Abstract
Introduction
Inhaled low-dose methoxyflurane is approved in Europe for emergency relief of moderate-to-severe trauma-related pain in adults, but data versus active comparators are sparse. The phase IIIb Methoxyflurane in Emergency Department in ITAly (MEDITA) trial investigated the analgesic efficacy, practicality and safety of methoxyflurane versus standard analgesic treatment (SAT) for acute trauma pain.
Methods
This was a randomised, active-controlled, parallel-group, open-label trial conducted in 15 Italian emergency units. Adults with limb trauma and pain score ≥ 4 on numerical rating scale (NRS) were randomised 1:1 to inhaled methoxyflurane 3 mL or SAT [intravenously administered (IV) morphine 0.1 mg/kg for severe pain (NRS ≥ 7); IV paracetamol 1 g or IV ketoprofen 100 mg for moderate pain (NRS 4–6)]. The primary endpoint was overall change in visual analogue scale (VAS) pain intensity from baseline (time of randomisation) to 3, 5 and 10 min. Non-inferiority and superiority of methoxyflurane versus SAT were concluded if the upper 95% confidence interval (CI) for the treatment comparison (methoxyflurane–SAT) was less than 1 and less than 0, respectively.
Results
Between 8 February 2018 and 8 February 2019, 272 patients were randomised (136 per treatment group). A total of 270 patients (mean age 51 years; 49% male; 34% with severe pain; mean baseline VAS 67 mm) were treated and analysed for efficacy and safety. Superiority of methoxyflurane was demonstrated for moderate-to-severe pain (adjusted mean treatment difference − 5.94 mm; 95% CI − 8.83, − 3.06 mm), moderate pain (− 5.97 mm; 95% CI − 9.55, − 2.39 mm) and severe pain (− 5.54 mm; 95% CI − 10.49, − 0.59 mm). Median onset of pain relief was 9 min for methoxyflurane and 15 min for SAT. Practicality of methoxyflurane treatment was rated “Excellent”, “Very Good” or “Good” by 90% of clinicians vs. 64% for SAT. Adverse events (all non-serious) were reported by 17% of methoxyflurane-treated patients and 3% of SAT-treated patients.
Conclusion
Methoxyflurane provided superior pain relief to SAT in patients with moderate-to-severe trauma pain and may offer a simple, fast, effective non-opioid treatment option.
Trial registration
Trial registered with EudraCT (2017-001565-25) on 2 March 2018 and ClinicalTrials.gov (NCT03585374) on 13 July 2018.
Funding
Mundipharma Pharmaceuticals S.r.l.
Electronic supplementary material
The online version of this article (10.1007/s12325-019-01055-9) contains supplementary material, which is available to authorized users.
Keywords: Acute pain, Analgesic, Emergency department, Methoxyflurane, Morphine, Non-steroidal anti-inflammatory drug, Paracetamol, Penthrox, Prehospital, Trauma
Introduction
Pain is the most common complaint of trauma patients attending the emergency department (ED), with prevalence of 70% reported in the prehospital setting and 91% in EDs [1, 2]. However, undertreatment of pain (oligoanalgesia) remains a widespread problem in the emergency setting [3–6]. Various reports have attributed oligoanalgesia to failure to assess/underestimation of pain by healthcare workers [7–9], lack of national/institutional guidelines for pain management [8–10] and limitations of currently available therapies [9].
Treatment options for acute pain management include paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs) and weak opioids for mild to moderate pain, and strong opioid analgesics for severe pain [11], although there is considerable heterogeneity in the therapeutic approach across Europe. Paracetamol and NSAIDs are weak analgesics with slow onset of action when administered orally, and risk of overdose if the patient has previously self-medicated. Furthermore, NSAIDs can be associated with gastrointestinal problems, nephropathy, cardiovascular disorders, and reduced fracture healing. Strong opioid analgesics are controlled drugs that require patient monitoring for potential side effects such as respiratory and central nervous system depression, nausea and vomiting. Intravenously administered (IV) analgesia, while aiding onset of action, requires additional healthcare resource, can be distressing for the patient and may be difficult in emergency rescue situations. There is rising use of alternative non-opioid and multimodal analgesic therapies such as subdissociative-dose ketamine and nitrous oxide [12]. Nitrous oxide (50:50 with oxygen) has the benefit of being non-invasive and non-narcotic with quick onset of action, rapid reversibility and few side effects [13–15], but the gas canisters are cumbersome and impractical when attending emergency situations in the field [16] and it should not be used if the patient has any condition where air is trapped in the body and expansion would be dangerous [17]. Thus, there is an unmet need for a rapid-acting, safe, effective and easy-to-use treatment for trauma pain in the emergency setting.
Methoxyflurane is a volatile inhalational analgesic that provides rapid short-term analgesia using a portable hand-held inhaler device (Penthrox®, 3 mL dose; Medical Developments International, Scoresby, Australia) and may provide an effective non-narcotic option for emergency pain management in the prehospital and ED setting. Methoxyflurane is self-administered by the patient, has a rapid onset of action (within around 4 min or 6–10 inhalations [18, 19]), its effects are quickly reversed (within 3–20 min after inhalation stops) and there are no reported drug interactions at analgesic doses [19]. These characteristics make methoxyflurane suitable for use as a sole agent, or as a bridging agent to other analgesia, and its use may have an opioid-sparing effect. One inhaler (3 mL methoxyflurane) provides 25–30 min of analgesia with continuous inhalation [19]; or approximately 1 h of analgesia under intermittent inhalation conditions [20]. Low-dose methoxyflurane analgesia has a well-established safety profile: no respiratory depression or clinically significant effects on vital signs have been reported and adverse events (AEs) are usually transient and self-limiting [18–21].
Methoxyflurane has been used extensively in Australia and New Zealand as emergency and procedural analgesia for more than 40 years [21–23], and is now licensed in Europe and other territories for the emergency relief of moderate-to-severe pain associated with trauma in conscious adult patients [24]. Its use as emergency analgesia is supported by robust data in the literature [21–23]. To date, key data in Europe are from STOP!, a randomised, double-blind, placebo-controlled UK study in 300 patients with acute trauma pain (baseline score of 4–7 on the 11-point numeric rating scale, NRS) [18, 25, 26]. STOP! showed a significantly greater reduction in visual analogue scale (VAS) pain scores overall for methoxyflurane vs. placebo (− 30.2 vs. − 15.2 mm, p < 0.0001) with a median time to first pain relief of 4 vs. 10 min [18]. More recently, the InMEDIATE study demonstrated a significantly greater decrease in NRS pain intensity over the first 20 min of treatment for methoxyflurane vs. standard analgesic treatment chosen by the treating physician according to local protocols (− 2.5 vs. − 1.4, p < 0.001), with a median time to first pain relief of 3 vs. 10 min (p < 0.001) in 305 trauma patients in Spanish EDs [18]. Both studies showed high patient and healthcare professional (HCP) satisfaction with methoxyflurane treatment [18]. However, additional comparative studies versus the best currently available therapeutic options are needed to fully elucidate the place of methoxyflurane in emergency analgesia, including in extreme conditions such as helicopter rescue, which is the subject of an ongoing interventional study [27].
The aim of the present study was to compare the short-term pain relief (up to 30 min after randomisation) and safety with inhaled methoxyflurane versus standard analgesic treatment (SAT) in adult patients presenting to Italian emergency medical centres with acute trauma-related pain. SAT comprised IV morphine for severe pain (NRS ≥ 7) and IV paracetamol or IV ketoprofen for moderate pain (NRS 4–6), consistent with Italian recommendations on pain management in the emergency setting [28].
