Abstract
Background
To evaluate the effects of Macuprev® supplementation on macular function and structure in intermediate age-related macular degeneration (AMD) along 6 months of follow-up.
Methods
In this double-blind, monocentric, randomized, and prospective study, 30 patients with intermediate AMD were enrolled and randomly divided into two age-similar groups: 15 patients (AMD-M group; mean age 68.50 ± 8.79 years) received 6-month oral daily supplementation with Macuprev® (Farmaplus Italia s.r.l., Italy, two tablets/day on an empty stomach, before meals; contained in total lutein 20 mg, zeaxanthin 4 mg, N-acetylcysteine 140 mg, bromelain 2500GDU 80 mg, vitamin D3 800 IU, vitamin B12 18 mg, alpha-lipoic acid 140 mg, rutin 157 mg, vitamin C 160 mg, zinc oxide 16 mg, Vaccinium myrtillus 36% anthocyanosides 90 mg, Ganoderma lucidum 600 mg) and 15 patients (AMD-P group; mean age 70.14 ± 9.87) received two tablets of placebo daily on an empty stomach, before meals. A total of 28 eyes, 14 from each AMD group, completed the study. Multifocal electroretinogram (mfERG) and spectral domain-optical coherence tomography (SD-OCT) were assessed at baseline and after 6 months.
Results
At 6-month follow-up, AMD-M eyes showed a significant increase of mfERG response amplitude density (RAD) recorded from the central macular areas (ring 1, 0–2.5°; ring 2, 2.5–5°), whereas non-significant changes of retinal and choroidal SD-OCT parameters were found when values were compared to baseline. Non-significant correlations between functional and structural changes were found. In AMD-P eyes, non-significant differences for each mfERG and SD-OCT parameters were observed at 6 months.
Conclusions
In intermediate AMD, Macuprev® supplementation increases the function of the macular pre-ganglionic elements, with no associated retinal and choroidal ultra-structural changes.
Trial Registration
ClinicalTrials.gov identifier, NCT03919019.
Funding
Research for this study was financially supported by the Italian Ministry of Health and Fondazione Roma. Article processing charges were funded by Farmaplus Italia s.r.l., Italy.
Keywords: Carotenoid and antioxidant supplementation, Intermediate age-related macular degeneration, mfERG, OCT, Ophthalmology
Introduction
Age-related macular degeneration (AMD) is a chronic, debilitating, and progressive degenerative disease affecting central vision of patients aged 55 years or older in industrialized countries [1]. AMD includes a wide spectrum of clinical presentations, from early to advanced forms, with an increasing degree of visual acuity (VA) and central visual field impairment [2, 3]. Patients affected by early AMD usually show normal VA, but ophthalmoscopic signs, such as central drusen, pigmentary abnormalities of retinal pigment epithelium (RPE), and extrafoveal geographic atrophy (GA), are already visible. Drusen correspond to the focal depositions of extracellular material, principally lipid-derived, between basal lamina of RPE and the inner collagenous layer of Bruch’s membrane. Intermediate AMD is characterized by at least one large drusen (size ≥ 125 µm) or multiple soft intermediate drusen (≥ 63 µm, ≤ 125 µm) and the presence of extrafoveal GA, and corresponds to AREDS 3 grade classification [2].
In clinical daily practice, macular involvement due to different stages of AMD can be functionally and structurally evaluated using electrophysiological tests (i.e., focal or multifocal electroretinogram, mfERG) and optical coherence tomography (OCT), respectively.
MfERG is an electrophysiological method that allows one to detect selectively the bioelectrical responses originating from photoreceptors and bipolar cells (pre-ganglionic elements) in localized central retina areas [4–8]. Abnormal mfERG responses have been recorded in the early or intermediate stages of AMD, thus suggesting early dysfunction of the macular pre-ganglionic elements [5–8].
OCT evaluation is a routine structural test for quantitative and qualitative assessment of retina and choroid [9–18]. In early AMD, thinning of all retinal layers (from ganglion cell to RPE) associated with outer retinal complex changes has been observed [9–12]. On spectral domain-OCT (SD-OCT), drusen appear as hyperreflective dome-shaped mound deposits under the RPE, possibly associated with additional retinal abnormalities, such as reticular pseudodrusen (RPD) [13–15]. The degenerative process extends also to the choriocapillary. Indeed, choroidal dropout and thinning have been observed in early stages of AMD, and the number and density of subretinal drusenoid deposits are linearly related to the disease progression [16–18].
Considering increasing life expectancy and to ameliorate quality of life in the elderly, an important goal for ophthalmologists is to offset AMD progression by adopting adequate therapeutic strategies. In this context, several studies reported that regular intake of lutein and zeaxanthin (constituents of the macular pigment) can reduce the risk of developing advanced AMD and their supplementation may induce a stabilization/increase of VA and of the contrast sensitivity with consequent improvement of the quality of life in patients with AMD [19, 20].
MfERG is an interesting tool for assessing functional changes (increase of the function of the pre-ganglionic elements) after supplementation with zeaxanthin and lutein in AMD patients [5]. In contrast, there is a lack of evidence about possible changes of the macular chorio-retinal structure in patients treated with the aforementioned molecules proven to induce functional benefit in pre-ganglionic macular elements (see above).
Therefore, the aim of our study was to assess whether oral supplementation with carotenoids and antioxidants, including lutein and zeaxanthin, as well as rutin, N-acetylcysteine, bromelain, alpha-lipoic acid, vitamin C, and vitamin D3 would induce functional (evaluated by mfERG) and/or morphological (assessed by SD-OCT) changes of the macular region in patients affected by intermediate AMD.
