Abstract
Introduction
Diroximel fumarate (DRF) is a novel oral fumarate in development for patients with relapsing forms of multiple sclerosis (MS). Clinical findings from the DRF development program suggest that rates of gastrointestinal (GI) treatment-emergent adverse events (TEAEs) and discontinuation due to GI TEAEs are low, based on clinical and real-world observations of other fumaric acid esters, including dimethyl fumarate (DMF). The incidence of GI TEAEs varies from 40 to 88% in clinical and real-world studies of DMF. The objective of this study is to present GI tolerability findings from the EVOLVE-MS-1 study and present biologic hypotheses for the improved GI properties of DRF.
Methods
GI TEAEs and treatment discontinuation because of GI TEAEs were assessed in DRF-treated patients with relapsing-remitting MS who were participating in the ongoing, 96-week, open-label, phase 3 EVOLVE-MS-1 study.
Results
As of March 30, 2018, a total of 696 patients were enrolled in EVOLVE-MS-1. GI TEAEs were reported in 30.9% (215/696) of patients; the vast majority (96%; 207/215) experienced events that were mild or moderate in severity. When GI AEs did occur, they occurred early in treatment, resolved (88.8%; 191/215), and were of short duration [median 7.5 (range 1–87) days] in most patients. GI TEAEs led to < 1% of patients discontinuing treatment.
Conclusions
We suggest that the distinct chemical structure of DRF contributes to the observed low rates of GI TEAEs and GI-associated treatment discontinuations, possibly due to a combination of several factors. We hypothesize that these factors may include less reactivity with off-target proteins and/or lower production of a methanol leaving group that may contribute to GI irritation. A direct comparison of GI tolerability with DRF versus DMF is being evaluated in the EVOLVE-MS-2 study.
Trial Registration
ClinicalTrials.gov number NCT02634307.
Funding
Alkermes Inc. (Waltham, MA, USA) and Biogen (Cambridge, MA, USA).
Electronic supplementary material
The online version of this article (10.1007/s12325-019-01085-3) contains supplementary material, which is available to authorized users.
Keywords: Dimethyl fumarate, Diroximel fumarate, Fumaric acid ester, Gastrointestinal, Multiple sclerosis, Neurology, Relapsing-remitting multiple sclerosis, Tolerability
Introduction
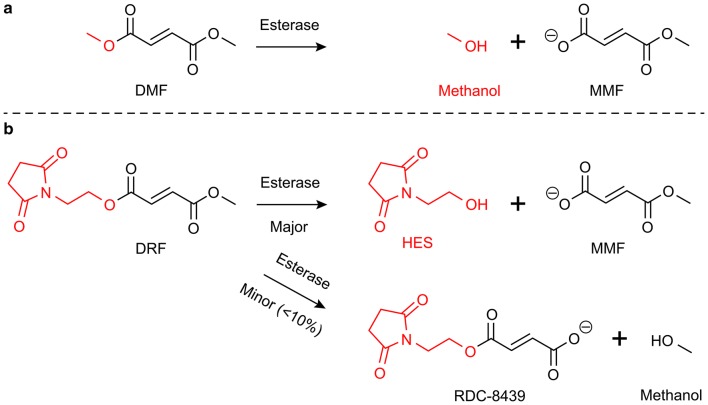
Delayed-release dimethyl fumarate (DMF) is a fumaric acid ester (FAE) approved as an oral therapy for relapsing forms of multiple sclerosis (MS). DMF has been shown to be effective in significantly reducing clinical and radiologic measures of disease activity in patients with relapsing-remitting MS (RRMS) in clinical trials as well as in patients with MS treated in real-world studies [1–5]. DMF undergoes esterase cleavage to monomethyl fumarate (MMF) prior to reaching systemic circulation in the blood (Fig. 1a). DMF therapy is thought to act by modulating cell-signaling pathways that produce neuroprotective and immunomodulatory effects [6–8].
Fig. 1.
Fumaric acid ester metabolism. a Upon oral administration, DMF undergoes esterase cleavage before systemic circulation to produce the major metabolites MMF and methanol. b DRF undergoes esterase cleavage to produce the major metabolites MMF and HES and the minor metabolites RDC-8439 and methanol. DMF dimethyl fumarate, DRF diroximel fumarate, HES 2-hydroxyethyl succinimide, MMF monomethyl fumarate
Through clinical trial and real-world experience, the benefits of DMF treatment for patients with relapsing MS and its safety profile have been well established [1–5]. Gastrointestinal (GI) side effects are commonly reported in patients receiving DMF therapy, including nausea, vomiting, diarrhea, and upper abdominal pain [9–11]. During phase 3 trials, GI adverse events (AEs) were reported in 40% of patients treated with DMF compared with 30% of patients treated with placebo [1, 2]. The incidence of GI AEs was 88% in a real-world study in which patients self-reported symptoms using eDiaries [10]. Although GI events are generally mild in severity and typically resolve within the first 2 months of treatment, these issues may impact patient quality of life and ultimately medication adherence. GI AEs are a common cause of discontinued DMF treatment [1, 2, 10, 12, 13].