Methods
Study Design
Methoxyflurane in Emergency Department in ITAly (MEDITA) was a phase IIIb, randomised, active-controlled, parallel-group, open-label study conducted at 15 emergency medical centres (both prehospital and ED settings) in Italy. Adult patients with moderate-to-severe trauma-related pain were randomised 1:1 to receive methoxyflurane or SAT. All study procedures were performed on the day of enrolment, with a follow-up telephone call 14 ± 2 days later to record any AEs and concomitant therapies. The full methodology for this study has previously been reported [29–31].
The study was approved by the Italian Medicines Agency (AIFA). The co-ordinating ethics committee, Comitato Etico Regione Toscana—Area Vasta Centro, Florence, Italy, approved the trial protocol on 1 December 2017. In addition, all study documents and procedures were reviewed and approved by the appropriate ethics committees at each centre (see Table S2 in the electronic supplementary material for list of all ethics committees). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Given the emergency setting and the requirement for rapid analgesia, if the patient was unable to provide written informed consent, witnessed verbal consent was obtained, with the patient signing the informed consent as soon as they were able.
Participants
Patients aged at least 18 years with trauma (fracture, dislocation, crushing, contusion) to a single limb and an NRS pain score ≥ 4 presenting at the hospital for triage or rescued in the prehospital environment through the Italian emergency medical service were eligible for the study. Patients had to be medically stable, alert and collaborative, and able to communicate with the investigator to perform the study activities, including providing informed consent. Exclusion criteria included ongoing analgesic treatment for chronic pain or use of any other analgesic in the previous 5 h (8 h for diclofenac), pregnancy or lactation, dynamics of at-risk trauma, and contraindications to methoxyflurane administration as per the Summary of Product Characteristics (SPC) [19] or to any of the SAT. Full inclusion and exclusion criteria have previously been reported [29].
Randomisation and Blinding
Patients were randomised to study treatment (methoxyflurane or SAT) via a centralised Interactive Web Response System that guaranteed allocation concealment, set up within the electronic case report form (eCRF). Randomisation was in a block randomisation scheme of four without stratification. As a result of the different routes of administration, need for rapid analgesia and characteristic fruity smell of methoxyflurane, study treatment was administered in an open-label fashion, commencing as soon as possible after randomisation.
Description of the Interventions
Patients randomised to methoxyflurane received one hand-held inhaler with a 3-mL vial of methoxyflurane. Following patient allocation to the experimental group, the methoxyflurane inhaler (Penthrox) was prepared by trained study HCP by adding the methoxyflurane liquid in the vial to the inhaler via a one-way valve, where it is absorbed by a polypropylene wick. Once absorbed, the liquid vaporizes and the patient inhales the vapour through the mouthpiece. Methoxyflurane was self-administered by the patient under the supervision of a trained study HCP. The patient was instructed to inhale intermittently from the device and could control their own level of analgesia by inhaling more or less frequently, or covering the diluter hole at the mouthpiece end with their index finger to obtain greater analgesia. The patient was also instructed to exhale back into the mouthpiece, so that any exhaled methoxyflurane was adsorbed by the activated charcoal chamber, preventing release of methoxyflurane in the vicinity of the patient. One inhaler (3 mL methoxyflurane) was expected to provide approximately 1 h of analgesia under suggested intermittent inhalation conditions, or 25 min with continuous inhalation [19, 20].
Patients randomised to SAT received analgesic medications that currently comprise standard analgesic treatment in Italy, determined on the basis of the intensity of the patient’s pain. Patients with severe pain (NRS ≥ 7) received IV morphine (0.10 mg/kg). Patients with moderate pain (NRS 4–6) received IV paracetamol (1 g) or IV ketoprofen (100 mg), chosen by the investigator on the basis of availability and local practice, and considering any prior history of allergy in the patient. The IV treatment was diluted and infused over 10 min as soon as possible after allocation of the patient to the control group. Venous access was obtained prior to randomization according to local clinical practice.
Rescue medication was permitted for patients in either treatment group from 25 min after randomisation (after measurement of pain relief for this time point), or earlier at the investigator’s discretion if the patient’s pain worsened, or improvement was insufficient. Rescue medication was administered according to local practice.
Outcomes
The primary outcome was the change in VAS pain intensity from baseline (time of randomisation) to 3, 5 and 10 min, with a secondary outcome of the change in VAS pain intensity from baseline to 15, 20, 25 and 30 min. Pain intensity was measured by asking the patient to respond to the question “How much pain do you feel at this moment?” by marking a 0–100 mm VAS, where 0 = no pain and 100 = maximum pain. Pain intensity assessments ceased if rescue medication was administered. Given the emergency/rescue setting and the acute nature of the endpoint, a specially trained HCP could assist the patient in completing the VAS, if required, in which case the patient authenticated the recording with a signature and date as soon as they were able. While the NRS was considered adequate for enrolment, VAS pain intensity was chosen as the primary endpoint because it is evaluated on a continuous scale, thus increasing the sensitivity and power of the study.
Further secondary outcomes were the use of rescue medication, the time of onset of pain relief as subjectively reported by the patient, ratings of study treatment efficacy by the patient, and ratings of the practicality of study treatment by the treating HCP. The patient rated the overall efficacy of the study treatment and the HCP rated the practicality of using the study treatment on a 5-point Likert qualitative scale (“Poor”, “Fair”, “Good”, “Very Good” or “Excellent”) at 30 min after randomisation. An exploratory outcome was the proportion of patients in the methoxyflurane group resorting to closure of the diluter hole (to administer a higher concentration of methoxyflurane).
Secondary safety outcomes were the incidence of AEs, and vital sign measurements at baseline and 10 and 30 min after randomisation. AEs (not related to the trauma presentation) were recorded from the time of randomisation until the safety follow-up telephone call at 14 ± 2 days after treatment. The final diagnosis of the trauma category (fracture, dislocation, crushing, contusion) was also recorded during the safety follow-up telephone call. All data collected during the trial were entered into an eCRF system accessible via the internet and fully monitored by clinical research associates to ensure data quality; in addition, the three highest-recruiting centres underwent external audit.
Objectives
The primary objective of the trial was to demonstrate non-inferiority of methoxyflurane compared to SAT in Italy (IV morphine/ketoprofen/paracetamol) for the treatment of moderate-to-severe acute pain in terms of the primary outcome in all study patients, with a secondary objective to demonstrate superiority of methoxyflurane compared to SAT for the same endpoint. The co-primary objective was to demonstrate superiority of methoxyflurane compared to SAT (IV ketoprofen/paracetamol) for the treatment of moderate acute pain in terms of the primary outcome in patients with baseline NRS 4–6. All other study objectives are detailed in the protocol publication [29].
Sample Size
A sample size of 108 patients per treatment group was estimated to provide 90% power to determine non-inferiority of methoxyflurane versus SAT for the primary outcome measure, assuming a non-inferiority margin of 1.0, a standard deviation of 2.5 [18] and a significance level of 0.05. Allowing for 20% of patients being non-evaluable, it was planned to randomise a total of 136 patients per treatment group. The co-primary objective was not considered in the sample size calculation because enrolment was not balanced by baseline pain severity; therefore, the number of patients enrolling with moderate pain was unpredictable.
