Abstract
Purpose
To determine whether magnetic resonance (MR) imaging evaluation of key morphologic tumor characteristics can improve patient selection for radical trachelectomy.
Materials and Methods
The institutional review board approved and waived informed consent for this study of 62 patients (mean age, 32 years; age range, 23–42 years) with International Federation of Gynecology and Obstetrics stage IB1 cervical carcinoma who underwent attempted radical trachelectomy between November 2001 and January 2011 and had preoperative MR imaging. Retrospectively, two radiologists reviewed MR images for tumor presence and size, distance between tumor and internal os, and presence of deep cervical stromal invasion. Associations between MR imaging findings and surgery type were tested.
Results
Sensitivity and specificity of tumor detection were, respectively, 87% and 100% (reader 1) and 76% and 95% (reader 2). Six of six patients with negative cone biopsy margins and no tumor at postconization MR imaging were without tumor at trachelectomy pathologic analysis. Mean differences between MR imaging and histologic tumor sizes were 0.7 mm (range, −15 to 11 mm) for reader 1 and 2.2 mm (range, −9 to 15 mm) for reader 2. Sensitivities for deep cervical stromal invasion were 75% (reader 1) and 50% (reader 2). For each reader, nine of nine (100%) patients with tumor 5 mm or less from the internal os and three of five (60%) patients with tumor 6–9 mm from the internal os at MR imaging needed radical hysterectomy. For both readers, tumor size of 2 cm or larger (P < .001) and deep cervical stromal invasion (P ≤ .003) at MR imaging were associated with increased chance of radical hysterectomy.
Conclusion
Pretrachelectomy MR imaging can help identify high-risk patients likely to need radical hysterectomy or confirm the absence of residual tumor in the cervix after a cone biopsy with negative margins.
© RSNA, 2013
Introduction
Cervical cancer screening programs have improved detection of early invasive cervical carcinomas in women of childbearing age (1,2). Traditionally, treatment for International Federation of Gynecology and Obstetrics (FIGO) stage IB disease has been radical hysterectomy and pelvic lymphadenectomy with en bloc removal of the uterus and parametria. In the late 1980s, radical trachelectomy with pelvic lymph node dissection was introduced as an alternative for young women with early stage cervical carcinoma who wanted to preserve their fertility (3). This procedure involves radical removal of the uterine cervix, adjacent parametria, and a cuff of the vagina. Ideally, a 1-cm disease-free margin is achieved and healthy upper endocervical stroma of 1 cm or larger is preserved to increase the chance of the patient maintaining pregnancy (4). Both transabdominal and transvaginal forms of radical trachelectomy are effective for treatment of early stage cervical cancers and result in acceptable rates of successful pregnancies (5).
Candidates for fertility-preserving surgery must be carefully selected. When first introduced, radical trachelectomy was only considered when the tumor diameter was less than 2 cm, the distance between the tumor and the internal os was 1 cm or greater, and cervical stromal invasion was less than 50%. However, some centers (including our center) will perform radical trachelectomy if the tumor size is 4 cm or less (especially if the tumor is exophytic), the endocervical resection margin is as little as 5 mm, and any degree of cervical stromal invasion is present (4,6–8). Therefore, patient selection criteria for radical trachelectomy remain under ongoing investigation.
Preoperative magnetic resonance (MR) imaging allows for noninvasive evaluation of the tumor size, cranial extent, and parametrial involvement (4,9). Although many practitioners consider pretrachelectomy MR imaging mandatory, few studies have evaluated the diagnostic value of MR imaging and its ability to help predict what surgical procedure will ultimately be performed (10–12).
The purpose of our study was to determine whether MR imaging evaluation of key morphologic tumor characteristics can improve patient selection for radical trachelectomy.
Materials and Methods
Eligibility
This retrospective single-institution study complied with the Health Insurance Portability and Accountability Act and was approved by our institutional review board, with a waiver of informed consent.
The study population was selected from a prospectively maintained database of 105 patients scheduled to under-go radical trachelectomy between November 2001 and January 2011. The inclusion criteria were as follows: age 18 years or older (it was believed the tumor-to-internal os distance in pediatric patients might be smaller and skew the results), FIGO stage IB1 cervical carcinoma, preoperative MR imaging, and surgery with the intent to perform radical trachelectomy. Preoperative MR imaging was used by the surgeons to exclude tumor size greater than 4 cm, to rule out parametrial invasion, and to exclude obvious nodal metastases. The surgical approach was guided entirely by intraoperative frozen slices (eg, presence of adequate endocervical margin [at least 5 mm] and lack of nodal metastases).
Patients were excluded from the study because they were younger than 18 years of age (n = 4), because they had FIGO stage IA2 disease or less (n = 17), because they did not have preoperative MR imaging available for review (n = 11), and because their MR examinations did not satisfy minimal technical standards described below (n = 11). Thus, our study included 62 patients. Clinical records were retrospectively reviewed for demographic information, details of surgery, and pathologic findings. Patients with adenosquamous carcinoma and adenocarcinoma were grouped together.
Patients from our study were also included in previously published studies that did not assess MR imaging: 22 patients in Abu-Rustum et al (13), 27 patients in Sonoda et al (14), 30 patients in Diaz et al (15), 31 patients in Einstein et al (16), 58 patients in Abu-Rustum et al (6), and 62 patients in Kim et al (17).
MR Imaging Technique
Preoperative MR examinations were performed at our institution (33 of 62 MR examinations) or elsewhere and digitized into a picture archiving and communication system (Centricity; GE Medical Systems, Milwaukee, Wis). Intravenous contrast material was used in 42 of 62 MR examinations.
At our institution, MR examinations were performed with systems that were 1.5 T or greater (Signa; GE Medical Systems). A body coil was used for excitation, and a pelvic phased-array coil was used for signal reception. All studies included a combination of axial, axial oblique, sagittal, and coronal T2-weighted fast spin-echo images (repetition time msec/echo time msec, 4000–6500/100; bandwidth, 16 kHz; section thickness, 5 mm; intersection gap, 1 mm; field of view, 16–20 cm; ≥256 × 192 matrix) and axial T1-weighted spin-echo images (typical parameters: 500/10; bandwidth, 15 kHz; section thickness, 5 mm; intersection gap, 1 mm; field of view, 28–36 cm). All in-house MR examinations performed after 2006 also included three-dimensional spoiled gradient-recalled images (typical parameters: flip angle, 12°; bandwidth, 62.5 kHz; section thickness, 3 mm with no intersection gap; field of view, 32–36 cm) obtained before and 1, 2, and 3 minutes after administration of intravenous gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Montville, NJ). The contrast material was administered at a dose of 0.1 mmol/kg of body weight with a bolus injection rate of 2 mL/sec by using an automatic injector (Medrad, Warrendale, Pa), which was followed by a 20-mL saline bolus injection.
All outside MR imaging examinations met or exceeded standards agreed to by the investigators. These standards required a 1.5-T or greater field strength, phased-array surface coils, acquisition of axial and sagittal T2-weighted images, and spin-echo or gradient-echo axial T1-weighted images. For all sequences, the parameters were the following: field of view, 20–28 cm; section thickness, 5 mm or less; 256 × 192 or greater matrix; and signal average, two or more.
