Abstract
Surface modifying additives (SMAs), which may be readily blended into silicones to improve anti-fouling behavior, must have excellent surface migration potential and must not leach into the aqueous environment. In this work, we evaluated the efficacy of a series of poly(ethylene oxide) (PEO)-based SMA amphiphiles which varied in terms of crosslinkability, siloxane tether length (m) and diblock versus triblock architectures. Specifically, crosslinkable, diblock PEO-silane amphiphiles with two oligodimethylsiloxane (ODMS) tether lengths [(EtO)3Si-(CH2)3-ODMSm-PEO8, m = 13 and 30] were compared to analogous non-crosslinkable, diblock (H-Si-ODMSm-PEO8) and triblock (PEO8-ODMSm-PEO8) SMAs. Prior to water conditioning, while all modified silicone coatings exhibited a high degree of water-driven surface restructuring, that prepared with the non-crosslinkable diblock SMA (m = 13) was the most hydrophilic. After conditioning, all modified silicone coatings were similarly hydrophilic and remained highly protein resistant, with the exception of PEO8-ODMS30-PEO8. Notably, despite twice the PEO content, triblock SMAs were not superior to diblock SMAs. For diblock SMAs, it was shown that water uptake and leaching were also similar whether or not the SMA was crosslinkable.
Keywords: ANTI-FOULING, POLYMERIC MATERIALS, SURFACE MODIFICATION
1. Introduction
While silicones favorably exhibit non-toxicity, biostability and elastomeric mechanical properties,1, 2 their poor anti-fouling behavior limits their efficacy for blood-contacting medical devices as well as non-toxic marine coatings. It is the extreme hydrophobicity of silicones which gives rise to its adhesiveness to a variety of biofoulers, such as proteins3–5 and marine organisms.6, 7 When silicones contact blood, the rapid adsorption of plasma proteins (e.g. fibrinogen) induces platelet adhesion and activation which leads to thrombus formation.4, 5, 8 For devices such as dialysis catheters, thrombosis can obstruct blood flow and facilitate infection.9–11 Biofouling of ship hulls generally results from the adhesion and accumulation of proteins followed by bacteria, diatoms, and other microorganisms, resulting in increased hydrodynamic drag and subsequently greater fuel consumption and maintenance costs.6, 12–14 Historically, marine biofouling has been attempted to be controlled through the use of toxic, ablative coatings which negatively impact non-target marine life and ecosystems. Towards reducing biofouling for these two applications, a non-toxic strategy to produce protein resistance is highly sought after, as this is typically indicative of broader anti-fouling behavior. While silicones are adhesive to proteins and other biofoulers, hydrophilization of silicone with physical, chemical, and combined strategies has been generally shown to improve anti-biofouling behavior.5, 15–19
Silicone modification with poly(ethylene oxide) (PEO) [or poly(ethylene glycol) (PEG)] has been studied towards increasing surface hydrophilicity and protein resistance.20–25 This is motivated by the exceptional ability of PEO to resist protein adsorption which is attributed to the formation of a repulsive hydration layer, steric repulsion and blockage of surface adsorption sites.26–29 The protein resistance of PEO has been largely demonstrated when applied to model substrates such as gold,30–32 glass,33, 34 and silicon wafer.35–38 While considered biocompatible39 and oxidatively stable in biological conditions,40 the in vivo protein resistance of PEO-modified polymeric materials has been observed to be limited and inconsistent.41–43 Thus, the method of incorporation of PEO into polymers such as silicones is critical to achieving surface hydrophilicity and protein resistance.
“Surface modifying additives” (SMAs), while incorporated into a base polymer in relatively small amounts via simple bulk modification (i.e. blending), are intended to migrate to the air or solution interface to affect surface modification.44, 45 SMA strategies are an attractive option for surface modification as they avoid the complications associated with poor adhesion of the coatings to substrates and complex surface grafting methods. SMAs have been reported for several base polymers and are typically amphiphilic diblock or triblock oligomers where the hydrophobic block has an affinity to the base polymer.44, 46 A PEO-based SMA for silicones must give rise to an adequately high PEO surface concentration,32, 36, 47, 48 specifically at the aqueous (i.e. biological) interface where protein adsorption occurs. In the case of the aforementioned “model PEO surfaces”, PEO chains are retained at the surface whether in air or in an aqueous environment. Silicones, however, are able to undergo substantial surface reorganization depending on their exposure to an air or aqueous environment.49 This process has been mostly studied in the case of hydrophobic recovery such as when plasma-treated silicone surfaces return to a hydrophobic state if maintained in air but remain hydrophilic if stored in water.50 This behavior stems from the low surface energy51 and high chain flexibility52 of silicones. Hydrophobic recovery is also observed for silicones bulk modified with conventional PEO-silanes such as triethoxysilylpropyl PEO monomethyl ether [(EtO)3Si-(CH2)3-PEOn-OCH3]20, 21 and allyl PEO monomethyl ether [CH2=CHCH2-PEOn-OCH3].22 Following hydrophobic recovery, PEO may have a limited potential to migrate to the aqueous interface.
To induce surface hydrophilicity and protein resistance, a PEO-based SMA incorporated into silicones must undergo appreciable water-driven restructuring to the surface. We have shown that for RTV silicones bulk-modified with conventional PEOn-silanes (n = 3, 8 and 16), poor water-driven surface restructuring of PEO was observed leading to high protein adsorption similar to that of unmodified silicone.53 In contrast, PEO-silane amphiphiles comprised of an oligodimethylsiloxane (ODMS) tether and crosslinkable triethoxysilane (TEOS) group [α-(EtO)3Si-(CH2)3-ODMS13-block-PEO8-OCH3] dramatically increased water-driven surface hydrophilicity and reduced the adhesion of proteins53–55 and other biofoulers.56, 57 Extensive atomic force microscopy (AFM) analysis was used to verify the water-driven migration of PEO to the silicone surface.58 For this class of PEO-silane amphiphiles, we have previously shown that siloxane tether length (m)59 as well as PEO segment length (n)55 impacts the extent of surface modification, with hydrophilicity and protein resistance generally the greatest when m = 13 and n = 8.
SMA efficacy may be limited by its ability to restructure to the aqueous interface as well as their tendency to leach from the base polymer during prolonged exposure to water.44, 45 While the PEO-silane amphiphile [α-(EtO)3Si-(CH2)3-ODMS13-block-PEO8-OCH3] demonstrated excellent efficacy as a silicone SMA, questions remain regarding its capacity to retain protein resistance during prolonged aqueous exposure. Moreover, the contributions of its diblock architecture and crosslinkable TEOS end group are not understood. Thus, in this work, a series of three PEO-amphiphile SMAs, which varied in terms of architecture and crosslinkability and each with a PEO segment length of m = 13 and 30, were evaluated in silicone (Figure 1). Thus, the previously studied crosslinkable, diblock PEO-silane amphiphile (“XL diblock, m = 13”) and that with a longer siloxane tether (“XL diblock, m = 30”) were compared to the non-crosslinkable SMA analogues (“diblock, m = 13” and “diblock, m = 30”) (i.e. no TEOS group). Additionally, triblock SMA analogues were also evaluated (“triblock, m = 13” and “triblock, m = 30”). For these triblock SMAs, they are not only non-crosslinkable but contain twice the PEO content as the diblock type SMAs. The increase of the siloxane tether length (m) was hypothesized to increase the affinity of the SMA to the silicone to potentially reduce leaching and potentially diminish the need for covalent crosslinking (i.e. TEOS end group). For each SMA modified silicone, the water-driven surface hydrophilicity and resistance to fibrinogen adsorption were measured before and during prolonged water conditioning. In addition, water uptake and leaching from modified silicones were also measured.
