Abstract
Introduction
The new direct acting antiviral (DAA) therapies are able to effectively treat chronic hepatitis C (CHC). This study elicited the preferences of CHC patients for treatment attributes of new DAAs.
Methods
An online discrete choice experiment survey was designed to collect data from adult CHC patients in the USA, UK, France, Germany, Spain, and Italy. Patients were asked to choose from alternative hypothetical DAA options, defined by differing levels of nine attributes [i.e., treatment duration, tablet count and packaging, cure rate, required office visits when on treatment, modifications to statins or to proton pump inhibitors (PPIs), and risks of diarrhea, headache and nausea]. Logistic regression was used to assess preference for the treatment options.
Results
A total of 328 patients with CHC completed the survey (USA, n = 227; European countries, n = 101), with a mean age of 47.7 years (SD = 14.4) and an average 11.2 years since CHC diagnosis; 51% of patients were female. More than half (60%) of the patients had treatment for CHC. Patients significantly preferred a DAA regimen with higher cure rate, shorter treatment duration, lower risks of diarrhea, headache, and nausea (all p < 0.001), reduced need for office visits when on treatment (p = 0.044), and without requiring dose reduction or timing change in PPIs (p = 0.032). Tablet counts were not found to be statistically significant.
Conclusion
Given the overall high cure rates of new DAAs, CHC patients’ preferences for therapy may be influenced by treatment attributes other than cure rates and tolerability. Treatments that are more convenient and require less disruption to their daily life (e.g., shorter treatment duration, no modification in PPI use, and fewer office visits when on treatment) are important to patients with CHC and should be considered when making treatment decisions.
Funding
AbbVie.
Keywords: Chronic hepatitis C virus, DDA, Direct acting antiviral therapies, HCV, Infectious diseases, Patient preferences
Introduction
Hepatitis C is a type of blood-borne viral infection [1] affecting more than 185 million people worldwide [2, 3]. The majority (75–85%) of patients infected with hepatitis C virus (HCV) develop chronic hepatitis C (CHC), which is associated with an increased risk of extrahepatic complications such as type 2 diabetes mellitus, cardiovascular disease, and chronic kidney disease, as well as many potentially life-threatening hepatic complications [1, 2, 4].
The main goal in the treatment of CHC is to achieve sustained virologic response (SVR), defined as an undetectable or unquantifiable level of HCV RNA at 12–24 weeks after completing treatment [5, 6]. When patients achieve sustained virologic response, they are considered clinically cured of CHC. Over the past decade, the use of direct acting antiviral agents (DAAs) combined with pegylated interferon alpha and ribavirin (P/R) has significantly advanced the management of CHC [5, 7, 8]. Since 2013, the approval of the first all-oral interferon-free DAAs has expanded the treatment options for CHC providing ease of use, favorable tolerability, and cure rates of 90% or higher in some HCV genotypes [6, 7, 9]. More recently, newer-generation all-oral interferon-free DAAs have further extended these clinical benefits to CHC patients with all HCV genotypes [7].
While sustained virologic response (i.e., cure rate) and tolerability remain the core outcomes of interest, given comparable high cure rates demonstrated both in clinical trials [10–13] and in the real-world setting [14–16], it is of value to know what other attributes, such as dosing schedule, may influence therapy decisions from the patient’s perspective. These convenience-related attributes, which may interfere with a patient’s daily routine, might very well play a key role given their potentially positive impact on adherence and clinical outcomes [16]. Given the increasing focus on patient-centered care, it is important to appreciate the value that patients place on CHC treatment attributes pertaining to benefits and risks to allow other key stakeholders (e.g., physicians, payers, policymakers) to take into account the patients’ perspective and more meaningfully contribute to shared decision-making [17, 18]. To shed light on this issue, we conducted a multi-country survey study eliciting the preferences of CHC patients for attributes of new DAAs.
Methods
Study Population
Study participants consisted of adult patients with self-reported CHC in the USA and five European countries (UK, France, Germany, Spain, and Italy). All patients were existing panel members of a well-established market research firm, Survey Sampling Internal (now Dynata), with a global presence for healthcare surveys. To be eligible for the study, patients were required to be at least 18 years old, have a self-reported diagnosis of HCV infection based on one of the following diagnostic assessments (blood test, liver biopsy, liver ultrasound scan, computed tomography scan, magnetic resonance imaging, or Home Access Hepatitis C Check kit), and have at least 6 years of school education. The targeted sample size was 300 participants, based on the empirical recommendation by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Research Practices for Conjoint Analysis Task Force [19]. Each study participant consented to participation in the study prior to proceeding to complete the survey questions and was compensated with panel points in the amount that was equivalent to $5 upon completion of the survey. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments and was granted an exemption status from a full review by the New England Institutional Research Board in December 2016.
