Abstract
The long cytoplasmic tail (CT) isoforms of carcinoembryonic Ag-related cell adhesion molecule 1 (CEACAM1) are expressed on activated human T cells and possess two ITIM motifs in the CT. These isoforms of CEACAM1 are inhibitory for T cell responses initiated by the TCR/CD3 complex with the inhibition dependent upon the ITIMs of CEACAM1 and Src homology 2 domain-containing phosphatase 1 (SHP-1). However, the mechanism by which this inhibition occurs in T cells is unknown. We demonstrate here that the Src family kinase, Lck, and the ability of CEACAM1 to bind homophilically are required for the ITIM phosphorylation of CEACAM1 that is a prerequisite for CEACAM1 association with SHP-1. We further show that CEACAM1 associates with and recruits SHP-1 to the TCR/CD3 complex leading to decreased phosphorylation of CD3-ζ and ZAP-70 and consequently decreased activation of the elements downstream of ZAP-70. This is physiologically relevant because extinction of SHP-1 expression or blockade of homophilic binding by CEACAM1 using a Fab that specifically recognizes the homophilic binding region of human CEACAM1 increases the cytolytic function initiated by the TCR/CD3 complex. These studies show that long CT isoforms of CEACAM1 orchestrate an inhibitory program that abrogates extremely proximal events downstream of the TCR/CD3 complex by focusing on the activation of ZAP-70.
The T cell activation and T cell-mediated immune responses are regulated by coordinate signaling emanating from the TCR/CD3 complex and coactivating and coinhibitory receptors (1,2). The most well studied examples of such coreceptors are the CD28/CTLA-4 family members (3, 4). Additional regulatory molecules that function as T cell coactivating or coinhibitory receptors have been described (2). We and others have recently defined carcinoembryonic Ag (CEA)3 related cell adhesion molecule 1 (CEACAM1) as a T cell inhibitory receptor which down-regulates TCR/CD3 signaling and T cell-mediated inflammation (5–10).
CEACAM1, also known as CD66a or biliary glycoprotein, belongs to the CEA family of Ig-like receptors (11, 12). CEACAM1 is expressed in polarized epithelia, blood vessel endothelia, granulocytes, macrophages, B cells, NK cells, and platelets. CEACAM1 expression is induced on the surface of all activated human and mouse T cells when examined ex vivo with limited cell surface expression in the absence of activation (13–15). CEACAM1 proteins consist of a membrane distal N-domain, variable numbers of extracellular Ig-like domains, a common transmembrane domain, and either a 10 amino acid short (S) cytoplasmic tail (CT) or a 72 amino acid long (L) CT. The N-domain contains a motif that mediates homophilic interactions with CEACAM1 either homotypically between the same types of cells or heterotypically between different types of cells as well as heterophilic interactions with other CEACAM family members and a certain group of pathogens. Through these interactions, CEACAM1 has been shown to regulate a variety of cellular functions that include angiogenesis, epithelial cell differentiation and polarization, and hematopoietic cell activity (12, 14, 16–21).
CEACAM1 L CT isoforms have shown the general property of inhibiting a variety of cell types, that include intestinal epithelial cells, B cells, NK cells, and T cells (10, 22–24). Deletion of CEACAM1 or overexpression of CEACAM1 L CT isoforms specifically within T cells results in increased and decreased TCR signaling (7), respectively. Moreover, when examined in each of these cell types, this inhibition generally involves the ITIM domains in the CT and Src homology 2 domain phosphatase 1 (SHP-1) (5, 7, 8). However, the mechanism by which the interactions between the ITIM domains of CEACAM1 L isoforms and SHP-1 mediate inhibition is unexplained, and the molecular elements that precede and follow these interactions are not defined in T cells. In this study, we demonstrate that in T cells, ITIM phosphorylation of CEACAM1 and, consequently, association with SHP-1 requires p56 Lck kinase and the ability to bind homophilically. Moreover, we show that CEACAM1 associates with and recruits SHP-1 to the TCR signalosome where SHP-1 blocks ZAP-70 activation via dephosphorylation of CD3-ζ and/or ZAP-70. This is confirmed to be physiologically relevant because masking of the homophilic binding site of CEACAM1 with Fab that specifically interact with the homophilic binding motif leads to increased cytotoxicity and lymphocyte degranulation.
Materials and Methods
Cells and cell culture
Human peripheral blood T cells were isolated from leukopacks (Brigham and Women’s Hospital Blood Center) using a human (h) pan-T cell isolation kit (Miltenyi Biotec). Cells were cultured with RPMI 1640 supplemented with 100 U/ml rh-IL-2 (BD Pharmingen) and 2 μg/ml PHA. Clonally expanded hCD8+ and hCD4+ T cell lines were kindly provided by Dr. Bana Jabri (University of Chicago, Chicago, IL) and cultured as described previously (25). Jurkat T cells stably transfected with hCEACAM1–4L, hCEACAM1–4S were kindly provided by Dr. John Shively (Beckman Research Institute of the City of Hope, Duarte, CA). Human leukemia CD8+ T cell line TALL 104 (TALL) and J.cam1.6 T cells were purchased from American Type Culture Collection.
Abs and reagents
The mouse anti-hCEACAM1 mAbs 5F4 and 34B1 (IgG1) were generated as previously described (26). Biotinylated 5F4 was made with EZ-Link sulfo-NHS-Biotin reagent according to instruction of the manufacturer Pierce. 5F4 Fab were prepared as per the manufacturer’s instructions (Pierce). Mouse anti-hCD3, isotype control IgG, PE-conjugated mouse anti-hLamp1 (CD107a), and rh-IL-2 were purchased from BD Pharmingen. Anti-phosphotyrosine mAb 4G10 and active ZAP-70 recombinant protein were purchased from Upstate Biotechnology; F(ab′)2 goat anti-mouse was obtained from Jackson ImmunoResearch Laboratories. Mouse anti-ERK, phospho(p)-ERK, anti-hCD3-ζ, p-CD3-ζ, anti-TCR-β, and rabbit anti-TCR-α were from Santa Cruz Biotechnology. Rabbit anti-ZAP70, p-ZAP70, p-linker for activation of T cells (LAT), and SLP-76 were from Cell Signaling Technology. Rabbit anti-SHP-1 mAb was from Epitomics, and anti-p56 Lck was from Calbiochem.
Plasmids, short interfering RNA (siRNA), and transfection
The hCEACAM1–3L and −3S, as well as CEACAM1–3L-FF mutant constructs, were previously generated (5). Jurkat and Lck-deficient Jurkat (J.cam1.6) T cells were transfected with nucleofector kit V from Amaxa according to the manufacturer’s instructions. Homophilic binding deficient hCEACAM1–3L was generated by replacing 43 arginine (R) with glycine (G) and 44 glutamine (Q) with leucine (L) (R43G Q44L) using a site-directed mutagenesis kit from Stratagene. N-terminal Flag tagged wildtype (WT) and R43G Q44L mutant CEACAM1–3L constructs were prepared using the following primers: the leader peptide forward primer containing KpnI site 5′-AAAAGGTACCGCCACCATGGGGCACCTC TCAG-3′ and reverse primer containing Flag sequence 5′-CTTATCGTC GTCATCCTTGTAATCGGCAGTGGTGGGCGGGTTCCAG-3′; and the mature peptide forward primer containing a Flag sequence 5′-GATTA CAAGGATGACGACGATAAGCAGCTCAC TACTGAATCC-3′ and reverse primer containing XhoI site is 5′-GGGCTCGAGTTACTGCTTTTT TACTTCTGAATAAATTA-3′. The PCR products were used as templates for a second round of PCR using the leader peptide forward primer and mature peptide reverse primer to produce full-length cDNA PCR product. The PCR products were inserted into pcDNA3 vector at KpnI and XhoI sites. All constructs were sequenced at the Sequence Center of Brigham and Women’s Hospital. SHP-1 siRNA was purchased from Santa Cruz Biotechnology. The siRNA was transfected into TALL cells with a siRNA test kit and nucleofector kit V according to the manufacturer’s instructions.