Image Interpretation
All MR examinations were independently and retrospectively interpreted by two radiologists with 4 years (Y.L.) and 7 years (O.A.) of experience in gynecologic cancer imaging. The readers knew that all patients had biopsy-proved cervical carcinoma, but they were blinded to all other clinical information.
For each MR examination, all pulse sequences were reviewed together and evaluated for (a) tumor presence and size, (b) distance between the proximal aspect of the tumor and the internal os (tumor-to-internal os distance), and (c) maximal depth of tumor invasion into the cervical stroma.
Cervical carcinoma was diagnosed if we found a lesion that had higher T2 signal intensity (SI) than the adjacent cervical stroma (Figs 1–5). The presence of tumor was scored as follows: 1, definitely absent; 2, probably absent; 3, indeterminate (ie, impossible to distinguish residual tumor from postprocedural inflammation); 4, probably present; and 5, definitely present. Tumor, if present, was measured in three orthogonal planes by analyzing T2-weighted and, if available, contrast agent material–enhanced images. The largest tumor dimension was recorded.
Figure 1a:
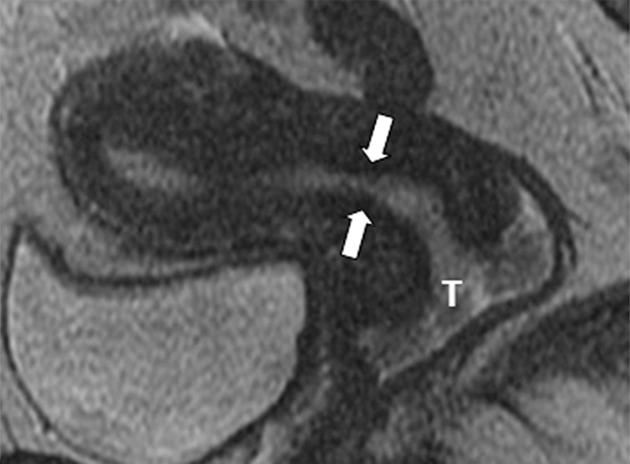
Detection of the tumor in the cervix and the location of the interval os. (a) Sagittal, (b) axial oblique at the internal os, (c) axial oblique at the external os, and (d) coronal oblique T2-weighted fast spin-echo images in a 28-year-old woman with stage IB1 endocervical adenosquamous carcinoma demonstrate predominantly exophytic intermediate T2-weighted SI tumor (T) adjacent to the low-SI cervical stroma (dashed arrow). The location of the internal cervical os (thick arrows) is defined as the entrance of the uterine vessels (thin arrows; best seen on axial oblique T2-weighted image) and narrowing of the uterine contour (best visualized on sagittal and coronal oblique T2-weighted images).
Figure 5:
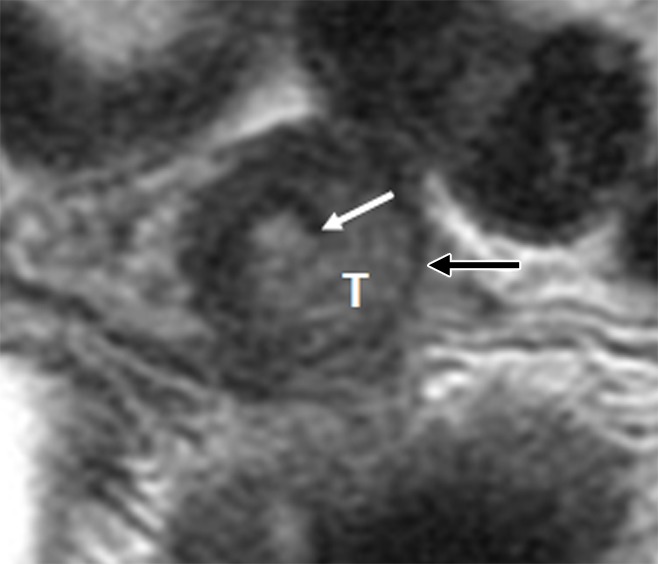
Invasive endocervical adenocarcinoma in a 34-year-old woman. Axial oblique T2-weighted fast spin-echo image illustrates intermediate-T2-SI tumor (T) disrupting inner (white arrow) and thinning outer cervical stroma (black arrow). At final pathologic analysis, the depth of cervical stromal invasion by tumor was 15 mm, while the total cervical stromal thickness was 18 mm.
Figure 1b:
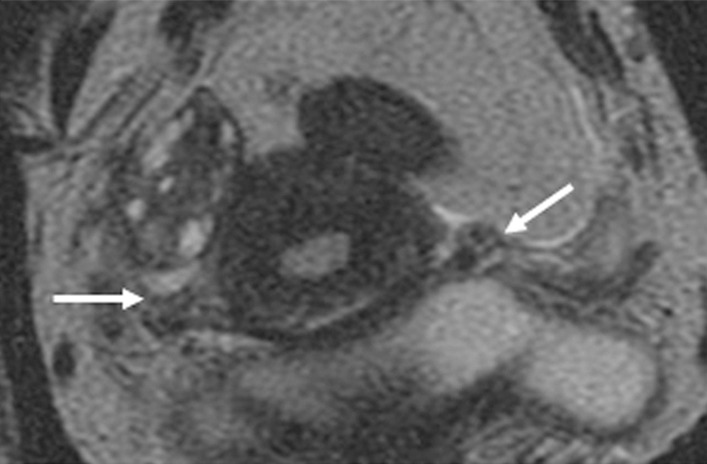
Detection of the tumor in the cervix and the location of the interval os. (a) Sagittal, (b) axial oblique at the internal os, (c) axial oblique at the external os, and (d) coronal oblique T2-weighted fast spin-echo images in a 28-year-old woman with stage IB1 endocervical adenosquamous carcinoma demonstrate predominantly exophytic intermediate T2-weighted SI tumor (T) adjacent to the low-SI cervical stroma (dashed arrow). The location of the internal cervical os (thick arrows) is defined as the entrance of the uterine vessels (thin arrows; best seen on axial oblique T2-weighted image) and narrowing of the uterine contour (best visualized on sagittal and coronal oblique T2-weighted images).