Survey Design
A cross-sectional survey was designed to collect information from USA and European patients with CHC on the participants’ characteristics, CHC-related medical history, and preferences for new all-oral CHC treatments. The online survey was pretested through phone interviews with simultaneous online screen-sharing with five patients in the USA to assess the clarity of the questions and the relevance of the treatment attributes (described below) and to evaluate user experience. The final version of the survey was based on the feedback received during these interviews. The survey was developed in English and subsequently translated into French, German, Spanish, and Italian. Translated versions of the survey were reviewed by native speakers to ensure clarity and consistency of the questions. Survey test launch was conducted with data collection from two patients meeting the same set of eligibility criteria for survey in each of the participating countries (a total of ten patients) to confirm the clarity prior to the full launch for data collection.
Participant Characteristics
When a patient received the invitation to participate in the survey through email, the patient was asked to review the background for the study intent and confirmed voluntary participation before going through the eligibility criteria. After confirming eligibility for participation, the first part of the survey collected information on patient demographics (age, gender, country of origin, level of education, and employment status), comorbidities, CHC medical history (HCV genotype, duration of disease, prior injectable recreational drug use), and treatment history (treatment experience, cure status as reported by a physician, proton pump inhibitors [PPI] and statin use, and the Patient Activation Measure [PAM-13]) [20]. PAM-13 measures a patient’s ability to manage own health and healthcare decisions [20, 21].
Patient Preferences
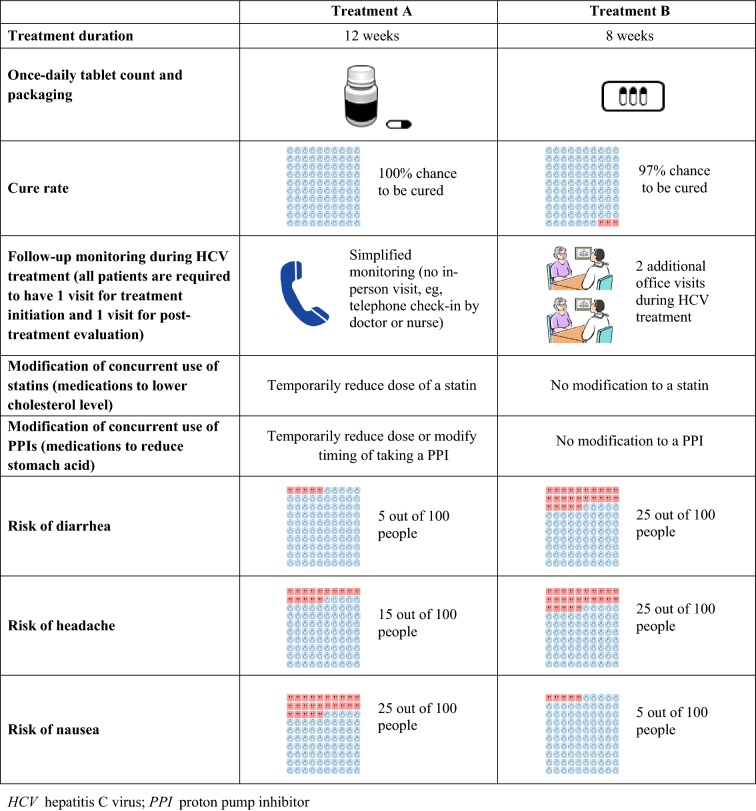
The second part of the survey consisted of questions pertaining to patient preferences for a new oral DAA treatment, designed using the discrete choice experiment (DCE) approach, a well-established and widely used methodology for preference elicitation [22]. The DCE design is frequently used to elicit patient preferences for hypothetical treatment scenarios with alternative options (e.g., treatment A vs. treatment B). Different scenarios are presented in the form of choice cards describing competing levels of attributes for each hypothetical treatment, and survey participants are asked to choose their preferred scenario. An example of choice card used in this study is presented in Fig. 1.
