.7) |
361 | 2.0 (1.0 to 5.0) |
3.3 (2.9 to 3.7) |
0.25 (−0.53 to 1.03) |
1.01 (0 to 1.18) |
.02 |
| Secondary Outcome: Platelet Transfusions Within 24 h After Cardiopulmonary Bypass | |||||||||
| Primary analysis set | 372 | 8.0 (4.0 to 12.0) |
9.1 (8.3 to 9.9) |
363 | 8.0 (4.0 to 12.0) |
9.7 (8.9 to 10.5) |
−0.46 (−1.70 to 0.78) |
0.94 (−∞ to 1.06) |
<.001 |
| Per-protocol set | 364 | 8.0 (4.0 to 12.0) |
9.1 (8.3 to 10.0) |
361 | 8.0 (4.0 to 12.0) |
9.6 (8.8 to 10.5) |
−0.31 (−1.56 to 0.93) |
0.95 (−∞ to 1.08) |
<.001 |
| Secondary Outcome: Plasma Transfusions Within 24 h After Cardiopulmonary Bypass | |||||||||
| Primary analysis set | 372 | 2.0 (0.0 to 6.0) |
3.9 (3.5 to 4.4) |
363 | 3.0 (0.0 to 6.0) |
4.1 (3.6 to 4.6) |
−0.04 (−0.97 to 0.89) |
0.96 (−∞ to 1.15) |
.008 |
| Per-protocol set | 364 | 2.0 (0.0 to 6.0) |
4.0 (3.5 to 4.5) |
361 | 3.0 (0.0 to 6.0) |
4.0 (3.6 to 4.6) |
0.04 (−0.91 to 0.98) |
0.98 (−∞ to 1.17) |
.01 |
| Secondary Outcome: Cumulative Allogeneic Blood Components Transfused Within 7 d After Start of Surgery | |||||||||
| Primary analysis set | 372 | 15.5 (6.0 to 29.5) |
22.5 (20.5 to 24.7) |
363 | 17.0 (9.0 to 28.0) |
22.3 (20.3 to 24.5) |
0.21 (−3.14 to 3.55) |
Unadjusted 1.01 (0 to 1.15) |
.005 |
| Adjusted 0.96 (−∞ to 1.09)c |
<.001 | ||||||||
| Per-protocol set | 364 | 15.5 (6.0 to 29.0) |
22.5 (20.5 to 24.8) |
361 | 17.0 (9.0 to 28.0) |
22.2 (20.2 to 24.5) |
0.31 (−3.07 to 3.70) |
Unadjusted 1.01 (0 to 1.16) |
.007 |
| Adjusted 0.96 (−∞ to 1.09)c |
<.001 | ||||||||
Abbreviation: LS, least-squares.
Units of allogenic blood components counted as follows: each red blood cell unit = 1 unit, each 250-mL plasma unit = 1 unit, each 500-mL plasma unit = 2 units, each platelet dose = 4 units.
Per-protocol set excluded patients who did not undergo cardiac surgery with cardiopulmonary bypass, received the incorrect treatment, received less than 80% of the planned dose, or received the first treatment more than 24 hours after cardiopulmonary bypass.
Adjusted for critical state before surgery (post hoc analysis).
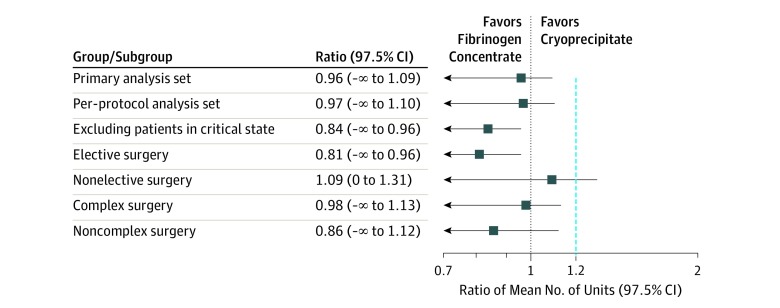
Figure 2. Ratio of Mean Number of Allogeneic Blood Components Transfused in the 24 Hours After Cardiopulmonary Bypass for the Primary Analysis Set, Per-Protocol Analysis Set, and A Priori–Defined Subgroups.