Figure 1.
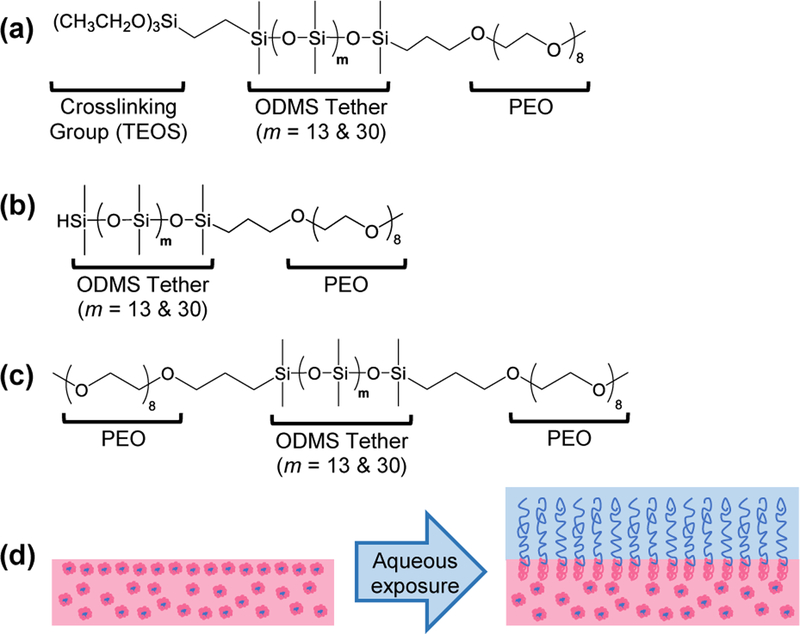
Surface-modifying additive (SMA) amphiphile structures: (a) “XL diblock amphiphile”, (b) “diblock amphiphile”, and (c) “triblock amphiphile”. (d) Upon aqueous exposure, silicones modified with these amphiphilic SMAs can undergo rapid, water-driven surface restructuring to present PEO chains to the surface and form a hydrophilic, protein resistant surface. The image shown represents restructuring of the “XL diblock” and “diblock” SMAs.
2. Materials and methods
2.1. Materials
Allyl methyl PEO [Polyglykol AM 450, Mn = 292–644 g mol−1 per manufacturer’s specifications; Mn = 424 g mol−1 per 1H NMR end group analysis; 1H NMR (δ, ppm): 3.35 (s, 3H, OCH3), 3.51–3.66 (m, 32H, OCH2CH2), 4.00 (d, J = 5.4 Hz, 2H, CH2=CHCH2O), 5.13–5.28 (m, 2H, CH2=CHCH2O) and 5.82–5.96 (m, 1H, CH2=CHCH2O)] was kindly provided by Clariant. Octamethylcyclotetrasiloxane, tetramethyldisiloxane, vinyltriethoxysilane (VTEOS), Pt-divinyltetramethyldisiloxane complex (Karstedt’s catalyst) in xylene, and α,ω-bis-(SiH)oligodimethylsiloxane13 [ODMS13; Mn = 1000–1100 g mol−1 per manufacturer’s specifications; Mn = 1096 g mol−1 per 1H NMR end group analysis; 1H NMR (δ, ppm): 0.05–0.10 (m, 78H, SiCH3), 0.19 (d, J = 2.7 Hz, 12H, OSi[CH3]2H) and 4.67–4.73 (m, 2H, SiH)] were purchased from Gelest. ODMS30 [Mn = 2354 g mol−1 per 1H NMR end group analysis] was prepared as reported;1H NMR (δ, ppm): 0.05–0.11 (m, 180H, SiCH3), 0.19 (d, J = 2.7 Hz, 12H, OSi[CH3]2H) and 4.67–4.73 (m, 2H, SiH).60 Triflic acid, RhCl(Ph3P)3 (Wilkinson’s catalyst), hexamethyldisilazane (HMDS), and solvents were obtained from Sigma-Aldrich. All solvents were dried over 4Å molecular sieves prior to use for hydrosilylation reactions and film casting. Glass microscope slides (75 × 25 × 1 mm) were purchased from Fisher Scientific. Medical-grade condensation-cure room-temperature-vulcanizing (RTV) silicone elastomer (MED-1137) was purchased from NuSil Technology. Per manufacturer’s specifications, MED-1137 is comprised of α,ω-bis(Si-OH)PDMS, silica (11–21%), methyltriacetoxysilane (<5%), ethyltriacetoxysilane (<5%) and trace amounts of acetic acid. Polystyrene 24-well well plates were purchased from Corning. Human fibrinogen (HF) was purchased from Calbiochem. Tris buffered saline with Tween 20 (TBS-T20), goat anti-fibrinogen horse radish peroxidase (HRP)-conjugated polyclonal detection antibody, and ultra 3,3’,5,5’-tetramethylbenzidine dihydrochloride (TMB di-HCl) substrate solution were purchased from Thermo Fisher Scientific.
2.2. Synthetic approach
Reactions were all run under a N2 atmosphere with a Teflon-covered stir bar. Chemical structures were confirmed with nuclear magnetic resonance (NMR) spectroscopy using a Mercury 300 MHz spectrometer operating in the Fourier transform (FT) mode and using CDCl3 as the standard.
2.2.1. Synthesis of crosslinkable, diblock amphiphile (“XL diblock, m = 13”, “XL diblock, m = 30”)
Crosslinkable (i.e. “XL”) diblock amphiphiles were synthesized as previously reported using a two-step hydrosilylation protocol.53, 60 Briefly, the ODMSm (m = 13 or 30) underwent Wilkinson’s-catalyzed regioselective hydrosilylation with vinyltriethoxysilane (1:1 molar ratio). Next, each product was reacted with allyl methyl PEO8 (1:1 molar ratio) via a Karstedt’s-catalyzed hydrosilylation reaction.
2.2.2. Synthesis of non-crosslinkable, diblock amphiphiles (“diblock, m = 13”, “diblock, m = 30”)
Non-crosslinkable diblock amphiphiles (m = 13 and 30) (i.e. lacking a TEOS terminal group) were prepared using a Wilkinson’s-catalyzed regioselective hydrosilylation procedure. Allyl methyl PEO8 and ODMSm (1:1 molar ratio) were dissolved in toluene in a sealed round bottom (rb) flask with Wilkinson’s catalyst (10 mg) and heated to 80 °C. After 16 h, the catalyst was removed from the reaction mixture by adding activated charcoal and heating at 90 °C for 2 h. The mixture was then cooled to room temperature (RT) and filtered to remove the charcoal. After filtration, the volatiles were removed under reduced pressure to yield a colorless liquid.