Fig. 1.
Example of choice card
Treatment attributes and levels used in the choice cards were determined on the basis of a targeted literature review of the characteristics of new DAAs, either approved or in clinical development at the time of this study in early 2017. The selected attributes and the associated levels reflected the factors that were most relatable to HCV patients and most likely played a role in patients’ preference for a treatment. The relevance and importance of the attributes and levels were confirmed by the HCV patients when being debriefed through survey pretests. Broadly, the nine selected attributes can be classified as relating to efficacy, convenience, co-therapy management, and common mild side effects for safety/tolerability, with each attribute having three to four levels (Table 1).
Table 1.
Attributes and levels
| Attributes | Levels |
|---|---|
| Efficacy | |
| Cure rate | 95%; 97%; 100% |
| Convenience | |
| Once-daily tablet count and packaging |
1 tablet from a prescription bottle 1 tablet in a single-dose blister pack 3 tablets in a single-dose blister pack |
| Duration of treatment (weeks) | 8; 12; 16; 24 |
| Office visits for HCV treatment (all patients required to have 1 visit for treatment initiation and 1 visit for post-treatment viral evaluation) |
Simplified monitoring during HCV treatment (e.g., telephone check-in by doctor or nurse) One additional office visit during HCV treatment Two additional office visits during HCV treatment |
| Co-therapy management | |
| Modification of concurrent statin usea |
No modification to a statin Temporarily reduce dose of a statin Temporarily stop taking a statin Switch to a different medication |
| Modification of concurrent PPI useb |
No modification to a PPI Temporarily reduce dose or modify timing of taking a PPI Temporarily stop taking a PPI Switch to a different medication |
| Mild common side effects | |
| Risk of diarrhea | 5%; 15%; 25% |
| Risk of headache | 5%; 15%; 25%; 35% |
| Risk of nausea | 5%; 15%; 25% |
HCV hepatitis C virus, PPI proton pump inhibitor
aThe levels “temporarily reduce dose of a statin” and “temporarily stop taking a statin” were pooled in the analysis, as they both reflect similar, temporary modifications of use
bThe levels “temporarily reduce dose or modify timing of taking a PPI” and “temporarily stop taking a PPI” were pooled in the analysis, as they both reflect similar temporary modifications of use
Seventy-two choice cards were generated using the SAS macro DCE package (SAS Institute, Cary, NC, USA) to achieve an orthogonal, balanced, and efficient design [17], assigning two hypothetical treatment scenarios to each choice card (Fig. 1). The 72 choice cards were randomly divided into eight blocks of nine cards each and approximately the same numbers of patients were randomly assigned to respond to one of the blocks. Each block also included two extra choice cards for assessing the test–retest internal validity of patients’ responses. These two choice cards had the same pair of treatment options but shown in reverse order (i.e., treatment A in one choice card was treatment B in the other choice card), with one treatment designed to be a dominant preferred option over the other treatment.
To ensure that all patients had sufficient background knowledge on CHC treatments and on requirements in the tasks for making a preferred choice decision, a brief tutorial was provided. The tutorial summarized the typical treatment journey of a CHC patient, described the attributes and associated levels of the new oral CHC treatments considered in the survey in plain language, and provided examples of choice cards to familiarize patients with the process of selecting treatment scenarios.
Statistical Analyses
Patient demographics, clinical characteristics, and treatment history were summarized using counts and proportions for categorical variables, and means and standard deviations for continuous variables. Multivariable logistic regression models with generalized estimating equations were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) and used to obtain point estimates and 95% confidence intervals for the preference weights. Attributes were modeled using dummy coding. Coefficients from the regression analysis were also used to assess the relative importance of treatment attributes in patient preferences.
Subgroup analyses were conducted in patients who self-reported as former or current users of injection drugs, given its high prevalence in HCV patients. With the test–retest choice card design, patients who made logically consistent choice selections between these two cards were considered to have passed the test–retest assessment. The proportion of patients failing the test–retest assessment of internal validity was documented, and sensitivity analyses limited to those patients who passed the test–retest assessment of internal validity were also conducted.