Statistical Analysis
The primary outcome measure was analysed using a linear mixed-effect model for repeated measures adjusted for baseline VAS score, and the interaction between time point and treatment. The statistical model was used to calculate the treatment difference (methoxyflurane − SAT) and associated 95% confidence interval (CI) at 3, 5 and 10 min for all patients, and patients with moderate pain (NRS 4–6) at baseline. The primary analysis was the overall test for treatment effect considering all three time points; non-inferiority was to be concluded if the estimated upper 95% confidence limit was below 1.0 and superiority was to be concluded if the estimated upper 95% confidence limit was below 0. The mean change from baseline with 95% CI was estimated for each group at 15, 20, 25 and 30 min after randomisation (secondary outcome).
It was planned to compare the percentage of patients who resorted to rescue medication between the treatment groups using a Z test; however, as a result of the low number of patients resorting to additional analgesia, the non-parametric Fisher’s exact test was used. Kaplan–Meier curves were used to present the time from randomisation to the onset of pain relief for each treatment group and, if appropriate, the treatment groups were compared using a Cox proportional hazards model. For patient efficacy ratings and HCP practicality ratings, the percentage of patients in each response category was compared between the treatment groups using a Mann–Whitney U test. The proportion of patients in the methoxyflurane treatment group who resorted to closure of the diluter hole was evaluated as a relative frequency with 95% CI (Clopper–Pearson).
AEs were coded using the Medical Dictionary for Regulatory Activities version 22.0 and summarised descriptively (using absolute frequencies and percentages) for each treatment group. All efficacy and safety analyses were performed using the intention-to-treat (ITT) population. No imputation of missing data was performed; therefore, only patients with available data were considered for each analysis. Randomisation, or the time at which treatment was assigned, represents the baseline time to which all the times of observation for the study endpoints refer.
Results
Study Participants
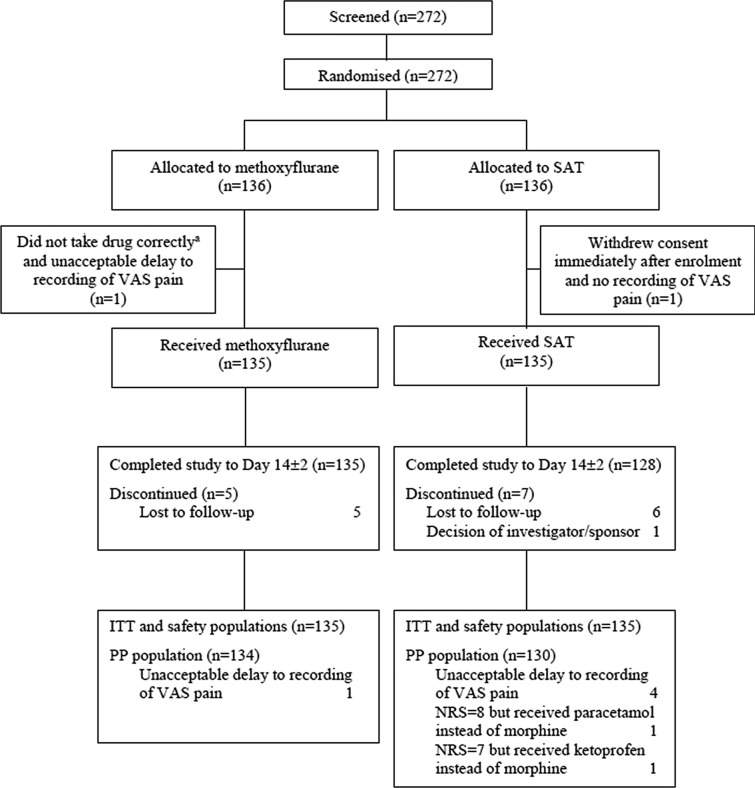
Between 8 February 2018 and 8 February 2019, 272 patients were screened and randomised. Of these, 270 patients (135 per treatment group) received study treatment and were included in the efficacy (ITT) and safety analyses (Fig. 1). All except 5 patients (3.7%) in the methoxyflurane group and 7 patients (5.2%) in the SAT group completed the study to day 14 ± 2. Six patients in the ITT population had major protocol deviations and were excluded from the per-protocol population; results were very similar for both analysis populations; therefore, only the results for the ITT population are presented. Demographic and baseline characteristics were comparable for both treatment groups (Table 1), with an overall mean age of 51 years (range 18–95 years), an even gender split and 95% of patients Caucasian. Approximately two-thirds of patients had moderate pain (NRS 4–6) and one-third had severe pain (NRS ≥ 7) at inclusion. In the SAT group, 80/91 (88%) patients with moderate pain received IV paracetamol and 11/91 (12%) received IV ketoprofen, while 42/44 (95%) patients with severe pain received IV morphine (one received paracetamol and one received ketoprofen in error). Patients most commonly presented with contusion, fracture or dislocation (Table 1). Compliance with VAS pain assessments was high, with at least 99% of patients completing the assessment at each specified time point.
Fig. 1.
Study flow chart. Note: patients may have had more than one reason for exclusion from the PP population. a Methoxyflurane was added to the wrong inhaler hole (in the carbon chamber not the base of the inhaler). ITT intention-to-treat, NRS numeric rating scale, PP per-protocol, SAT standard analgesic treatment, VAS visual analogue scale
Table 1.