Diroximel fumarate (DRF) is a novel oral fumarate in development for patients with relapsing forms of MS. Like DMF, DRF is metabolized to MMF, but with the methanol leaving group substituted with an inert leaving group, 2-hydroxyethyl succinimide (HES; Fig. 1b) [14]. At therapeutic doses, DRF and DMF produce bioequivalent systemic exposure of MMF, which is thought to drive efficacy in patients with MS. However, the other metabolite profiles of DMF and DRF are different. The first step of DRF metabolism produces two major metabolites, MMF and HES, and two minor metabolites, RDC-8439 and methanol (Fig. 1b). This is in contrast to DMF, where the first step of metabolism produces MMF and methanol as the major metabolites (Fig. 1a). Because of its chemical structure and corresponding metabolites, DRF is expected to confer an efficacy and safety profile consistent with the experience of DMF, but with an improved GI tolerability profile.
The effects of DRF, including its GI tolerability profile, are being assessed as part of the DRF clinical development program, which comprises ten completed phase 1 studies (nine conducted in healthy volunteers; one conducted in healthy volunteers and patients with varying degrees of renal impairment) and two phase 3 studies in patients with RRMS; EVOLVE-MS-1 and EVOLVE-MS-2. Over 1500 participants have received DRF as part of the clinical development program, including 696 patients (representing 685 patient-years of exposure) enrolled in the ongoing EVOLVE-MS-1 study as of March 2018. Clinical findings from the DRF development program suggest that rates of GI events and discontinuation due to GI events are low. The objective of this article is to present GI tolerability findings from the phase 3 EVOLVE-MS-1 study and to present biologic hypotheses for why rates of GI AEs with DRF appear low.
Methods
EVOLVE-MS-1 Study
Two phase 3 studies are assessing patients with RRMS treated with DRF. EVOLVE-MS-1 (NCT02634307) is an ongoing, global, open-label, single-arm, phase 3 study assessing long-term safety, tolerability, and treatment effects of DRF 462 mg twice daily over 96 weeks. The EVOLVE-MS-1 study design, end points, and analysis populations have been described previously (R.T. Naismith et al., Multiple Sclerosis Journal, accepted, 2019). EVOLVE-MS-1 enrollment is ongoing, with 800–1000 patients planned for this study. The EVOLVE-MS-1 study population includes patients who rolled over from the 5-week EVOLVE-MS-2 (NCT03093324) study and those who were newly enrolled in the DRF clinical development program. EVOLVE-MS-2 is a randomized, double-blind, phase 3 study to evaluate the tolerability of DRF versus DMF over 5 weeks.
EVOLVE-MS-1 Patients and Dosing
DRF 462 mg was administered twice daily by oral capsules. Patients receiving DRF for the first time were given a titrated dose of DRF 231 mg twice daily for the first study week followed by 462 mg twice daily thereafter; all other patients received DRF 462 mg twice daily over the entire 96-week study period.
The EVOLVE-MS-1 study protocol did not include specific instructions on management strategies for patients with GI TEAEs. However, dose reduction to 231 mg twice daily was permitted at the investigator’s discretion starting on day 8 of treatment for patients unable to tolerate the 462 mg twice-daily dose. If a patient continued to be unable to tolerate the 462 mg twice-daily dose after 1 month of treatment, further dose reduction was not permitted and that patient was discontinued from the study. Concomitant medications for treating GI symptoms were not prohibited.
Patients participating in the phase 3 EVOLVE-MS-1 study were 18–65 years of age, with a confirmed diagnosis of RRMS [15] and no evidence of relapse within 30 days of screening. Patients were not eligible if they had significant recurring or active GI symptoms within 3 months of screening, including symptoms that required the initiation of or change in symptomatic medical treatment; clinically significant gastrointestinal and/or other major disease that would preclude participation in a clinical trial, prior history of DMF discontinuation because of tolerability issues or lack of efficacy; or a clinically significant medical condition that prevented participation in the opinion of the investigator.
The EVOLVE-MS-1 study protocol was approved by the institutional review board at each study site. The study was conducted in accordance with the Declaration of Helsinki and International Council of Harmonisation Good Clinical Practice guidelines. All study participants provided written, informed consent.