Figure 1c:
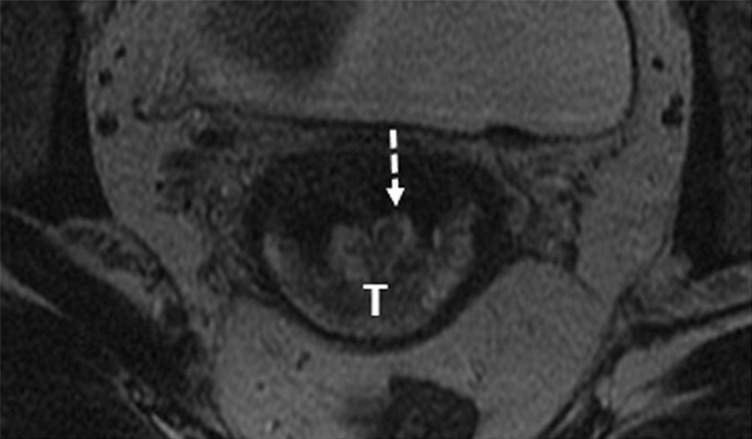
Detection of the tumor in the cervix and the location of the interval os. (a) Sagittal, (b) axial oblique at the internal os, (c) axial oblique at the external os, and (d) coronal oblique T2-weighted fast spin-echo images in a 28-year-old woman with stage IB1 endocervical adenosquamous carcinoma demonstrate predominantly exophytic intermediate T2-weighted SI tumor (T) adjacent to the low-SI cervical stroma (dashed arrow). The location of the internal cervical os (thick arrows) is defined as the entrance of the uterine vessels (thin arrows; best seen on axial oblique T2-weighted image) and narrowing of the uterine contour (best visualized on sagittal and coronal oblique T2-weighted images).
Figure 1d:
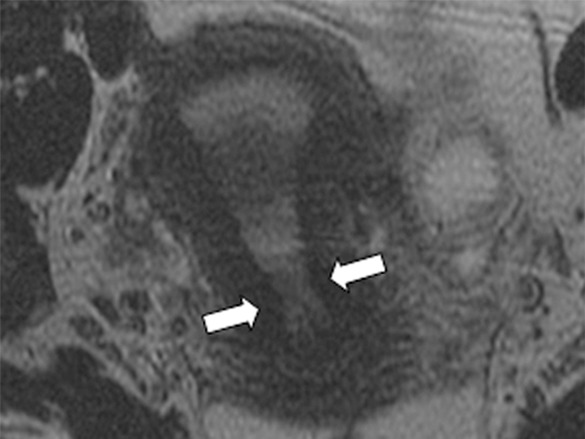
Detection of the tumor in the cervix and the location of the interval os. (a) Sagittal, (b) axial oblique at the internal os, (c) axial oblique at the external os, and (d) coronal oblique T2-weighted fast spin-echo images in a 28-year-old woman with stage IB1 endocervical adenosquamous carcinoma demonstrate predominantly exophytic intermediate T2-weighted SI tumor (T) adjacent to the low-SI cervical stroma (dashed arrow). The location of the internal cervical os (thick arrows) is defined as the entrance of the uterine vessels (thin arrows; best seen on axial oblique T2-weighted image) and narrowing of the uterine contour (best visualized on sagittal and coronal oblique T2-weighted images).
Figure 2b:
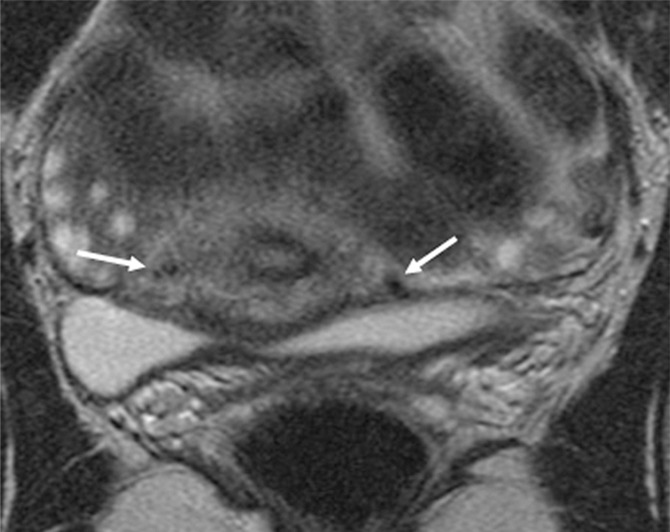
Tumor-to-internal os distance and presence of deep cervical stromal invasion. (a) Sagittal and (b) axial oblique T2-weighted fast spin-echo images in a 23-year-old woman with stage IB1 invasive adenocarcinoma of the uterine cervix demonstrate intermediate T2-weighted SI tumor (T) within 3 mm of the internal os. The internal os is seen as the narrowing of the uterine contour, where the low-SI fibrous cervical stroma changes to the intermediate-SI uterine myometrium (thick arrows; best seen on the sagittal T2-weighted image) and the entrance of the uterine vessels (thin arrows; best seen on axial oblique T2-weighed image). The tumor disrupts low-T2-SI inner cervical stroma (white dashed arrow) and extends up to the outer cervical stroma.
Figure 3:
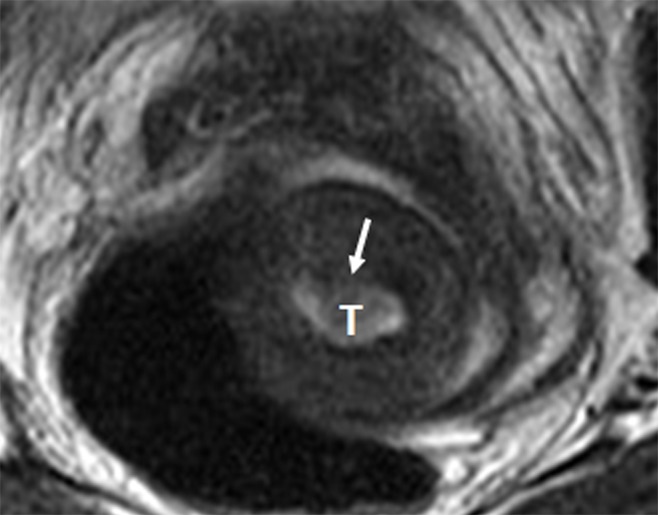
Invasive adenosquamous carcinoma of the cervix in a 30-year-old woman. Axial oblique T2-weighted fast spin-echo image shows superficial cervical stromal invasion by tumor (T) with subtle disruption of the inner cervical stroma (arrow). At final trachelectomy pathologic analysis, the depth of cervical stromal was 4 mm, while the total cervical stromal thickness was 18 mm.
Figure 4:
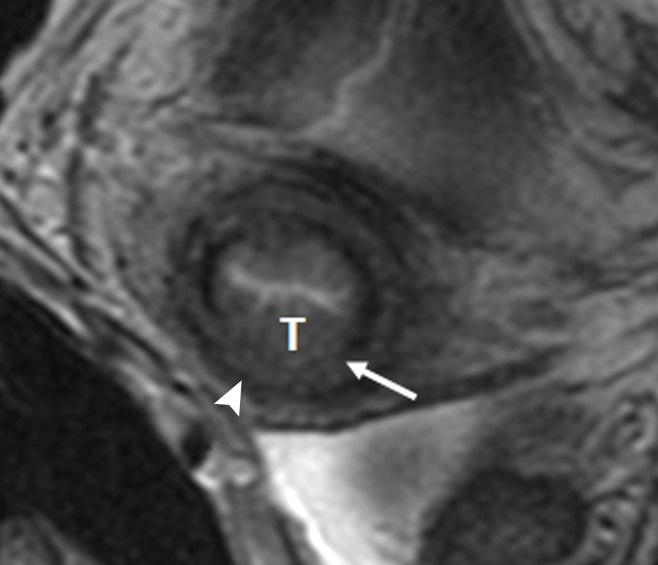
Invasive squamous cell carcinoma of the cervix in a 25-year-old woman. Axial oblique T2-weighted fast spin-echo image shows invasion of the middle third of the cervical stroma by tumor (T). Inner cervical stroma is interrupted (arrow), while the outer cervical stroma is preserved (arrowhead). At final trachelectomy pathologic analysis, the depth of cervical stromal invasion was 8 mm, while the total cervical stromal thickness was 18 mm.