“Diblock, m = 13”.
Allyl methyl PEO8 (3.09 g, 7.29 mmol) and ODMS13 (8.00 g, 7.30 mmol) and Wilkinson’s catalyst were reacted in 50 mL toluene to yield the product (8.98 g, 81% yield). 1H NMR (δ, ppm): 0.04–0.10 (m, 84H, SiCH3), 0.18–0.19 (d, J = 2.7 Hz, 6H, OSi[CH3]2H), 0.48–0.54 (m, 2H, SiCH2CH2CH2), 1.55–1.66 (m, 2H, SiCH2CH2CH2), 3.37 (s, 3H, OCH3), 3.38–3.43 (t, J = 7.1 Hz, 2H, SiCH2CH2CH2), 3.53–3.71 (m, 32H, CH2CH2O), and 4.67–4.73 (m, 1H, SiH).
“Diblock, m = 30”.
Allyl methyl PEO8 (2.48 g, 5.85 mmol) and ODMS30 (13.79 g, 5.86 mmol) and Wilkinson’s catalyst were reacted in 50 mL toluene to yield the product (13.00 g, 80% yield). 1H NMR (δ, ppm): 0.05–0.10 (m, 186H, SiCH3), 0.18–0.19 (d, J = 2.7 Hz, 6H, OSi[CH3]2H), 0.45–0.54 (m, 2H, SiCH2CH2CH2), 1.55–1.66 (m, 2H, SiCH2CH2CH2), 3.37 (s, 3H, OCH3), 3.38–3.43 (t, J = 7.4 Hz, 2H, SiCH2CH2CH2), 3.53–3.66 (m, 32H, CH2CH2O), and 4.67–4.73 (m, 1H, SiH).
2.2.3. Synthesis of non-crosslinkable, triblock amphiphiles (“triblock, m = 13”, “triblock, m = 30”)
Non-crosslinkable triblock amphiphiles (m = 13 and 30) (i.e. no TEOS group) were prepared using a Karstedt’s-catalyzed hydrosilylation procedure. Allyl methyl PEO8 and ODMSm (2:1 molar ratio) were dissolved in toluene in a sealed rb flask with Karstedt’s catalyst and heated to 80 °C. After 16 h, the catalyst was removed by adding activated charcoal to the reaction mixture and heating at 90 °C for 2 h. The mixture was then cooled to RT and filtered. The volatiles were then removed under reduced pressure to yield the colorless liquid.
“Triblock, m = 13”.
Allyl methyl PEO8 (3.21 g, 7.57 mmol) and ODMS13 (4.15 g, 3.79 mmol) and Karstedt’s catalyst (25 μL) were reacted in 30 mL toluene to yield the product (3.35 g, 46% yield). 1H NMR (δ, ppm): 0.04–0.09 (m, 90H, SiCH3), 0.48–0.54 (m, 4H, SiCH2CH2CH2), 1.55–1.66 (m, 4H, SiCH2CH2CH2), 3.37 (s, 6H, OCH3), 3.37 (t, J = 7.1 Hz, 4H, SiCH2CH2CH2), and 3.53–3.71 (m, 64H, CH2CH2O).
“Triblock, m = 30”.
Allyl methyl PEO8 (1.49 g, 3.51 mmol) and ODMS30 (4.12 g, 1.75 mmol) and Karstedt’s catalyst (25 μL) were reacted in 30 mL toluene to yield the product (3.67 g, 65% yield). 1H NMR (δ, ppm): 0.05–0.09 (m, 192H, SiCH3), 0.48–0.54 (m, 4H, SiCH2CH2CH2), 1.55–1.67 (m, 4H, SiCH2CH2CH2), 3.37 (s, 6H, OCH3), 3.37–3.43 (t, J = 7.1 Hz, 4H, SiCH2CH2CH2), and 3.53–3.71 (m, 64H, CH2CH2O).
2.3. Film preparation
Glass microscope slides were sequentially rinsed with dichloromethane (DCM) and acetone followed by drying in a 120 °C oven overnight. Casting solutions were prepared by combining MED-1137 with hexane (1:3 wt/wt) and mixing with a vortexer until a homogenous solution was obtained. For modification of silicone, each SMA amphiphile was added to individual casting solutions at 50 µmol of SMA per 1 g of silicone. Solutions were solvent-cast onto leveled glass slides (1.5 mL per slide; 2.0 mL for leaching study) and covered with a Petri dish. For protein testing, solutions were solvent-cast into 24-well plates (0.25 mL per well). All films were allowed to cure for one week at RT and then promptly used for designated analyses.
2.4. Water-driven surface restructuring
Water-driven surface restructuring of silicones was characterized with static water contact angle (θstatic) measurements using a CAM-200 goniometer (KSV Instruments) equipped with an autodispenser, video camera, and drop-shape analysis software (Attension Theta). “Non-conditioned” films (i.e. not conditioned in water) were measured immediately after one week of curing. A 5 μL deionized (DI) water droplet was placed on the film and θstatic was iteratively measured over a 5 min period. The reported θstatic values are an average and standard deviation of three measurements each made on different areas of the same film. An equivalent set of films were subjected to conditioning in DI water and subsequent contact angle measurement. For these, a coated glass slide was sequentially submerged in ~30 mL of fresh DI water in plastic Petri dish, removed after a given time period (t = 2 h, 6 h, 24 h, 48 h, 72 h, 6 days, 10 days, and 14 days), quickly dried under a stream of nitrogen and θstatic, 5 min measured as above. On the 14th day, each conditioned film was dried under reduced pressure overnight (30 in. Hg, 50 °C) and θstatic, 5 min was measured.
2.5. Water uptake
Triplicate films were prepared and subsequently submerged in 30 mL DI water in plastic Petri dishes. After 24 h, 7 days and 14 days, the films were removed from water and briefly dried with stream of air and blotted with a paper towel. The water content of the film was measured by thermal gravimetric analysis (TGA). A 20 ± 2 mg segment of film was removed from the glass slide by razor blade and placed in a platinum TGA pan. Using a TA Instruments Q50, weight loss was measured as the sample was heated from RT to 150 °C at a rate of 10 °C min-1. Water loss was observed as a peak in the mass loss derivative curve that occurred between RT and approximately 100 °C. Wt% water content of each film was then determined by measuring the percent mass loss over the bounds of that peak. After each measurement, the films were re-submerged in fresh DI water to advance to the next time period. The reported values are the average water contents and standard deviation of three identically-prepared films at the same submersion time.