Results
Characteristics of Survey Participants
Data were collected from 328 adult patients with CHC, 227 (69.2%) from the USA and 101 (30.8%) from the five European countries (20 each from the UK, Germany, Spain, and France, and 21 from Italy) in January through March 2017. On average, it took 19 min for a patient to complete the survey. Patient characteristics are summarized in Table 2. Patients had a mean age of 47.7 years and 51.2% were female. Most patients had more than 12 years of education (68.6%) and 53.7% of the patients were employed. Approximately half of all patients (49.4%) were either former or current users of injectable recreational drugs. Among patients who knew their genotype (62.8%), 35.9% were genotype 1, 24.8% were genotype 2, 27.7% were genotype 3, 10.2% were genotype 4, 1.5% were genotype 5, and none were genotype 6. The self-reported average time since CHC diagnosis was 11.2 years.
Table 2.
Characteristics of survey participants
| CHC patientsa N = 328 |
|
|---|---|
| Age (years), mean ± SD | 47.7 ± 14.4 |
| Female, n (%) | 168 (51.2) |
| Country/region, n (%) | |
| USA | 227 (69.2) |
| European countriesb | 101 (30.8) |
| Highest level of formal education, n (%) | |
| Completed 6–12 years of education | 103 (31.4) |
| Completed > 12 years of education | 225 (68.6) |
| Employment status, n (%) | |
| Employed | 176 (53.7) |
| Not working | 140 (42.7) |
| Student | 8 (2.4) |
| Declined to answer | 4 (1.2) |
| HCV genotype among patients reporting genotype (n = 206), n (%)c | |
| Genotype 1 | 74 (35.9) |
| Genotype 2 | 51 (24.8) |
| Genotype 3 | 57 (27.7) |
| Genotype 4 | 21 (10.2) |
| Genotype 5 | 3 (1.5) |
| Genotype 6 | 0 (0.0) |
| Time since HCV diagnosis, n (%) | |
| Within the last 1 year | 18 (5.5) |
| More than 1 year, but less than 3 years | 43 (13.1) |
| Between 3 and 5 years | 40 (12.2) |
| More than 5 years | 227 (69.2) |
| Time since HCV diagnosis (years), mean ± SD | 11.2 ± 7.2 |
| Treatment history, n (%) | |
| Treatment-naïve | 131 (39.9) |
| Treatment-experienced | 197 (60.1) |
| Told cured by doctor, n (%) | 163 (49.7) |
| Selected key chronic comorbidities, n (%) | |
| Anxiety/depression | 149 (45.4) |
| Cardiovascular disease (e.g., ischemic heart disease) | 29 (8.8) |
| Cirrhosis | 47 (14.3) |
| Diabetes/insulin resistance | 59 (18.0) |
| Fibrosis | 33 (10.1) |
| Gastroesophageal reflux disease | 77 (23.5) |
| Hepatitis B virus infection | 32 (9.8) |
| HIV infection/AIDS | 23 (7.0) |
| Kidney disease | 24 (7.3) |
| Liver cancer | 17 (5.2) |
| Liver transplant | 20 (6.1) |
| Injectable recreational drug use, n (%) | |
| Never used | 154 (47.0) |
| Former/current user | 162 (49.4) |
| Decline to answer | 12 (3.7) |
| Concurrent medications, n (%) | |
| Receiving PPIs | 59 (18.0) |
| Receiving statins | 67 (20.4) |
| Patient motivation level (by PAM-13), n (%) | |
| Level 1 (not yet believe patients have active/important role) | 27 (8.4) |
| Level 2 (lack confidence/knowledge to take action) | 34 (10.6) |
| Level 3 (beginning to take action) | 148 (46.0) |
| Level 4 (maintaining behavior over time) | 113 (35.1) |
HCV hepatitis C virus, HIV human immunodeficiency virus, AIDS acquired immunodeficiency syndrome, PPI proton pump inhibitor
aInclude patients from the USA and European countries
bUK, France, Germany, Spain, and Italy
cCounts for categorical variables may not sum to 328 because of missing data or patients declining to answer certain questions
More than half of the patients (n = 197, 60.1%) had prior exposure to or were currently on CHC treatment as of their time of participation in this study in early 2017 (Table 2). Of those who were treatment-experienced, 31.0% (n = 61) had exposure to both interferon-containing and interferon-free treatments and one-third only received interferon-free treatments (n = 68, 34.5%). The most frequently reported chronic comorbidities included anxiety/depression (45.4%), cirrhosis or fibrosis (24.4%), gastroesophageal reflux disease (23.5%), and diabetes/insulin resistance (18.0%). Approximately 18% and 20% of patients were on PPIs and statins, respectively. At the time of the survey, approximately half of the patients were told by their doctors that they were cured of CHC (49.7%). The majority of the patients (81.1%) were motivated, either beginning to take action or having maintained behavior over time in managing their own health. Among the participating patients, 83.2% patients provided consistent responses to the test–retest questions.