RT-PCR
The primers to determine CEACAM1 isoforms were 5′-GCTCTACCA CAAGAAAATGG-3′ (forward) and 5′-CATTGGAGTGGTCCTGAG-3′(reverse). They amplified the L isoform cDNA to produce a 197 bp fragment and the S isoform to produce a 143 bp fragment.
Immunoprecipitation (IP) and Western blotting
IP and Western blotting were performed as previously described (5) by using specific Abs as indicated in results and legends.
Confocal microscopy
TALL cells were incubated with FITC-anti-CD3 on ice for 1 h. The cells were transferred to 37°C for 5 min for surface cap staining or 30 min for internalized vesicle staining. Staining with indicated Abs in legends was performed as described previously (27). The slides were analyzed on a workstation of a Nikon TE2000-E inverted microscope coupled to a PerkinElmer spinning disk confocal unit and an Orca AG cooled CCD camera (Hamamatsu) with a plexiglass chamber enclosing the stage for control of the sample environment (temperature and humidity).
Flow cytometry assay
For CEACAM1 expression analysis, cells were incubated with 5F4 for 20 min followed by FITC-conjugated rat anti-mouse IgG1. For Lamp1 surface translocation, cells were seeded in a 96-well plate coated with Abs as indicated, incubated in 5% CO2 37°C incubator for 4 h, and stained with PE-conjugated anti-Lamp1. Stained cells were analyzed on a FACscan platform (BD Biosciences).
Serine esterase release and cytotoxicity assay
Serine esterase assay and calculation of released esterase percentage was performed following standard protocol with modification (28). Ab redirected Ab-dependent cell mediated cytotoxicity and calculation of specific 51Cr release was performed as previously described (25, 26).
Statistics
The student’s t test was used to determine significance. A value of p < 0.05 was considered as significant.
Results
Stimulation of T cells induces expression of L and S CT containing isoforms of CEACAM1
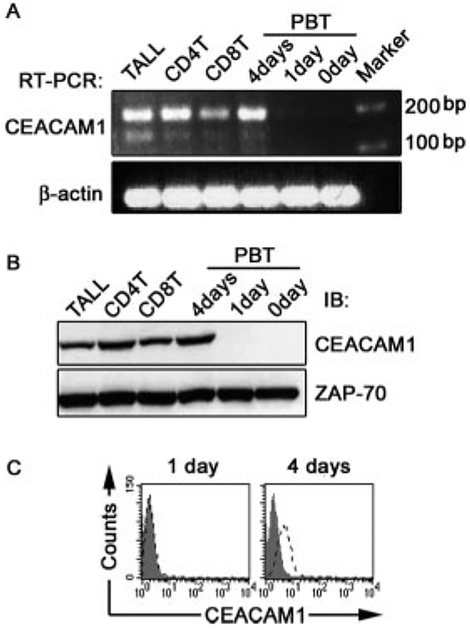
The specific isoforms of CEACAM1 expressed by human T cells is controversial, with some studies showing that human T cells only express L CT isoforms (15). Therefore, to define which isoforms of CEACAM1 are expressed in human T cells, we established primers which amplify a 143 bp fragment from all S isoform cDNAs and a 197 bp fragment from all L isoform cDNAs (data not shown). Using this unique set of primers, we analyzed freshly isolated peripheral blood T (PBT) cells, clonally expanded intestinal intraepithelial lymphocyte (IEL) CD4+ T cells, IEL CD8+ T cell lines, and the CD8+ T cell leukemia TALL 104 (TALL) cell line. The results in Fig. 1A show that all continuously cultured T cells lines expressed high levels of CEACAM1 L isoforms and low levels of CEACAM1 S isoforms. In contrast, unstimulated primary PBT cells expressed negligible levels of both isoforms of CEACAM1. However, after 4 days of culture with IL-2 and PHA, the CEACAM1 mRNA levels in PBT cells reached those of the clonally expanded IEL T cell lines and TALL cells constantly maintained in IL-2 media with predominant expression of long-CT isoforms of CEACAM1. These results show that increased CEACAM1 transcripts are induced in primary human T cells by activation with IL-2 and PHA, and that the predominant isoforms expressed are those that contain a L CT.
FIGURE 1.
CEACAM1 expressing a L CT is expressed by human T cells upon activation. A, mRNA expression of CEACAM1 in human T cells. The mRNA was isolated from various types of human T cells as indicated. RT-PCR was performed. The larger band (197 bp) represents the L isoform and smaller band (143 bp) the S isoform of CEACAM1. Equal mRNA content was verified by β-actin. B, CEACAM1 protein expression in different T cells. The cell lysates were immunoprecipitated by anti-CEACAM1 (5F4). The precipitates were analyzed by western blot (IB) by 5F4. An equal amount of proteins used for IP was verified by monitoring ZAP-70 in 20 μl aliquot of each lysate. C, Surface expression of CEACAM1 on PBT cells. PBT cells cultured with rhIL-2 plus PHA for 0 or 4 days were stained by 5F4 (open line) or isotype control Ig (solid) followed by FITC-anti-mouse IgG1. All results are representative of three experiments.
To determine whether the CEACAM1 mRNAs that are induced by IL-2 or IL-2 plus PHA result in increased CEACAM1 protein expression, we immunoprecipitated and immunoblotted (IB) CEACAM1 from various T cell lysates using the monoclonal antihuman CEACAM1 Ab (5F4) (26). Fig. 1B shows that CEACAM1 protein could not be detected in resting human PBT cells. However, after 4 days of culture with IL-2 plus PHA, CEACAM1 could be detected at levels that were similar to T cell lines continuously maintained in IL-2. Increasing the culture period to 7 days had no further effect on CEACAM1 mRNA or protein levels of primary human PBT cells (data not shown). Flow cytometry analysis also showed undetectable levels of CEACAM1 on the cell surface of primary human PBT cells (Fig. 1C). However, CEACAM1 surface expression was evident after 4 days of culture in IL-2 and PHA (Fig. 1C). These studies confirm that CEACAM1 is an activation-induced cell surface protein in T cells and, that the predominant forms of CEACAM1 expressed by human T cells are those containing a L CT.
CEACAM1 ITIM phosphorylation and subsequent association with SHP-1 requires Lck and homophilic binding
CEACAM1-L ITIM phosphorylation is required for association with SHP-1 and inhibition of TCR signaling because deletion of critical tyrosine residues within the ITIMs abrogates the inhibitory functions of mouse and human CEACAM1 (7, 14, 29). In addition, phosphorylated, but not nonphosphorylated, CEACAM1 CT containing fusion proteins can associate with SHP-1 in mice (14). We therefore examined tyrosine phosphorylation of endogenous CEACAM1 in T cells cultivated in IL-2. Fig. 2A shows that all activated T cells examined contain tyrosine phosphorylated CEACAM1. Because phosphorylation of CEACAM1 could be due to IL-2 stimulation and/or homophilic ligation of CEACAM1, we pursued the following studies in Jurkat T cells that do not normally express CEACAM1 (8). We transiently transfected Jurkat T cells with WT CEACAM1–3L (3L-WT), CEACAM1–3S (3S-WT), or CEACAM1–3L-FF (3L-FF) in which the two tyrosines in ITIMs were replaced with conservative phenylalanine substitutions as previously described (5). Given that Jurkat T cells do not require IL-2 and do not spontaneously produce IL-2 in culture, it was interesting to observe that CEACAM1 3L-WT was phosphorylated in Jurkat T cells without stimulation (Fig. 2B). In contrast, neither CEACAM1 3S-WT, nor 3L-FF, which are otherwise identical except for the CT, were not phosphorylated. The latter studies confirm that phosphorylation of CEACAM1 3L-WT occurs on the ITIM tyrosines.