Patient characteristics (ITT population)
| Characteristic | Methoxyflurane (N = 135) | Standard analgesic treatment (N = 135) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 50.8 (18.35) | 51.5 (19.25) |
| Range | 18–91 | 18–95 |
| Gender [n (%)] | ||
| Male | 70 (51.9) | 62 (45.9) |
| Female | 65 (48.1) | 73 (54.1) |
| Race [n (%)] | ||
| Caucasian | 127 (94.1) | 130 (96.3) |
| Asian | 4 (3.0) | 0 |
| Black | 3 (2.2) | 2 (1.5) |
| Other | 1 (0.7) | 3 (2.2) |
| Baseline pain group [n (%)] | ||
| Moderate [NRS 4–6] | 86 (63.7) | 91 (67.4) |
| Severe [NRS ≥ 7] | 49 (36.3) | 44 (32.6) |
| Suspected injury type at inclusion [n (%)] | ||
| Contusion | 72 (53.3) | 60 (44.4) |
| Fracture | 38 (28.1) | 43 (31.9) |
| Dislocation | 19 (14.1) | 21 (15.6) |
| Crushing | 6 (4.4) | 11 (8.1) |
| Final diagnosis [n (%)] | ||
| Fracture | 64 (47.4) | 60 (44.4) |
| Contusion | 39 (28.9) | 38 (28.1) |
| Dislocation | 29 (21.5) | 32 (23.7) |
| Crushing | 3 (2.2) | 5 (3.7) |
ITT intent-to-treat, NRS numeric rating scale, SD standard deviation
Efficacy Results
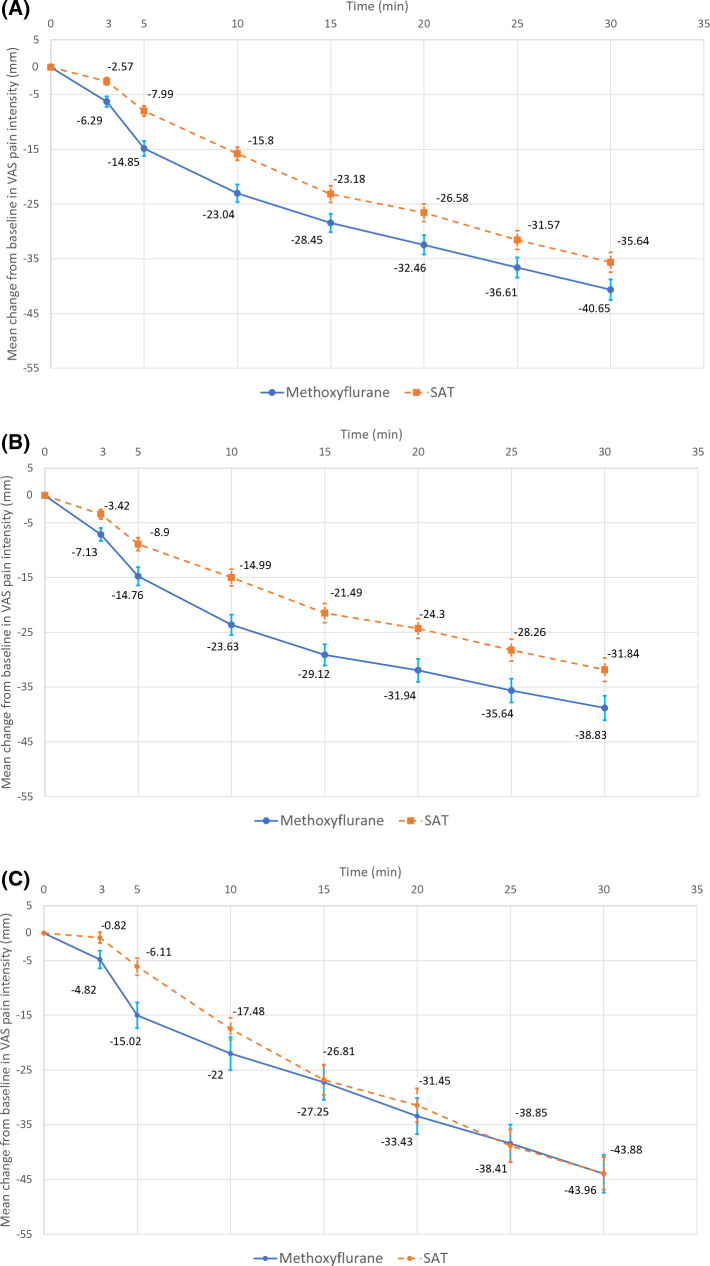
The overall change in VAS pain intensity in the first 10 min (all patients) was significantly greater in the methoxyflurane group compared with the SAT group (adjusted mean treatment difference − 5.94 mm; 95% CI − 8.83, − 3.06 mm; p < 0.001), demonstrating both non-inferiority (upper 95% CI < 1, primary objective) and superiority (upper 95% CI < 0, secondary objective) of methoxyflurane versus SAT (Table 2). Similar results were obtained for patients with moderate pain (− 5.97 mm; 95% CI − 9.55, − 2.39 mm; p = 0.001, co-primary objective) and patients with severe pain (− 5.54 mm; 95% CI − 10.49, − 0.59 mm; p = 0.029, post hoc exploratory analysis) (Table 2). The estimates at each individual observation time (3, 5, 10 min) also demonstrated non-inferiority and superiority of methoxyflurane versus SAT in all patients and patients with moderate pain; in patients with severe pain, non-inferiority was demonstrated at 3 min and superiority was shown at 5 min (Table 2). Analysis of changes in VAS pain intensity from baseline to 15, 20, 25 and 30 min (all patients) showed a significant treatment difference in favour of methoxyflurane at 15 min (− 5.27 mm; 95% CI − 9.71, − 0.83 mm; p = 0.020), 20 min (− 5.89 mm; 95% CI − 10.60, − 1.17 mm; p = 0.015) and 25 min (− 5.04 mm; 95% CI − 9.99, − 0.08 mm; p = 0.046), but not 30 min (− 5.01 mm; 95% CI − 10.14, 0.13 mm; p = 0.056). The mean change from baseline in VAS pain intensity at each time point up to 30 min is presented graphically for all patients, patients with moderate pain and patients with severe pain in Fig. 2. Exploratory analysis of the primary outcome measure by injury type (contusion/dislocation/fracture) showed that the change from baseline was significantly lower than 0 at all time points up to 30 min in both treatment groups, except at 3 min in the methoxyflurane group for the dislocation subgroup, and at 3 min in the SAT group for the fracture subgroup. According to the statistical analysis plan, a head-to-head comparison according to injury type was not performed. However, the results of this analysis support the significant trend in favour of methoxyflurane independently of the injury type, with a slightly larger effect in contusions and dislocations than in fractures, as clinically expected. Table S1 in the electronic supplementary material shows change in VAS pain intensity by injury type.
Table 2.
Analysis of change from baseline in VAS pain intensity up to 10 min (ITT population)
| Population | Time point (min) | Estimated mean change from baseline (95% confidence interval) | Estimated treatment effect (95% confidence interval) | |
|---|---|---|---|---|
| Methoxyflurane | Standard analgesic treatment | |||
| All patients (N = 270) | 3 | − 6.29 (− 7.94, − 4.64) | − 2.56 (− 4.21, − 0.91) | − 3.73 (− 6.06, − 1.40)a |
| 5 | − 14.85 (− 17.16, − 12.54) | − 7.99 (− 10.30, − 5.68) | − 6.86 (− 10.13, − 3.59)a | |
| 10 | − 23.04 (− 25.84, − 20.23) | − 15.80 (− 18.60, − 13.00) | − 7.24 (− 11.20, − 3.27)a | |
| Primary endpoint | Overall | − 14.73 (− 16.77, − 12.69) | − 8.78 (− 10.82, − 6.75) | − 5.94 (− 8.83, − 3.06)a |
| Moderate pain (N = 177) | 3 | − 7.07 (− 9.16, − 4.99) | − 3.45 (− 5.48, − 1.42) | − 3.63 (− 6.54, − 0.72)a |
| 5 | − 14.70 (− 17.58, − 11.82) | − 8.96 (− 11.75, − 6.16) | − 5.75 (− 9.76, − 1.73)a | |
| 10 | − 23.57 (− 26.97, − 20.18) | − 15.04 (− 18.34, − 11.75) | − 8.53 (− 13.26, − 3.80)a | |
| Co-primary endpoint | Overall | − 15.12 (− 17.68, − 12.55) | − 9.15 (− 11.64, − 6.66) | − 5.97 (− 9.55, − 2.39)a |
| Severe pain (N = 93) | 3 | − 4.69 (− 7.27, − 2.11) | − 0.96 (− 3.68, 1.76) | − 3.73 (− 7.48, 0.024) |
| 5 | − 14.89 (− 18.80, − 10.99) | − 6.26 (− 10.38, − 2.13) | − 8.64 (− 14.32, − 2.96)a | |
| 10 | − 21.87 (− 26.93, − 16.81) | − 17.62 (− 22.96, − 12.28) | − 4.25 (− 11.61, 3.12) | |
| Exploratory endpoint | Overall | − 13.82 (− 17.22, − 10.42) | − 8.28 (− 11.87, − 4.69) | − 5.54 (− 10.49, − 0.59)a |
ITT intent-to-treat, VAS visual analogue scale
aMean difference significant at the 0.05 level (Bonferroni adjustment for multiple comparisons)
Fig. 2.