Patient Preferences for HCV Treatment
CHC treatment attributes significantly and strongly preferred by patients were higher cure rate, lower risks for common mild side effects including diarrhea, headache, and nausea, and shorter treatment duration (all p < 0.01) (Table 3). In addition, treatments requiring only one additional visit while on CHC treatment were favored over those requiring two additional visits (p = 0.044). Treatments requiring no modification to PPIs were favored over those requiring a temporary dose reduction, change in timing, or temporarily stop in PPI (p = 0.032) though patients did not seem to mind changing to a different PPI. Of all attributes considered, two of them were not significant in patients’ preference decision towards a new oral CHC treatment: patients were found to be indifferent between having one tablet or three tablets in terms of pill count and for having modification or change to a different statin vs. no change in statin.
Table 3.
Patient preferences for treatment attributes (overall populationa)
| Treatment attributes | Estimate | 95% CI | p value |
|---|---|---|---|
| Efficacy | |||
| Cure rate (per % change) | 0.311 | (0.267, 0.356) | < 0.001* |
| Convenience | |||
| Treatment duration (per week) | |||
| 8 weeks vs. 12 weeks | 0.255 | (0.090, 0.419) | 0.002* |
| 16 weeks vs. 12 weeks | − 0.061 | (− 0.219, 0.097) | 0.447 |
| 24 weeks vs. 12 weeks | − 0.321 | (− 0.505, − 0.138) | < 0.001* |
| Once-daily tablet count and packaging | |||
| 1 tablet from a prescription bottle vs. 1 tablet in a single-dose blister pack | 0.090 | (− 0.035, 0.216) | 0.159 |
| 3 tablets in a single-dose blister pack vs. 1 tablet in a single-dose blister pack | − 0.034 | (− 0.171, 0.103) | 0.624 |
| Visits during HCV treatments | |||
| Simplified monitoring (no in-person visit, e.g., telephone checking in) vs. two additional office visits | 0.131 | (− 0.036, 0.298) | 0.124 |
| One additional office visit vs. two additional office visits | 0.170 | (0.005, 0.336) | 0.044* |
| Co-therapy management | |||
| Modification of statins | |||
| Temporarily reduce dose or stop taking vs. no modification | − 0.105 | (− 0.249, 0.039) | 0.152 |
| Switch to a different medication vs. no modification | − 0.129 | (− 0.296, 0.038) | 0.131 |
| Modification of PPIs | |||
| Temporarily reduce dose/timing or stop taking vs. no modification | − 0.171 | (− 0.326, − 0.015) | 0.032* |
| Switch to a different medication vs. no modification | − 0.125 | (− 0.298, 0.047) | 0.155 |
| Mild common side effects | |||
| Risk of adverse events (per % change) | |||
| Diarrhea | − 0.033 | (− 0.040, − 0.025) | < 0.001* |
| Headache | − 0.028 | (− 0.034, − 0.022) | < 0.001* |
| Nausea | − 0.028 | (− 0.036, − 0.019) | < 0.001* |
aAll patients from the USA and the five European countries (UK, France, Germany, Spain, and Italy)
*p value < 0.05
Among the subgroup of patients who self-reported as former or current injectable drug users, treatment attributes preferred were generally similar to the overall patients, with strong preference for higher cure rate, lower side effect risks, and shorter treatment duration. Among these patients, treatments requiring simplified monitoring without in-person office visit (p = 0.023) or only one additional office visit (p = 0.084) were preferred over those requiring two additional office visits.
As a sensitivity analysis, the model was also run using data from patients who passed the test–retest questions (N = 273; 83.2%). The results were consistent except that all coefficient estimates became stronger and that no in-person visits became also significantly favored over two additional office visits (p = 0.026), along with favoring one additional visit over two additional office visits (p = 0.009).