The internal os (inner aspect of the cervix) was defined as the entrance of the uterine vessels (assessed on axial and, if available, axial oblique and coronal oblique T2-weighted images) and the narrowing of the uterine contour where the low-SI cervical stroma changes to the intermediate-SI uterine myometrium (assessed on sagittal and, if available, coronal oblique T2-weighted images) (Figs 1, 2) (10–12,18). Tumor extension into the lower uterus was diagnosed if tumor extended across the internal os into the uterine myometrium and disrupted its normal SI. The tumor-to-internal os distance was recorded in millimeters (Fig 2).
Figure 2a:
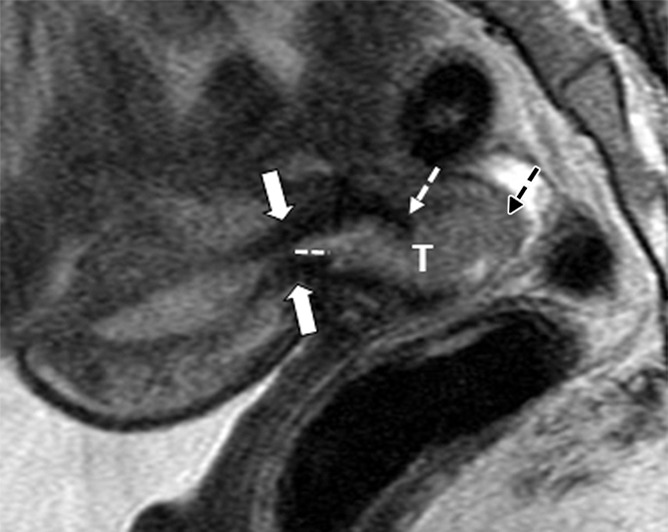
Tumor-to-internal os distance and presence of deep cervical stromal invasion. (a) Sagittal and (b) axial oblique T2-weighted fast spin-echo images in a 23-year-old woman with stage IB1 invasive adenocarcinoma of the uterine cervix demonstrate intermediate T2-weighted SI tumor (T) within 3 mm of the internal os. The internal os is seen as the narrowing of the uterine contour, where the low-SI fibrous cervical stroma changes to the intermediate-SI uterine myometrium (thick arrows; best seen on the sagittal T2-weighted image) and the entrance of the uterine vessels (thin arrows; best seen on axial oblique T2-weighed image). The tumor disrupts low-T2-SI inner cervical stroma (white dashed arrow) and extends up to the outer cervical stroma.
Cervical stroma was considered normal if it demonstrated homogeneous low SI on T2-weighted images (Fig 1). Cervical stromal invasion was diagnosed if tumor disrupted the normal low-SI stroma (18) (Figs 2–5). The maximal thickness of the cervical stroma at or adjacent to the tumor and the maximal depth of tumor invasion into the stroma were measured in millimeters (19). The radiologist who interpreted the images chose the imaging plane used for these measurements. Deep cervical stromal invasion was diagnosed if the tumor involved the outer third of the cervical stroma (Fig 5).
Standard of Reference
All gynecologic specimens were reviewed by specially trained gynecologic pathologists as a part of patient care. The specimens were analyzed for tumor presence, tumor size, length of endocervical margin (trachelectomy specimens), tumor-to-internal os distance (hysterectomy specimens), depth of cervical stromal invasion, and presence of nodal metastases.
Statistical Analysis
Interobserver agreement for a reading with two categories was analyzed with the Cohen κ statistic, while that for a reading with more than two categories was analyzed with weighted κ statistics by using quadratic weights (20). The agreement for continuous variables was assessed by using the intraclass correlation coefficient (ICC) (21). The κ statistic was interpreted as follows: κ less than 0, no agreement; κ= 0–0.20, slight agreement; κ= 0.21–0.40, fair agreement; κ= 0.41–0.60, moderate agreement; κ= 0.61–0.80, substantial agreement; and κ= 0.81–1.0, almost perfect agreement (22). We estimated 95% confidence intervals (CIs) for κ statistics and ICCs.
Measurements of accuracy were performed by using final surgical pathologic examinations as the reference standard. For each reader, the imaging impression regarding the presence of tumor was indexed as a three-level result. The levels were (a) tumor absent (combination of scores, 1–2); (b) tumor present (combination of scores, 4–5); or (c) indeterminate (score of 3). Because of the presence of indeterminate results, the sensitivity and specificity of MR imaging were calculated by using definitions based on conditional probability, and conventional diagnostic likelihood ratios were estimated for each reader (23,24). The sensitivity, specificity, positive predictive value, and negative predictive value of MR imaging for detection of deep cervical stromal invasion were calculated for each reader.
MR imaging and histologic-derived tumor sizes were compared by using separate scatter plots for each reader. The associations between MR imaging findings of tumor-to-internal os distance (0–5 mm, 6–9 mm, or ≥10 mm), tumor size (<2 cm or ≥2 cm), presence of deep cervical invasion, and the surgical procedure ultimately performed were tested by using the Fisher exact test. A test with the P value of less than .05 indicated statistical significance.
All statistical analyses were performed by using statistical software packages (SAS 9.2, SAS Institute, Cary, NC; R version 2.13, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
Table 1 summarizes patients’ characteristics and surgical outcomes.
Table 1.
Patient Characteristics and Surgical Outcomes
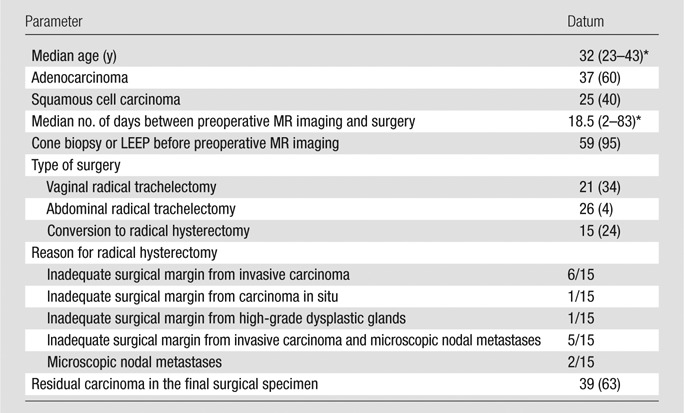
Note.—Unless otherwise indicated, data are the number of patients and data in parentheses are percentages. LEEP = loop electrosurgical excision procedure.
Data in parentheses are the range.
Tumor Detection at MR Imaging
At final surgical pathologic analysis, 23 of 62 (37%) patients had no tumors (all tumors had been removed during initial biopsy and/or conization). Of the 39 with tumors at pathologic examination, 15 (38%) had tumors less than 10 mm.