All patients in critical state were in the nonelective subgroup. Blue dashed line at x = 1.2 indicates the noninferiority margin.
Noninferiority was also observed for the secondary outcomes of individual 24-hour and cumulative 7-day blood component transfusions (Table 3), as well as in the post hoc outcome of cumulative transfusions measured from product administration to 24 hours after termination of cardiopulmonary bypass (mean units, 8.6 [95% CI, 7.5 to 9.9] in the fibrinogen concentrate group vs 8.9 [95% CI, 7.8 to 10.2] in the cryoprecipitate group; mean ratio, 0.97 [1-sided 97.5% CI, −∞ to 1.18; P = .02 for noninferiority) and after accounting for treatment × site interaction (P < .001) (eFigure 1 in Supplement 2) on the primary outcome (mean ratio, 1.00 [1-sided 97.5% CI, −∞ to 1.13; P = .003 for noninferiority). Noninferiority was also observed for all defined subgroups except for the nonelective group, which included all patients in critical state before surgery (Figure 2; eTable 2 in Supplement 2).
Fibrinogen Levels
Timing and number of doses of investigational product were similar between groups. Fibrinogen response was slightly greater in the fibrinogen concentrate group (median increase, 0.9 [IQR, 0.6-1.2] g/L vs 0.7 [IQR, 0.5–1.0] g/L; P < .001) (Table 2; eTable 1 in Supplement 2).
Adverse Events and Other Outcomes
Treatment-emergent adverse event profiles were similar (Table 4; eTable 3 in Supplement 2), as were the duration of intubation, intensive care unit stay, and hospital stay (Table 4). There were 35 deaths (9.4%) in the fibrinogen concentrate group and 27 (7.4%) in the cryoprecipitate group (unadjusted hazard ratio, 1.28 [95% CI, 0.77 to 2.12]; P = .35) (Kaplan-Meier curves shown in eFigures 2 and 3 in Supplement 2). Death rates were also not significantly different when stratified by critical state before surgery (fibrinogen concentrate vs cryoprecipitate: 19/63 [30.2%] vs 12/38 [31.6%] in critical state patients; 16/309 [5.2%] vs 15/325 [4.6%] in remaining patients) and after risk adjustment for critical state (post hoc analysis hazard ratio, 1.03 [95% CI, 0.62 to 1.70]; P = .92) (results from post hoc analyses reported in eTables 4 and 5 in Supplement 2). Thromboembolic adverse events were observed in 26 patients (7.0%) in the fibrinogen group and 35 patients (9.6%) in the cryoprecipitate group (unadjusted odds ratio, 0.70 [95% CI, 0.42 to 1.20]) (Table 4).
Table 4. Treatment-Emergent Adverse Events and Other Measured Outcomes at 28-Day Follow-up.
| Outcome | No. (%) | |
|---|---|---|
| Fibrinogen Concentrate (n = 372) | Cryoprecipitate (n = 363) | |
| Any adverse event | 248 (66.7) | 264 (72.7) |
| No. of events | 623 | 673 |
| Any serious adverse event | 117 (31.5) | 126 (34.7) |
| No. of events | 224 | 264 |
| Thromboembolic adverse eventsa | 26 (7.0) | 35 (9.6) |
| No. of events | 27 | 39 |
| Stroke/TIA | 17 (4.6) | 18 (5.0) |
| DVT/PE | 5 (1.3) | 9 (2.5) |
| Myocardial infarction | 3 (0.8) | 4 (1.1) |
| Other vessel thrombosis | 0 | 7 (1.9) |
| Amaurosis fugax | 0 | 1 (0.3) |
| Disseminated intravascular coagulation | 1 (0.3) | 0 |
| Thrombophlebitis | 1 (0.3) | 0 |
| Acute kidney injuryb | 48 (12.9) | 48 (13.2) |
| Hepatobiliary disordersc | 32 (8.6) | 37 (10.2) |
| Duration of mechanical ventilation, median (IQR), d | 1.3 (0.7-5.0) [n = 337] | 1.3 (0.7-4.2) [n = 342] |
| Duration of intensive care unit stay, median (IQR), d | 2.9 (1.4-5.7) [n = 352] | 2.8 (1.2-5.6) [n = 345] |
| Duration of hospitalization, median (IQR), d | 8.2 (6.3-13.0) [n = 314] | 9.0 (6.3-13.3) [n = 308] |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; TIA, transient ischemic attack.