2.6. Water-induced mass loss
Coated slides were weighed (Wi) and then each soaked in DI water (30 mL) at RT for two weeks. The coated slides were subsequently dried at RT under reduced pressure (30 in. Hg) and weighed (Wf). The weight of the uncoated glass slide was subtracted from Wi and Wf before calculating the water-induced mass loss (i.e. water-extractable content). Measurements were performed on triplicate films. Mass loss is defined as:
| (Equation 1) |
2.7. Protein adsorption
Human fibrinogen (HF) adsorption was measured using a modified immunosorbent assay.61 Three replicate coated wells of each composition were exposed to 0.15 mL of HF solution prepared in phosphate buffered saline (PBS) (3.0 mg/mL) and statically incubated for 1 h at 37 °C. The protein solution was removed and each well was rinsed three times with PBS before the addition of TBS-T20 (0.50 mL), which was incubated for 30 min at 37 °C. Wells were then rinsed three times with TBS-T20. Next, to each well was added 0.5 mL goat anti-fibrinogen (HRP)-conjugated polyclonal detection antibody (1:50,000 dilution in TBS-T20) and statically incubated for 1 h at 37 °C. Wells were then rinsed three times with TBS-T20. TMB di-HCl substrate solution (0.5 mL) was added and allowed to incubate for 30 min at 37 °C. To stop the reaction, 2 M H2SO4 was added to each well and plates were shaken on an orbital shaker at RT for 15 min. To quantify the amount of adsorbed HF on each surface, 0.15 mL of each resulting solution was transferred to a 96-well plate, absorbance was measured at 450 nm using a spectrophotometer (Tecan Safire2), and the value was compared to a HF standard curve (0.01 to 1000 ng/mL).
3. Results and discussion
3.1. Water-driven surface restructuring: before, during, and after conditioning in water
The efficacy of amphiphiles as SMAs to improve silicone protein resistance is predicted to be governed by their ability to undergo water-driven surface reorganization to form a hydrophilic, PEO-enriched surface. Previously, this process was directly observed for silicones modified with “XL diblock, m = 13”.53–55 The temporal measurement of the decrease in water droplet θstatic values was utilized to monitor the relative rate and extent of PEO migration to the silicone surface-water interface. Thus, the θstatic values of water droplets deposited onto surfaces were measured over a 5 min period (Figure 2, Table S1). While the unmodified silicone expectedly remained very hydrophobic (θstatic = ~106 °), all SMA modified silicones exhibited initially similar hydrophobicity but rapidly underwent significant water-driven restructuring to form very hydrophilic surfaces. Temporal θstatic value profiles were dependent on SMA structure, including the siloxane tether length. For instance, for silicones modified with “XL diblock, m =13” and “triblock, m =13”, θstatic, 5 min values were somewhat similar to those of silicones modified with the corresponding SMA of greater siloxane tether length (m = 30). In contrast, “diblock, m = 13” (θstatic, 5 min = ~6 °) produced a modified silicone of significantly greater hydrophilicity versus “diblock, m = 30” (θstatic, 5 min = ~26 °) as well as “XL diblock, m = 13” (θstatic, 5 min = ~28 °) and “triblock, n =13” (θstatic, 5 min = ~21°). The enhanced water-driven surface migration potential of “diblock, m = 13” versus “XL diblock, m = 13” is attributed to the former’s greater chain mobility, being not limited by covalent attachment to the silicone network. Despite the similar lack of crosslinking, “diblock, m = 30” appeared to be more limited in its surface migration potential versus “diblock, m = 13”, perhaps due to the longer siloxane tethers’ higher affinity of the silicone matrix and increased steric constraints. Notably, while “triblock, m = 13” contained twice the amount of PEO, it produced lesser hydrophilicity versus “diblock, m = 13”. This reduced migration potential may also be due to steric constraints of an overall higher molecular weight triblock chain.
Figure 2.
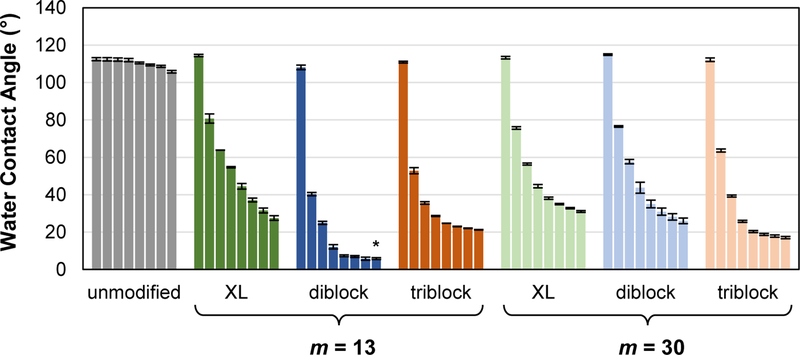
Following one-week cure, θstatic measured over a 5 min period on silicones bulk-modified with SMA amphiphiles (“XL diblock”, “diblock” and “triblock”; m = 13 and 30 for each). Bars are organized as time after the initial drop placement from left to right as follows: 0 s, 15 s, 30 s, 1 min, 2 min, 3 min, 4 min, and 5 min. Statistical analysis was done using single factor Anova. The “diblock, m = 13” (t = 5 min) was compared against all other compositions at t = 5 min (* indicates p < 0.01).
Critical to their sustained anti-fouling behavior is the capacity of modified silicones containing SMAs to retain their surface hydrophilicity during prolonged exposure to water. Thus, over a two-week conditioning period in DI water, θstatic, 5 min values were periodically measured (Figure 3, Table S2). Interestingly, after two weeks, all modified silicones exhibited similar hydrophilicity, having θstatic, 5 min values between ~36 and 43 °. However, these θstatic, 5 min values were somewhat higher than at t = 0, indicating migration of PEO away from the water-interface. Versus prior to conditioning in water (i.e. “t = 0 h”), modified silicones exhibited a modest increase in θstatic, 5 min of at least ~8 ° by two weeks. The lowest increase in Δθstatic, 5 min was observed for the silicone modified with “XL diblock, m = 30” (~8 ° increase) whereas the highest increase was observed with “diblock, m = 13” (~30 ° increase). The silicone modified with “diblock, = 30” exhibited a modest increase (~15 °), much lower than for “diblock, m = 13” and similar to that for silicone modified with “XL diblock, m = 13”. Intermediate water uptake values were observed for silicones modified with triblock SMAs. Based on these results, stability of surface hydrophilicity during water exposure is best for silicones modified with diblock SMA having a crosslinkable group and a longer siloxane tether (m = 30). However, the crosslinkable group may be eliminated without large reductions in surface hydrophilicity for diblock SMAs with the longer siloxane tether (m = 30).
Figure 3.
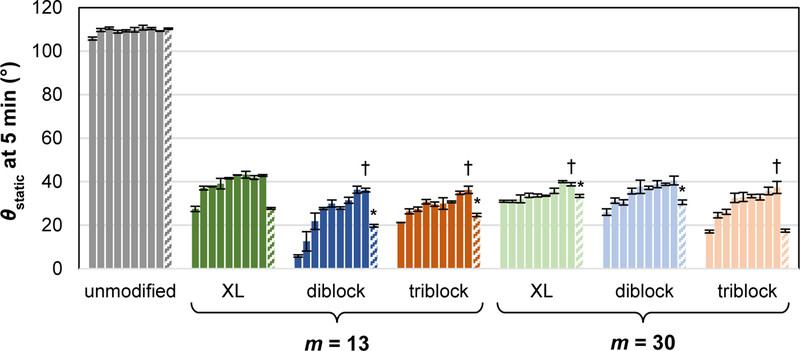
During a two-week exposure to DI water, θstatic was measured over a 5 min period on silicones bulk-modified with SMA amphiphiles (“XL diblock”, “diblock” and “triblock”; m = 13 and 30 for each). Bars are organized in terms of time of duration of water conditioning from left to right: 0 h, 2 h, 6 h, 24 h, 48 h, 72 h, 6 days, 10 days, and 14 days. The final “patterned bar” in each series represents θstatic, 5 min following drying of the “14 day” water-conditioned specimens. (Note: 0 h time point corresponds to 5 min θstatic value in Figure 2.) Statistical analysis was done using single factor Anova. First, the t = 0 h time point was compared to the final “patterned bar” in each series (*indicates p < 0.05). Second, the t = 14 day time point of each SMA were compared to the t = 14 day time point of “XL diblock, m = 13” († indicates p < 0.05).