Assessments and Analysis Populations
Safety was evaluated based on the incidence of treatment-emergent AEs (TEAEs), incidence of serious AEs, and AEs leading to discontinued treatment. TEAEs were reported and coded according to medical dictionary for regulatory activities (MedDRA) system organ class (SOC). GI TEAEs were coded according to the MedDRA preferred terms within the SOC for GI disorders, which included nausea, diarrhea, upper abdominal pain, vomiting, constipation, abdominal pain, flatulence, gastroesophageal reflux disease, abdominal discomfort, and 34 other GI-related events. As patients with prior history of significant GI disease were excluded from the study, herein we report GI events that occurred during the treatment period (GI TEAEs). Safety analyses were performed using the safety population, which was defined as all patients who received at least one dose of study drug. Summary statistics were provided for safety data.
Results
Patients
As of March 30, 2018, a total of 696 patients were enrolled in the ongoing phase 3 EVOLVE-MS-1 study and received at least one dose of DRF. Enrolled patients were mean (SD) 41.9 (11.0) years of age; 72.6% were female, 91.7% were white, 40.7% were enrolled in the United States, and 64.9% had received prior disease-modifying therapy. Mean (SD) time since diagnosis was 7.6 (7.3) years.
GI TEAEs and Treatment Discontinuation Because of GI TEAEs
At the time of the data cut (March 30, 2018), patients had received DRF for a median of 59.9 (range, 0.1–98.9) weeks. Of the 696 enrolled patients, 83.2% were on treatment with DRF, 1.9% completed the study, and 14.9% discontinued treatment; 6.3% discontinued DRF because of AEs. Less than 1% of patients discontinued DRF specifically because of GI TEAEs (0.7%; 5/696; one patient each with anal incontinence, diarrhea, dyspepsia, irritable bowel syndrome, and peptic ulcer).
GI TEAEs were reported in 30.9% (215/696) of the overall population, the most common (occurring in at least 2% of patients) of which were diarrhea (10.8%; 75/696), nausea (6.8%; 47/696), upper abdominal pain (4.5%; 31/696), vomiting (3.6%; 25/696), constipation (3.4%; 24/696), abdominal pain (2.4%; 17/696), flatulence (2.2%; 15/696), and gastroesophageal reflux disease (2.0%; 14/696; Table 1). Among patients with GI TEAEs, the vast majority, 96% (207/215), had events that were mild or moderate in severity; the severity of specific GI events is shown in Table 1. Serious GI AEs were reported in 0.4% (3/696) of patients (Table 1).
Table 1.
Incidence and severity of gastrointestinal TEAEs and serious gastrointestinal events occurring in patients in EVOLVE-MS-1
| TEAE, n (%) | Phase 3 EVOLVE-MS-1, N = 696 | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | Total | |