Attributes Relative Importance
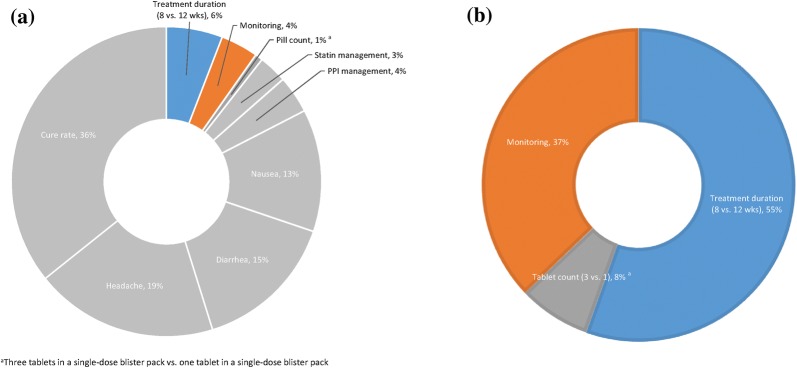
The relative importance of the treatment attributes is illustrated in Fig. 2. Overall, cure rate had the highest impact on HCV treatment choice (36%), followed by side effects (13–19%), statin and PPI medication management (7%), treatment duration of 8 weeks instead of 12 weeks (6%), and monitoring during treatment (4%). Pill count had the least impact on preference (1%). Altogether, convenience attributes were valued more than co-therapy management (11% vs. 7%). All other attributes held equal, among the convenience attributes, the 8-week treatment duration was the most valued by patients when compared to the 12-week treatment duration (55%), followed by monitoring involving one fewer visit when compared to two additional office visits (37%), and tablet counts for 3 vs. 1 (8%), although the coefficient of pill counts indicates that this attribute does not significantly impact patients’ choices in therapies. When we focus entirely on non-clinical attributes, i.e., treatment duration and pill counts, 87 out of 100 patients would prefer the 8-week treatment duration when compared to the 12-week treatment duration (87%). Thirteen percent of patients would prefer tablet counts, although the coefficient of pill counts indicates that it is not a statistically significant factor in patients’ treatment choice decisions for HCV.
Fig. 2.
Relative importance of treatment attributes. a Relative importance of all attributes for all-oral HCV treatment choice. b Relative importance of convenience attributes
Discussion
The treatment of CHC has undergone significant advancement over the past decade. In particular, the cure rate with new DAAs has climbed to over 90% across genotypes, with some of the newest DAAs achieving a cure rate of 100% in certain genotypes [9, 23, 24]. Given that the difference in cure rate for the newest DAAs is relatively small, other treatment characteristics may in turn have a substantial impact on which treatment patients may prefer. This study found that attributes in each of the four categories, i.e., efficacy, safety/tolerability, co-therapy management, and convenience, can play a significant role in patient’s preference decisions for CHC treatment. Not surprisingly, the most important treatment attributes were efficacy (i.e., cure rate) and safety/tolerability (i.e., risks of common mild side effects). Cure rate identified as the attribute of a DAA that CHC patients value the most is in line with previous studies [16, 25–28].
Among the remaining attributes for convenience and co-therapy management in the present study, treatment duration (i.e., 8 weeks vs. 12 weeks) was found to be the most important characteristic in preference decision, followed by preferring no modification to temporarily reducing dose/timing or stopping taking PPI, and having one, instead of two, additional office visits. Among all attributes and levels considered, having three tablets once daily vs. one tablet once daily was the least important and not a significant factor in patients’ preference for a CHC treatment. With all other attributes held equal and if patients were to choose between the two non-clinical attributes of treatment duration and tablet counts, 87 out of 100 patients will choose a therapy with a shorter treatment duration of 8 weeks vs. 12 weeks.
The findings on the monitoring attribute were also worth noting. While patients strongly preferred having one additional office visit over two additional office visits during their HCV treatment, the least preferred option was having no in-person office visits at all and only talking with a physician or a nurse over the phone. We speculate that it is possible while patients appreciate the opportunity to reduce interruptions to their daily life (e.g., due to an additional visit to their physician, instead of two), they feel more comfortable with not altogether eliminating in-person physician visits while on treatment. This could be a reasonable consideration, given that many patients with CHC had various types of comorbidities. In our study, nearly half of the patients reported having anxiety or depression. Patients who had co-therapy such as statin or PPI to manage their blood cholesterol level or gastric reflux problem could require modification to those therapies and thus might also prefer to see their physician to ensure their treatments are being managed appropriately. Interestingly, patients who self-reported as current or previous injection drug users had stronger preferences for having none or one fewer visit rather than two visits, suggesting that these patients may prefer fewer in-person visits and consider phone-based monitoring sufficient for management of their HCV treatment.