The interreader agreement was almost perfect for tumor detection (κ = 0.84; 95% CI: 0.74, 0.94). Overall sensitivity, specificity, and diagnostic likelihood ratios for tumor detection on MR images are reported in Table 2. Reader 1 assigned a score of 3 (indeterminate) to 11 of 62 (18%) patients; at surgical pathologic examination, two of these patients had inflammation without tumor and nine patients had tumors that were smaller than 10 mm.
Table 2.
Accuracy of Preoperative MR Imaging in Tumor Detection
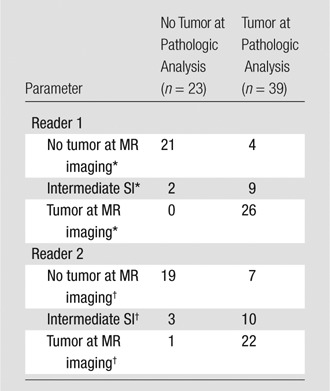
Note.—Data are the number of patients
Sensitivity, 0.87 (95% CI: 0.69, 0.96); specificity, 1.00 (95% CI: 0.84, 1.00); positive diagnostic likelihood ratio, ∞; negative diagnostic likelihood ratio, 0.11 (95% CI: 0.106, 0.12).
Sensitivity, 0.76 (95% CI: 0.56, 0.90); specificity, 0.95 (95% CI: 0.75, 0.99); positive diagnostic likelihood ratio, 13.0 (95% CI: 9.9, 17.0); negative diagnostic likelihood ratio, 0.22 (95% CI: 0.20, 0.23).
Reader 2 assigned a score of 3 (indeterminate) to 13 of 62 (21%) patients; at surgical pathologic examination, three of these patients had inflammation without tumor, seven patients had tumors smaller than 10 mm, and three patients had tumors 10 mm or larger.
For each reader, six of six patients with negative cone biopsy margins and no tumor at postconization MR imaging had no residual carcinoma on trachelectomy pathologic examination.
Tumor Size at MR Imaging
Tumors were found in 39 of 62 (63%) patients at surgery. Largest tumor diameter at pathologic examination ranged from 0.1 to 3.5 cm (mean, 1.47 cm ± 0.96 [standard deviation]).
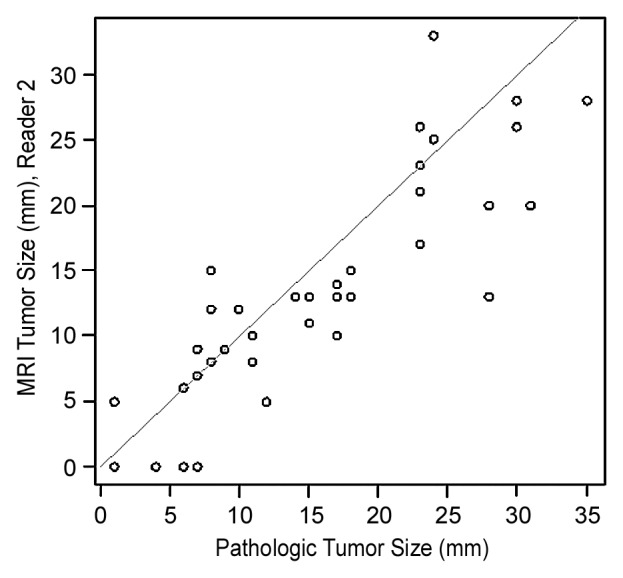
Interreader agreement for the largest tumor diameter at MR imaging was good (ICC = 0.87; 95% CI: 0.81, 0.93). The ICC between pathologic and MR imaging tumor sizes was 0.84 (95% CI: 0.72, 0.91) for reader 1 and 0.86 (95% CI: 0.70, 0.93) for reader 2. Mean differences between tumor sizes derived from pathologic examination and MR imaging were 0.7 mm (range, −15 to 11 mm) for reader 1 and 2.2 mm (range, −9 to 15 mm) for reader 2 (Fig 6) For both readers, 50% of tumor sizes at MR imaging were within 4.5 mm of tumor sizes at pathologic examination.
Figure 6a:
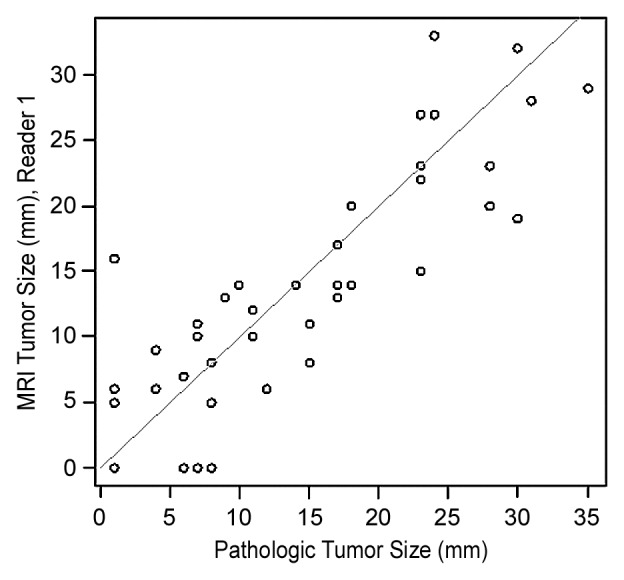
Scatter plots show differences in tumor sizes derived from MR imaging and pathologic analysis for (a) reader 1 and (b) reader 2.
Figure 6b:
Scatter plots show differences in tumor sizes derived from MR imaging and pathologic analysis for (a) reader 1 and (b) reader 2.
For tumors smaller than 20 mm at pathologic examination, mean differences between tumor sizes derived from pathologic examination and MR imaging were 0.1 mm (range, −15 to 8 mm) for reader 1 and 1.7 mm (range, −7 to 7 mm) for reader 2. For tumors 20 mm or larger at pathologic examination, the mean differences between tumor sizes derived from pathologic examination and MR imaging were 2.0 mm (range, −9 to 11 mm) for reader 1 and 3.5 mm (range, −9 to 15 mm) for reader 2.
For both readers, based on univariate analyses, patients with tumors 20 mm or larger at MR imaging were more likely to require radical hysterectomy compared with patients who had smaller or no residual tumors (P < .001, Table 3).
Table 3.
Associations between MR Imaging Features and Surgery Type
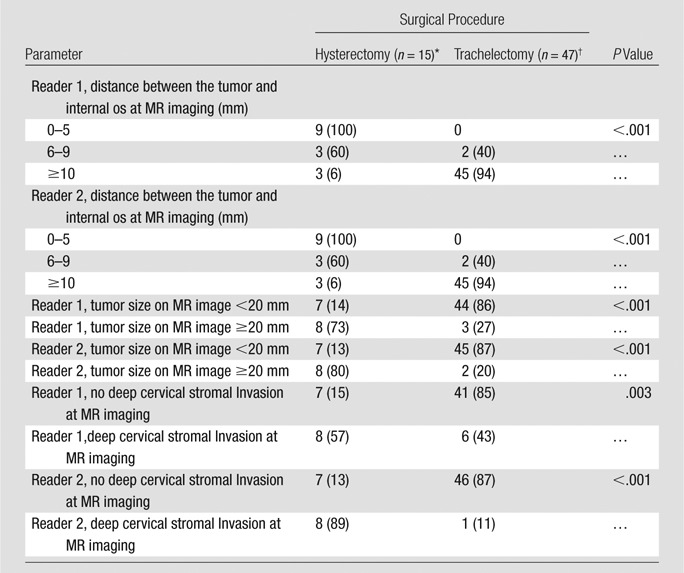
Note.—Data are the number of patients and data in parentheses are percentages.