| Gastrointestinal disordersa | 147 (21.1) | 60 (8.6) | 8 (1.1) | 215 (30.9) |
| Diarrhea | 48 (6.9) | 24 (3.4) | 3 (0.4) | 75 (10.8) |
| Nausea | 39 (5.6) | 8 (1.1) | 0 | 47 (6.8) |
| Upper abdominal pain | 27 (3.9) | 4 (0.6) | 0 | 31 (4.5) |
| Vomiting | 14 (2.0) | 10 (1.4) | 1 (0.1) | 25 (3.6) |
| Constipation | 21 (3.0) | 2 (0.3) | 1 (0.1) | 24 (3.4) |
| Abdominal pain | 6 (0.9) | 10 (1.4) | 1 (0.1) | 17 (2.4) |
| Flatulence | 14 (2.0) | 1 (0.1) | 0 | 15 (2.2) |
| Gastroesophageal reflux disease | 9 (1.3) | 5 (0.7) | 0 | 14 (2.0) |
| Abdominal discomfort | 13 (1.9) | 0 | 0 | 13 (1.9) |
| Dyspepsia | 10 (1.4) | 1 (0.1) | 1 (0.1) | 12 (1.7) |
| Dental caries | 2 (0.3) | 3 (0.4) | 1 (0.1) | 6 (0.9) |
| Dysphagia | 4 (0.6) | 2 (0.3) | 0 | 6 (0.9) |
| Dry mouth | 4 (0.6) | 1 (0.1) | 0 | 5 (0.7) |
| Gastrointestinal disorders, serious events | 3 (0.4) | |||
| Abdominal pain | – | – | – | 1 (0.1) |
| Inguinal hernia | – | – | – | 1 (0.1) |
| Peptic ulcer | – | – | – | 1 (0.1) |
Data cutoff date is March 30, 2018. "–" indicates not applicable
TEAE treatment-emergent adverse event
aEvents occurring in at least five patients; data are based on the Medical Dictionary for Regulatory Activities system organ class and preferred terms
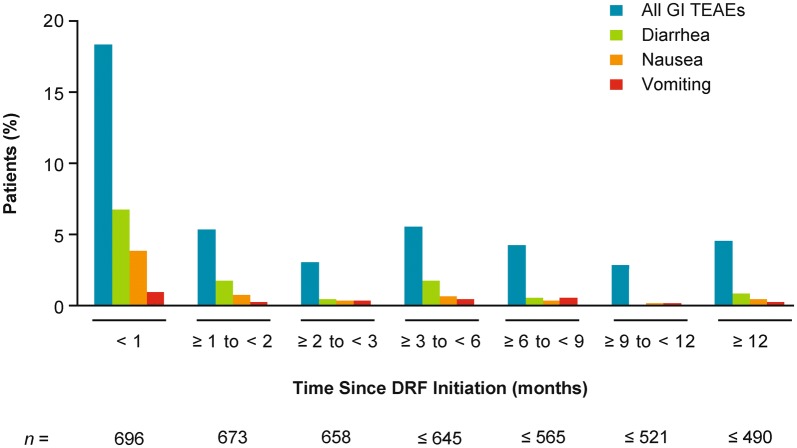
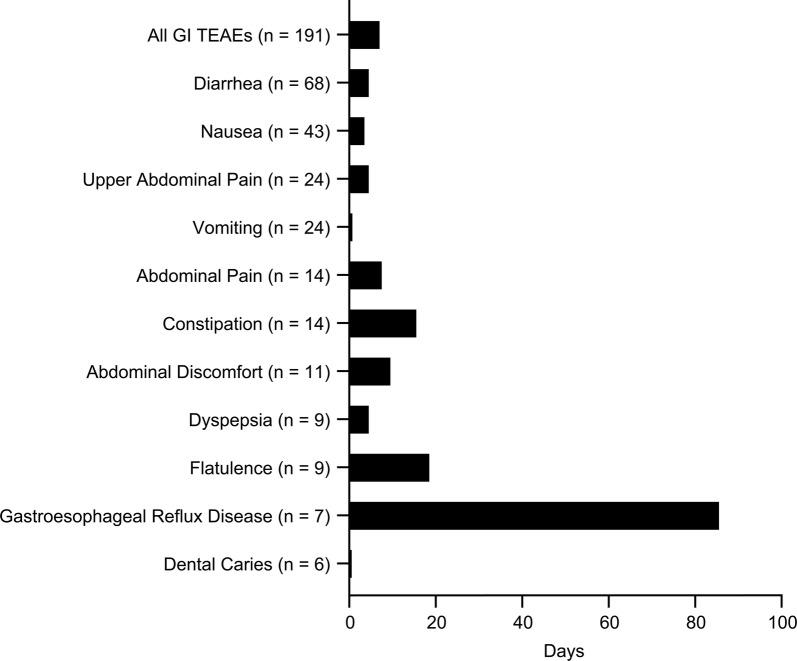
GI TEAEs occurred early in treatment, typically within 1 month of initiating DRF. Among the 214 patients with GI TEAEs with complete start and end dates recorded, 60.3% (129/214) experienced their first event at < 1 month, 9.8% (21/214) had first events from ≥ 1 to < 2 months, 5.1% (11/214) had events from ≥ 2 to < 3 months, 9.3% (20/214) had events from ≥ 3 to < 6 months, 7.5% (16/214) had events from ≥ 6 to < 9 months, 3.7% (8/214) had events from ≥ 9 to < 12 months, and 4.2% (9/214) had events at ≥ 12 months. Median (25th–75th percentile) time to onset for first GI TEAE was 19 (5–83) days. Incidence of overall and key individual GI events by time interval is shown in Fig. 2. Of patients who experienced GI TEAEs, events resolved in 88.8% (191/215) of patients with a median (10th–90th percentile) duration of 7.5 (1–87) days; median (10th–90th percentile) duration of diarrhea was 5 (1–63) days, nausea was 4 (1–86) days, upper abdominal pain 5 (1–94) days, and vomiting 1 (1–14) day. Durations of additional individual GI events are shown in Table 2 and Fig. 3.
Fig. 2.
GI TEAEs occurred early in treatment. Incidence of all GI TEAEs, diarrhea, nausea, and vomiting were reported by time interval. Patients with multiple events in a time interval were counted only once for that interval. Patients who experienced events in multiple time intervals were counted with each interval. DRF diroximel fumarate, GI gastrointestinal, TEAE treatment-emergent adverse event
Table 2.