Patients who experienced more than 1 event are counted only once in the total.
Acute kidney injury was defined as greater than a 2-fold increase in creatinine or kidney failure requiring hemodialysis within 28 days of surgery.
Hepatobiliary disorders were defined by the Medical Dictionary for Regulatory Activities (version 21.1) system of nomenclature.
Discussion
In this study of patients who experienced bleeding after cardiac surgery and required fibrinogen replacement as part of routine clinical practice, the primary finding was that fibrinogen concentrate was noninferior to cryoprecipitate as measured by the number of blood components transfused.
The study protocol, which did not modify clinical practice and used a delayed (postintervention) consent process, successfully randomized the small proportion of patients with bleeding who were ordered fibrinogen replacement (comprising approximately 5% of patients who underwent cardiac surgery at participating centers during the study period). The groups were well balanced for baseline variables except for critical state before surgery, an important prognostic variable that was more prevalent in the fibrinogen concentrate group. The occurrence of adverse events was comparable between the groups, although there was a nonsignificant difference of 2.0% in mortality. This seemed to be related to the between-group imbalance in the number of patients in critical state before surgery.
The study objective was to compare the 2 currently recommended therapies for fibrinogen replacement4,5 under usual conditions of clinical care. Thus, it did not include a placebo group to determine the efficacy of fibrinogen replacement per se, nor did it attempt to identify the appropriate threshold for fibrinogen replacement.16 A recent systematic review comparing fibrinogen concentrates with cryoprecipitate in patients with bleeding10 identified 1 randomized trial (with high risk for bias)19 and 3 observational studies25,26,27 and concluded that there was insufficient evidence to recommend one product over the other. Another review of trauma, obstetric, and gastrointestinal bleeding populations reached similar conclusions.9 This study provides further data regarding the comparative efficacy and adverse event profile of these 2 products.
The noninferiority finding can inform the choice of cryoprecipitate or fibrinogen concentrate for treatment of bleeding related to acquired hypofibrinogenemia. A core tenet and major regulatory driver in transfusion medicine is the precautionary principle that requires risk-mitigation strategies to be instituted even if there is only a theoretical risk of harm.28 Consistent with this principle, the US Food and Drug Administration blood safety strategy recommends the use of pathogen-reduced blood products when feasible.28 Currently, there are no known impending infectious threats for recipients of cryoprecipitate; however, the history of blood pathogens suggests that plasma-derived products have transmitted pathogens in the past and may do so in the future.29 In addition, mathematical models of emerging pathogens suggest that the association of plasma-derived products (such as cryoprecipitate) with outcomes and costs may be substantial.30 In this regard, fibrinogen concentrate may be preferred to cryoprecipitate because it is pathogen-reduced. Another advantage of fibrinogen concentrate is that in most situations it can be logistically simpler to deliver to the bedside of a patient with bleeding. One important consideration is the cost differential that currently favors cryoprecipitate, but this varies across regions, and the most recent economic analysis failed to include the costs of future emerging pathogens and did not include comprehensive activity-based costing.31
Limitations
This study has several limitations. First, since the aim was to compare the 2 products when used under usual conditions of care, it was not logistically possible to institute a standardized transfusion protocol across the sites or to blind clinicians to treatment assignment. Although a post hoc analysis showed that there were treatment × site interactions, accounting for the interactions did not have a material effect on the primary outcome. Moreover, postoperative hemoglobin, platelet count, and coagulation measures were similar between groups (Table 2), suggesting that transfusion practice was consistent in both groups. In addition, the results were consistent when transfusions were measured from beginning of surgery to postoperative day 7 (a priori analysis) or after administration of investigational product (post hoc analysis), which suggests that lack of blinding did not influence the timing of transfusions relative to product administration. Of note, to ensure that all transfusions and adverse events were collected without bias, patients and outcome assessors were blinded to treatment allocation and independent monitors reviewed all transfusion and adverse events. Second, while the study had few exclusion criteria, it included only patients undergoing cardiac surgery with bleeding, and its findings may therefore not be generalizable to other settings where fibrinogen replacement is required. This would primarily be other types of surgery, trauma, and postpartum hemorrhage,32 but most evidence suggests that the role of fibrinogen is similar in these settings.33,34 Third, because of the pragmatic design of the study, strict timing of laboratory assessments could not be enforced. However, only less than 5% of patients had no measured fibrinogen levels before product administration. Related to this, a small proportion of patients had fibrinogen values above recommended thresholds when treatment order was received. However, this is consistent with recent experience in major bleeding,32 and ongoing bleeding from sample collection to therapy means that actual fibrinogen levels at the time of therapy were likely lower than measured values. Fourth, fibrinogen content is standardized in fibrinogen concentrate but is highly variable in cryoprecipitate. As a result, some patients in the cryoprecipitate group likely received much less or much more than 4 g of fibrinogen. While this is one of the inherent limitations of cryoprecipitate, observed differences in pretreatment and posttreatment fibrinogen levels suggest that on average both groups received clinically similar amounts of fibrinogen.