FIGURE 2.
CEACAM1 tyrosine phosphorylation and association with SHP-1. A, Endogenous CEACAM1 tyrosine phosphorylation. CEACAM1 was immunoprecipitated by 5F4. The precipitates were analyzed by IB with anti-phosphotyrosine (p-Tyr) Ab (4G10). The membrane was re-blotted by 5F4 to verify CEACAM1. B, Constitutive tyrosine phosphorylation of CEACAM1 ITIM. The WT CEACAM1–3L (3L-WT) and its corresponding S isoform (3S-WT) and mutant 3L-FF were transfected into Jurkat T cells. CEACAM1 was immunoprecipitated by 5F4 Ab. The p-Tyr of CEACAM1 was determined and total CEACAM1 verified. C, CEACAM1–3L R43G Q44L mutant (3L-MU) is normally expressed on cell surface. 3L-WT and 3L-R43G Q44L isoform surface expression in Jurkat T cells was evaluated by flow cytometry with an anti-Flag Ab. D, Homophilic interaction is responsible for CEACAM1 tyrosine phosphorylation. Flag tagged 3L-WT and 3L-R43G Q44L (3L-MU) isoforms were transfected into Jurkat T cells. CEACAM1 was immunoprecipitated by anti-Flag and p-Tyr of CEACAM1 was evaluated by 4G10. The membrane was re-blotted with anti-Flag to assess total CEACAM1. E, Src kinase is required for CEACAM1 ITIM phosphorylation. 3L-WT was transiently transfected into Jurkat T cells. Cells were treated or not by 2 μM of indicated inhibitors for 24 h. CEACAM1 was immunoprecipitated, p-Tyr of CEACAM1 was determined, and total CEACAM1 was verified by IB. F, Lck is required for CEACAM1 ITIM phosphorylation. 3L-WT was transiently transfected CEACAM1 CT (CT)-GST fusion protein (GST-CT) pull-down assay. Upper panel, Coomassie into Lck-deficient Jurkat T cells (J.cam1.6) and WT Jurkat T cells. CEACAM1 was immunoprecipitated, p-Tyr of CEACAM1 was evaluated, and total CEACAM1 verified by IB. G, CEACAM1 CT (CT)-GST fusion protein (GST-CT) pull-down assay. Upper panel, Coomassieblue staining that shows GST-CT and GST proteins. Lower panel, The associated Lck that was pulled-down by the respective fusion protein as defined by IB with anti-Lck. H, Association of endogenous CEACAM1 and Lck. TALL cells were treated or not by 10 μg anti-CD3 followed by F(ab′)2 goat anti-mouse IgG for 2 min. The cell lysates were immunoprecipitated with 5F4. The immunoprecipitates were analyzed by IB with an anti-Lck. The membrane was re-blotted with 5F4 to verify CEACAM1. Stim, Stimulation.
These studies suggested that homophilic interactions are sufficient to induce CEACAM1-L tyrosine phosphorylation. To provide further confirmation, we prepared N-terminal Flag-tagged WT CEACAM1–3L and mutated CEACAM1–3L in which the homophilic binding site was deleted by replacing CEACAM1–3L 43 arginine (R) with glycine (G) and 44 glutamine (Q) with leucine (L) (R43G Q44L) as previously described (30). Such mutations of human CEACAM1 abrogate homophilic binding and heterophilic binding of CEACAM1 to the mouse anti-human CEACAM1 Ab 5F4 (30). Although CEACAM1–3LR43G Q44L was able to reach the cell surface at levels similar to that observed with WT CEACAM1–3L (Fig. 2C), there was no evidence for tyrosine phosphorylation of CEACAM1–3LR43G Q44L (Fig. 2D). Thus, tyrosine phosphorylation of CEACAM1 is regulated by homophilic ligation of CEACAM1 in the absence of T cell activation.
In non-T cell types, CEACAM1 has been shown to associate with Src family tyrosine kinases (29). To define which kinase in T cells is responsible for phosphorylating the CEACAM1-L ITIM tyrosines, we performed the following studies. We transiently expressed human CEACAM1–3L in Jurkat T cells. The cells were then treated with a Src tyrosine kinase inhibitor (PP2). As expected, treatment of Jurkat T cells that transiently expressed CEACAM1–3L with PP2 profoundly reduced CEACAM1-L phosphorylation. In contrast, treatment with a vehicle control containing a Src tyrosine kinase-irrelevant inhibitor (AG1478) specific for the epidermal growth factor receptor, which is not expressed by Jurkat T cells (31) and which has been shown to tyrosine-phosphorylate CEACAM1-L (32), had no effect on CEACAM1-L phosphorylation. To identify the specific tyrosine kinase required for CEACAM1 phosphorylation in T cells, we transiently transfected CEACAM1 3L-WT into WT and Lck-deficient (J.cam1.6) Jurkat T cells. In the absence of Lck, both CEACAM1 phosphorylation and association with SHP-1 were blocked (Fig. 2F). These results indicate that Lck is required for CEACAM1 phosphorylation and SHP-1 association.
To demonstrate an interaction between human CEACAM1 and Lck, we created a GST fusion protein containing the CT of the L isoform CEACAM1 (GST-CT). These studies showed that GST-CT, but not GST, could coimmunoprecipitate Lck from Jurkat T cell lysates (Fig. 2G). Furthermore, when primary PBT cell lysates were immunoprecipitated by an anti-CEACAM1 Ab and the immune-complexes analyzed by immunoblot with an anti-Lck Ab, we observed an association between endogenous CEACAM1 and Lck (Fig. 2H). Similar results were obtained using clonally expanded IEL and TALL cells (data not shown). Interestingly, the association between CEACAM1 and Lck was observed to decrease shortly after TCR engagement with anti-CD3 (Fig. 2H), suggesting that CEACAM1 association with Lck was mainly due to an association with nonphosphorylated CEACAM1 (see Fig. 2G) or that Lck was displaced by an association with SHP-1 (see following results).
CEACAM1 recruits SHP-1 to deactivate ZAP-70
To define the immediate elements that are influenced by the interaction of the ITIMs of CEACAM1 and SHP-1, we evaluated the effect of CEACAM1 ligation on CD3-ζ and ZAP-70 tyrosine phosphorylation. TALL cells were treated with anti-CD3-ε with or without an anti-CEACAM1 Ab that binds to the homophilic binding site (5, 26, 30, 33) followed by crosslinking with a F(ab′)2 goat anti-mouse IgG for 2 min. Phosphorylation of CD3-ζ and ZAP-70 was then assessed by IB with corresponding phosphotyrosine specific Abs. The results in Fig. 3A show that coligation of CEACAM1 caused a profound decrease in TCR stimulation-induced CD3-ζ and ZAP-70 phosphorylation. However, the total CD3-ζ and ZAP-70 were not changed (shown herein is only ZAP-70). Similar results were obtained with clonally expanded IEL cells (data not shown).
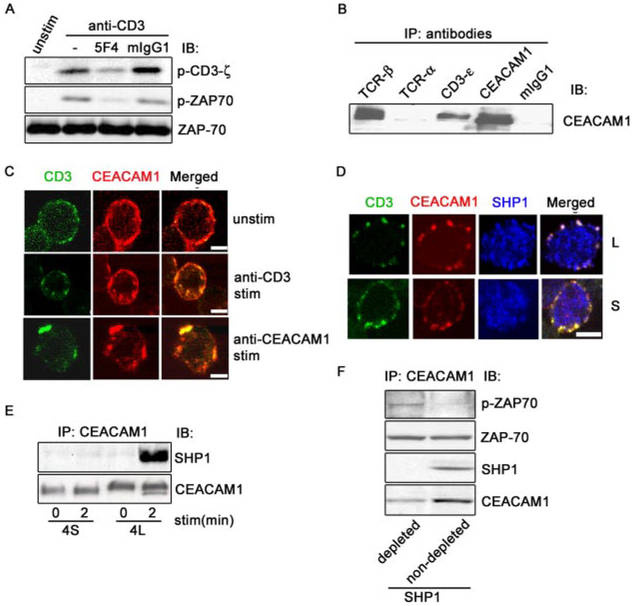
FIGURE 3.