Change from baseline in VAS pain intensity ± standard error of the mean (ITT population). a All patients (N = 270). b Patients with moderate pain (NRS 4–6) at baseline (N = 177). c Patients with severe pain (NRS ≥ 7) at baseline (N = 93). ITT intention-to-treat, NRS numerical rating scale, SAT standard analgesic treatment, VAS visual analogue scale
The time to onset of pain relief was reported by 110 patients (82.7%) in the methoxyflurane group and 105 patients (78.9%) in the SAT group. The median time to onset of pain relief was shorter in the methoxyflurane group (9 min; 95% CI 7.72, 10.28 min) compared with the SAT group (15 min; 95% CI 14.17, 15.83 min). The Kaplan–Meier curves confirm a better onset of action with methoxyflurane up to approximately 20 min (Fig. 3); however, after this time point the curves converged; therefore, the Cox proportional hazards model was not used to compare treatment groups since the assumption of proportional risks was not met. The Gehan–Breslow–Wilcoxon test was used to examine the difference between the Kaplan–Meier curves, showing a significant treatment difference in favour of methoxyflurane (p < 0.001). Rescue medication use was low in both treatment groups, with three (2.2%) methoxyflurane-treated patients and five (3.7%) SAT-treated patients resorting to rescue medication (p = 0.722). The proportion of patients in the methoxyflurane group who resorted to closure of the diluter hole (to obtain greater analgesia) was 13.0% (95% CI 9.2, 17.6%).
Fig. 3.
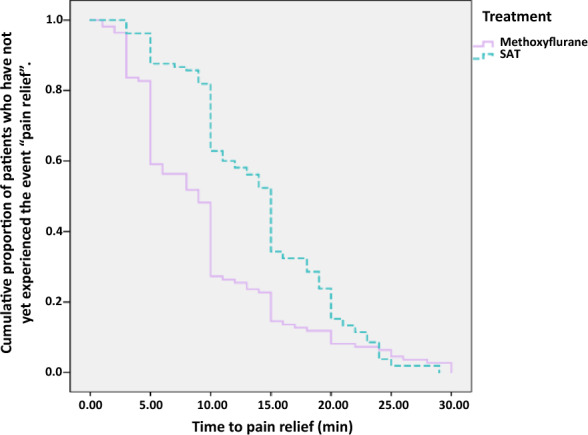
Kaplan–Meier plot of time to onset of pain relief. The Kaplan–Meier curve represents an estimate of the cumulative proportion of patients who have not yet experienced onset of pain relief. Higher curves indicate longer time to pain relief. No censoring was performed
The overall efficacy of study treatment was rated “Excellent”, “Very Good” or “Good” by significantly more patients in the methoxyflurane group compared to the SAT group (72.7% vs. 60.9%; p = 0.001; Fig. 4a). Similarly, significantly more HCPs rated the practicality of using study treatment as “Excellent”, “Very Good” or “Good” for methoxyflurane than SAT (90.3% vs. 64.4%; p < 0.001; Fig. 4b).
Fig. 4.
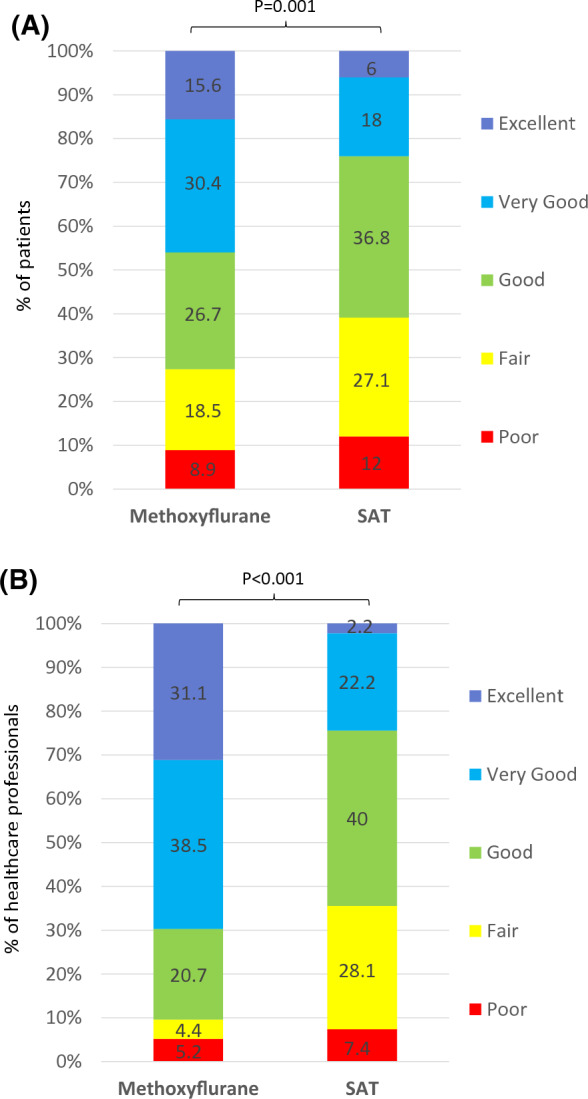
Patient and healthcare professional-reported outcomes (ITT population). a Overall treatment efficacy evaluated by the patient. b Practicality of using study treatment evaluated by the healthcare professional
Safety Results
AEs are summarised in Table 3. Twenty-three patients (17.0%) in the methoxyflurane group and four patients (3.0%) in the SAT group experienced AEs; none were serious. All except two AEs (bronchitis in the methoxyflurane group and pruritus in the SAT group, both unrelated to study treatment) resolved by the follow-up telephone call. Eight patients discontinued study treatment because of AEs [euphoria (two patients), nausea (two patients), feeling drunk, vertigo, dizziness and diplopia) and two patients had a temporary discontinuation of study treatment due to AEs (somnolence and dysgeusia); all were in the methoxyflurane group. Special safety events of interest were pregnancy, misuse and abuse; no events of pregnancy or abuse and one case of misuse (methoxyflurane not taken correctly because of error in preparing inhaler) were recorded. There were no clinically notable changes from baseline in mean vital sign parameters (electronic supplementary material).
Table 3.