Methods
Patients
Seventy patients (35 male and 35 female, mean age 70.34 ± 9.82 years) affected by AMD were screened for enrollment in the study.
Clinical diagnosis of AMD was based on slit-lamp and indirect ophthalmoscopic examination using + 90D or + 78D non-contact lens (Volk Optical, Mentor, OH) after pupillary dilatation with tropicamide 1% drops. Stereoscopic color fundus photographs (30° centered on the fovea) were also taken, and independently analyzed and graded by two masked observers (MT and MV) in accordance with the AREDS classification [2]. Macular features included drusen number, size and confluence, focal hyperpigmentation, or hypopigmentation of RPE.
Only eyes with AREDS category 3 features (intermediate AMD) were selected for this study. Inclusion criteria for the selected eyes were as follows: VA ≥ 20/32 [0.2 logarithm of the minimum angle of resolution (logMAR)], 74 letters of the Early Treatment Diabetic Retinopathy Study (ETDRS) charts], extensive (as measured by drusen area) intermediate (≥ 63 µm, < 125 µm) drusen, at least one large (≥ 125 µm) drusen or GA not involving the center of the macula. In addition, AMD patients were never supplemented with carotenoids or antioxidants.
Exclusion criteria, based on the fact that several pathologies may influence the bioelectrical responses derived from the macular region [21], were moderate to dense lens opacity, implanted intraocular lens, corneal opacities, previous history of refractive surgery, glaucoma or ocular hypertension, previous history of intraocular inflammation such as anterior or posterior uveitis, previous history of retinal detachment or laser treatment for peripheral retinal diseases, diabetes or systemic hypertension under medical treatment, previous history of ocular trauma, usage of systemic treatments with known toxic effects on the macula (e.g., chloroquine, oxazepam), neurological diseases, or presence of any signs of advanced AMD (choroidal neovascularization or central GA) in the study eye.
When both eyes fulfilled the inclusion criteria, the eye with the best VA was selected; when both eyes had the same VA, the right eye was chosen for analysis.
As a result, 30 eyes with intermediate AMD from 30 patients (9 male and 21 female, mean age 69.32 ± 9.21 years) were enrolled in the study.
Study Design
This study has been designed as a monocentric, randomized, prospective, and double blind (placebo vs active treatment). The research followed the tenets of the Helsinki Declaration (1964 and further revisions) and the study was approved by the local ethics committee (Comitato Etico Centrale IRCCS Lazio, Sezione IFO/Fondazione Bietti, Rome, Italy). The present study was registered at ClinicalTrials.gov (NCT03919019). Upon recruitment, executed from February to July 2017, at the IRCCS Fondazione Bietti, each patient signed an informed consent.
Baseline
All enrolled AMD patients were randomly divided into two age-similar groups, each made up of 15 patients:
AMD-M group: 15 enrolled patients providing 15 eyes; mean age 68.50 ± 8.79 years
AMD-P group: 15 enrolled patients providing 15 eyes; mean age 70.14 ± 9.87 years
The random separation of AMD patients (screened by MT and MP) was performed by an electronically generated randomization system on the basis of age, gender, and mfERG ring 1 response amplitude density (RAD) (see below).
Months 0–6
Throughout a 6-month period, AMD-M patients received two tablets per day on an empty stomach, before meals of a dietary complementary supplement containing in total lutein (20 mg), zeaxanthin (4 mg), N-acetylcysteine (140 mg), bromelain 2500GDU (80 mg), vitamin B12 (18 mg), vitamin D3 (800 IU), alpha-lipoic acid (140 mg), rutin (157 mg), vitamin C (160 mg), zinc oxide (16 mg), Vaccinium myrtillus 36% anthocyanosides (90 mg), Ganoderma lucidum (600 mg) (Macuprev®, Farmaplus Italia s.r.l., Italy). AMD-P patients received a daily dietary complementary supplement of placebo (two tablets per day on an empty stomach, before meals) containing microcrystalline cellulose (885 mg), talcum (28 mg), calcium phosphate tribasic (688 mg), vegetable magnesium stearate (14 mg), and calcium carbonate (344 mg).
During this period, one eye belonging to the AMD-M group was excluded for lack of compliance and therefore 14 AMD-M eyes out of 15 eyes completed the study. One eye belonging to the AMD-P group was excluded on the basis of IOP increase (> 21 mmHg and < 24 mmHg) and therefore 14 AMD-P eyes out of 15 eyes completed the study.
Following a criterion previously used in other published works [5], in order to evaluate mfERG and OCT data, independently from the clinical conditions and patients’ assignment group, all examinations were performed at baseline and after 6 months of follow-up in the presence of four operators (DM, PG, LZ, and VVF; see Acknowledgements), who were masked for each patient evaluation.
At month 6, compliance to administration (active or placebo treatment) was assessed by evaluating the ratio between the number of tablets delivered at baseline and the number of tablets returned from each AMD patient after 6 months. “Good compliance” was considered as a ratio > 90%. This value was reached by all AMD enrolled patients but one (from the AMD-M group, see above) that was excluded from the analysis at follow-up.
The key was opened to all the investigators at the end of the follow-up.
Electrophysiological Examination (mfERG Recordings)
In AMD-M and AMD-P eyes, mfERG was recorded according to the standard ISCEV [22], and to our previously published method [5, 6, 21].
In the analysis of mfERG responses, we analyzed the average RAD (measured in nV/degree2) between the first negative peak, N1, and the first positive peak, P1, obtained in five concentric annular retinal regions (rings) centered on the fovea. Therefore, we analyzed the N1–P1 RADs derived from 0° to 2.5° (ring 1, R1), from 2.5° to 5° (ring 2, R2), from 5° to 10° (ring 3, R3), from 10° to 15° (ring 4, R4), and from 15° to 20° (ring 5, R5).