Summary of duration of gastrointestinal TEAEs that resolved in patients in EVOLVE-MS-1
| TEAEs occurring in at least 2% of patients | Overall population, N = 696 | ||
|---|---|---|---|
| All events, n (%) | Events that resolveda, n (%) |
Duration in days, median (10th–90th percentile) | |
| Gastrointestinal TEAEs, all events | 215 (30.9) | 191 (88.8) | 7.5 (1–87) |
| Diarrhea | 75 (10.8) | 68 (90.7) | 5.0 (1–63) |
| Nausea | 47 (6.8) | 43 (91.5) | 4.0 (1–86) |
| Upper abdominal pain | 31 (4.5) | 24 (77.4) | 5.0 (1–94) |
| Vomiting | 25 (3.6) | 24 (96.0) | 1.0 (1–14) |
| Constipation | 24 (3.4) | 14 (58.3) | 16.0 (3–120) |
| Abdominal pain | 17 (2.4) | 14 (82.4) | 8.0 (1–52) |
| Flatulence | 15 (2.2) | 9 (60.0) | 19.0 (1–120) |
| Gastroesophageal reflux disease | 14 (2.0) | 7 (50.0) | 86.0 (1–167) |
Data cutoff date March 30, 2018
TEAE treatment-emergent adverse event
aOf events with complete start and end dates
Fig. 3.
Median duration of overall and individual GI TEAEs that resolved in the overall population. Events occurring in ≥ 5 patients were included. GI gastrointestinal, TEAE treatment-emergent adverse event
Patient Management
Patients who experienced GI TEAEs with DRF treatment were allowed to reduce their DRF dose temporarily and/or receive concomitant therapy to manage GI symptoms. GI TEAEs led to dose reduction in 1.9% (13/696) patients. Thirty-nine percent (84/215) of patients who experienced GI TEAEs received transient concomitant therapy to treat GI symptoms.
Discussion
Interim findings from the ongoing, 96-week, open-label EVOLVE-MS-1 study suggest that DRF is a well-tolerated treatment option for patients with RRMS. Patients in EVOLVE-MS-1 who reported GI TEAEs typically experienced events that were mild, transient (89% resolved), and of short duration (median, 7.5 days). Most events occurred within 1 month of treatment initiation. The timing and severity of GI TEAEs with DRF are similar to patterns observed in clinical trials of DMF [1, 2, 10]. However, the overall proportion of patients experiencing GI TEAEs (approximately 31%) and the extremely low discontinuation rate due to GI TEAEs (< 1%) over the 96-week treatment period in EVOLVE-MS-1 suggest that this novel oral fumarate has improved GI tolerability compared with DMF. The EVOLVE-MS-2 study will allow for a more complete evaluation of the GI tolerability profiles of DRF versus DMF.
Although the mechanisms driving GI tolerability of FAEs are unclear, there is evidence to suggest that subtle differences in FAEs could greatly affect tolerability. For example, recent studies have demonstrated that FAEs have differing irritant capacities based upon their chemical structure [16]. Lammintausta et al. further explored the irritant nature of FAEs by conducting skin patch testing [17]. Although the compounds tested in this series (including DMF) were all very similar in chemical structure, they observed a wide range of irritation associated with exposure to the compounds, providing evidence that the irritant nature of these compounds can be altered based upon minor changes to the fumarate ester structure. From a scientific rationale standpoint, DRF is well positioned to have an improved GI tolerability profile compared to DMF.
Release of a Less Irritating Promoiety: HES Versus Methanol
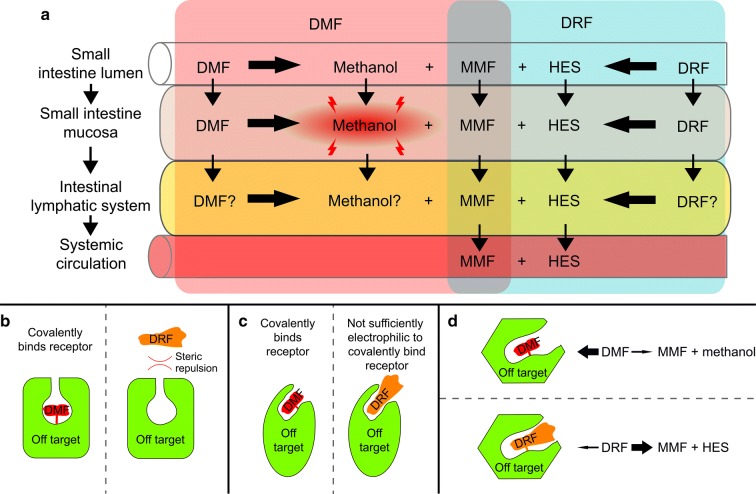
The first step in DMF metabolism, esterase hydrolysis, generates stoichiometric quantities of MMF and methanol in a 1:1 ratio. In contrast, in the first step of DRF metabolism, MMF and methanol are generated in approximately a 9:1 ratio, causing a significantly lower exposure of methanol with DRF treatment compared with DMF treatment (Fig. 1a, b). The release of methanol upon the first step of DMF metabolism may be a contributing factor to DMF-associated tolerability issues. At high systemic concentrations, methanol consumption is known to cause GI AEs [14, 18]. Formic acid is the main driver of methanol side effects and is generated by the enzymes alcohol dehydrogenase (ADH) and formaldehyde dehydrogenase during methanol metabolism [19, 20]. ADHs, depending upon the isoform, are exclusively expressed within the duodenum, small intestine, and liver (e.g., ADH1A or ADH4), or expressed in numerous tissues (ADH1C), whereas aldehyde dehydrogenases are highly expressed throughout most human tissues [21]. Therefore, locally elevated levels of methanol and the GI-irritating metabolite formic acid within the small intestine may subsequently play a role in the mechanism of GI issues and contribute to GI events (Fig. 4a). Because DRF generates significantly less methanol, this potential mechanism of irritation would be mitigated.