Adverse events (safety population)
| Number (%) of patients | Methoxyflurane (N = 135) | Standard analgesic treatment (N = 135) | ||
|---|---|---|---|---|
| All AEs | Related AEsa | All AEs | Related AEsa | |
| Any adverse event | 23 (17.0) | 17 (12.6) | 4 (3.0) | 2 (1.5) |
| Euphoric mood | 5 (3.7) | 5 (3.7) | 0 | 0 |
| Somnolence | 4 (3.0) | 4 (3.0) | 0 | 0 |
| Nausea | 3 (2.2) | 3 (2.2) | 1 (0.7) | 0 |
| Dysgeusia | 3 (2.2) | 3 (2.2) | 0 | 0 |
| Feeling abnormal | 3 (2.2) | 3 (2.2) | 0 | 0 |
| Pyrexia | 2 (1.5) | 0 | 0 | 0 |
| Vertigo | 2 (1.5) | 2 (1.5) | 0 | 0 |
| Presyncope | 1 (0.7) | 0 | 2 (1.5) | 1 (0.7) |
| Bronchitis | 1 (0.7) | 0 | 0 | 0 |
| Diplopia | 1 (0.7) | 1 (0.7) | 0 | 0 |
| Dizziness | 1 (0.7) | 1 (0.7) | 0 | 0 |
| Feeling drunk | 1 (0.7) | 1 (0.7) | 0 | 0 |
| Headache | 1 (0.7) | 0 | 0 | 0 |
| Oral discomfort | 1 (0.7) | 1 (0.7) | 0 | 0 |
| Sedation | 1 (0.7) | 1 (0.7) | 0 | 0 |
| Vomiting | 0 | 0 | 2 (1.5) | 1 (0.7) |
| Constipation | 0 | 0 | 1 (0.7) | 0 |
| Hyperhidrosis | 0 | 0 | 1 (0.7) | 0 |
| Hypotension | 0 | 0 | 1 (0.7) | 1 (0.7) |
| Pruritis | 0 | 0 | 1 (0.7) | 0 |
Data are presented as number (%) of patients. Adverse events (AEs) are presented by MedDRA preferred term in decreasing order of frequency in the methoxyflurane group, followed by the standard analgesic treatment group
aEvents considered possibly or probably related to study treatment by the investigator
Discussion
This trial demonstrated both non-inferiority and superiority of methoxyflurane compared to SAT in terms of the decrease in pain intensity achieved in the first 10 min post baseline. Methoxyflurane was superior to standard treatment for both moderate pain (IV paracetamol or ketoprofen) and severe pain (IV morphine). Given the intravenous administration of the comparator treatment, the change in the first 10 min post baseline reflected the most challenging comparison for methoxyflurane, and was directly relevant to the indication for methoxyflurane of emergency relief of moderate-to-severe trauma pain [19]. Previous studies have shown that methoxyflurane is effective at the 20-min time point [18, 25, 26, 32], thus the 10-min endpoint utilized in this study provides additional information about the efficacy profile of inhaled methoxyflurane. The secondary analysis of the change in VAS pain intensity to later time points also showed significantly larger reductions in pain intensity for methoxyflurane versus SAT up to 25 min. Although in the present study patients were provided with only one vial (3 mL) of methoxyflurane, ensuring approximately 25–60 min of effect, depending on frequency of inhalation [19, 20], in clinical practice a second 3-mL dose could be administered if a longer analgesic effect is required [19]. The absolute mean change from baseline in VAS pain intensity at 10 min for methoxyflurane in this study (− 23 mm from a baseline mean of 67 mm) is likely to represent a clinically meaningful change according to previous estimates of minimum clinically important difference in acute pain of approximately 20% reduction on the NRS [33] or approximately 13 mm on the VAS [34, 35].
The median time from randomisation to onset of pain relief was significantly shorter for methoxyflurane (9 min) compared with SAT (15 min) in this trial. This result was confirmed by the Gehan–Breslow–Wilcoxon test comparing the Kaplan–Meier curves. Given that the standard analgesic treatments were all administered intravenously, this may reflect the time taken to administer SAT as well as the onset of analgesic action. The time of randomisation was chosen as baseline with the specific intention of investigating not only the intrinsic efficacy but also the speed of drug administration in an emergency context, characterized by the need to intervene as quickly as possible to relieve the patient’s pain while being able to continue with the diagnostic-therapeutic process. The InMEDIATE study reported that the median time to onset of pain relief from the start of treatment was significantly shorter for methoxyflurane (3 min) than SAT (10 min), suggesting that the quicker onset of action of methoxyflurane in the present study is not solely due to the difference in time taken to administer the treatment [32]. Similar to the present study, most patients in the SAT group in the InMEDIATE study received IV analgesics, although the majority of patients with severe pain received first-step analgesics rather than opioids. Median time to onset of pain relief for methoxyflurane in the placebo-controlled UK STOP! study was 4 min [18] (5 min in the adult subgroup [25]).
The effectiveness as a short-term treatment option for acute trauma pain, the ease of preparation and administration of methoxyflurane, and the fact that patients can self-administer treatment and control their own level of analgesia have the advantage of lightening the care burden in the ED compared with IV treatments. This is supported by the HCP ratings of the practicality of using study treatment, which was rated as “Excellent”, “Very Good” or “Good” in 90.3% of cases for methoxyflurane and 64.4% for SAT. Methoxyflurane does not require physiological monitoring during use, unlike opioid analgesics, and does not have the associated administration costs of IV morphine, which have been estimated at €14–22 in terms of workforce costs alone, rising considerably when costs of morphine- and IV-related AEs are included [36]. Inhaled methoxyflurane may also be less stressful for the patient than intravenous administration, and more practical in the out-of-hospital environment.
Although more AEs were reported in the methoxyflurane group (17%) compared with the SAT group (3%), most of them were minor and transient, and only eight patients (5.9%) in the methoxyflurane group discontinued treatment because of AEs. The STOP! and InMEDIATE studies also reported higher AE rates for methoxyflurane vs. placebo and active treatment, respectively, but patient-reported outcomes in these studies indicated high patient acceptance of treatment. Indeed, 78% of adult patients in STOP! rated methoxyflurane treatment as excellent, very good or good [25], and patients in InMEDIATE rated methoxyflurane a median of 9 out of 10 for pain control, comfort of treatment and safety (AEs) [32]. No effects of methoxyflurane on vital sign parameters were observed, consistent with reports from other clinical trials in Europe [18, 25, 26, 32] and findings of two observational studies including 1217 patients treated with methoxyflurane in the prehospital setting in Australia [20, 37]. No cases of methoxyflurane abuse were identified in our study.
Strengths and Limitations
The study strengths include the multicentre, randomised design, allocation concealment (reflected by the homogeneous baseline data) and comparison with an active comparator treatment that reflects current practice in Italy [28]. However, national- and local-level variations in pain management guidelines may limit the generalizability of the results to some other countries, and data from ongoing and recently completed studies in other countries are awaited [38, 39]. The current study results are robust; consistent results were obtained for the primary outcome measure across analyses of all patients, patients with moderate pain treated with IV paracetamol/ketoprofen, and patients with severe pain treated with IV morphine. Secondary efficacy results for the onset of pain relief and patient ratings of efficacy also supported the conclusion of improved efficacy with methoxyflurane versus SAT. The study population included a broad range of adult patients with acute trauma pain in terms of age, gender, pain severity and injury type, reflecting a “real-world” emergency setting.
A limitation of this trial is the open nature of treatment administration. Although this design presents an intrinsic bias, it was considered the only practical option due to the nature of the trial setting and of the treatments being studied. A double-blind study, while ideal from a methodological perspective, would not have been feasible because of the different routes of administering the two treatments, unless using a double-dummy design. A double-dummy design is difficult to apply in the context of emergency medicine because of extremely tight time frames and may also raise ethical issues, as it could result in delayed administration of the active drug and a prolongation of pain for the patient with trauma. Finally, considering that the literature reports that the intravenous route is generally associated with a greater placebo effect, and that the medications which constitute SAT have widely been shown to be effective options for the management of moderate-to-severe trauma pain, the risk of bias in patient assessment is believed to be very limited in this specific case. The choice of SAT for moderate pain (IV ketoprofen or paracetamol) was discretional; however, most patients (88%) received paracetamol, thus providing a relatively consistent comparative treatment. We did not perform laboratory safety tests in this trial because of the setting and the lack of opportunity for in-person follow-up with patients; however, previous clinical studies have identified no renal or liver concerns arising from clinical laboratory evaluations performed at baseline and follow-up (14 ± 2 days) [18, 32].