As noted, during conditioning, water absorption by modified silicones may contribute to their decreased hydrophilicity by inducing migration of the PEO from the water-interface and back into the bulk. If this is the case, subsequent drying of water conditioned specimens should restore the surface hydrophilicity to that initially observed (i.e. θstatic, 5 min at t = 0). Thus, after two weeks of conditioning in water, the specimens were subsequently dried (36 mm Hg, 50 °C, overnight) and θstatic, 5 min measured (Figure 3, Table S2). Indeed, following drying, most modified silicones exhibited θstatic, 5 min values similar that at t = 0. The only exception was silicone modified with “diblock, m = 13” whose θstatic, 5 min was ~20 ° after drying compared to ~6 ° before water conditioning (t = 0). As noted above, this modified silicone experienced the highest increase in Δθstatic, 5 min following exposure to water. Its irreversible change in surface hydrophilicity was considered to possibly be indicative of leaching from the silicone and is considered below. Overall, however, water uptake into the films contributed to the observed decrease in hydrophilicity upon prolonged exposure to water. Still, following two weeks of water conditioning, all modified silicones remained quite hydrophilic.
3.2. Water uptake
As noted above, water uptake contributed to the decrease in surface hydrophilicity of modified silicones. Thus, the amount of water absorbed by each modified silicone was quantified when conditioned in water for 24 h, one week and two weeks (Figure 4, Table S3). The unmodified silicone expectedly absorbed little water (<0.1 wt%) due to its hydrophobicity. After just 24 h of conditioning, silicones modified with SMA amphiphiles absorbed more water (between ~0.8 and ~2.8 wt%) due to their greater hydrophilicity imparted by PEO. Given the ability of block copolymers to undergo local aggregation within polymer matrixes,62 these SMA amphiphiles are hypothesized to form micelle-like structures in the silicone to contain the absorbed water. Water uptake into modified silicones increased from 24 h to one week of conditioning, but did not increase substantially at two weeks. Final wt% water values of modified silicones depending on SMA structure and ranged modified from ~1.6 – 6 wt%. SMAs with a longer siloxane tether reduced water absorption, even when not crosslinkable. In this way, the least amount of water adsorbed (< 2 wt%) at two weeks was observed for the silicone modified with “XL diblock, m = 30” and “diblock, m = 30”. For diblock SMAs with the shorter siloxane tether (m = 13), a lack of crosslinkability led to higher water adsorption (“XL diblock, m = 13”; ~3% and “diblock, m = 13”; ~6 %). Due to it increased PEO content, the silicone modified with “triblock, m = 30” produced a modified silicone with higher water absorption (~6 wt%). Thus, diblock SMAs having a longer siloxane tether, even in the absence of a crosslinkable group, are the most efficient at reducing water uptake into modified silicones.
Figure 4.
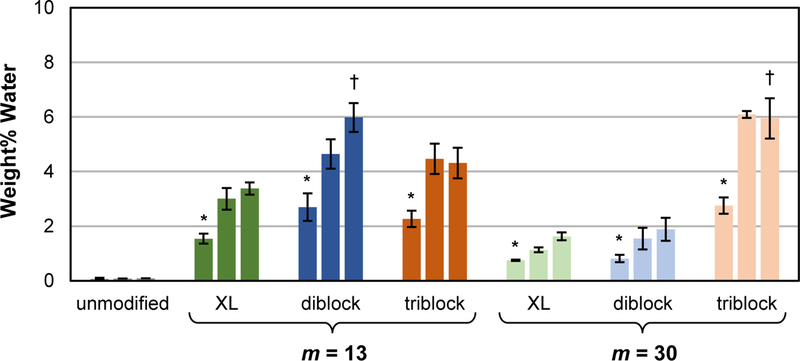
Wt% water absorbed by silicones modified with SMA amphiphiles (“XL diblock”, “diblock” and “triblock”; m = 13 and 30 for each). Bars are organized in terms of duration of water conditioning from left to right: 1 day, 1 week, and 2 weeks. Statistical analysis was done using single factor Anova. First, following 24 h of conditioning, wt% water absorbed by modified silicones were compared against the unmodified silicone control (* indicates p < 0.05). Second, following 2 weeks of conditioning, wt% water absorbed by silicones modified with a given SMA (m = 13) were compared to that modified with the corresponding SMA (m = 30) († indicates p < 0.05).
3.3. Water-induced mass loss
During exposure to water, mass loss measurements following two weeks of exposure to DI water was used to determine if significant mass loss occurred which could indicate leaching of the SMA amphiphile from the modified silicone. For the unmodified silicone control, a ~0.7 wt% mass loss was observed (Figure 5, Table S4). For modified silicones, wt% mass loss increased only slightly, to ~0.9 – 1.4 wt%. Also, while all SMA amphiphiles were added at 50 µmol to silicones, the corresponding wt% values are appreciably higher than the wt% loss following conditioning in water. As noted above and in Figure 3, initial surface hydrophilicity (i.e. θstatic, 5 min at t = 0) was recovered when modified silicones which were dried following conditioning in water. The only exception was for the silicone modified with “diblock, m = 13”. However, since a substantial increase in weight loss was not observed, leaching of the “diblock, m = 13” cannot be the source of this observation. We speculate that this modified silicone may have retained some residual water during drying, reducing its recovery of initial surface hydrophilicity.
Figure 5.
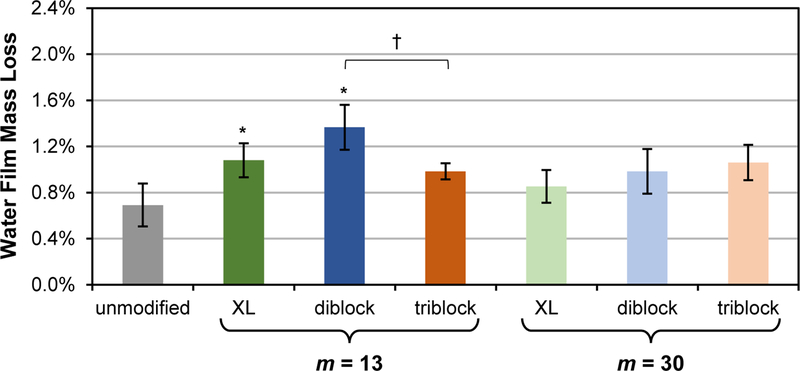
Following exposure to water for 2 weeks, wt% mass loss from silicones modified with SMA amphiphiles (“XL diblock”, “diblock” and “triblock”; m = 13 and 30 for each). Each bar represents an average percent mass loss of three identically prepared films with the error bar representing the standard deviation. Statistical analysis was done using single factor Anova. Mass loss of bulk-modified films were compared against the unmodified control (* indicates p < 0.05). Mass loss of bulk-modified films within a tether length series (m = 13 or m = 30) were also compared († indicates p < 0.05).