CEACAM1 association with SHP-1 dephosphorylates ZAP-70. A, CEACAM1 coligation decreases CD3-ζ and ZAP-70 phosphorylation induced by anti-CD3. T cells were treated with Abs as indicated, followed by crosslinking with F(ab′)2 anti-mouse IgG for 2 min. CD3-ζ and ZAP-70 activation was evaluated by IB with corresponding specific p-Tyr Abs. Protein loading was verified by re-blotting the membrane by anti-ZAP-70. B, CEACAM1 associates with TCR/CD3 complex. CEACAM1 was immunoprecipitated from lysates from TALL cells with 5 μg of anti-TCR-α, anti-TCR-β, anti-CD3-ε, anti-CEACAM1 (5F4), and mouse IgG, respectively. The precipitates were analyzed by IB with anti-CEACAM1 (5F4). C, TCR/CD3 complex induced CEACAM1 colocalization. TALL cells were incubated with FITC-anti-CD3 or biotinylated 5F4, respectively, at 4°C for 1 h, then maintained at 4°C or transferred to 37°C for 30 min. CEACAM1 or the TCR/CD3 complex was visualized by biotinylated 5F4 followed by Texas red-streptavidin or FITC-anti-CD3, respectively. Yellow spots show colocalization (scale bar represents 5 micrometers). D, SHP-1 colocalization with CEACAM1 and TCR/CD3 complex. Jurkat T cells either stably expressing CEACAM1–4L or CEACAM1–4S isoforms were incubated with FITC- anti-CD3 at 4°C for 1 h, then transferred to 37°C for 5 min. CEACAM1 was visualized as above and SHP-1 was viewed with rabbit anti-SHP-1 followed by cy5-anti-rabbit IgG. White spots show triple colocalization (scale bar represents 5 micrometers). E, L CT isoform of CEACAM1 association with SHP-1. Jurkat T cells stably expressing 4L or 4S isoforms of CEACAM1 were treated or not with anti-CD3 followed by F(ab′)2 goat anti-mouse IgG for 2 min, respectively. The cell lysates were immunoprecipitated with 5F4. The immunoprecipitates were analyzed by IB with anti-SHP-1. The membrane was re-blotted with 5F4 to assess CEACAM1. F, CEACAM1-associated SHP-1 dephosphorylates active ZAP-70. The lysates from T cells treated with anti-CD3 for 2 min were immunoprecipitated with rabbit anti-SHP-1 or rabbit IgG coupled protein G beads twice. The supernatants were further immunoprecipitated with 5F4. The final immunoprecipitates were incubated with active p-ZAP-70. The mixtures were analyzed by IB to assess p-ZAP-70 using anti-p-ZAP-70. Protein loading was verified by assessing total ZAP-70 with anti-ZAP-70. SHP-1 and CEACAM1 were detected by the corresponding specific Abs. stim, stimulation; and unstim, unstimulation.
To interrogate the mechanism by which CEACAM1 recruits SHP-1 to TCR signalosome, we performed coimmunoprecipitation assays and confocal microscopy. As shown in Fig. 3B, Abs against CEACAM1, CD3-ε-chain, and the TCR-β-chain coprecipitated CEACAM1 from TALL cell lysates, showing that CEACAM1 interacts with the endogenous TCR/CD3 complex. Interestingly, an Ab specific for the TCR-α-chain did not coimmunoprecipitate CEACAM1, suggesting thatCEACAM1 is mainly associated with the TCR-β- but not TCR-a-chain or that the TCR-α Ab used induced dissociation of CEACAM1. Fig. 3C shows that CEACAM1 and the TCR/CD3 localize on cell surface and exhibit significant colocalization. Upon stimulation of the TCR/CD3 complex, the colocalization was markedly increased especially intracellularly, indicating stimulation of the TCR/CD3 complex caused significant cointernalization with CEACAM1. When T cells were stimulated with an anti-CEACAM1 Ab, we also observed an intensive colocalization of CEACAM1 and the TCR/CD3 complex intracellularly. These studies indicate a physiological association of CEACAM1 and TCR/CD3 complex.
To determine whether CEACAM1 recruits SHP-1 to the TCR signalosome upon TCR stimulation, Jurkat T cells either stably expressing CEACAM-4L or CEACAM1–4S were stimulated with FITC-conjugated anti-CD3 for 5 min, fixed, and stained for CEACAM1 and SHP-1. The results shown in Fig. 3D indicate that under these conditions the TCR/CD3 complex, CEACAM1, and SHP-1 colocalize in punctate structures at the plasma membrane in CEACAM1–4L transfected cells. In contrast, in CEACAM1–4S transfected cells colocalization was observed only between the TCR/CD3 complex and CEACAM1 as revealed by confocal microscopy. In addition, Jurkat T cells stably expressing CEACAM1–4L, but not CEACAM1–4S, was observed to associate with SHP-1 within 2 min of anti-CD3 stimulation (Fig. 3E). Taken together with the microscopy studies (Fig. 3D), these studies indicate that the ITIMs of CEACAM1 recruit SHP-1 that in turn mobilizes SHP-1 to the TCR signalosome.
The kinetics of CEACAM1 association with SHP-1 (Fig. 3E) was reciprocal to that observed for Lck association (Fig. 2H) suggesting that CEACAM1 interacts sequentially with Lck and SHP-1 and may account for the dephosphorylation of ZAP-70. Thus, to demonstrate that the SHP-1, which is associated with CEACAM1, can dephosphorylate elements within the TCR signalosome, we incubated T cell lysates with anti-SHP-1 or isotype control IgG coupled to protein-G Sepharose beads twice to deplete SHP-1 and then isolated CEACAM1 by IP with 5F4. The CEACAM1 isolated in this manner that was depleted or not depleted of coassociated SHP-1 was then tested for its ability to dephosphorylate ZAP-70. As expected, when SHP-1 was depleted from lysates using a SHP-1 specific Ab (Fig. 3F), but not an IgG control, CEACAM1 lost the ability to dephosphorylate active ZAP-70. These studies indicate that a CEACAM1/SHP-1 complex in the TCR signalosome is able to dephosphorylate ZAP-70 and/or CD3-ζ.