Fig. 4.
Hypotheses for enhanced GI tolerability of DRF. Diester fumarates, such as DMF and DRF, are small-molecular-weight drugs capable of modifying the function of several proteins. These interactions can be considered on target when contributing to the efficacy of the drug and off target when contributing to the unwanted side effects, such as GI events. a DMF hydrolyzes to MMF and methanol (a known GI irritant). This localized methanol production within the GI tract may contribute to DMF GI tolerability issues. In contrast, DRF mainly hydrolyzes to HES (a biologically inert metabolite) and MMF. b DRF is a larger molecule than DMF; therefore, DRF may not fit into as many protein-binding pockets as DMF because of steric clashes between DRF and off-target proteins. c DRF could be less electrophilic; therefore, DRF may not react with certain off-target proteins compared with DMF. d The rate of hydrolysis to MMF may be slightly faster for DRF compared with DMF, thereby lowering the amount of DRF exposure to the GI tract and causing less covalent binding to off-target proteins compared with DMF. DMF dimethyl fumarate, DRF diroximel fumarate, GI gastrointestinal, HES 2-hydroxyethyl succinimide, MMF monomethyl fumarate
To put the systemic exposure of methanol after taking DMF or DRF into context, pharmacokinetic studies have demonstrated that blood levels of methanol and formic acid in patients taking DMF or DRF were either undetectable or significantly below the levels known to cause any systemic adverse effects. Consumption of compounds containing methyl esters is common in many diets. For example, one of the most frequently consumed molecules containing a methyl ester is aspartame, the artificial sweetener in soft drinks and other food items. The US Food and Drug Administration has set the acceptable maximal daily intake of aspartame at 50 mg/kg [22]. For a 70-kg person, that would be the equivalent of approximately 18–22 cans of 12-oz diet soft drinks. The total amount of methanol generated from a 240 mg dose of DMF is equivalent to that generated after consumption of approximately five to six cans of aspartame-containing 12-oz diet soft drinks [23]. In summary, even though a local elevated concentration of methanol within the GI tract may be causing GI tolerability issues, the overall exposure of methanol from DMF treatment is well below the acceptable safe limits for methanol ingestion and does not cause any systemic issues in patients.
DRF Is Less Reactive Toward Pre-Systemic Off-Target Proteins Compared with DMF
The small molecular weight (144.1 g/mol) of DMF and therefore small physical size allow it to access numerous receptors and proteins within the GI tract and GI wall [11, 24–26]. These interactions are beneficial when they are on target, thereby contributing to the efficacy of the drug. Alternatively, if they are off target, they could cause GI issues such as diarrhea, nausea, and vomiting. There is ambiguity surrounding which cell type or receptor is driving the GI events associated with FAE treatment. DMF could be reacting with the microbiota, analogous to how antibiotics cause GI events, or it could be reacting with pre-systemic proteins/receptors, analogous to how acarbose or opioids modulate GI function [27, 28]. It is hypothesized that DRF will have fewer off-target interactions with GI receptors/proteins (located either in the microbiota or GI tract) compared with DMF because of potential differences in molecular size, electrophilicity, and/or the half-life of the molecules (Fig. 4b–d).
A key determinant of whether a molecule can interact with a protein or receptor is its ability to physically fit into a binding cleft; this is governed by the van der Waals radii of the molecule (which are directly proportional to the size and number of atoms in the compound) and whether the filled chemical space bumps into and has steric clashes with the structure of the protein or receptor. DRF is almost double the molecular weight of DMF and occupies a significantly larger molecular volume; therefore, it is likely that DRF occupies fewer proteins/receptors (including off-target proteins/receptors) than DMF because of steric clashes. In addition to the increase in volume, DRF has significantly more molecular complexity than DMF because the methyl group of DMF is substituted with HES for DRF. This substitution adds five carbons and three heteroatoms (one nitrogen and two oxygen atoms) to DRF, significantly altering the hydrophobic and hydrophilic requirements for an energetically favorable binding to a target. Consequently, DRF has more restraints on the proteins with which it can form complexes compared with DMF and therefore likely reacts with fewer off-target proteins (Fig. 4b).