Tumor-to-Internal Os Distance at MR Imaging
For each reader, the visualization of the internal os was adequate for interpretation in all cases. The interreader agreement was almost perfect for tumor-to-internal os distance (κ = 0.91; 95% CI: 0.84, 0.98). On the basis of univariate analyses, the tumor-to-internal os distance at MR imaging was significantly associated with the surgery type for each reader (P < .001, Table 3).
Each reader identified nine patients with a tumor-to-internal os distance of 5 mm or less at MR imaging. Seven of nine patients were the same for both readers. For each reader, radical hysterectomy was required for nine of nine (100%; 95% CI: 66%, 100%) patients with a tumor-to-internal os distance of 5 mm or less. Among the nine patients identified by reader 1, the intraoperative reason or reasons for radical hysterectomy were inadequate endocervical margins in six patients and inadequate margins and microscopic pelvic nodal metastases in three patients. Among the nine patients identified by reader 2, the reasons were inadequate margins five patients and inadequate margins and microscopic pelvic nodal metastases in four patients.
Each reader found five patients with a tumor-to-internal os distance of 6–9 mm. Three of five patients were the same for both readers. For each reader, three of five patients (60%; 95% CI: 15%, 95%) with a tumor-to-internal os distance of 6–9 mm at MR imaging required radical hysterectomy, one patient because of an insufficient surgical margin and two patients because of microscopic pelvic nodal metastases.
Both readers identified the same 48 patients with a tumor-to-internal os distance of 10 mm or greater at MR examination. Three of 48 patients (6%; 95% CI: 1%, 17%) underwent radical hysterectomy, one patient because of highly dysplastic cervical glands extending to the internal os, one patient because of carcinoma in situ within 1 mm of the internal os, and one patient because of microscopic pelvic nodal metastases.
Deep Cervical Stromal Invasion at MR Imaging
Interreader agreement on the presence of deep cervical stromal invasion was substantial (κ = 0.63; 95% CI: 0.39, 0.88). Table 4 lists sensitivity, specificity, positive predictive value, and negative predictive value for detection of deep cervical stromal invasion (present in 12 of 62 [19%] patients at surgical pathologic examination). At univariate analysis, for both readers, patients with deep cervical stromal invasion at MR imaging were more likely to undergo radical hysterectomy (reader 1, P = .003; reader 2, P < .001; Table 3).
Table 4.
Accuracy of Preoperative MR Imaging for Detection of Deep Cervical Stromal Invasion
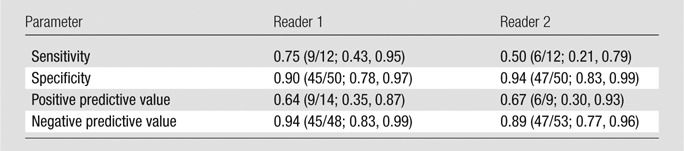
Note.—Data in parentheses are numerator/denominator and 95% CI range.
Discussion
Despite careful preoperative patient evaluation, 10%–12% of patients are found to have more extensive than expected endocervical disease or positive lymph nodes during attempted fertility-sparing surgery, which results in the abandonment of radical trachelectomy in favor of radical hysterectomy or adjuvant radiation therapy (9,25–28). We aimed to determine whether patient selection for radical trachelectomy could be improved by using MR imaging to evaluate tumor presence and size, the distance between the tumor and the internal os, and the presence of deep cervical stromal invasion.
Few studies have specifically investigated preoperative MR imaging evaluation of the tumor-to-internal os distance. One retrospective study (11) of 150 patients with early stage cervical carcinoma found that for detection of internal os involvement, MR imaging had sensitivity of 90%, specificity of 98%, positive predictive value of 86%, and negative predictive value of 98%. In another retrospective study (12) of 27 patients who underwent hysterectomy for FIGO stage IB1 cervical carcinoma and had tumors of less than 20 mm, the ICC for MR imaging and pathologic measurements of the tumor-to-internal os distance was 0.792. A prospective study of 30 patients with early stage cervical cancer who underwent radical hysterectomy (n = 27) or radical trachelectomy (n = three) found that for detecting extension beyond the internal os, preoperative MR imaging had sensitivity of 100%, specificity of 96%, and positive predictive value of 83% (10).
To our knowledge, no prior study has examined the association between tumor-to-internal os distance at MR imaging and the surgery type. Our results suggest that tumor distance of 5 mm or less from the internal os at MR imaging is a statistically significant adverse imaging feature that identifies patients who are likely to require radical hysterectomy. Thus, measurement of the tumor-to-internal os distance at preoperative MR imaging may improve pretreatment counseling and ensure that patients have realistic expectations of probable surgical outcomes should radical trachelectomy be attempted. Knowledge of this measurement may encourage a surgeon to pursue alternative treatment options, such as neoadjuvant chemotherapy, in the hopes of shrinking the tumor and making fertility-sparing surgery possible (29–33).
A growing body of evidence suggests that patients with early invasive cervical carcinoma (FIGO stages IA2 through early IB1) are perhaps overtreated by radical trachelectomy because the chance of tumor spreading to the parametria is exceedingly low (29); 62%–67% of patients who have had a diagnostic cone biopsy have no residual carcinoma in the final trachelectomy specimen (25,26,28). The chances of finding residual cancer are further reduced when cone biopsy margins are negative. We found that six of six patients with negative cone biopsy margins and no tumor at postconization MR imaging had no tumor at final trachelectomy pathologic examination. Further studies are needed to determine whether a less radical procedure (eg, a simple trachelectomy or a large cold knife cone combined with sentinel lymph node sampling) may effectively treat the cancer and spare fertility in this group of low-risk patients.
Several prior studies found correlations between tumor volumes and sizes derived from MR imaging and pathologic examination (11,34–36). In the largest study, preoperative MR examinations of patients with early stage cervical carcinoma who underwent radical hysterectomy (n = 94) or radical trachelectomy (n = 56) were retrospectively reviewed; at MR imaging, 95% of tumors larger than 10 mm were within 8 mm of pathologic size (11). We similarly found close correlation between tumor sizes derived from MR imaging and pathologic analysis. At univariate analysis, we found a significant association between tumor size and surgical procedure: patients with tumors 2 cm or larger were more likely to require radical hysterectomy compared with patients with smaller lesions or no residual tumors (both readers, P < .001).