CEACAM1 engagement prevents activation of ZAP-70-related signaling pathways
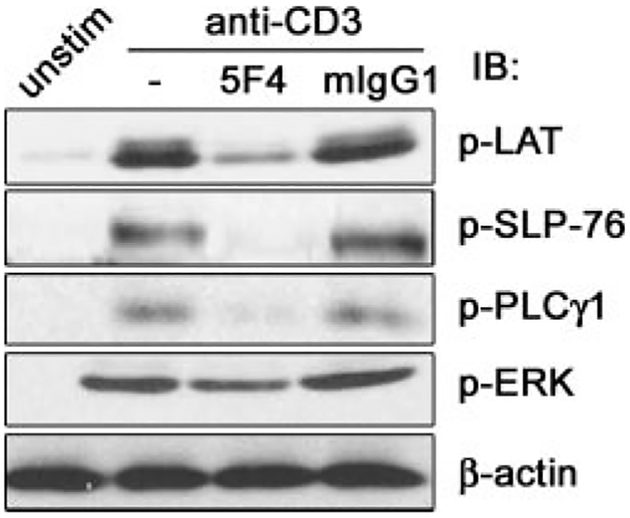
Based on the results presented above, we predicted that coengagement of CEACAM1 would inhibit activation of other signaling elements downstream of ZAP-70 that are induced by TCR/CD3 complex stimulation. To confirm this prediction, TALL cells were treated with anti-CD3 with or without the anti-CEACAM1 mAb 5F4 or an isotype control IgG followed by crosslinking with F(ab′)2 goat anti-mouse IgG for 2 min. Activation of LAT, PLCγ1 and ERK was evaluated by immunoblot of whole cell lysates with the corresponding anti-phospho-specific Abs. SLP-76 activation was assessed by IP of SLP-76 followed by immunoblot with an anti-p-Tyr Ab. Coengagement of the TCR/CD3 complex and CEACAM1 decreased the phosphorylation and thus the activation of LAT, SLP-76, PLCγ1 and ERK compared with that induced by TCR/CD3 stimulation alone (Fig. 4).
FIGURE 4.
CEACAM1 engagement impairs activation of ZAP-70-related signaling elements. TALL cells were stimulated or not (unstim) with anti-CD3 (—), anti-CD3 plus 5F4 (5F4), or anti-CD3 plus mIgG1 (mIgG1) as indicated. The lysates were analyzed by IB with the indicated anti-p-Tyr. The p-SLP-76 was detected by IP with SLP-76 Ab followed by IB with anti-p-Tyr. Protein loading was verified by re-blotting the membranes with anti-LAT, SLP-76, PLCγ1, ERK, and β-actin Abs, respectively. Only β-actin IB is shown. The results were representative of three independent experiments. Similar results were obtained with clonally expanded IEL and PBT cells.
CEACAM1 engagement inhibits TCR-mediated cytotoxicity by blocking granule exocytosis
Previous studies have shown that CEACAM1 engagement of stimulated CD8+ IELs (26) and tumor infiltrating CD8+ T cells (9) inhibits cytolytic function. ZAP-70 signaling is involved in TCR cytolytic function (34, 35). We therefore confirmed that CEACAM1 engagement inhibited anti-CD3 redirected target cell lysis of P815 cells (Fig. 5, A–C) in clonally expanded IEL CD8+ T cells and the TALL T cell line. We next investigated the mechanism for CEACAM1-mediated inhibition of TCR cytolytic function. As seen in Fig. 5D, anti-CEACAM1, but not mouse IgG, inhibited the anti-CD3-induced release of serine esterases. Neither the CEACAM1-specific Ab 5F4 nor control IgG alone induced release of serine esterases from T cell granules. These results indicate that CEACAM1 engagement blocks TCR-induced granule exocytosis. To confirm this, granule movement from the cytoplasm to the surface membrane was assessed by Lamp1 staining of the cell surface after CD3 stimulation in the presence or absence of increasing concentrations of the CEACAM1-specific mAb 5F4 or control mouse IgG1. As shown in Fig. 5E, the 5F4 mAb, but not an isotype control, caused a significant reduction of Lamp1 accumulation on the surface. These studies show that coengagement of CEACAM1 with the TCR/CD3 complex results in inhibition of granule exocytosis and consequently diminished cytotoxicity, functions that are downstream of ZAP-70.
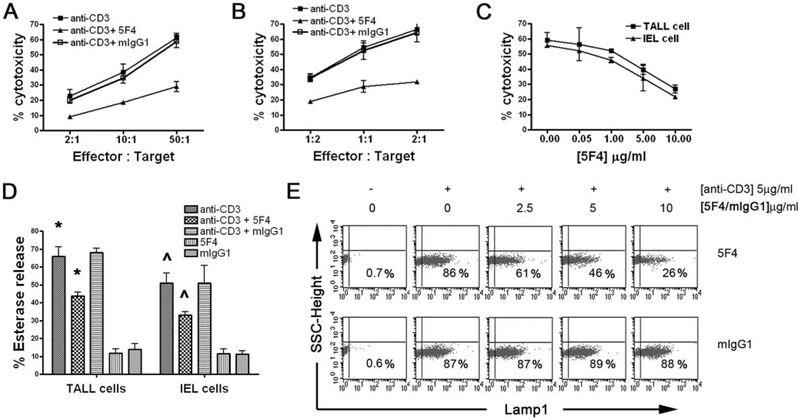
FIGURE 5.
Coligation of TCR/CD3 complex and CEACAM1 inhibits degranulation of CTLs. A and B, CEACAM1 coligation inhibited anti-CD3 re-directed cytolysis. TALL cells (A) or IEL CD8+ T cells (B) were pretreated with 5F4 or mIgG1 for 30 min followed by incubation of anti-CD3 Ab and analysis of cytotoxicity. % specific cytotoxicity is shown at the indicated E:T ratios. C, Dose-dependent inhibition of anti-CD3 re-directed lysis by anti-CEACAM1 Ab. TALL cells or IEL CD8+ T cells were pretreated with different concentrations of 5F4 as indicated for 30 min. Effector/target ratio was 2:1 for TALL cells and 50:1 for IEL CD8+ T cells. D, CEACAM1 coligation inhibits serine esterase release induced by anti-CD3 stimulation. The 96-well plates were coated with 1 μg 5F4, 1 μg mIgG1, 0.5 μg anti-CD3, 0.5 μg anti-CD3 plus 1 μg 5F4, or 0.5 μg anti-CD3 plus 1 μg mIgG1 in 100 μl, respectively. TALL or IEL cells were seeded and incubated for 4 h at 37°C. The serine esterase activity released into the supernatant was measured. * and ^ indicate statistically significantly differences between the anti-CD3 and anti-CD3 plus 5F4 conditions (p < 0.05). E, Dose-dependent inhibition of 5F4 Ab on Lamp1 surface expression induced by anti-CD3. TALL cells were seeded into 96-well plates, precoated with the indicated concentrations of 5F4 Ab or mIgG1 with or without anti-CD3, and incubated for 3 h at 37°C. Lamp1 surface expression was evaluated by flow cytometry with PE-anti-Lamp1 Ab. Similar results were achieved with IEL CD8+ T cells. The results are representative of three independent experiments.
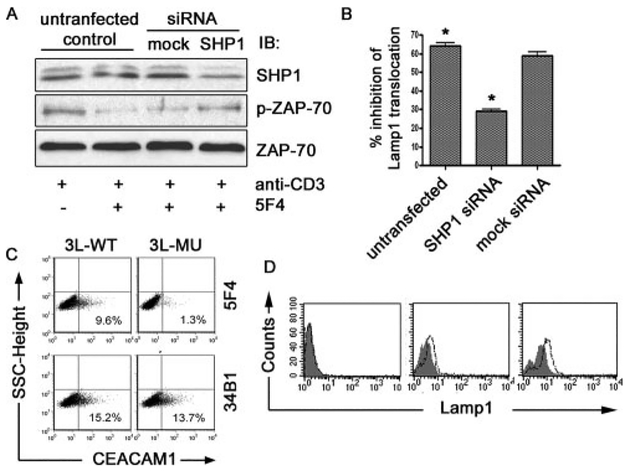
Silencing of SHP-1 and blockade of the CEACAM1 N-domain reverses CEACAM1-mediated inhibition
To determine whether CEACAM1-mediated inhibition of TCR signaling is physiologically affected by SHP-1-mediated dephosphorylation of ZAP-70, we silenced SHP-1 expression in T cells by transfection of TALL cells with SHP-1-specific siRNA. As shown in Fig. 6A, treatment of TALL cells with SHP-1 siRNA resulted in decreased SHP-1 protein expression in comparison to that observed in cells treated with a mock siRNA. In comparison, treatment of TALL cells with SHP-1 siRNA did not affect expression of ZAP-70 (Fig. 6A). We next evaluated the effects of SHP-1 silencing on ZAP-70 phosphorylation induced by ligation with anti-CD3 or anti-CD3 in the presence of CEACAM1 ligation. Whereas ZAP-70 phosphorylation induced by anti-CD3 ligation was inhibited in control cells treated with anti-CEACAM1 in the presence of mock siRNA, CEACAM1-mediated inhibition of anti-CD3-induced ZAP-70 phosphorylation was reversed in cells treated with SHP-1 siRNA (Fig. 6A). We next investigated the effects of SHP-1 silencing on Lamp1 surface accumulation upon TCR/CD3 complex engagement in the presence or absence of CEACAM1 ligation. As shown in Fig. 6B, redistribution of Lamp1 to the cell surface induced by anti-CD3 was inhibited by coengagement of CEACAM1 with the 5F4 mAb. This inhibition of Lamp1 redistribution to the cell surface by coligation of CD3 and CEACAM1 was not affected by mock siRNA. In comparison, ligation of CEACAM1 by the 5F4 was unable to effect suppression of Lamp1 expression on the cell surface when SHP-1 expression was silenced by treatment with specific siRNA (Fig. 6B). These results are consistent with previous studies showing that SHP-1 siRNA can reverse suppression of TCR-induced NFAT activation by CEACAM1 (5), and that CEACAM1–4L overexpression in normal T cells, but not in SHP-1 deficient T cells, can inhibit T cell transfer colitis (7).