3.4. Protein adsorption
The adsorption of HF protein on modified silicones was evaluated before and after two weeks of conditioning in water (Figure 6, Table S5). As expected, unmodified silicone films, due to their hydrophobicity, adsorbed a relatively large amount of HF before and after water conditioning (~125 and ~138 ng cm−2, respectively). For all silicones modified with SMA amphiphiles, protein resistance was very high at t = 0 and adsorbed HF concentrations were dramatically reduced to ~2 – 12 ng cm-2. As noted above, conditioning of modified silicones in water for two weeks led to a reduction in surface hydrophilicity (i.e. increased θstatic, 5 min) (Figure 3, Table S2). Thus, the impact of water conditioning on modified silicones’ resistance to HF was assessed by also measuring protein adsorption immediately following two weeks of aqueous exposure. Modified silicones exhibited either similar or a very minor increase in HF adsorption following water conditioning. The one exception was for the silicone modified with “triblock, m =30” which exhibited a substantial increase in HF adsorption (~74 ng cm−2). For the silicone modified with this triblock SMA, surface hydrophilicity after two weeks of conditioning was not significantly different versus silicones modified with other amphiphiles (Figure 3), nor did this silicone show any evidence of amphiphile leaching (Figure 5). Thus, while it is not expected that surface hydrophilicity (i.e. θstatic, 5 min) and protein adsorption correlate in a scalable fashion, this result does highlight that other factors influence protein resistance. In total, the ability of diblock SMAs, with or without crosslinking, to maintain protein resistance following water conditioning is notable.
Figure 6.
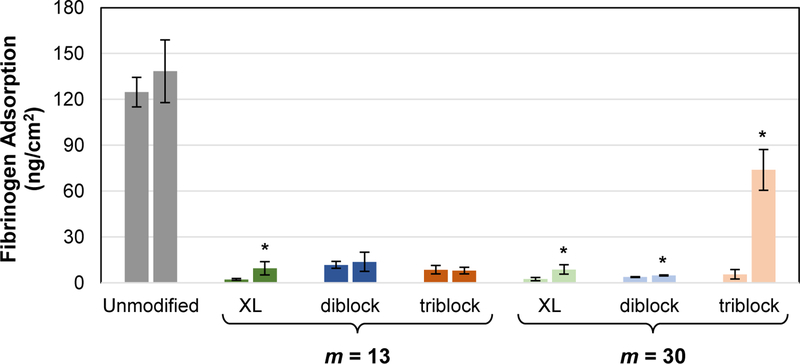
Fibrinogen adsorption (concentration = 3 mg/mL; exposure time = 1 h) onto silicones modified with SMA amphiphiles (“XL diblock”, “diblock” and “triblock”; m = 13 and 30 for each) before (t = 0) and after 2 weeks exposure to water (t = 2 weeks). For each composition fibrinogen adsorption was measured before and after water-equilibration as represented by the left and right bars, respectively. Each individual bar represents the average fibrinogen adsorption of three identically prepared replicate wells with the error bars representing the standard deviations. Statistical analysis was done using single factor Anova. Fibrinogen adsorption values were compared at t = 0 versus t = 2 weeks (* indicates p < 0.05).
4. Conclusion
Silicones, due to their extreme hydrophobicity, exhibit poor anti-fouling behavior which limits their efficacy in medical devices as well as for non-toxic marine coatings. PEO-based SMAs represent a potentially convenient way to modify silicones to impart migration of PEO to the aqueous (i.e. biological) interface resulting in increased surface hydrophilicity and a reduction in protein adsorption and other biofouling processes. By reducing protein adsorption and biofouling of silicones, PEO-based SMAs can allow for safe, effective, and non-toxic silicones for medical devices and environmentally friendly marine coatings. Previously, we have described a PEO-silane amphiphile SMAs having a diblock architecture and being comprise of a siloxane tether and crosslinkable TEOS group [α-(EtO)3Si-(CH2)3-ODMS13-block-PEO8-OCH3] (“XL diblock, m = 13”). When used to bulk modify RTV silicones, exposure to water led to a rapid and dramatic increase in surface hydrophilicity due to migration of PEO to the water interface and a substantial reduction in biofouling. In contrast, conventional PEO-silanes [(EtO)3Si-(CH2)3-PEO8-OCH3] acted as a poor SMAs in silicones. Given the effectiveness of the PEO-silane amphiphile as a SMA, this work sought to identify whether such modified silicones would retain their anti-fouling behavior during prolonged exposure to water. Additionally, the contributions of its diblock architecture and crosslinkable TEOS end group were examined by comparing to non-crosslinkable diblock and triblock analogues. Given its affinity to the silicone matrix, the length of the siloxane tether (m) was also increased to 30. Thus, a series of six PEO-based amphiphile SMAs, which varied in terms of architecture and crosslinkability and each with a siloxane tether length of m = 13 and 30, were evaluated in silicone: crosslinkable, diblock SMAs (“XL diblock, m = 13” and “XL diblock, m = 30”), non-crosslinkable, diblock SMAs (“diblock, m = 13” and “diblock, m = 30”) and non-crosslinkable, triblock SMAs (“triblock, m = 13” and “triblock, m = 30”). After just 5 minutes of aqueous exposure, while all modified silicones were very hydrophilic, the silicone modified with “diblock, m = 13” was the most hydrophilic (i.e. lowest θstatic, 5 min). However, following two weeks of aqueous conditioning, all modified silicones exhibited rather similar surface hydrophilicity. After subsequent drying of these water conditioned silicones, most were able to achieve their original surface hydrophilicity (i.e. θstatic, 5 min at t = 0) with the exception to the silicone modified with “diblock, m = 13”. Among modified silicones, water uptake was lowest and similar for those prepared with diblock SMAs having longer siloxane tethers (“XL diblock, m = 30” and “diblock, m = 30”). Leaching studies confirmed that, even for the silicone modified with “diblock, m =13”, SMAs were not lost at appreciable levels (between ~0.9 and 1.4 wt%). Adsorption of fibrinogen protein before and after two weeks of water conditioning remained very low with the exception of “triblock, m = 30”. These studies confirm that a diblock architecture is more effective versus triblock for these SMA amphiphiles, despite twice the PEO content in triblock SMAs. Reducing the decrease in surface hydrophilicity during water conditioning and minimizing water uptake as well as leaching was best controlled for diblock SMAs when the siloxane tether length is increased from m = 13 to m = 30. Moreover, the need for a TEOS crosslinking group is no longer necessary as the longer siloxane tether’s affinity to the silicone is enhanced. These results will be useful for the modification of silicones with PEO-based SMAs for the reduction of biofouling.
Supplementary Material
Acknowledgements
The authors thank the Texas Engineering and Experiment Station (TEES) for financial support of this research. M. A. Rufin gratefully acknowledges support from the NIH (3R01DK95101-02S1). S. J. Stafslien gratefully acknowledges support from the Office of Naval Research (ONR) (N00014-15-1-2323).