MfERGs were performed three times on three different days in each AMD patient. The recording with the highest R1–R5 N1–P1 RADs was considered in the statistical analysis (see below).
Morphological Evaluation (SD-OCT Assessment)
All patients underwent a structural SD-OCT scan using Heidelberg Spectralis (version 1.10.4.0, Heidelberg Engineering, Heidelberg, Germany). The SD-OCT imaging protocol, after pupil dilation, consisted of at least 20° × 15° volume scans of the macula area with 19 B-scans. Furthermore, the enhanced depth imaging (EDI)-OCT scans were acquired to better visualize the choroid.
All B-scan images were checked for errors in automatic segmentation and manual adjustments were used if uncorrected automatic segmentation occurred. Central macular thickness (CMT), inner retinal layer (IRL) and outer retinal layer (ORL) thickness and volume were automatically measured in the macular map centered on the fovea, through inbuilt software of Heidelberg Spectralis (version 1.10.2.0). The IRL included the sum of inner plexiform and nuclear layers thickness, whereas the ORL thickness corresponded to the sum of outer plexiform and nuclear layers thickness.
Two experienced operators (EC and DM) manually measured the choroidal thickness (CT) in EDI-OCT scans, as the distance between hyperreflective inferior limit of RPE and the hyperreflective sclera-choroidal junction, using a caliper integrated in the device. Interobserver agreement was calculated (Cohen’s kappa 0.98 and 0.92), and measurements from the first operator (EC) were used for the study analysis. The CT was measured at the fovea (SCT), 1000 µm nasally (NCT) and 1000 µm temporally (TCT) to the fovea.
In the analysis on SD-OCT we considered the following parameters: CRT (micron), central macular volume (CMV, mm3), central inner retinal thickness and volume (C-IRT, micron and C-IRV, mm3, respectively), central outer retinal thickness and volume (C-ORT, micron and C-ORV, mm3, respectively), subfoveal choroidal thickness (SCT, micron), temporal choroidal thickness (TCT, micron), and nasal choroidal thickness (NCT, micron).
Morpho-Functional Correlations
In order to evaluate whether the possible electrofunctional changes induced by the active treatment were dependent or not on the morphological changes, we correlated mfERG and OCT data derived from the same central macular areas. Therefore, in these correlations we compared exclusively the R1 and R2 mfERG results with corresponding SD-OCT data.
Statistics
Sample size estimates were obtained from pilot evaluations of mfERG recordings performed in 10 non-advanced AMD patients other than those included in the current study (unpublished results), using R1 RAD as the main outcome measure. With a power of 90% at an alpha of 0.01, to detect an expected difference of 35% in mfERG RAD, a sample size of 10 subjects was obtained.
A dropout rate of 40% was estimated; thus, the number of subjects required was 14 for both groups.
Test–retest data of mfERG results were expressed as the mean difference between two recordings obtained in separate sessions ± the standard deviation (SD) of this difference. The Anderson–Darling and Kolmogorov–Smirnov tests were applied to verify that the data were normally distributed. Indeed 95% confidence limits of test–retest variability in patients were established assuming a normal distribution. In AMD patients, test–retest data were calculated considering the entire cohort of enrolled patients that completed the study (28 AMD eyes).
At baseline, the differences of mfERG and SD-OCT values detected in the groups (AMD-P and AMD-M eyes) were evaluated by one-way analysis of variance (ANOVA).
At the end of follow-up, individual changes (values detected at 6 months minus those detected at baseline) of mfERG data detected in AMD-P and AMD-M were calculated by performing a logarithmic transformation.
Mean values of absolute changes in mfERG and SD-OCT data observed in AMD-P and AMD-M eyes after 6 months were compared to baseline values by one-way ANOVA.
The linear correlations between the individual differences (difference of logarithmic transformation of values at 6 months minus logarithmic transformation of values at baseline) of mfERG and SD-OCT data were assessed by Pearson’s test. All statistical analyses were performed by using MedCalc V.13.0.4.0 (MedCalc, Mariakerke, Belgium), and a p value less than 0.05 was considered as statistically significant.
Results
Electrophysiological (mfERG) Data
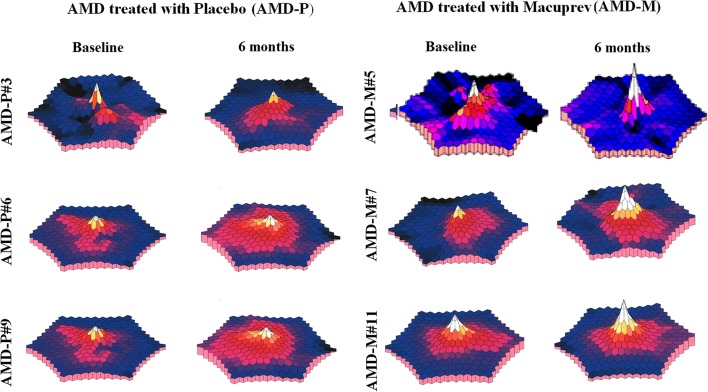
Figure 1 shows examples of a tri-dimensional mfERG plot recorded in three different AMD-P and AMD-M eyes at baseline conditions and after 6 months.
Fig. 1.
Examples of multifocal electroretinogram (mfERG) tri-dimensional plots, recorded from six eyes with intermediate age-related macular degeneration (AMD) at baseline and after 6-month follow-up. AMD patients were treated with placebo (AMD-P eyes) or with Macuprev® (AMD-M eyes). At 6 months, with respect to baseline, the tri-dimensional plot in AMD-P eyes showed unmodified or worsened central localized amplitudes, whereas AMD-M eyes showed improved central localized amplitudes
At baseline, AMD-P and AMD-M eyes showed non-significantly (p > 0.05) different R1–R5 RAD values. Mean RAD values and relative statistical analysis are reported in Table 1 (baseline).