In a treatment landscape where an increasing number of new pan-genotypic DAAs may offer the same or similar high cure rates, policymakers’ consideration for insurance coverage and reimbursement decisions and physicians’ prescribing decisions are likely to increasingly rely on patient preferences. A better understanding of patient preferences is also key to designing future DAAs that may further enhance patients’ adherence to the CHC therapy and thus improve clinical outcomes of the treatment. Indeed, DAAs that offer patients greater ease of use, convenience, and better safety profiles are likely to result in increased adherence [29, 30]. Financial burden associated with medical services in general and pharmaceutical products specifically has drawn increasing attention in recent years. Our study did not include costs of treatment as an attribute because out-of-pocket costs vary substantially within the USA (e.g., government payer vs. commercial payers) and across the countries along with the currency difference. Moreover, costs are more an extrinsic factor; the current study focus was on attributes relating specifically to patients’ day-to-day experience of these treatments that would be consistent across regions.
Some limitations should be considered when evaluating the results of this study. First, as a general limitation of all preference research, the preferred choices that patients made in the DCE exercise may not represent their actual CHC treatment decisions in the real world. Second, the patients who participated in the survey were part of existing market research panels, patients were recruited on the basis of self-reported CHC, and the study was completed through online survey. The findings of this study may not be fully generalizable to the broader CHC patient population though efforts were made so that this study included participants from six countries, including the USA and five European countries. Third, recall bias of disease and treatment-related medical history may have been present. We attempted to minimize this potential bias by providing patients with background information on CHC and treatment through a mini tutorial. Lastly, factors independent of attributes of a treatment may also impact on the preference decision of the patients, such as patients’ knowledge of CHC, experiences with prior CHC treatments, and the financial burden associated with a treatment. These are not the focus of the current study but should be considered in future research.
Conclusions
Given the overall high cure rates achieved by the latest generation of the new all-oral DAAs, patients with CHC may prefer one treatment over another on the basis of attributes other than cure rate. Indeed, besides cure rate and safety/tolerability, the treatment attributes valued most by patients are added convenience and no or less disruption to their daily routine (i.e., shorter treatment duration, one fewer office visits during treatment, and no modification of PPI use). It is interesting to find that patients are not at all concerned about the number of tablets for a once-daily oral medication while less time spent on managing their disease, be it fewer encounters with healthcare system or an 8-week treatment duration instead of 12, is most valued. The results of this study provide a better understanding of patient preferences for the attributes of newer-generation DAAs and may improve the efficiency in the shared decision-making process when managing these patients.
Acknowledgements
The authors wish to thank the contribution from all of the study participants.
Funding
Sponsorship for this study, the journal’s article processing charges, and open access fee were funded by AbbVie. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The design, study conduct, and financial support for the study were provided by AbbVie (Grant no. 1). AbbVie participated in the interpretation of data, review, and approval of the publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Cinzia Metallo, PhD, an employee of Analysis Group, Inc. The authors wish to thank David Kinrich and Yao Wang, employees of Analysis Group, Inc., for conducting statistical analysis for the study. AbbVie provided funding for this work.
Prior Presentation
This manuscript was based on a poster that was previously presented at the American Association for the Study of Liver Disease 68th Annual Meeting, October 20–24, 2017, Washington, DC. Additional analysis was conducted for the manuscript as well.
Disclosures
Tania M. Welzel is a member of advisory committees or review panels for Novartis, Janssen, Gilead, AbbVie, Boehringer-Ingelheim, and BMS. Brett Pinsky is a stock shareholder and employee of AbbVie. Yanjun Bao is a stock shareholder and employee of AbbVie. Yaozhu J. Chen was an employee of AbbVie until September 2018 and owns stocks of Abbvie. Min Yang is an employee of Analysis Group, Inc., which has received consultancy fees from AbbVie for this study. Gautam Sajeev is an employee of Analysis Group, Inc., which has received consultancy fees from AbbVie for this study. Eric Q. Wu is an employee of Analysis Group, Inc., which has received consultancy fees from AbbVie for this study. Douglas Dieterich has served as a consultant to AbbVie, and has received research funding and speaker fees from AbbVie.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments and was granted an exemption from a full review by the New England Institutional Research Board. All survey participants consented to participation in the study prior to proceeding to completing the survey questions.