Only three studies, published in the 1990s, have examined the value of MR imaging for assessment of deep cervical stromal invasion, a known independent risk factor for recurrence and mortality (37). These studies (34,38,39) assessed the detection of full-thickness cervical stromal invasion. Sensitivities were 56%–94%, specificities were 62%–91%, and negative predictive values were greater than 90%. Our results suggest that, despite technological advances, detection of deep cervical stromal invasion by MR imaging remains a challenge. We speculate that the performance of MR imaging is limited primarily by the difficulty of distinguishing tumor from postprocedural inflammation, and that accuracy for detection of deep cervical stromal invasion might improve if MR imaging were performed before conization. At univariate analysis, we found a statistically significant association between the presence of deep cervical stromal invasion at MR imaging and the surgery type: Patients with deep cervical stromal invasion were more likely to require radical hysterectomy (reader 1, P = .003; reader 2, P < .001).
Our study had several limitations. To begin with, it was a retrospective analysis of a small population accumulated over 10 years. Additionally, there was a relatively long time period between preoperative MR imaging and surgery for some patients. Only patients scheduled to undergo radical trachelectomy were included, and this may have introduced a selection bias. Another limitation was that preoperative MR imaging was performed over a 10-year period by using various MR techniques, hardware, and software. The potential effects of these changes in clinical practice on performance of the readers are unknown. Furthermore, by only including patients with technically adequate MR examinations we may have selected a group of patients who differed in a meaningful way from patients whose MR examinations did not meet the technical standards that were agreed to by study investigators.
The radiologists in our study had expertise in gynecologic cancer imaging, and thus our results may not be broadly generalizable. However, the selection of two experienced radiologists was justified by the paucity of published information on the topic and uncertainty regarding the value of MR imaging in the setting assessed. If interpretations of inexperienced radiologists had been compared, we would not have known whether the differences were because of variable experience levels or inherent limitations of MR imaging. Further studies will be required to determine the influence of reader experience on the reproducibility of the results.
Another limitation of our study was that, in keeping with current clinical practice, most patients who were included underwent conization before MR imaging, which complicated image interpretation, particularly the processes of distinguishing tumor from postprocedural inflammation and detecting deep cervical stromal invasion. Additionally, we were not able to assess the effect of other confounding factors (eg, cervicitis, deep nabothian cysts, tunnel clusters, and endocervical glandular hyperplasia) on the accuracy of MR imaging interpretations.
Finally, we were unable to perform a multivariate logistic regression because no patients with tumor-to-internal os distance of 5 mm or less were able to undergo radical trachelectomy. Therefore, it is uncertain if all variables predictive of surgery type at univariate analysis would remain predictive of surgical procedure at multivariate analysis.
In summary, our study indicated that a distance of 5 mm or less between the tumor and the internal os at pretrachelectomy MR imaging identifies patients who are likely to need radical hysterectomy. Tumor size of 2 cm or larger and presence of deep cervical stromal invasion at MR imaging also lessen the chance of successful radical trachelectomy. Furthermore, MR imaging after a conization with negative margins is useful for confirmation of the absence of tumor in the remaining cervix. This information may profoundly affect patient counseling and selection for fertility-sparing treatments. Prospective studies are needed to further assess the value of MR imaging to improve patient selection for fertility-sparing surgery.
Advances in Knowledge.
• A distance of 5 mm or less between the tumor and the internal os at pretrachelectomy MR imaging identifies high-risk patients likely to require radical hysterectomy; for each reader, radical trachelectomy was aborted in favor of radical hysterectomy in nine of nine patients with this imaging feature.
• Interobserver agreement for measurement of the distance between the tumor and the internal os at MR imaging was almost perfect (weighted κ value for both readers, 0.91; 95% confidence interval: 0.84, 0.98).
• Patients with tumors that were 2 cm or larger at MR imaging were more likely to require radical hysterectomy compared with patients with smaller or no residual tumors (both readers, P < .001), and patients with deep cervical stromal invasion at MR imaging were also more likely to undergo radical hysterectomy (P ≤ .03 for both readers).
• Tumor size measurements at MR imaging and pathologic analysis were very similar, with mean differences of 0.7 mm (range, −15 to 11 mm) for reader 1 and 2.2 mm (range, −9 to 15 mm) for reader 2.
• MR imaging after a conization with negative margins may help to confirm the absence of residual cervical carcinoma (six of six patients with negative cone biopsy margins and no tumor at postconization MR imaging were without tumors at trachelectomy pathologic analysis).
Implications for Clinical Care.
• Evaluation of the distance between the tumor and the internal os, tumor size, and cervical stromal invasion at pretreatment MR may substantially improve patient counseling and treatment selection in young women with International Federation of Gynecology and Obstetrics stage IB1 cervical cancer who want to preserve their fertility.
• MR after a conization with negative margins may help to confirm the absence of residual tumor in the cervix and identify low-risk patients who may benefit from fertility-sparing surgery that is less extensive than radical trachelectomy.
Disclosures of Conflicts of Interest: Y.L. No relevant conflicts of interest to disclose. O.A. No relevant conflicts of interest to disclose. K.J.P. No relevant conflicts of interest to disclose. D.M.S. No relevant conflicts of interest to disclose. J.Z. No relevant conflicts of interest to disclose. D.A.G. No relevant conflicts of interest to disclose. M.J.S. No relevant conflicts of interest to disclose. C.S.M. No relevant conflicts of interest to disclose. Y.S. No relevant conflicts of interest to disclose. H.H. No relevant conflicts of interest to disclose. N.R.A.R. No relevant conflicts of interest to disclose.
Acknowledgments
The authors thank Ada Muellner, MS, for editing the manuscript.
Received August 14, 2012; revision requested October 18; revision received January 22, 2013; accepted February 20; final version accepted April 4.