Table 1.
MfERG data at baseline and during follow-up
| Baseline | ANOVA vs AMD-P | 6 months | ANOVA vs baseline | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | f (1,27) | p | Mean | SD | f (1,27) | p | |
| R1 RAD (nV/degree2) | ||||||||
| AMD-P (n = 14) | 65.24 | 14.06 | 65.12 | 20.19 | 0.001 | 0.991 | ||
| AMD-M (n = 14) | 61.52 | 15.22 | 0.43 | 0.516 | 85.02 | 27.91 | 7.64 | 0.010 |
| R2 RAD (nV/degree2) | ||||||||
| AMD-P (n = 14) | 26.93 | 5.43 | 28.08 | 8.21 | 0.17 | 0.685 | ||
| AMD-M (n = 14) | 26.65 | 7.47 | 0.02 | 0.886 | 34.78 | 12.46 | 4.48 | 0.044 |
| R3 RAD (nV/degree2) | ||||||||
| AMD-P (n = 14) | 16.07 | 3.94 | 18.58 | 4.25 | 2.91 | 0.099 | ||
| AMD-M (n = 14) | 15.24 | 4.55 | 0.21 | 0.651 | 18.39 | 4.98 | 3.07 | 0.092 |
| R4 RAD (nV/degree2) | ||||||||
| AMD-P (n = 14) | 11.42 | 3.61 | 12.98 | 3.61 | 1.34 | 0.258 | ||
| AMD-M (n = 14) | 11.91 | 2.73 | 0.17 | 0.691 | 12.81 | 3.24 | 0.63 | 0.434 |
| R5 RAD (nV/degree2) | ||||||||
| AMD-P (n = 14) | 8.24 | 3.06 | 8.42 | 2.37 | 0.03 | 0.864 | ||
| AMD-M (n = 14) | 7.91 | 3.21 | 0.42 | 0.524 | 8.81 | 2.10 | 0.77 | 0.388 |
Mean ± standard deviation (SD) of multifocal electroretinogram (mfERG) R1–R5 N1–P1 response amplitude density (RAD) values detected at baseline and after 6 months of follow-up in AMD eyes treated with placebo (AMD-P eyes) or with Macuprev® (AMD-M eyes). R1–R5 refers to localized mfERG responses averaged in five eccentricity areas between the fovea and mid-periphery: 0–2.5° (R1), 2.5–5° (R2), 5–10° (R3), 10–15° (R4), and 15–20° (R5). Statistics: analysis of variance (ANOVA) between AMD-P and AMD-M groups at baseline and between AMD-P and AMD-M groups at 6 months vs baseline; n number of eyes
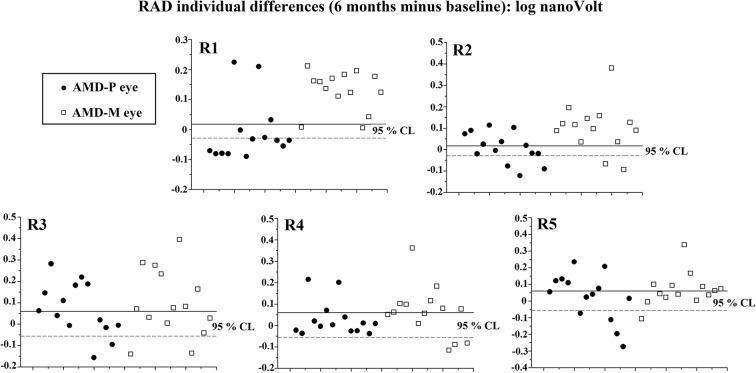
At 6-month follow-up, a great percentage of AMD-M eyes (> 85%) showed increased R1 and R2 RAD values, whereas an increase of RADs was found in R3–R5 in a percentage ranging from 42.86% to 57.14%. In AMD-P eyes, reduced RAD values were detected in R1 in a great percentage of eyes (57.14%), whereas in the other rings (R2–R4) RAD values were unmodified in a percentage of eyes ranging from 42.86% to 78.57%. The individual changes observed at 6 months in AMD-P and AMD-M eyes with respect to baseline are reported in Table 2 and Fig. 2.
Table 2.
MfERG changes during follow-up
| Worsened | Unmodified | Improved | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| R1 RAD | ||||||
| AMD-P | 8 | 57.14 | 3 | 21.43 | 3 | 21.43 |
| AMD-M | 0 | 0 | 2 | 14.29 | 12 | 85.71 |
| R2 RAD | ||||||
| AMD-P | 3 | 21.43 | 6 | 42.86 | 5 | 35.71 |
| AMD-M | 2 | 14.29 | 0 | 0 | 12 | 85.71 |
| R3 RAD | ||||||
| AMD-P | 2 | 14.29 | 6 | 42.86 | 6 | 42.86 |
| AMD-M | 2 | 14.29 | 4 | 28.57 | 8 | 57.14 |
| R4 RAD | ||||||
| AMD-P | 0 | 0 | 11 | 78.57 | 3 | 21.43 |
| AMD-M | 3 | 21.43 | 4 | 28.57 | 7 | 50 |
| R5 RAD | ||||||
| AMD-P | 4 | 28.57 | 4 | 28.57 | 6 | 42.86 |
| AMD-M | 1 | 7.143 | 7 | 50 | 6 | 42.86 |
Individual changes after 6 months of follow-up of multifocal electroretinogram (mfERG) R1–R5 N1–P1 response amplitude density (RAD) values observed in AMD eyes treated with placebo (AMD-P eyes) or with Macuprev® (AMD-M eyes). R1–R5 refers to localized mfERG responses averaged in five eccentricity areas between the fovea and the mid-periphery: 0–2.5° (R1), 2.5–5° (R2), 5–10° (R3), 10–15° (R4), and 15–20° (R5). Unmodified = within the 95% confidence test–retest limit; improved = increase in values of mfERG RAD that exceeded the 95% confidence test–retest limit; worsened = reduction in values of mfERG RAD that exceeded the 95% confidence test–retest limit; n number of eyes
Fig. 2.