The putative mechanism of action for fumarate esters is through a Michael addition of a protein nucleophile, typically a cysteine thiol functional group, to the alpha-beta unsaturated ester of the fumarate ester (DRF, DMF, or MMF). The reactivity of the alkene (carbon–carbon double bond) dictates which thiols within the proteome it will covalently modify. This is governed by the interplay between the chemical structure of the fumarate ester and the protein microenvironment to which it binds. Based on the chemical structure differences between DRF and DMF, it is possible that DRF is less electrophilic (i.e., has a less reactive alkene) and therefore would react with fewer off-target receptors (Fig. 4c).
Additionally, the rate of hydrolysis to MMF may be a contributing factor for GI tolerability. The half-life of the reaction of DMF with physiologic thiols, such as glutathione, is approximately 9 min [29]. Given the rapid, irreversible covalent modification of proteins by DMF, the biologic effects of DMF are established within the first minutes of exposure. The systemic pharmacokinetic profiles of DMF and DRF are well established and have demonstrated bioequivalent exposure of MMF following hydrolysis. However, if the pre-systemic rate of hydrolysis to MMF is slightly faster for DRF compared with DMF, this may reduce the time of exposure of DRF to off-target proteins compared with DMF and possibly mitigate GI side effects (Fig. 4d). In summary, DRF may have fewer off-target interactions with GI receptors/proteins (located either in the microbiota or GI tract) compared with DMF because of potential differences in molecular size, electrophilicity, and/or half-life of the molecules.
Conclusions
DRF is a novel oral fumarate with a distinct chemical structure in development for patients with relapsing forms of MS. It is hypothesized that DRF may produce a differentiated GI tolerability profile through less direct irritation within the GI tract (due to formation of less methanol) and possibly because of less reactivity with off-target receptors as a result of its distinct chemical structure. Clinical implications of these hypotheses are unknown; however, clinical data to date suggest DRF has favorable GI tolerability, particularly when considering the tolerability profiles of other FAEs such as DMF. Importantly, the GI tolerability of DRF compared with DMF is being evaluated in the head-to-head EVOLVE-MS-2 trial; in addition to real-world data, this will further inform the GI tolerability profile of DRF.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the EVOLVE-MS-1 study patients, investigators, and staff.
Funding
This study was funded by Alkermes Inc. (Waltham, MA, USA) and Biogen (Cambridge, MA, USA). Rapid service and open access fees were funded by Biogen. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Biogen provided funding for medical writing support in the development of this manuscript; Susan Chow, PhD, from Excel Scientific Solutions wrote sections of the first draft of the manuscript based on input from authors, and Miranda Dixon from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. The authors had full editorial control of the manuscript and provided their final approval of all content.
Disclosures
Michael J. Palte is a full-time employee of and holds stock/stock options in Biogen. At the time of the study Angela Wehr was an employee of Alkermes. Angela Wehr is a current employee of Sage Therapeutics and holds stock/stock options in Alkermes Inc. and Biogen. Mark Tawa is a full-time employee of and holds stock/stock options in Alkermes Inc. Kristopher Perkin is a full-time employee of and holds stock/stock options in Alkermes Pharma Ireland Ltd. Richard Leigh-Pemberton is a full-time employee of and holds stock/stock options in Alkermes Inc. Jerome Hanna is a full-time employee of and holds stock/stock options in Biogen. Catherine Miller is a full-time employee of and holds stock/stock options in Biogen. Natasha Penner is a full-time employee of and holds stock/stock options in Biogen.
Compliance with Ethics Guidelines
The EVOLVE-MS-1 study protocol was approved by central and local ethics committees at each study site (see supplementary material for full details) and is being conducted in accordance with the International Council on Harmonization guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written, informed consent.
Data Availability
EVOLVE-MS-1 was registered with ClinicalTrials.gov (NCT02634307). Study data will be shared in accordance with applicable regulations and laws.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9736163.
References
- 1.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 2.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 3.Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler. 2017;23(2):253–265. doi: 10.1177/1352458516649037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: a multicenter retrospective observational study (STRATEGY) Mult Scler Relat Disord. 2018;22:27–34. doi: 10.1016/j.msard.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Kresa-Reahl K, Repovic P, Robertson D, Okwuokenye M, Meltzer L, Mendoza JP. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther. 2018;40(12):2077–2087. doi: 10.1016/j.clinthera.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Parodi B, Rossi S, Morando S, et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015;130(2):279–295. doi: 10.1007/s00401-015-1422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Assmann JC, Krenz A, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Investig. 2014;124(5):2188–2192. doi: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. TECFIDERA summary of product characteristics. https://ec.europa.eu/health/documents/community-register/2018/20180528141209/anx_141209_en.pdf. Accessed 19 Feb 2019.