Conclusions
In patients undergoing cardiac surgery who develop clinically significant bleeding and hypofibrinogenemia after cardiopulmonary bypass, fibrinogen concentrate is noninferior to cryoprecipitate with regard to number of blood components transfused in a 24-hour period post bypass. Use of fibrinogen concentrate may be considered for management of bleeding in patients with acquired hypofibrinogenemia in cardiac surgery.
Study Protocol and Statistical Analysis Plan
FIBRES Committees and Investigators
eMethods
eFigure 1. Estimate of Site-Specific Mean Ratio (1-Sided 97.5% CI) of the Number of Allogeneic Blood Component Units Transfused within 24 hours Post Bypass in the Fibrinogen Concentrate to Cryoprecipitate Treatment Groups
eFigure 2. Kaplan-Meier Curve for Overall Survival (Primary Analysis Set)
eFigure 3. Kaplan-Meier Curve for Overall Survival (Per-Protocol Population)
eTable 1. Details of Intervention, Laboratory Values and Fibrinogen Levels, and Bleeding Status in the Per-Protocol Population
eTable 2. Allogeneic Transfusion Components Within 24 hours After Cardiopulmonary Bypass in Assessed Subgroups
eTable 3. Adverse Events Occurring in More Than 1 Patient for the Primary Analysis Set
eTable 4. Survival Stratified by Preoperative Critical State (Primary Analysis Set)
eTable 5. Survival Stratified by Preoperative Critical State (Per-Protocol Population)
Data Sharing Statement
References
- 1.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544-2552. doi: 10.1161/CIRCULATIONAHA.107.698977 [DOI] [PubMed] [Google Scholar]
- 2.Faraoni D, Willems A, Savan V, Demanet H, De Ville A, Van der Linden P. Plasma fibrinogen concentration is correlated with postoperative blood loss in children undergoing cardiac surgery: a retrospective review. Eur J Anaesthesiol. 2014;31(6):317-326. doi: 10.1097/EJA.0000000000000043 [DOI] [PubMed] [Google Scholar]
- 3.Karkouti K, Callum J, Crowther MA, et al. The relationship between fibrinogen levels after cardiopulmonary bypass and large volume red cell transfusion in cardiac surgery: an observational study. Anesth Analg. 2013;117(1):14-22. doi: 10.1213/ANE.0b013e318292efa4 [DOI] [PubMed] [Google Scholar]
- 4.Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23(1):98. doi: 10.1186/s13054-019-2347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boer C, Meesters MI, Milojevic M, et al. ; Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA) . 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32(1):88-120. doi: 10.1053/j.jvca.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 6.Callum JL, Karkouti K, Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev. 2009;23(3):177-188. doi: 10.1016/j.tmrv.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 7.Dunbar NM, Olson NJ, Szczepiorkowski ZM, et al. Blood component transfusion and wastage rates in the setting of massive transfusion in three regional trauma centers. Transfusion. 2017;57(1):45-52. doi: 10.1111/trf.13880 [DOI] [PubMed] [Google Scholar]
- 8.Yazer MH, Dunbar NM, Cohn C, et al. ; Biomedical Excellence for Safer Transfusion (BEST) Collaborative . Blood product transfusion and wastage rates in obstetric hemorrhage. Transfusion. 2018;58(6):1408-1413. doi: 10.1111/trf.14571 [DOI] [PubMed] [Google Scholar]
- 9.Novak A, Stanworth SJ, Curry N. Do we still need cryoprecipitate? cryoprecipitate and fibrinogen concentrate as treatments for major hemorrhage—how do they compare? Expert Rev Hematol. 2018;11(5):351-360. doi: 10.1080/17474086.2018.1458610 [DOI] [PubMed] [Google Scholar]