Conclusion
Methoxyflurane provided superior pain relief to SAT in patients with moderate-to-severe trauma pain. Exploratory analysis also demonstrated superiority of methoxyflurane versus IV morphine in patients with severe pain over the first 10 min. Low-dose inhaled methoxyflurane may therefore offer a simple, fast and effective non-narcotic treatment option for the short-term relief of moderate-to-severe trauma pain. Given the heterogeneity of pain and pain management, additional studies are needed that compare methoxyflurane with other analgesic modalities to help further inform physicians regarding the appropriate use of methoxyflurane in emergency medicine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Contract Research Organization YGHEA, Division of Ecol Studio Bioikos S.r.l., Bologna, for the operational support and in particular Eleonora Romagnoli for protocol writing, Vanessa Bacchi for trial start-up, Elisa Lani and Francesca Bressan for clinical monitoring, Carolina Cerrè for data management and Francesca Cannoletta for project management. Penthrox® is a registered trade mark of Medical Developments International (MDI) Limited and used under licence.
Funding
The study was funded by Mundipharma Pharmaceuticals S.r.l. The study sponsor was involved in the study design and data interpretation but had no role in data collection or analysis. The sponsor reviewed the report and provided funding for medical writing assistance. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The journal’s Rapid Service and Open Access Fee were funded by Mundipharma Pharmaceuticals S.r.l.
Editorial Assistance
Editorial assistance in the preparation of this manuscript was provided by Karen Mower of Scientific Editorial and funded by Mundipharma Research Limited.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
List of Investigators
The investigators of the MEDITA Study Group are Germana Ruggiano, Guido Tota, Michela Zerini, Alessandra Semino, Sara Rasla, Elena Mecatti, Gabriele Panci, Catia Fronduti, Patrizia Signoroni, Alessandro Corradini, Alessandra Benucci (Emergency Medicine Department, Santa Maria Annunziata Hospital, Florence, Italy); Isabella Bartoli, Davide Messina (SUES 118 Catania-Ragusa-Siracusa, Cannizzaro Hospital, Catania, Italy); Giuseppe Carpinteri, Paola Noto, Chiara Giraffa (Department of Emergency Medicine, Vittorio Emanuele University Hospital, Catania, Italy); Andrea Fabbri (Department of Emergency Medicine, Morgagni-Pierantoni Hospital, Forlì, Italy); Francesco Bermano; Cristina Lapini (118 Service, San Martino Hospital, Genova. Italy); Maurizio Chiesa; Beatrice Sabini (Emergency Department, S. Antonio Hospital—ULSS 16, Padova, Italy); Mario Oppes, Vincenzo Pretti (Emergency Department, Sassari University Hospital, Sassari, Italy); Peppino Masciari, Antonietta Comito, Lucia Orlando (Emergency Department, Pugliese-Ciaccio Hospital, Catanzaro, Italy); Davide Torti, Giorgio Carbone, Gianfrancesco Alberto (Emergency Department, Gradenigo Hospital, Torino, Italy); Vittorio Iorno (Anesthesia, Resuscitation and Emergency Department, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico di Milano, Milan, Italy); Antonio Voza, Alessandro Barraco, Fabio Intelligente, Monica Suardi, Claudio Addari (Emergency Department, IRCCS Humanitas Research Teaching Hospital, Rozzano, Milan, Italy); Piero Paolini, Nicola Bertocci (118 Unit, Azienda USL Toscana Centro, Pistoia, Italy); Sossio Serra, Stefano Tranelli, Pasquale Di Conza, Alessandro Valentino, Patrizia Cuppini, Elisabetta Lucchi, Carlo Garaffoni (Emergency Department, Maurizio Bufalini Hospital, Cesena, Italy); Gianfilippo Gangitano, Giuseppe D’Antuono, Lorena Bertozzi, Giulia Montanari (Emergency Department, Infermi Hospital, Rimini, Italy), Raffaella Francesconi (Faenza Hospital, Faenza, Italy).
Prior Presentation
This work was previously published as a preprint: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3420404.
Disclosures
Elisabetta Bonafede is an employee of the clinical research organisation that conducted the study. Antonella Sblendido is an employee of Mundipharma Pharmaceuticals S.r.l. Amedeo Soldi is an employee of Mundipharma Pharmaceuticals S.r.l. Alberto Farina is an employee of Mundipharma Pharmaceuticals S.r.l. Sebastiano Mercadante, Antonio Voza, Sossio Serra, Germana Ruggiano, Andrea Fabbri, Giuseppe Carpinteri, Gianfilippo Gangitano and Fabio Intelligente have nothing to disclose.
Compliance with Ethics Guidelines
The trial was approved by the Italian Medicines Agency (AIFA). The co-ordinating ethics committee, Comitato Etico Regione Toscana—Area Vasta Centro, Florence, Italy, approved the trial protocol on 1 December 2017. In addition, all trial documents and procedures were reviewed and approved by the appropriate ethics committees at each centre (see Table S2 in the electronic supplementary material for list of all ethics committees). Informed consent was obtained from all individual participants included in the study. Given the emergency setting and the requirement for rapid analgesia, if the patient was unable to provide written informed consent, witnessed verbal consent was obtained, with the patient signing the informed consent as soon as they were able.
Data Availability
The datasets generated, analyzed and reported within this manuscript may be requested in accordance with the Data Sharing Policy of Mundipharma Research Limited available from www.mundipharma-rd.eu.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9228326.
References
- 1.Berben SAA, Schoonhoven L, Meijs THJM, van Vugt AB, van Grunsven PM. Prevalence and relief of pain in trauma patients in emergency medical services. Clin J Pain. 2011;27:587–592. doi: 10.1097/AJP.0b013e3182169036. [DOI] [PubMed] [Google Scholar]
- 2.Berben SA, Meijs TH, van Dongen RT, et al. Pain prevalence and pain relief in trauma patients in the accident and emergency department. Injury. 2008;39:578–585. doi: 10.1016/j.injury.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Karwowski-Soulie F, Lessenot-Tcherny S, Lamarche-Vadel A, et al. Pain in an emergency department: an audit. Eur J Emerg Med. 2006;13:218–224. doi: 10.1097/01.mej.0000217975.31342.13. [DOI] [PubMed] [Google Scholar]
- 4.Todd KH, Ducharme J, Choiniere M, et al. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007;8:460–466. doi: 10.1016/j.jpain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht E, Taffe P, Yersin B, Schoettker P, Decosterd I, Hugli O. Undertreatment of acute pain (oligoanalgesia) and medical practice variation in prehospital analgesia of adult trauma patients: a 10 yr retrospective study. Br J Anaesth. 2013;110:96–106. doi: 10.1093/bja/aes355. [DOI] [PubMed] [Google Scholar]
- 6.Pierik JG, Ijzerman MJ, Gaakeer MI, et al. Pain management in the emergency chain: the use and effectiveness of pain management in patients with acute musculoskeletal pain. Pain Med. 2015;16:970–984. doi: 10.1111/pme.12668. [DOI] [PubMed] [Google Scholar]
- 7.Pierik JGJ, Ijzerman MJ, Gaakeer MI, Vollenbroek-Hutten MMR, Doggen CJM. Painful discrimination in the emergency department: risk factors for underassessment of patients’ pain by nurses. J Emerg Nurs. 2017;43:228–238. doi: 10.1016/j.jen.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Motov SM, Khan AN. Problems and barriers of pain management in the emergency department: are we ever going to get better? J Pain Res. 2008;2:5–11. [PMC free article] [PubMed] [Google Scholar]
- 9.Dißmann PD, Maignan M, Cloves PD, Gutierrez Parres B, Dickerson S, Eberhardt A. A review of the burden of trauma pain in emergency settings in Europe. Pain Ther. 2018;7:179–192. doi: 10.1007/s40122-018-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decosterd I, Hugli O, Tamchès E, et al. Oligoanalgesia in the emergency department: short-term beneficial effects of an education program on acute pain. Ann Emerg Med. 2007;50:462–471. doi: 10.1016/j.annemergmed.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO’s cancer pain ladder for adults 1986. http://www.who.int/cancer/palliative/painladder/en/. Accessed 1 Jul 2019.