Individual changes after 6-month follow-up with respect to baseline of multifocal electroretinogram (mfERG) R1–R5 N1–P1 response amplitude density (RAD) values observed in AMD eyes treated with placebo (AMD-P eyes) or treated with Macuprev® (AMD-M eyes). R1–R5 refers to localized mfERG responses averaged in five eccentricity areas between the fovea and mid-periphery: 0–2.5° (R1), 2.5–5° (R2), 5–10° (R3), 10–15° (R4), and 15–20° (R5). The percentage of unmodified eyes (within the 95% confidence test–retest limit—values within solid and dashed line), eyes with improvement (values over the 95% confidence test–retest limit—solid line), and eyes with worsening (values under the 95% confidence test–retest limit—dashed line) are reported in Table 2
On average, at 6-month follow-up in comparison with baseline, AMD-P eyes showed non-significant (p > 0.05) changes in R1–R5 RAD values. In AMD-M eyes, a significant (p < 0.05) increase of RAD values was found in R1 and R2, whereas non-significant (p > 0.05) RAD changes were observed in R3–R5. Mean data and relative statistical analyses of mfERG responses are shown in Table 1 (6 months).
SD-OCT Data
At baseline, AMD-P and AMD-M eyes showed non-significantly (p > 0.05) different values for each SD-OCT parameter. Mean values and relative statistical analysis are reported in Table 3.
Table 3.
SD-OCT data at baseline and during follow-up
| Baseline | ANOVA vs AMD-P | 6 months | ANOVA vs baseline | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | f (1,27) | p | Mean | SD | f (1,27) | p | |
| Central macular thickness (CRT, micron) | ||||||||
| AMD-P (n = 14) | 273.85 | 24.68 | 275.00 | 26.10 | 0.01 | 0.909 | ||
| AMD-M (n = 14) | 281.86 | 20.88 | 0.83 | 0.370 | 281.36 | 22.20 | 0.00 | 0.952 |
| Central macular volume (CMV, mm3) | ||||||||
| AMD-P (n = 14) | 0.21 | 0.014 | 0.21 | 0.016 | 0.06 | 0.805 | ||
| AMD-M (n = 14) | 0.22 | 0.016 | 2.01 | 0.169 | 0.22 | 0.019 | 0.00 | 1.000 |
| Central inner retinal thickness (C-IRT, micron) | ||||||||
| AMD-P (n = 14) | 41.00 | 7.95 | 39.46 | 7.64 | 0.25 | 0.620 | ||
| AMD-M (n = 14) | 42.00 | 5.71 | 0.14 | 0.709 | 40.57 | 6.73 | 0.37 | 0.550 |
| Central inner retinal volume (C-IRV, mm3) | ||||||||
| AMD-P (n = 14) | 0.031 | 0.01 | 0.032 | 0.01 | 0.07 | 0.793 | ||
| AMD-M (n = 14) | 0.036 | 0.01 | 1.75 | 0.197 | 0.031 | 0.01 | 1.75 | 0.197 |
| Central outer retinal thickness (C-ORT, micron) | ||||||||
| AMD-P (n = 14) | 116.85 | 16.37 | 119.46 | 13.89 | 0.19 | 0.664 | ||
| AMD-M (n = 14) | 122.14 | 15.02 | 0.77 | 0.389 | 123.71 | 12.33 | 0.09 | 0.765 |
| Central outer retinal volume (C-ORV, mm3) | ||||||||
| AMD-P (n = 14) | 0.089 | 0.01 | 0.091 | 0.01 | 0.28 | 0.601 | ||
| AMD-M (n = 14) | 0.096 | 0.01 | 3.43 | 0.075 | 0.097 | 0.01 | 0.07 | 0.793 |
| Subfoveal choroidal thickness (SCT, micron) | ||||||||
| AMD-P (n = 14) | 228.31 | 50.34 | 228.46 | 58.77 | 0.00 | 0.994 | ||
| AMD-M (n = 14) | 227.86 | 44.26 | 0.00 | 0.980 | 230.64 | 48.12 | 0.03 | 0.875 |
| Temporal choroidal thickness (TCT, micron) | ||||||||
| AMD-P (n = 14) | 239.31 | 64.66 | 232.15 | 56.69 | 0.09 | 0.767 | ||
| AMD-M (n = 14) | 237.00 | 35.24 | 0.01 | 0.908 | 237.50 | 32.48 | 0.00 | 0.969 |
| Nasal choroidal thickness (NCT, micron) | ||||||||
| AMD-P (n = 14) | 217.77 | 59.69 | 210.00 | 69.94 | 0.09 | 0.763 | ||
| AMD-M (n = 14) | 238.64 | 48.84 | 1.00 | 0.328 | 231.57 | 48.20 | 0.15 | 0.703 |
Mean ± standard deviation (SD) of spectral domain-optical coherence tomography (SD-OCT) values detected at baseline condition and after 6 months of follow-up in AMD eyes treated with placebo (AMD-P eyes) or with Macuprev® (AMD-M eyes). Statistics: one-way analysis of variance (ANOVA) between AMD-P and AMD-M groups at baseline and in AMD-P and AMD-M groups at 6 months vs baseline; n number of eyes
At 6-month follow-up, on average, all SD-OCT values were not significantly (p > 0.05) modified in comparison with those observed at baseline in both AMD groups.