- 10.Jensen NH, Stensballe J, Afshari A. Comparing efficacy and safety of fibrinogen concentrate to cryoprecipitate in bleeding patients: a systematic review. Acta Anaesthesiol Scand. 2016;60(8):1033-1042. doi: 10.1111/aas.12734 [DOI] [PubMed] [Google Scholar]
- 11.Li JY, Gong J, Zhu F, et al. Fibrinogen concentrate in cardiovascular surgery: a meta-analysis of randomized controlled trials. Anesth Analg. 2018;127(3):612-621. doi: 10.1213/ANE.0000000000003508 [DOI] [PubMed] [Google Scholar]
- 12.Karkouti K, Callum J, Rao V, et al. Protocol for a phase III, non-inferiority, randomised comparison of a new fibrinogen concentrate versus cryoprecipitate for treating acquired hypofibrinogenaemia in bleeding cardiac surgical patients: the FIBRES trial. BMJ Open. 2018;8(4):e020741. doi: 10.1136/bmjopen-2017-020741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Institutes of Health Research Introducing TCPS 2 (2018)—Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. Government of Canada website. http://www.pre.ethics.gc.ca/eng/nr-cp_2019-06-05.html. Published August 15, 2019. Accessed October 8, 2019.
- 14.Erdoes G, Gerster G, Colucci G, Kaiser H, Alberio L, Eberle B. Prediction of post-weaning fibrinogen status during cardiopulmonary bypass: an observational study in 110 patients. PLoS One. 2015;10(5):e0126692. doi: 10.1371/journal.pone.0126692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mace H, Lightfoot N, McCluskey S, et al. Validity of thromboelastometry for rapid assessment of fibrinogen levels in heparinized samples during cardiac surgery: a retrospective, single-center, observational study. J Cardiothorac Vasc Anesth. 2016;30(1):90-95. doi: 10.1053/j.jvca.2015.04.030 [DOI] [PubMed] [Google Scholar]
- 16.Bilecen S, de Groot JA, Kalkman CJ, et al. Effect of fibrinogen concentrate on intraoperative blood loss among patients with intraoperative bleeding during high-risk cardiac surgery: a randomized clinical trial. JAMA. 2017;317(7):738-747. doi: 10.1001/jama.2016.21037 [DOI] [PubMed] [Google Scholar]
- 17.Nascimento B, Callum J, Tien H, et al. Fibrinogen in the initial Resuscitation of Severe Trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117(6):775-782. doi: 10.1093/bja/aew343 [DOI] [PubMed] [Google Scholar]
- 18.Winearls J, Wullschleger M, Wake E, et al. Fibrinogen Early In Severe Trauma studY (FEISTY): study protocol for a randomised controlled trial. Trials. 2017;18(1):241. doi: 10.1186/s13063-017-1980-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galas FR, de Almeida JP, Fukushima JT, et al. Hemostatic effects of fibrinogen concentrate compared with cryoprecipitate in children after cardiac surgery: a randomized pilot trial. J Thorac Cardiovasc Surg. 2014;148(4):1647-1655. doi: 10.1016/j.jtcvs.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 20.Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol. 2018;181(1):54-67. doi: 10.1111/bjh.15167 [DOI] [PubMed] [Google Scholar]
- 21.Callum JL, Pinkerton PH, Lima A, et al. Bloody Easy 4: Blood Transfusions, Blood Alternatives and Transfusion Reaction. 4th ed Toronto, Ontario, Canada: Ontario Regional Blood Coordinating Network; 2016. [Google Scholar]
- 22.Dyke C, Aronson S, Dietrich W, et al. Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg. 2014;147(5):1458-1463. doi: 10.1016/j.jtcvs.2013.10.070 [DOI] [PubMed] [Google Scholar]
- 23.Karkouti K, Callum J, Wijeysundera DN, et al. ; TACS Investigators . Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. 2016;134(16):1152-1162. doi: 10.1161/CIRCULATIONAHA.116.023956 [DOI] [PubMed] [Google Scholar]
- 24.Fleming TR, Harrington DP, O’Brien PC. Designs for group sequential tests. Control Clin Trials. 1984;5(4):348-361. doi: 10.1016/S0197-2456(84)80014-8 [DOI] [PubMed] [Google Scholar]