- 12.Todd KH. A review of current and emerging approaches to pain management in the emergency department. Pain Ther. 2017;6:193–202. doi: 10.1007/s40122-017-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducassé JL, Siksik G, Durand-Béchu M, et al. Nitrous oxide for early analgesia in the emergency setting: a randomized, double-blind multicenter prehospital trial. Acad Emerg Med. 2013;20:178–184. doi: 10.1111/acem.12072. [DOI] [PubMed] [Google Scholar]
- 14.Porter KM, Siddiqui MK, Sharma I, Dickerson S, Eberhardt A. Management of trauma pain in the emergency setting: low-dose methoxyflurane or nitrous oxide? A systematic review and indirect treatment comparison. J Pain Res. 2017;11:11–21. doi: 10.2147/JPR.S150600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young A, Ismail M, Papatsoris AG, et al. Entonox® inhalation to reduce pain in common diagnostic and therapeutic outpatient urological procedures: a review of the evidence. Ann R Coll Surg Engl. 2012;94:8–11. doi: 10.1308/003588412X13171221499702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komessaroff D. Pre-hospital pain relief: penthrane or entonox. Aust J Emerg Care. 1995;2:28–29. [Google Scholar]
- 17.Entonox summary of product characteristics. https://www.drugs.com/uk/entonox-medicinal-gas-leaflet.html. Accessed 1 Jul 2019.
- 18.Coffey F, Wright J, Hartshorn S, et al. STOP!: a randomised, double-blind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute pain. Emerg Med J. 2014;31:613–618. doi: 10.1136/emermed-2013-202909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penthrox® summary of product characteristics. https://www.medicines.org.uk/emc/medicine/31391. Accessed 1 Jul 2019.
- 20.Oxer HF. Effects of Penthrox® (methoxyflurane) as an analgesic on cardiovascular and respiratory functions in the pre-hospital setting. J Mil Veterans Health. 2016;24:14–20. [Google Scholar]
- 21.Porter KM, Dayan AD, Dickerson S, Middleton PM. The role of inhaled methoxyflurane in acute pain management. Open Access Emerg Med. 2018;10:149–164. doi: 10.2147/OAEM.S181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jephcott C, Grummet J, Nguyen N, Spruyt O. A review of the safety and efficacy of inhaled methoxyflurane as an analgesic for outpatient procedures. Br J Anaesth. 2018;120:1040–1048. doi: 10.1016/j.bja.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Blair HA, Frampton JE. Methoxyflurane: a review in trauma pain. Clin Drug Investig. 2016;36:1067–1073. doi: 10.1007/s40261-016-0473-0. [DOI] [PubMed] [Google Scholar]
- 24.Medical Developments International. Penthrox: European update, 07 February 2017. https://www.medicaldev.com/wp-content/uploads/2017/02/ASX-Announcement-Penthrox-European-Update-7-Feb-2017.pdf. Accessed 1 Jul 2019.
- 25.Coffey F, Dissmann P, Mirza K, Lomax M. Methoxyflurane analgesia in adult patients in the emergency department: a subgroup analysis of a randomized, double-blind, placebo-controlled study (STOP!) Adv Ther. 2016;33:2012–2031. doi: 10.1007/s12325-016-0405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartshorn S, Dissmann P, Coffey F, Lomax M. Low-dose methoxyflurane analgesia in adolescent patients with moderate-to-severe trauma pain: a subgroup analysis of the STOP! study. J Pain Res. 2019;12:689–700. doi: 10.2147/JPR.S188675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marinangeli F, Reggiardo G, Sblendido A, Soldi A, Farina A, METEORA Group Prospective, multicentre trial of methoxyflurane for acute trauma-related pain in helicopter emergency medical systems and hostile environments: METEORA protocol. Adv Ther. 2018;35:2081–2092. doi: 10.1007/s12325-018-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raccomandazioni Intersocietarie Italiane (SIAARTI, SIMEU, SIS 118, AISD, SIARED, SICUT, IRC) sulla gestione del dolore in emergenza. http://www.aisd.it/e107_files/downloads/raccintersocietarie_it_complete31052014.pdf. Accessed 1 Jul 2019.
- 29.Fabbri A, Carpinteri G, Ruggiano G, et al. Methoxyflurane versus standard of care for acute trauma-related pain in the emergency setting: protocol for a randomised, controlled study in Italy (MEDITA) Adv Ther. 2019;36:244–256. doi: 10.1007/s12325-018-0830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montassier E, Freund Y. A rigorous evaluation of methoxyflurane is needed: comment on “methoxyflurane versus standard of care for acute trauma-related pain in the emergency setting: protocol for a randomised, controlled study in Italy (MEDITA)”. Adv Ther. 2019;36:1241–1242. doi: 10.1007/s12325-019-00936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabbri A, Carpinteri G, Ruggiano G, et al. Response to “a rigorous evaluation of methoxyflurane is needed: comment on ‘methoxyflurane versus standard of care for acute trauma-related pain in the emergency setting: protocol for a randomised, controlled study in Italy (MEDITA)’”. Adv Ther. 2019;36:1243–1245. doi: 10.1007/s12325-019-00937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borobia AM, García Collado S, Carballo Cardona C, et al. Inhaled methoxyflurane provides greater analgesia and faster onset of action versus standard analgesia in patients with trauma pain. InMEDIATE: a randomized controlled trial in Emergency Departments. Ann Emerg Med. (In press). [DOI] [PubMed]
- 33.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. doi: 10.1016/S0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 34.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–489. doi: 10.1016/S0196-0644(96)70238-X. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–638. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 36.Casamayor M, DiDonato K, Hennebert M, Brazzi L, Prosen G. Administration of intravenous morphine for acute pain in the emergency department inflicts an economic burden in Europe. Drugs Context. 2018;7:212524. doi: 10.7573/dic.212524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston S, Wilkes GJ, Thompson JA, Ziman M, Brightwell R. Inhaled methoxyflurane and intranasal fentanyl for prehospital management of visceral pain in an Australian ambulance service. Emerg Med J. 2011;28:57–63. doi: 10.1136/emj.2009.078717. [DOI] [PubMed] [Google Scholar]
- 38.ClinicalTrials.gov. Efficacy and safety of Penthrox combined with a standard analgesia (SoC) in adult patients admitted to the emergency department with moderate to severe pain associated with trauma (Pen ASAP). NCT03798899.https://clinicaltrials.gov/ct2/show/NCT03798899?term=methoxyflurane&rank=12. Accessed 1 Jul 2019.
- 39.ClinicalTrials.gov. A phase IV real world study on the use of low dose methoxyflurane (PENTHROX™) for the treatment of moderate to severe trauma pain in the Canadian emergency department. NCT03868436.https://clinicaltrials.gov/ct2/show/NCT03868436?term=methoxyflurane&rank=10. Accessed 1 Jul 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated, analyzed and reported within this manuscript may be requested in accordance with the Data Sharing Policy of Mundipharma Research Limited available from www.mundipharma-rd.eu.