FIGURE 6.
Masking of CEACAM1 homophilic binding site reverses the inhibition of CEACAM1 on TCR function. A, Suppression of SHP-1 expression prevents ZAP-70 dephosphorylation induced by CEACAM1 engagement. TALL cells were transfected with mock siRNA or SHP-1 specific siRNA and stimulated as above with anti-CD3 with or without 5F4 or mIgG1. Cellular lysates were analyzed by IB with anti-SHP-1 or anti-p-ZAP-70 Abs, respectively. Protein loading was verified by stripping and re-blotting the membrane with anti-ZAP-70. B, SHP-1 siRNA decreases inhibition of CEACAM1 engagement on Lamp1 surface translocation induced by anti-CD3. TALL cells that were untransfected or transfected with either mock siRNA or SHP-1 specific siRNA were stimulated with anti-CD3 alone or anti-CD3 with 5F4. Lamp1 surface expression was assessed. The percentage of inhibition on Lamp1 surface expression was calculated by the formula: (Lamp1 positive percentage of cells treated by anti-CD3 alone — Lamp1 positive percentage of cells treated with anti-CD3 and 5F4)/Lamp1 positive percentage of cells treated by anti-CD3 alone × 100. * indicates statistically significantly differences between untransfected (un-trans) and SHP1 siRNA (p < 0.05). C, 5F4 binds to CEACAM1 homophilic binding motif. Flag tagged 3L-WT and 3L-R43G Q44L isoforms were transfected into Jurkat T cells. CEACAM1–3L surface expression was evaluated by flow cytometry with anti-CEACAM1 34B1 and 5F4 as indicated. D, Fab of 5F4 enhances TCR/CD3-induced Lamp1 surface expression. TALL cells were pretreated with 5F4 Fab (10 μg/ml; open histograms) or PBS (solid histograms) for 20 min at 37°C and seeded into 96-well plates precoated with PBS (left), 0.5 μg/ml anti-CD3 (middle), or 2 μg/ml anti-CD3 (right) for 3 h. Lamp1 surface accumulation was assessed by flow cytometry with PE-anti-Lamp1. The results are representative of three independent experiments.
Finally, to confirm that homophilic ligation is involved in CEACAM1-mediated inhibition and to identify an antagonist that can specifically block CEACAM1 homophilic binding, we generated Fab fragments (data not shown) of the human CEACAM1-specific mAb, 5F4, that binds to the homophilic binding motif of CEACAM1 (30, 33). Fig. 6C confirms previously published work of Markel and colleagues (30) that the 5F4 Ab interacts with the homophilic binding site of the CEACAM1 N-domain as staining of T cells expressing the CEACAM1–3L R43G Q44L mutant is lost in comparison to staining of the WT CEACAM1–3L isoform. In comparison, the mouse anti-human CEACAM1 mAb 34B1 (26), which binds outside of the homophilic binding site (33), stains both the WT and mutant CEACAM1–3L isoforms (Fig. 6C). The IgG control Ab bound neither WT nor mutated CEACAM1 (data not shown). Fab of the 5F4 mAb were therefore tested for their ability to regulate the T cell function. Anti-CD3 treatment of TALL cells induced an increase in Lamp1 accumulation on cell surface as seen in Fig. 6D (solid histograms; middle and right panels) relative to that observed in untreated cells (solid histogram; left panel). Pretreatment of TALL cells with the Fab of 5F4 significantly increased the accumulation of Lamp1 on the cell surface beyond that induced by anti-CD3 stimulation alone (Fig. 6D; open histograms). Importantly, the Fab alone did not induce Lamp1 accumulation on the cell surface in the absence of TCR stimulation (Fig. 6D, left panel). These studies confirm CEACAM1 as a coinhibitory receptor and indicate that CEACAM1-medi-ated inhibition occurs by homophilic engagement.
Discussion
The inhibitory function of CEACAM1 is dependent on the ITIM domains within the CT of CEACAM1 and SHP-1 because deletion of either abrogates CEACAM1-mediated inhibition. The tyrosine phosphorylation of the ITIMs of CEACAM1 is a prerequisite for CEACAM1 association with SHP-1 in mouse T cells (14) and SHP-1 as well as SHP-2 in epithelia cells (36, 37). However, there exists three fundamental unanswered questions by which CEACAM1 functions in T cells that are what initiates CEACAM1 ITIM phosphorylation, which tyrosine kinase phosphorylates CEACAM1 ITIMs, and how does SHP-1 association with CEACAM1 inhibit TCR/CD3 complex signaling. We now demonstrate that the tyrosine phosphorylation of CEACAM1 L isoforms is initiated by the ability of CEACAM1 to exhibit homophilic binding through its N-domain. Physiologically, this is predicted to occur at high T cell densities with other CEACAM1 bearing cells. In addition, we show that Lck is the tyrosine kinase that is required for phosphorylation of the CEACAM1 ITIMs. In Lck-deficient T cells, CEACAM1 cannot be phosphorylated at tyrosine residues and consequently CEACAM1 lacks the ability to associate with SHP-1. This is consistent with previous observations that the tyrosines within the ITIMs of CEACAM1 can be phosphorylated by c-Src kinase in epithelial cells (29, 32), Btk kinase in B cells (24), Lyn in neutrophils (38, 39), and insulin receptor tyrosine kinase as well as epidermal growth factor receptor tyrosine kinase (40, 41). Specific dependence on Lck for CEACAM1 tyrosine phosphorylation in T cells is consistent with a similar role for Lck in mediating tyrosine phosphorylation of FcγRIIb in NK cells (42). We further show that Lck likely directly phosphorylates the CEACAM1 ITIM tyrosines because endogenous CEACAM1 constitutively associates with Lck. In addition, a GST fusion protein containing the CEACAM1 CT can associate with Lck. However, it is still possible that tyrosine phosphorylation of CEACAM1 is mediated by another tyrosine kinase that requires Lck in T cells. Taken together, these studies suggest that the cell surface of CEACAM1 may induce association of itself with Lck, which is further enhanced by TCR/CD3 complex activation.