Data Availability
This manuscript has no associated data or the data will not be deposited.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8241509.
References
- 1.Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyagi I, Koirala J. Hepatitis C. Treasure Island: StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK430897/. Accessed Sep 2018.
- 3.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150:1599–1608. doi: 10.1053/j.gastro.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis. 2015;15:19. doi: 10.1186/s12879-015-0748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrault NA, Hassanein TI. Management of the patient with SVR. J Hepatol. 2016;65:S120–S129. doi: 10.1016/j.jhep.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Kish T, Aziz A, Sorio M. Hepatitis C in a new era: a review of current therapies. Pharm Ther. 2017;42:316–329. [PMC free article] [PubMed] [Google Scholar]
- 8.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 9.Horner SM, Naggie S. Successes and challenges on the road to cure hepatitis C. PLoS Pathog. 2015;11:e1004854. doi: 10.1371/journal.ppat.1004854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeuzem S, Foster GR, Wang S, et al. Glecaprevir–pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378:354–369. doi: 10.1056/NEJMoa1702417. [DOI] [PubMed] [Google Scholar]
- 11.Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17:1062–1068. doi: 10.1016/S1473-3099(17)30496-6. [DOI] [PubMed] [Google Scholar]
- 12.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 13.Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 14.D’Ambrosio R, Pasulo L, Puoti M, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol. 2019;70(3):379–87. [DOI] [PubMed]
- 15.Belperio PS, Shahoumian TA, Loomis TP, Mole LA, Backus LI. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. 2019;70:15–23. doi: 10.1016/j.jhep.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Muhlbacher A, Bethge S. First and foremost battle the virus: eliciting patient preferences in antiviral therapy for hepatitis C using a discrete choice experiment. Value Health. 2016;19:776–787. doi: 10.1016/j.jval.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16:3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 18.FDA. Advancing use of patient preference information as scientific evidence in medical product evaluation. https://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm574320.htm. Accessed Sep 2018. [DOI] [PubMed]
- 19.Bridges JF, Hauber AB, Marschal D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho M, Saha A, McCleary KK, et al. A framework for incorporating patient preferences regarding benefits and risks into regulatory assessment of medical technologies. Value Health. 2016;19:746–750. doi: 10.1016/j.jval.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Su F, Beste LA, Green PK, Berry K, Ioannou GN. Direct-acting antivirals are effective for chronic hepatitis C treatment in elderly patients: a real-world study of 17,487 patients. Eur J Gastroenterol Hepatol. 2017;29:686–693. doi: 10.1097/MEG.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalgard O, Weiland O, Noraberg G, et al. Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections—a Scandinavian real-life study. PLoS One. 2017;12:e0179764. doi: 10.1371/journal.pone.0179764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evon DM, Golin CE, Stoica T, et al. What’s important to the patient? Informational needs of patients making decisions about hepatitis C treatment. Patient. 2017;10:335–344. doi: 10.1007/s40271-016-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuchowski JL, Hamilton AB, Pyne JM, et al. Qualitative analysis of patient-centered decision attributes associated with initiating hepatitis C treatment. BMC Gastroenterol. 2015;15:124. doi: 10.1186/s12876-015-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauf TL, Mohamed AF, Hauber AB, Fetzer D, Ahmad A. Patients’ willingness to accept the risks and benefits of new treatments for chronic hepatitis C virus infection. Patient. 2012;5:265–278. doi: 10.1007/BF03262498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlbacher AC, Bridges JF, Bethge S, et al. Preferences for antiviral therapy of chronic hepatitis C: a discrete choice experiment. Eur J Health Econ. 2017;18:155–165. doi: 10.1007/s10198-016-0763-8. [DOI] [PubMed] [Google Scholar]
- 29.Re VL, Amorosa VK, Localio AR, et al. Adherence to hepatitis C virus therapy and early virologic outcomes. Clin Infect Dis. 2009;48:186–193. doi: 10.1086/595685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong SW, Kim JD, Woo HY, et al. Impact of adherence to peginterferon-ribavirin combination therapy in chronic hepatitis C patients on achieving a sustained virologic response. Korean J Hepatol. 2009;15:338–349. doi: 10.3350/kjhep.2009.15.3.338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript has no associated data or the data will not be deposited.