Abbreviations:
- CI
- confidence interval
- FIGO
- International Federation of Gynecology and Obstetrics
- ICC
- intraclass correlation coefficient
- SI
- signal intensity
References
- 1.Saraiya M, Ahmed F, Krishnan S, Richards TB, Unger ER, Lawson HW. Cervical cancer incidence in a prevaccine era in the United States, 1998-2002. Obstet Gynecol 2007;109(2 Pt 1):360–370. [DOI] [PubMed] [Google Scholar]
- 2.Mathews TJ, Miniño AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics 2011;127(1):146–157. [DOI] [PubMed] [Google Scholar]
- 3.Plante M, Renaud MC, Hoskins IA, Roy M. Vaginal radical trachelectomy: a valuable fertility-preserving option in the management of early-stage cervical cancer. A series of 50 pregnancies and review of the literature. Gynecol Oncol 2005;98(1):3–10. [DOI] [PubMed] [Google Scholar]
- 4.Rob L, Skapa P, Robova H. Fertility-sparing surgery in patients with cervical cancer. Lancet Oncol 2011;12(2):192–200. [DOI] [PubMed] [Google Scholar]
- 5.Roman LD. Pregnancy after radical vaginal trachelectomy: maybe not such a risky undertaking after all. Gynecol Oncol 2005;98(1):1–2. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Rustum NR, Sonoda Y. Fertility-sparing surgery in early-stage cervical cancer: indications and applications. J Natl Compr Canc Netw 2010;8(12):1435–1438. [DOI] [PubMed] [Google Scholar]
- 7.Dursun P, LeBlanc E, Nogueira MC. Radical vaginal trachelectomy (Dargent’s operation): a critical review of the literature. Eur J Surg Oncol 2007;33(8):933–941. [DOI] [PubMed] [Google Scholar]
- 8.Covens A. Preserving fertility in early cervical cancer with radical trachelectomy. Contemp Obstet Gynecol 2003;2:46–66. [Google Scholar]
- 9.Plante M. Vaginal radical trachelectomy: an update. Gynecol Oncol 2008;111(2 Suppl):S105–S110. [DOI] [PubMed] [Google Scholar]
- 10.Peppercorn PD, Jeyarajah AR, Woolas R, et al. Role of MR imaging in the selection of patients with early cervical carcinoma for fertility-preserving surgery: initial experience. Radiology 1999;212(2):395–399. [DOI] [PubMed] [Google Scholar]
- 11.Sahdev A, Sohaib SA, Wenaden AE, Shepherd JH, Reznek RH. The performance of magnetic resonance imaging in early cervical carcinoma: a long-term experience. Int J Gynecol Cancer 2007;17(3):629–636. [DOI] [PubMed] [Google Scholar]
- 12.Bipat S, van den Berg RA, van der Velden J, Stoker J, Spijkerboer AM. The role of magnetic resonance imaging in determining the proximal extension of early stage cervical cancer to the internal os. Eur J Radiol 2011;78(1):60–64. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Rustum NR, Neubauer N, Sonoda Y, et al. Surgical and pathologic outcomes of fertility-sparing radical abdominal trachelectomy for FIGO stage IB1 cervical cancer. Gynecol Oncol 2008;111(2):261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonoda Y, Chi DS, Carter J, Barakat RR, Abu-Rustum NR. Initial experience with Dargent’s operation: the radical vaginal trachelectomy. Gynecol Oncol 2008;108(1):214–219. [DOI] [PubMed] [Google Scholar]
- 15.Diaz JP, Sonoda Y, Leitao MM, et al. Oncologic outcome of fertility-sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinoma. Gynecol Oncol 2008;111(2):255–260. [DOI] [PubMed] [Google Scholar]
- 16.Einstein MH, Park KJ, Sonoda Y, et al. Radical vaginal versus abdominal trachelectomy for stage IB1 cervical cancer: a comparison of surgical and pathologic outcomes. Gynecol Oncol 2009;112(1):73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CH, Abu-Rustum NR, Chi DS, et al. Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol Oncol 2012;125(3):585–588. [DOI] [PubMed] [Google Scholar]
- 18.Hricak H, Lacey CG, Sandles LG, Chang YCF, Winkler ML, Stern JL. Invasive cervical carcinoma: comparison of MR imaging and surgical findings. Radiology 1988;166(3):623–631. [DOI] [PubMed] [Google Scholar]
- 19.Seki H, Azumi R, Kimura M, Sakai K. Stromal invasion by carcinoma of the cervix: assessment with dynamic MR imaging. AJR Am J Roentgenol 1997;168(6):1579–1585. [DOI] [PubMed] [Google Scholar]
- 20.Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 1973;33(3):613–619. [Google Scholar]
- 21.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45(1):255–268. [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159–174. [PubMed] [Google Scholar]
- 23.Simel DL, Feussner JR, DeLong ER, Matchar DB. Intermediate, indeterminate, and uninterpretable diagnostic test results. Med Decis Making 1987;7(2):107–114. [DOI] [PubMed] [Google Scholar]
- 24.Simel DL, Rennie D, Bossuyt PM. The STARD statement for reporting diagnostic accuracy studies: application to the history and physical examination. J Gen Intern Med 2008;23(6):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchiole P, Benchaib M, Buenerd A, Lazlo E, Dargent D, Mathevet P. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): a comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH). Gynecol Oncol 2007;106(1):132–141. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd JH, Spencer C, Herod J, Ind TE. Radical vaginal trachelectomy as a fertility-sparing procedure in women with early-stage cervical cancer-cumulative pregnancy rate in a series of 123 women. BJOG 2006;113(6):719–724. [DOI] [PubMed] [Google Scholar]
- 27.Beiner ME, Covens A. Surgery insight: radical vaginal trachelectomy as a method of fertility preservation for cervical cancer. Nat Clin Pract Oncol 2007;4(6):353–361. [DOI] [PubMed] [Google Scholar]
- 28.Plante M, Renaud MC, François H, Roy M. Vaginal radical trachelectomy: an oncologically safe fertility-preserving surgery. An updated series of 72 cases and review of the literature. Gynecol Oncol 2004;94(3):614–623. [DOI] [PubMed] [Google Scholar]
- 29.Maneo A, Landoni F, Caspani G, et al. Chemotherapy and conization for preserving fertility in stage IB1 cervical cancer [abstr]. Int J Gynecol Cancer 2004;14(Suppl):193. [Google Scholar]
- 30.Landoni F, Parma G, Peiretti M, et al. , Chemo-conization in early cervical cancer. Gynecol Oncol 2007;107(1, Suppl 1):S125–S126. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Akiyama F, Hasumi K. A case of successful pregnancy after treatment of invasive cervical cancer with systemic chemotherapy and conization. Gynecol Oncol 2006;100(1):213–215. [DOI] [PubMed] [Google Scholar]
- 32.Plante M, Lau S, Brydon L, Swenerton K, LeBlanc R, Roy M. Neoadjuvant chemotherapy followed by vaginal radical trachelectomy in bulky stage IB1 cervical cancer: case report. Gynecol Oncol 2006;101(2):367–370. [DOI] [PubMed] [Google Scholar]
- 33.Robova H, Pluta M, Hrehorcak M, Skapa P, Rob L. High-dose density chemotherapy followed by simple trachelectomy: full-term pregnancy. Int J Gynecol Cancer 2008;18(6):1367–1371. [DOI] [PubMed] [Google Scholar]
- 34.Subak LL, Hricak H, Powell CB, Azizi L, Stern JL. Cervical carcinoma: computed tomography and magnetic resonance imaging for preoperative staging. Obstet Gynecol 1995;86(1):43–50. [DOI] [PubMed] [Google Scholar]
- 35.Fischerova D, Cibula D, Stenhova H, et al. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. Int J Gynecol Cancer 2008;18(4):766–772. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell DG, Snyder B, Coakley F, et al. Early invasive cervical cancer: tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. J Clin Oncol 2006;24(36):5687–5694. [DOI] [PubMed] [Google Scholar]
- 37.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999;73(2):177–183. [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Choi BI, Han JK, et al. Preoperative staging of uterine cervical carcinoma: comparison of CT and MRI in 99 patients. J Comput Assist Tomogr 1993;17(4):633–640. [DOI] [PubMed] [Google Scholar]
- 39.Sironi S, De Cobelli F, Scarfone G, et al. Carcinoma of the cervix: value of plain and gadolinium-enhanced MR imaging in assessing degree of invasiveness. Radiology 1993;188(3):797–801. [DOI] [PubMed] [Google Scholar]