Correlations Between Electrophysiological and Morphological Data
In AMD-M, the logarithmic differences (6 months minus baseline) of mfERG parameters (R1 and R2 RADs) were not significantly correlated (p > 0.05) to the corresponding differences of morphological (SD-OCT) parameters. The values of these correlations are shown in Table 4.
Table 4.
Correlations between functional and morphological changes at 6-month follow-up
| AMD-M eyes | R1 RAD (R; p) | R2 RAD (R; p) |
|---|---|---|
| Central macular thickness | − 0.210; 0.471 | – |
| Central macular volume | 0.521; 0.056 | − 0.111; 0.704 |
| Central inner retinal thickness | − 0.487; 0.084 | − 0.254; 0.380 |
| Central inner retinal volume | − 0.318; 0.267 | 0.255; 0.378 |
| Central outer retinal thickness | 0.395; 0.162 | 0.014; 0.961 |
| Central outer retinal volume | 0.209; 0.471 | 0.190; 0.514 |
| Subfoveal choroidal thickness | − 0.276; 0.338 | – |
| Temporal choroidal thickness | 0.258; 0.373 | – |
| Nasal choroidal thickness | 0.135; 0.645 | – |
Pearson’s test results of linear correlations between the individual logarithmic differences (6 months minus baseline) of mfERG values (R1 and R2 RAD) and the individual logarithmic differences (6 months minus baseline) of SD-OCT parameters observed in AMD eyes treated with Macuprev® (AMD-M eyes)
Discussion
The aim of our study was to assess whether the oral supplementation with Macuprev® can induce an increase in the function of pre-ganglionic retinal elements, associated or not with morphological changes in macular chorio-retinal ultra-structure in patients with intermediate AMD.
This monocentric, randomized, prospective, and double-blind (placebo vs active treatment) study was conducted to assess retinal functional changes by mfERG recordings and macular structure by SD-OCT evaluation.
After 6 months of supplementation with Macuprev®, eyes with intermediate AMD showed increased mfERG R1 and R2 RADs, suggesting a functional improvement of the pre-ganglionic retinal elements located in the 0–5 central retinal degrees. Since in the more external retinal areas (5–20°, rings 3, 4, and 5), non-significant functional changes were observed, the effects induced by the oral intake can be consider as limited to the central macular areas. Despite these functional changes, no differences of the macular chorio-retinal structural parameters were detected at the end of follow-up, as demonstrated by the absence of any significant differences in OCT values of the AMD-M group. As a consequence, the increased function was not significantly correlated with the structural changes from the same tested retinal areas. It is worth noting that in eyes with intermediate AMD treated with placebo, no functional or morphological differences were seen at the end of follow-up.
Since the oral supplement contains different compounds (lutein, zeaxanthin, N-acetylcysteine, bromelain 2500GDU, vitamin B12, vitamin D3, alpha-lipoic acid, rutin, vitamin C, zinc oxide, Vaccinium myrtillus 36% anthocyanosides, Ganoderma lucidum), we are not able to define the specific effects of each of them.
Nevertheless, the observed increase of macular function (improved R1 and R2 mfERG RADs) in active-treated AMD eyes requires an explanation based on the available information on the mechanisms of action of the principal compounds of the supplement. The main and well-studied components are lutein and zeaxanthin, which are known to increase retinal function in healthy subjects [23] and in human pathological conditions [24–26]. Indeed, our results are in agreement with our previous study [5], in which similar mfERG changes, despite the lower concentration of lutein (10 mg) and zeaxanthin (1 mg), were found.
To explain why mfERG improvement in our active-treated AMD eyes was found exclusively in the most central retinal area, it is relevant that the highest concentration of lutein and zeaxanthin can be found in the fovea, as studied in cone-rich animal models [27, 28]. This is corroborated by the finding that these functional changes were not observed in areas external to the fovea (5–20°). Therefore, considering also the photoreceptor distribution and topography in human retina [29, 30], it is unlikely that the supplementation with lutein and zeaxanthin would induce an over-normal function in the extra-foveal areas.
Supplementation of AMD patients with lutein and zeaxanthin increases macular pigments and consequently prevents light-induced damage and aging oxidative injury [31]. These protective mechanisms, widely described in a review by Stahl and Sies [32], can explain analogous mfERG findings in AMD patients supplemented with similar content of lutein and zeaxanthin [33].
The relevance of dietary intake of lutein and zeaxanthin has been highlighted by a clinical epidemiological study [34] and by a study from AMD donor eyes [35], showing that food rich of these macular pigments, as well as high plasma levels of these two compounds, was associated with lower risk of AMD. The importance of dietary intake is supported by opposite data showing that drusen develop at the RPE level in monkeys fed with a xanthophyll-depleted diet [36] and by the finding that the concentration of zeaxanthin was inversely and significantly related to the number of light-induced apoptotic photoreceptors in quail retina [37]. In these animal models, lutein and zeaxanthin were considered to have significant antioxidant effects, preventing or delaying photoreceptor dysfunction or loss [36, 37].
Among the other components used in the current study, N-acetylcysteine, rutin, alpha-lipoic acid, and vitamin C may also play a significant role by reducing the oxidative injury [38–41] and decreasing photoreceptor impairment in AMD. Also bromelain, a cysteine protease enzyme extracted from the stems of pineapple, with its anti-inflammatory activities observed in several therapeutic fields [42], is likely to play a role in the reduction of the AMD photoreceptor dysfunction, possibly contributing to reduce the inflammation involved in the AMD degenerative process [43].