The interaction between CEACAM1 ITIMs and SHP-1 suppresses multiple different functions initiated by TCR/CD3 complex signaling including proliferation, cytokine secretion, and cytotoxicity (5, 8–10, 26). However, the final common pathway by which this occurs has been unknown. In this study, we show that coligation of CEACAM1 decreases phosphorylation of CD3-ζ and ZAP-70 induced by stimulation of the TCR/CD3 complex. CD3-ζ is a direct adaptor of the TCR and recruits ZAP-70 to immune synapses upon TCR stimulation. In the immune synapse, ZAP-70 is activated (43) and subsequently functions as a central organizer of several downstream signaling events (44, 45). The current study shows that CD3-ζ and/or ZAP-70 are the proximal elements that are influenced by the interaction of CEACAM1 and SHP-1. Consistent with this, we now show that CEACAM1 inhibits cytotoxicity by reducing degranulation; a process that is downstream of ZAP-70 (35). Although SHP-1 has been shown to inhibit TCR signaling through dephosphorylation of CD3-ζ and ZAP-70 (46, 47), SHP-1 is normally excluded from membrane lipid rafts (48, 49) where the TCR/CD3 complex localizes (50). Therefore, the mechanism by which SHP-1 accesses the TCR signalosome has been an open question. SHP-1 has been shown to associate with coinhibitory receptors, including killer inhibitory receptors (42), CTLA-4 (4, 51), and PD-1 (47). Nevertheless, it is not known whether these coinhibitory receptors can recruit SHP-1 to the TCR signalosome. Our study demonstrates that CEACAM1, which is able to shuttle between lipid rafts and nonlipid raft membranes, can associate with the TCR/CD3 complex. Furthermore, this association is increased by TCR/CD3 complex engagement and is relevant to TCR/CD3 complex function as we observed colocalization and cointernalization of the TCR/CD3 complex. These studies thus suggest that CEACAM1 facilitates entry of SHP-1 into the vicinity of the TCR/CD3 complex signalosome that is rich in lipid rafts (50) and provide evidence to explain previous observations that stimulation of T cells induces SHP-1 translocation to lipid rafts where it dephosphorylates ZAP-70 and/or CD3-ζ (46).
In addition, previous studies have demonstrated that both the anti-human CEACAM1 monoclonal body 5F4 that recognizes the homophilic binding site within the C-C’ loop of the human CEACAM1 N-domain and the 34B1 mAb that recognizes the N-domain outside of the C-C’ loop can induce CEACAM1 to transduce inhibitory signals in human T and/or NK cells (5, 26, 30, 33). This indicates that CEACAM1 may be able to bind multiple ligands that can trigger inhibitory signaling, which is consistent with CEACAM1-mediated inhibition induced by the murine hepatitis virus spike glycoprotein (6). CEACAM1 containing mutations in the Arg43 and Gln45 residues (R43GQ45L) loses the ability to bind to CEACAM1 itself as well as CEACAM5 and the 5F4, but not the 34B1, mAb and loses the ability to inhibit NK cell-mediated cytotoxicity (30). These studies indicate, in particular, that the 5F4 mAb binds within the homophilic binding site. Moreover, in line with these previous studies, masking the homophilic binding site of CEACAM1 with a Fab of the 5F4 mAb enhances the cytolytic function of T cells (Fig. 6D). Taken together, these studies now show that the 5F4 mAb binds within the homophilic binding site and is agonistic.
In summary, we show that expression of L CT containing isoforms of CEACAM1 in T cells is induced by activation and, furthermore, the activity of CEACAM1 is dependent upon homophilic binding. This ability to interact homophilically allows for association of CEACAM1 with and phosphorylation by the Src kinase, Lck, which is sequentially replaced by an association between CEACAM1 and SHP-1 which is brought into the vicinity of the TCR/CD3 complex where it dephosphorylates the most proximal signaling elements and associated adaptors of the TCR/CD3 complex, namely CD3-ζ and ZAP-70. These studies thus explain how CEACAM1 expression on T cells has such a profound impact on diverse cellular functions (6, 8, 9, 26, 52) making CEACAM1 of significant importance to a variety of immune responses as potentially diverse as inflammation, autoimmunity, and tumor immunity.
Acknowledgments
We owe thanks to Dr. John E. Shively for kindly providing the human CEACAM1–4L and −4S Jurkat T cell lines and to Dr. Bana Jabri for generously providing clonally expanded human CD4+ and CD8+ T cell clones. We thank Dr. Lewis Lanier for useful discussions and volunteer student Yiling Chen for assistance in completion of the GST-CT fusion protein. We also appreciate Dr. Nicolas Kuperwasser for help in preparing the figures.
This work was supported by National Institutes of Health Grants R01 DK53056, R01 DK51362, R01 DK44319, and P30 DK034854 (Harvard Digestive Diseases Center; to R.S.B.).
Abbreviations used in this paper:
- CEA
carcinoembryonic Ag
- CEACAM1
CEA Ag-related cell adhesion molecule 1
- CT
cytoplasmic tail
- cyt
cytoplasmic
- SHP-1
Src homology 2 domain-containing phosphatase 1
- S
short
- L
long
- H
human
- siRNA
short interfering RNA
- p
phospho
- IP
immunoprecipitation
- PBT
peripheral blood T
- IEL
intraepithelial lymphocyte
- IB
immunoblotted
- WT
wild type
- LAT
linker for activation of T cells
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Greenfield EA, Nguyen KA, and Kuchroo VK. 1998. CD28/B7 costimulation: a review. Crit. Rev. Immunol 18: 389–418. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe AH, and Freeman GJ. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol 2: 116–126. [DOI] [PubMed] [Google Scholar]
- 3.Lenschow DJ, Walunas TL, and Bluestone JA. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol 14: 233–258. [DOI] [PubMed] [Google Scholar]
- 4.Guntermann C, and Alexander DR. 2002. CTLA-4 suppresses proximal TCR signaling in resting human CD4+ T cells by inhibiting ZAP-70 Tyr(319) phosphorylation: a potential role for tyrosine phosphatases. J. Immunol 168: 4420–4429. [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, and Blumberg RS. 2004. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J. Immunol 172: 3535–3543. [DOI] [PubMed] [Google Scholar]
- 6.Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN, et al. 2004. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J. Exp. Med 199: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, Qiao SW, Chen Z, Glickman J, Najjar SM, Nakajima A, et al. 2006. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity 25: 769–781. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, and Shively JE. 2004. The cell-cell adhesion molecule carcinoembryonic antigen-related cellular adhesion molecule 1 inhibits IL-2 production and proliferation in human T cells by association with Src homology protein-1 and down-regulates IL-2 receptor. J. Immunol 172: 3544–3552. [DOI] [PubMed] [Google Scholar]
- 9.Markel G, Seidman R, Stern N, Cohen-Sinai T, Izhaki O, Katz G, Besser M, Treves AJ, Blumberg RS, Loewenthal R, et al. 2006. Inhibition of human tumor-infiltrating lymphocyte effector functions by the homophilic carcinoem-bryonic cell adhesion molecule 1 interactions. J. Immunol 177: 6062–6071. [DOI] [PubMed] [Google Scholar]
- 10.Boulton IC, and Gray-Owen SD. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol 3: 229–236. [DOI] [PubMed] [Google Scholar]
- 11.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarstrom S, Holmes KV, Karlsson A, Kuroki M, et al. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res 252: 243–249. [DOI] [PubMed] [Google Scholar]
- 12.Gray-Owen SD, and Blumberg RS. 2006. CEACAM1: contact-dependent control of immunity. Nat. Rev. Immunol 6: 433–446. [DOI] [PubMed] [Google Scholar]