Contributor Information
Marc A. Rufin, Department of Biomedical Engineering, Texas A&M University, College Station, TX, USA
Bryan Khai D. Ngo, Department of Biomedical Engineering, Texas A&M University, College Station, TX, USA
Mikayla E. Barry, Department of Biomedical Engineering, Texas A&M University, College Station, TX, USA
Vanessa M. Page, Department of Biomedical Engineering, Texas A&M University, College Station, TX, USA
Melissa L. Hawkins, Department of Biomedical Engineering, Texas A&M University, College Station, TX, USA
Shane J. Stafslien, Center for Nanoscale Science and Engineering, North Dakota State University, Fargo, ND, USA
Melissa A. Grunlan, Department of Biomedical Engineering and Department of Materials Science and Engineering, Texas A&M University, College Station, TX, USA, 5030 Emerging Technologies Building, College Station, TX 77843-3120
References
- 1.Curtis J, Colas A. (2004) Medical applications of silicones. In Biomaterials Science: An Introduction to Materials in Medicine Elsevier Academic Press, San Diego, CA: pp. 697–707. [Google Scholar]
- 2.VanDyke ME, Clarson SJ, Arshady R. (2003) Silicone biomaterials. In An Introduction to Polymeric Biomaterials Citrus Books, London: pp. 109–35. [Google Scholar]
- 3.Brash JL. (1977) Hydrophobic polymer surfaces and their interactions with blood. Annals of the New York Academy of Sciences 283(1): 356–71. [Google Scholar]
- 4.Bartzoka V, McDermott MR, Brook MA. (1999) Protein-silicone interactions. Advanced Materials 11(3): 257–9. [Google Scholar]
- 5.Hron P (2003) Hydrophilisation of silicone rubber for medical applications. Polymer International 52(9): 1531–9. [Google Scholar]
- 6.Callow JA, Callow ME. (2011) Trends in the development of environmentally friendly fouling-resistant marine coatings. Nature Communications 2: 244. [DOI] [PubMed] [Google Scholar]
- 7.Molino PJ, Wetherbee R. (2008) The biology of biofouling diatoms and their role in the development of microbial slimes. Biofouling 24(5): 365–79. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JM. (2001) Biological responses to materials. Annual Review of Materials Research 31(1): 81–110. [Google Scholar]
- 9.Lloyd DA, Shanbhogue LKR, Doherty PJ, Sunderland D, Hart CA, Williams DF. (1993) Does the fibrin coat around a central venous catheter influence catheter-related sepsis? Journal of Pediatric Surgery 28(3): 345–9. [DOI] [PubMed] [Google Scholar]
- 10.Raad I, Luna M, Khalil SM, Costerton JW, Lam C, Bodey GP. (1994) The relationship between the thrombotic and infectious complications of central venous catheters. JAMA 271(13): 1014–6. [PubMed] [Google Scholar]
- 11.Ratner BD, Horbett TA. (2013) Evaluation of blood–materials interactions. In Biomaterials Science (3rd Ed). Academic Press; pp. 617–34. [Google Scholar]
- 12.Bartzoka V, McDermott MR, Brook MA. (1999) Protein‐Silicone interactions. Advanced Materials 11(3): 257–9. [Google Scholar]
- 13.Chambers LD, Stokes KR, Walsh FC, Wood RJK. (2006) Modern approaches to marine antifouling coatings. Surface and Coatings Technology 201(6): 3642–52. [Google Scholar]
- 14.Yebra DM, Kiil S, Dam-Johansen K. (2004) Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Progress in Organic Coatings 50(2): 75–104. [Google Scholar]
- 15.Abbasi F, Mirzadeh H, Katbab A-A. (2001) Modification of polysiloxane polymers for biomedical applications: a review. Polymer International 50(12): 1279–87. [Google Scholar]
- 16.Bodas D, Khan-Malek C. (2006) Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectronic Engineering 83(4–9): 1277–9. [Google Scholar]
- 17.Yao K, Huang X-D, Huang X-J, Xu Z-K. (2006) Improvement of the surface biocompatibility of silicone intraocular lens by the plasma-induced tethering of phospholipid moieties. Journal of Biomedical Materials Research Part A 78A(4): 684–92. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Annich GM, Miskulin J, Osterholzer K, Merz SI, Bartlett RH, et al. (2002) Nitric oxide releasing silicone rubbers with improved blood compatibility: preparation, characterization, and in vivo evaluation. Biomaterials 23(6): 1485–94. [DOI] [PubMed] [Google Scholar]
- 19.Yeh S-B, Chen C-S, Chen W-Y, Huang C-J. (2014) Modification of silicone elastomer with zwitterionic silane for durable antifouling properties. Langmuir 30: 11386–93. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Brook MA, Chen Y, Sheardown H. (2005) Surface properties of PEO-silicone composites: reducing protein adsorption. Journal of Biomaterials Science, Polymer Edition 16(4): 531–48. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Brook MA, Sheardown H. (2004) Silicone elastomers for reduced protein adsorption. Biomaterials 25(12): 2273–82. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Zhang Z, Chen Y, Brook MA, Sheardown H. (2005) Protein repellant silicone surfaces by covalent immobilization of poly(ethylene oxide). Biomaterials 26(15): 2391–9. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Vörös J. (2005) An aqueous-based surface modification of poly(dimethylsiloxane) with poly(ethylene glycol) to prevent biofouling. Langmuir 21(25): 11957–62. [DOI] [PubMed] [Google Scholar]
- 24.Papra A, Bernard A, Juncker D, Larsen NB, Michel B, Delamarche E. (2001) Microfluidic networks made of poly(dimethylsiloxane), Si, and Au coated with polyethylene glycol for patterning proteins onto surfaces. Langmuir 17(13): 4090–5. [Google Scholar]
- 25.Thompson DB, Fawcett AS, Brook MA. (2008) Simple strategies to manipulate hydrophilic domains in silicones. In Silicon Based Polymers Springer; pp. 29–38. [Google Scholar]
- 26.Chen S, Li L, Zhao C, Zheng J. (2010) Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 51(23): 5283–93. [Google Scholar]
- 27.Jeon SI, Lee JH, Andrade JD, De Gennes PG. (1991) Protein—surface interactions in the presence of polyethylene oxide: I. Simplified theory. Journal of Colloid and Interface Science 142(1): 149–58. [Google Scholar]
- 28.Jeon SI, Andrade JD. (1991) Protein—surface interactions in the presence of polyethylene oxide: II. Effect of protein size. Journal of Colloid and Interface Science 142(1): 159–66. [Google Scholar]
- 29.Szleifer I (1997) Protein adsorption on surfaces with grafted polymers: a theoretical approach. Biophysical Journal 72: 595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman K, Hähner G, Spencer ND, Harder P, Grunze M. (1999) Probing resistance to protein adsorption of oligo(ethylene glycol)-terminated self-assembled monolayers by scanning force microscopy. Journal of the American Chemical Society 121(43): 10134–41. [Google Scholar]