In the present study, we also evaluated the possible changes induced by oral supplementation on the chorio-retinal structures of AMD eyes. Non-significant changes between baseline and the end of supplementation were found for each SD-OCT parameter, as described by the absence of differences in the inner and outer retinal layers’ thickness and volume and by the absence of changes in choroidal thickness during the follow-up. This also led us to believe that in our patients with intermediate AMD, the oral supplementation with the aforementioned components is not able to modify the chorio-retinal morphology, and no other findings available in the literature can confirm or contrast our results at this moment.
Conclusions
In intermediate AMD patients, supplementation with combined components of Macuprev® (lutein, zeaxanthin, N-acetylcysteine, bromelain 2500GDU, vitamin B12, vitamin D3, alpha-lipoic acid, rutin, vitamin C, zinc oxide, Vaccinium myrtillus 36% anthocyanosides, Ganoderma lucidum) increases the function of pre-ganglionic elements located in the most central macular areas, confirming functional improvement [5, 33] that is not associated with morphological changes.
Nevertheless, the present study presents the following limitations: our results, observed after a 6-month period of treatment with Macuprev®, need to be confirmed by long-term supplementation and, since combined compounds were administered, the specific role of each of them in improving macular function needs to be identified. However, our findings could constitute a rationale for further clinical studies, assessing whether the improvement of macular pre-ganglionic function obtained with Macuprev® can be associated or not with the already know changes in psychophysical (i.e., visual acuity, contrast sensitivity [20], and macular microperimetry [33]) measurements in AMD.
Acknowledgements
We thank the participants of the study. The authors also acknowledge Dr. Valter Valli Fiore for his technical help in the electrophysiological evaluations.
Funding
Research for this study was financially supported by the Italian Ministry of Health and Fondazione Roma. Article processing charges were funded by Farmaplus Italia s.r.l., Italy.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Mariacristina Parravano, Massimiliano Tedeschi, Daniela Manca, Eliana Costanzo, Antonio Di Renzo, Paola Giorno, Lucilla Barbano, Lucia Ziccardi, Monica Varano, and Vincenzo Parisi have nothing to disclose.
Compliance with Ethics Guidelines
The study was registered at ClinicalTrials.gov (NCT03919019) and was approved by the local ethics committee (Comitato Etico Centrale IRCCS Lazio, Sezione IFO/Fondazione Bietti, Rome, Italy—Registro Sperimentazioni Italiano approval number 59/17/FB). All procedures performed in this study were also in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
The original version of this article was revised due to retrospective open access.
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8243120.
Change history
9/19/2019
The article ‘‘Effects of Macuprev Supplementation in Age-Related Macular Degeneration: A Double-Blind Randomized Morpho-Functional Study Along 6 Months of FollowUp’’, written by Mariacristina Parravano, Massimiliano Tedeschi, Daniela Manca, Eliana Costanzo, Antonio Di Renzo, Paola Giorno, Lucilla Barbano, Lucia Ziccardi, Monica Varano, Vincenzo Parisi was originally published electronically on the publisher’s internet portal (currently SpringerLink) on June 25, 2019 without Open Access. The article has now been made Open Access.
References
- 1.Evans JR, Fletcher AE, Wormald RP, et al. Prevalence of visual impairment in people aged 75 years and older in Britain: results from the MRC trial of assessment and management of older people in the community. Br J Ophthalmol. 2002;86:795–800. doi: 10.1136/bjo.86.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Beckman initiative for macular research classification committee. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe GJ, Martin DF, Toth CA, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:1860–1870. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000;19:607–646. doi: 10.1016/S1350-9462(00)00013-6. [DOI] [PubMed] [Google Scholar]
- 5.Parisi V, Tedeschi M, Gallinaro G, et al. Carotenoids and antioxidants in age-related maculopathy Italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008;115:324–333. doi: 10.1016/j.ophtha.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Parisi V, Perillo L, Tedeschi M, et al. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina. 2007;27:879–890. doi: 10.1097/IAE.0b013e318042d6aa. [DOI] [PubMed] [Google Scholar]
- 7.Moschos MM, Nitoda E. The role of mf-ERG in the diagnosis and treatment of age-related macular degeneration: electrophysiological features of AMD. Semin Ophthalmol. 2018;33:461–469. doi: 10.1080/08820538.2017.1301496. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann-Vernaleken B, Palmowski AM, Allgayer R, Ruprecht KW. Comparison of different high resolution multifocal electroretinogram recordings in patients with age-related maculopathy. Graefes Arch Clin Exp Ophthalmol. 2001;239:556–561. doi: 10.1007/s004170100308. [DOI] [PubMed] [Google Scholar]
- 9.Rimayanti U, Kiuchi Y, Yamane K, et al. Inner retinal layer comparisons of eyes with exudative age-related macular degeneration and eyes with age-related macular degeneration and glaucoma. Graefes Arch Clin Exp Ophthalmol. 2014;252:563–570. doi: 10.1007/s00417-013-2496-z. [DOI] [PubMed] [Google Scholar]
- 10.Lee EK, Yu HG. Ganglion cell-inner plexiform layer and peripapillary retinal nerve fiber layer thicknesses in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3976–3983. doi: 10.1167/iovs.15-17013. [DOI] [PubMed] [Google Scholar]