- 13.Moller MJ, Kammerer R, Grunert F, and von Kleist S. 1996. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int. J. Cancer 65: 740–745. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima A, Iijima H, Neurath MF, Nagaishi T, Nieuwenhuis EE, Raychowdhury R, Glickman J, Blau DM, Russell S, Holmes KV, and Blumberg RS. 2002. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J. Immunol 168: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 15.Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, and Obrink B. 2002. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J. Immunol 168: 5139–5146. [DOI] [PubMed] [Google Scholar]
- 16.Wagener C, and Ergun S. 2000. Angiogenic properties of the carcinoembryonic antigen-related cell adhesion molecule 1. Exp. Cell Res 261: 19–24. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, Kilic E, Huland H, Friedrich M, and Ergun S. 2004. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res 64: 8932–8938. [DOI] [PubMed] [Google Scholar]
- 18.Kirshner J, Chen CJ, Liu P, Huang J, and Shively JE. 2003. CEACAM1–4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc. Natl. Acad. Sci. USA 100: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Simpson JF, Glackin C, Riethorf L, Wagener C, and Shively JE. 1998. Expression of biliary glycoprotein (CD66a) in normal and malignant breast epithelial cells. Anticancer Res 18: 3203–3212. [PubMed] [Google Scholar]
- 20.Virji M, Makepeace K, Ferguson DJ, and Watt SM. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol 22: 941–950. [DOI] [PubMed] [Google Scholar]
- 21.Mansson R, Lagergren A, Hansson F, Smith E, and Sigvardsson M. 2007. The CD53 and CEACAM-1 genes are genetic targets for early B cell factor. Eur. J. Immunol 37: 1365–1376. [DOI] [PubMed] [Google Scholar]
- 22.Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, and Mandelboim O. 2005. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J. Immunol 174: 6692–6701. [DOI] [PubMed] [Google Scholar]
- 23.Bradbury J 2002. Neisseria gonorrhoeae evades host immunity by switching off T lymphocytes. Lancet 359: 681. [DOI] [PubMed] [Google Scholar]
- 24.Pantelic M, Kim YJ, Bolland S, Chen I, Shively J, and Chen T. 2005. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect. Immun 73: 4171–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, et al. 2004. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21: 357–366. [DOI] [PubMed] [Google Scholar]
- 26.Morales VM, Christ A, Watt SM, Kim HS, Johnson KW, Utku N, Texieira AM, Mizoguchi A, Mizoguchi E, Russell GJ, et al. 1999. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J. Immunol 163: 1363–1370. [PubMed] [Google Scholar]
- 27.Chen Z, Dupre DJ, Le Gouill C, Rola-Pleszczynski M, and Stankova J. 2002. Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation: role of the C terminus and the (D/N)PXXY motif. J. Biol. Chem 277: 7356–7362. [DOI] [PubMed] [Google Scholar]
- 28.Takayama H, Trenn G, and Sitkovsky MV. 1987. A novel cytotoxic T lymphocyte activation assay: optimized conditions for antigen receptor triggered granule enzyme secretion. J. Immunol. Methods 104: 183–190. [DOI] [PubMed] [Google Scholar]
- 29.Brummer J, Neumaier M, Gopfert C, and Wagener C. 1995. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene 11: 1649–1655. [PubMed] [Google Scholar]
- 30.Markel G, Gruda R, Achdout H, Katz G, Nechama M, Blumberg RS, Kammerer R, Zimmermann W, and Mandelboim O. 2004. The critical role of residues 43R and 44Q of carcinoembryonic antigen cell adhesion molecules-1 in the protection from killing by human NK cells. J. Immunol 173: 3732–3739. [DOI] [PubMed] [Google Scholar]
- 31.Guo L, Yang K, Li Y, Situ HF, Chen WJ, Hong M, Lu JZ, and Lu SD. 2003. Construction of a non-viral vector H1s-EGFc and a preliminary study on its function. Zhonghua Yi Xue Za Zhi 83: 848–852. [PubMed] [Google Scholar]
- 32.Abou-Rjaily GA, Lee SJ, May D, Al-Share QY, Deangelis AM, Ruch RJ, Neumaier M, Kalthoff H, Lin SH, and Najjar SM. 2004. CEACAM1 modulates epidermal growth factor receptor-mediated cell proliferation. J. Clin. Invest 114: 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watt SM, Teixeira AM, Zhou GQ, Doyonnas R, Zhang Y, Grunert F, Blumberg RS, Kuroki M, Skubitz KM, and Bates PA. 2001. Homophilic adhesion of human CEACAM1 involves N-terminal domain interactions: structural analysis of the binding site. Blood 98: 1469–1479. [DOI] [PubMed] [Google Scholar]
- 34.Weiss A 1993. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell 73: 209–212. [DOI] [PubMed] [Google Scholar]
- 35.Abram CL, and Lowell CA. 2007. Convergence of immunoreceptor and integrin signaling. Immunol. Rev 218: 29–44. [DOI] [PubMed] [Google Scholar]
- 36.Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, and Beauchemin N. 1999. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J. Biol. Chem 274: 335–344. [DOI] [PubMed] [Google Scholar]
- 37.Izzi L, Turbide C, Houde C, Kunath T, and Beauchemin N. 1999. cis-Deter-minants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene 18: 5563–5572. [DOI] [PubMed] [Google Scholar]
- 38.Skubitz KM, Campbell KD, Ahmed K, and Skubitz AP. 1995. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J. Immunol 155: 5382–5390. [PubMed] [Google Scholar]
- 39.Nair KS, and Zingde SM. 2001. Adhesion of neutrophils to fibronectin: role of the cd66 antigens. Cell. Immunol 208: 96–106. [DOI] [PubMed] [Google Scholar]
- 40.Najjar SM 2002. Regulation of insulin action by CEACAM1. Trends Endocrinol. Metab 13: 240–245. [DOI] [PubMed] [Google Scholar]
- 41.Poy MN,Ruch RJ, Fernstrom MA, Okabayashi Y, and Najjar SM. 2002. Shc and CEACAM1 interact to regulate the mitogenic action of insulin. J. Biol. Chem 277: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 42.Binstadt BA, Brumbaugh KM, Dick CJ, Scharenberg AM, Williams BL, Colonna M, Lanier LL, Kinet JP, Abraham RT, and Leibson PJ. 1996. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity 5: 629–638. [DOI] [PubMed] [Google Scholar]
- 43.Duplay P, Thome M, Herve F, and Acuto O. 1994. p56lck interacts via its src homology 2 domain with the ZAP-70 kinase. J. Exp. Med 179: 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Oers NS, and Weiss A. 1995. The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes. Semin. Immunol 7: 227–236. [DOI] [PubMed] [Google Scholar]
- 45.Raab M, da Silva AJ, Findell PR, and Rudd CE. 1997. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR ζ/CD3 induction of interleukin-2. Immunity 6: 155–164. [DOI] [PubMed] [Google Scholar]
- 46.Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, and Thomas ML. 1996. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science 272: 1173–1176. [DOI] [PubMed] [Google Scholar]
- 47.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, and Chaudhary D. 2004. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3 ζ signalosome and downstream signaling to PKC0. FEBS Lett 574: 37–41. [DOI] [PubMed] [Google Scholar]
- 48.Su MW, Yu CL, Burakoff SJ, and Jin YJ. 2001. Targeting Src homology 2 domain-containing tyrosine phosphatase (SHP-1) into lipid rafts inhibits CD3-induced T cell activation. J. Immunol 166: 3975–3982. [DOI] [PubMed] [Google Scholar]
- 49.Kosugi A, Sakakura J, Yasuda K, Ogata M, and Hamaoka T. 2001. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity 14: 669–680. [DOI] [PubMed] [Google Scholar]
- 50.Harder T 2004. Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr. Opin. Immunol 16: 353–359. [DOI] [PubMed] [Google Scholar]
- 51.Walunas TL,Bakker CY, and Bluestone JA. 1996. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med 183: 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markel G, Wolf D, Hanna J, Gazit R, Goldman-Wohl D, Lavy Y, Yagel S, and Mandelboim O. 2002. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J. Clin. Invest 110: 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]