- 10.Fox EJ, Vasquez A, Grainger W, et al. Gastrointestinal tolerability of delayed-release dimethyl fumarate in a multicenter, open-label study of patients with relapsing forms of multiple sclerosis (MANAGE) Int J MS Care. 2016;18(1):9–18. doi: 10.7224/1537-2073.2014-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mrowietz U, Morrison PJ, Suhrkamp I, Kumanova M, Clement B. The pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol Sci. 2018;39(1):1–12. doi: 10.1016/j.tips.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Vollmer B, Ontaneda D, Bandyopadhyay A, et al. Discontinuation and comparative effectiveness of dimethyl fumarate and fingolimod in 2 centers. Neurol Clin Pract. 2018;8(4):292–301. doi: 10.1212/CPJ.0000000000000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirabella M, Prosperini L, Lucchini M, et al. Safety and efficacy of dimethyl fumarate in multiple sclerosis: an Italian, multicenter, real-world study. CNS Drugs. 2018;32(10):963–970. doi: 10.1007/s40263-018-0543-3. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Methanol: systemic agent U.S. Department of Health and Human Services. https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750029.html. Accessed 23 Apr 2019.
- 15.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Haan P, von Blomberg-van der Flier BM, de Groot J, Nieboer C, Bruynzeel DP. The risk of sensibilization and contact urticaria upon topical application of fumaric acid derivatives. Dermatology. 1994;188(2):126–130. doi: 10.1159/000247116. [DOI] [PubMed] [Google Scholar]
- 17.Lammintausta K, Zimerson E, Winhoven S, et al. Sensitization to dimethyl fumarate with multiple concurrent patch test reactions. Contact Dermat. 2010;62(2):88–96. doi: 10.1111/j.1600-0536.2009.01662.x. [DOI] [PubMed] [Google Scholar]
- 18.Moon CS. Estimations of the lethal and exposure doses for representative methanol symptoms in humans. Ann Occup Environ Med. 2017;29:44. doi: 10.1186/s40557-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liesivuori J, Savolainen H. Methanol and formic acid toxicity: biochemical mechanisms. Pharmacol Toxicol. 1991;69(3):157–163. doi: 10.1111/j.1600-0773.1991.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 20.Kraut JA, Mullins ME. Toxic alcohols. N Engl J Med. 2018;378(3):270–280. doi: 10.1056/NEJMra1615295. [DOI] [PubMed] [Google Scholar]
- 21.The Human Protein Atlas. Alcohol dehydrogenases. https://www.proteinatlas.org/search/alcohol+dehydrogenase. Accessed 23 Apr 2019.
- 22.United States Food and Drug Administration. Additional information about high-intensity sweetners permitted for use in food in the United States U.S. Department of Health and Human Services. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm. Accessed 23 Apr 2019.
- 23.Tsang WS, Clarke MA, Parrish FW. Determination of aspartame and its breakdown products in soft drinks by reverse-phase chromatography with UV detection. J Agric Food Chem. 1985;33(4):734–738. doi: 10.1021/jf00064a043. [DOI] [Google Scholar]
- 24.Haupt VJ, Daminelli S, Schroeder M. Drug promiscuity in PDB: protein binding site similarity is key. PLoS One. 2013;8(6):e65894. doi: 10.1371/journal.pone.0065894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Glehn F, Dias-Carneiro RPC, Moraes AS, et al. Dimethyl fumarate downregulates the immune response through the HCA2/GPR109A pathway: implications for the treatment of multiple sclerosis. Mult Scler Relat Disord. 2018;23:46–50. doi: 10.1016/j.msard.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Hosseini A, Masjedi A, Baradaran B, et al. Dimethyl fumarate: regulatory effects on the immune system in the treatment of multiple sclerosis. J Cell Physiol. 2019;234(7):9943–9955. doi: 10.1002/jcp.27930. [DOI] [PubMed] [Google Scholar]
- 27.Makins R, Ballinger A. Gastrointestinal side effects of drugs. Expert Opin Drug Saf. 2003;2(4):421–429. doi: 10.1517/14740338.2.4.421. [DOI] [PubMed] [Google Scholar]
- 28.Leong RW, Chan FK. Drug-induced side effects affecting the gastrointestinal tract. Expert Opin Drug Saf. 2006;5(4):585–592. doi: 10.1517/14740338.5.4.585. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt TJ, Ak M, Mrowietz U. Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cysteine—preparation of S-substituted thiosuccinic acid esters. Bioorg Med Chem. 2007;15(1):333–342. doi: 10.1016/j.bmc.2006.09.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
EVOLVE-MS-1 was registered with ClinicalTrials.gov (NCT02634307). Study data will be shared in accordance with applicable regulations and laws.