- 11.Wood A, Binns A, Margrain T, et al. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011;152:1030–1038. doi: 10.1016/j.ajo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 2009;116:488–496. doi: 10.1016/j.ophtha.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Zuo C, Xiao H, et al. Photoreceptor dysfunction in early and intermediate age-related macular degeneration assessed with mfERG and spectral domain OCT. Doc Ophthalmol. 2016;132:17–26. doi: 10.1007/s10633-016-9523-4. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007;91:354–359. doi: 10.1136/bjo.2006.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilde C, Patel M, Lakshmanan A, Morales MA, Dhar-Munshi S, Amoaku WM. Prevalence of reticular pseudodrusen in eyes with newly presenting neovascular age-related macular degeneration. Eur J Ophthalmol. 2016;26:128–134. doi: 10.5301/ejo.5000661. [DOI] [PubMed] [Google Scholar]
- 16.Laíns I, Wang J, Providência J, et al. Choroidal changes associated with subretinal drusenoid deposits in age-related macular degeneration using swept-source optical coherence tomography. Am J Ophthalmol. 2017;180:55–63. doi: 10.1016/j.ajo.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Keenan TD, Klein B, Agrón E, Chew EY, Cukras CA, Wong WT. Choroidal thickness and vascularity vary with disease severity and subretinal drusenoid deposit presence in non advanced age-related macular degeneration. Retina. 2019 doi: 10.1097/iae.0000000000002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yiu G, Chiu SJ, Petrou PA, et al. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol. 2015;159:617–626. doi: 10.1016/j.ajo.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 20.Piermarocchi S, Saviano S, Parisi V, et al. Carotenoids in Age-related Maculopathy Italian Study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol. 2011;22:216–225. doi: 10.5301/ejo.5000069. [DOI] [PubMed] [Google Scholar]
- 21.Parisi V, Ziccardi L, Centofanti M, et al. Macular function in eyes with open-angle glaucoma evaluated by multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2012;53:6973–6980. doi: 10.1167/iovs.12-10256. [DOI] [PubMed] [Google Scholar]
- 22.Hood DC, Bach M, Brigell M, et al. ISCEV standard for clinical multifocal electroretinography (2011 edition) Doc Ophthalmol. 2012;124:1–13. doi: 10.1007/s10633-011-9296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrow EJ, Bartlett HE, Eperjesi F. The effect of nutritional supplementation on the multifocal electroretinogram in healthy eyes. Doc Ophthalmol. 2016;132:123–135. doi: 10.1007/s10633-016-9532-3. [DOI] [PubMed] [Google Scholar]
- 24.Moschos MM, Dettoraki M, Tsatsos M, Kitsos G, Kalogeropoulos C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis (Lond) 2017;4:23. doi: 10.1186/s40662-017-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berrow EJ, Bartlett HE, Eperjesi F, Gibson JM. The effects of a lutein-based supplement on objective and subjective measures of retinal and visual function in eyes with age-related maculopathy—a randomised controlled trial. Br J Nutr. 2013;109:2008–2014. doi: 10.1017/S0007114512004187. [DOI] [PubMed] [Google Scholar]
- 26.Ma L, Dou HL, Huang YM, et al. Improvement of retinal function in early age-related macular degeneration after lutein and zeaxanthin supplementation: a randomized, double-masked, placebo-controlled trial. Am J Ophthalmol. 2012;154:625–634. doi: 10.1016/j.ajo.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Snodderly DM, Handelman GJ, Adler AJ. Distribution of individual macular pigment carotenoids in central retina of macaque and squirrel monkeys. Invest Ophthalmol Vis Sci. 1991;32:268–279. [PubMed] [Google Scholar]
- 28.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 29.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye (Lond) 2001;15:376–383. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 30.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 31.Obana A, Tanito M, Gohto Y, Okazaki S, Gellermann W, Bernstein PS. Changes in macular pigment optical density and serum lutein concentration in Japanese subjects taking two different lutein supplements. PLoS One. 2015;10:e0139257. doi: 10.1371/journal.pone.0139257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740:101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Dou H, Huang F, et al. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: a randomised, double-blind, placebo-controlled trial. Br J Ophthalmology. 2015;99:371–375. doi: 10.1136/bjophthalmol-2014-305503. [DOI] [PubMed] [Google Scholar]
- 34.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. J Am Med Assoc. 1994;272:1413–1420. doi: 10.1001/jama.1994.03520180037032. [DOI] [PubMed] [Google Scholar]
- 35.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case–control study. Invest Ophthalmol Vis Sci. 2001;42:235–240. [PubMed] [Google Scholar]
- 36.Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci. 1980;19:857–863. [PubMed] [Google Scholar]
- 37.Thomson LR, Toyoda Y, Langner A, et al. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest Ophthalmol Vis Sci. 2002;43:3538–3549. [PubMed] [Google Scholar]
- 38.Carver KA, Yang D. N-Acetylcysteine amide protects against oxidative stress-induced microparticle release from human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2016;57:360–371. doi: 10.1167/iovs.15-17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayama M, Aihara M, Chen YN, Araie M, Tomita-Yokotani K, Iwashina T. Neuroprotective effects of flavonoids on hypoxia-, glutamate-, and oxidative stress-induced retinal ganglion cell death. Mol Vis. 2011;17:1784–1793. [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YS, Kim M, Choi MY, et al. Alpha-lipoic acid reduces retinal cell death in diabetic mice. Biochem Biophys Res Commun. 2018;503:1307–1314. doi: 10.1016/j.bbrc.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 41.Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2012;14(11):CD00025440. doi: 10.1002/14651858.CD000254.pub3. [DOI] [PubMed] [Google Scholar]
- 42.de Lencastre Novaes LC, Jozala AF, Lopes AM, et al. Stability, purification, and applications of bromelain: a review. Biotechnol Prog. 2016;32:5–13. doi: 10.1002/btpr.2190. [DOI] [PubMed] [Google Scholar]
- 43.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73:1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.