- 25.Ahmed S, Harrity C, Johnson S, et al. The efficacy of fibrinogen concentrate compared with cryoprecipitate in major obstetric haemorrhage—an observational study. Transfus Med. 2012;22(5):344-349. doi: 10.1111/j.1365-3148.2012.01178.x [DOI] [PubMed] [Google Scholar]
- 26.Theodoulou A, Berryman J, Nathwani A, Scully M. Comparison of cryoprecipitate with fibrinogen concentrate for acquired hypofibrinogenaemia. Transfus Apher Sci. 2012;46(2):159-162. doi: 10.1016/j.transci.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Vuylsteke A, Gerrard C, Besser M, Baglin T. Postoperative fibrinogen level is associated with postoperative bleeding following cardiothoracic surgery and the effect of fibrinogen replacement therapy remains uncertain. J Thromb Haemost. 2013;11(8):1519-1526. doi: 10.1111/jth.12304 [DOI] [PubMed] [Google Scholar]
- 28.Leach Bennett J, Blajchman MA, Delage G, Fearon M, Devine D. Proceedings of a consensus conference: risk-based decision making for blood safety. Transfus Med Rev. 2011;25(4):267-292. doi: 10.1016/j.tmrv.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Stramer SL, Hollinger FB, Katz LM, et al. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49(suppl 2):1S-29S. doi: 10.1111/j.1537-2995.2009.02279.x [DOI] [PubMed] [Google Scholar]
- 30.Kleinman S, Cameron C, Custer B, et al. Modeling the risk of an emerging pathogen entering the Canadian blood supply. Transfusion. 2010;50(12):2592-2606. doi: 10.1111/j.1537-2995.2010.02724.x [DOI] [PubMed] [Google Scholar]
- 31.Okerberg CK, Williams LA III, Kilgore ML, et al. Cryoprecipitate AHF vs. fibrinogen concentrates for fibrinogen replacement in acquired bleeding patients—an economic evaluation. Vox Sang. 2016;111(3):292-298. doi: 10.1111/vox.12417 [DOI] [PubMed] [Google Scholar]
- 32.McQuilten ZK, Bailey M, Cameron PA, et al. Fibrinogen concentration and use of fibrinogen supplementation with cryoprecipitate in patients with critical bleeding receiving massive transfusion: a bi-national cohort study. Br J Haematol. 2017;179(1):131-141. doi: 10.1111/bjh.14804 [DOI] [PubMed] [Google Scholar]
- 33.McQuilten ZK, Wood EM, Bailey M, Cameron PA, Cooper DJ. Fibrinogen is an independent predictor of mortality in major trauma patients: a five-year statewide cohort study. Injury. 2017;48(5):1074-1081. doi: 10.1016/j.injury.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 34.Gillissen A, van den Akker T, Caram-Deelder C, et al. ; TeMpOH-1 Study Group . Coagulation parameters during the course of severe postpartum hemorrhage: a nationwide retrospective cohort study. Blood Adv. 2018;2(19):2433-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Statistical Analysis Plan
FIBRES Committees and Investigators
eMethods
eFigure 1. Estimate of Site-Specific Mean Ratio (1-Sided 97.5% CI) of the Number of Allogeneic Blood Component Units Transfused within 24 hours Post Bypass in the Fibrinogen Concentrate to Cryoprecipitate Treatment Groups
eFigure 2. Kaplan-Meier Curve for Overall Survival (Primary Analysis Set)
eFigure 3. Kaplan-Meier Curve for Overall Survival (Per-Protocol Population)
eTable 1. Details of Intervention, Laboratory Values and Fibrinogen Levels, and Bleeding Status in the Per-Protocol Population
eTable 2. Allogeneic Transfusion Components Within 24 hours After Cardiopulmonary Bypass in Assessed Subgroups
eTable 3. Adverse Events Occurring in More Than 1 Patient for the Primary Analysis Set
eTable 4. Survival Stratified by Preoperative Critical State (Primary Analysis Set)
eTable 5. Survival Stratified by Preoperative Critical State (Per-Protocol Population)
Data Sharing Statement