- 31.Pale-Grosdemange C, Simon ES, Prime KL, Whitesides GM. (1991) Formation of self-assembled monolayers by chemisorption of derivatives of oligo(ethylene glycol) of structure HS(CH2)11(OCH2CH2)mOH on gold. Journal of the American Chemical Society 113(1): 12–20. [Google Scholar]
- 32.Prime KL, Whitesides GM. (1993) Adsorption of proteins onto surfaces containing end-attached oligo(ethylene oxide): a model system using self-assembled monolayers. Journal of the American Chemical Society 115(23): 10714–21. [Google Scholar]
- 33.Jo S, Park K. (2000) Surface modification using silanated poly(ethylene glycol)s. Biomaterials 21(6): 605–16. [DOI] [PubMed] [Google Scholar]
- 34.Lee S-W, Laibinis PE. (1998) Protein-resistant coatings for glass and metal oxide surfaces derived from oligo(ethylene glycol)-terminated alkyltrichlorosilanes. Biomaterials 19(18): 1669–75. [DOI] [PubMed] [Google Scholar]
- 35.Papra A, Gadegaard N, Larsen NB. (2001) Characterization of ultrathin poly(ethylene glycol) monolayers on silicon substrates. Langmuir 17(5): 1457–60. [Google Scholar]
- 36.Sofia SJ, Premnath V, Merrill EW. (1998) Poly(ethylene oxide) grafted to silicon surfaces: grafting density and protein adsorption. Macromolecules 31(15): 5059–70. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Desai T, Ferrari M. (1998) Proteins and cells on PEG immobilized silicon surfaces. Biomaterials 19(10): 953–60. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Ferrari M. (1998) Hemocompatible polyethylene glycol films on silicon. Biomedical Microdevices 1(1): 81–9. [Google Scholar]
- 39.Harris JM, editor. (1992). Poly(ethylene glycol) chemistry: Biotechnical and biomedical applications Plenum Press, New York. [Google Scholar]
- 40.Browning MB, Cereceres SN, Luong PT, Cosgriff-Hernandez EM. (2014) Determination of the in vivo degradation mechanism of PEGDA hydrogels. Journal of Biomedical Materials Research Part A [DOI] [PMC free article] [PubMed]
- 41.Du YJ, Klement P, Berry LR, Tressel P, Chan AK. (2005) In vivo rabbit acute model tests of polyurethane catheters coated with a novel antithrombin-heparin covalent complex. Thrombosis and Haemostasis 94(2): 366–72. [DOI] [PubMed] [Google Scholar]
- 42.Hunt JA, Meijs G, Williams DF. (1997) Hydrophilicity of polymers and soft tissue responses: a quantitative analysis. Journal of Biomedical Materials Research 36(4): 542–9. [DOI] [PubMed] [Google Scholar]
- 43.Park K, Shim HS, Dewanjee MK, Eigler NL. (2000) In vitro and in vivo studies of PEO-grafted blood-contacting cardiovascular prostheses. Journal of Biomaterials Science, Polymer Edition 11(11): 1121–34. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Donaire ML, Santerre JP. (2014) Surface modifying oligomers used to functionalize polymeric surfaces: Consideration of blood contact applications. Journal of Applied Polymer Science 131(14). [Google Scholar]
- 45.Tanzi MC. (2005) Bioactive technologies for hemocompatibility. Expert Review of Medical Devices 2(4): 473–92. [DOI] [PubMed] [Google Scholar]
- 46.Ward R, White K, Hu C. (1984) Use of surface-modifying additives in the development of a new biomedical polyurethaneurea. In Polyurethanes in Biomedical Engineering Elsevier, New York: pp. 181–200. [Google Scholar]
- 47.Kingshott P, Thissen H, Griesser HJ. (2002) Effects of cloud-point grafting, chain length, and density of PEG layers on competitive adsorption of ocular proteins. Biomaterials 23(9): 2043–56. [DOI] [PubMed] [Google Scholar]
- 48.Malmsten M, Emoto K, Van Alstine JM. (1998) Effect of chain density on inhibition of protein adsorption by poly(ethylene glycol) based coatings. Journal of Colloid and Interface Science 202(2): 507–17. [Google Scholar]
- 49.Yasuda H, Sharma AK, Yasuda T. (1981) Effect of orientation and mobility of polymer molecules at surfaces on contact angle and its hysteresis. Journal of Polymer Science Part B: Polymer Physics 19(9): 1285–91. [Google Scholar]
- 50.Owen MJ, Smith PJ. (1994) Plasma treatment of polydimethylsiloxane. Journal of Adhesion Science and Technology 8(10): 1063–75. [Google Scholar]
- 51.Owen MJ. (1993) Surface chemistry and applications. In Siloxane Polymers Prentice Hall, Englewood Cliffs: pp. 309. [Google Scholar]
- 52.Lane TH, Burns SA. (1996) Silica, silicon and silicones Unraveling the mystery. In Immunology of Silicones Springer, Berlin: pp. 3–12. [DOI] [PubMed] [Google Scholar]
- 53.Rufin MA, Gruetzner JA, Hurley MJ, Hawkins ML, Raymond ES, Raymond JE, et al. (2015) Enhancing the protein resistance of silicone via surface-restructuring PEO-silane amphiphiles with variable PEO length. Journal of Materials Chemistry B 3(14): 2816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawkins ML, Grunlan MA. (2012) The protein resistance of silicones prepared with a PEO-silane amphiphile. Journal of Materials Chemistry 22(37): 19540–6. [Google Scholar]
- 55.Rufin MA, Barry ME, Adair PA, Hawkins ML, Raymond JE, Grunlan MA. (2016) Protein resistance efficacy of PEO-silane amphiphiles: Dependence on PEO-segment length and concentration. Acta Biomaterialia 41: 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faÿ FH, ML; Réhel K; Grunlan MA; Linossier I (2016) Non-toxic, anti-fouling silicones with variable PEO-silane amphiphile content. Green Materials 4(2): 53–62. [Google Scholar]
- 57.Hawkins MLF, F.; Cheverau E; Linossier I; Grunlan MA (2014) Bacteria and diatom resistance of silicone modified with PEO-silane amphiphiles. Biofouling 30(2): 247–58. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins ML, Rufin MA, Raymond JE, Grunlan MA. (2014) Direct observation of the nanocomplex surface reorganization of antifouling silicones containing a highly mobile PEO-silane amphiphile. Journal of Materials Chemistry B 2(34): 5689–97. [DOI] [PubMed] [Google Scholar]
- 59.Murthy R, Cox CD, Hahn MS, Grunlan MA. (2007) Protein-resistant silicones: Incorporation of poly(ethylene oxide) via siloxane tethers. Biomacromolecules 8(10): 3244–52. [DOI] [PubMed] [Google Scholar]
- 60.Hawkins ML. Antifouling silicone coatings prepared with PEO-silane amphiphiles College Station, TX: Texas A&M University; 2014. [Google Scholar]
- 61.Maitz MF, Gago R, Abendroth B, Camero M, Caretti I, Kreissig U. (2006) Hemocompatibility of low-friction boron–carbon–nitrogen containing coatings. Journal of Biomedical Materials Research Part B 77B(1): 179–87. [DOI] [PubMed] [Google Scholar]
- 62.Ruzette A-V, Leibler L. (2005) Block copolymers in tomorrow’s plastics. Nature Materials 4(1): 19–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


