Abstract
Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists represent a class of treatments for type 2 diabetes that offer multifactorial benefits, including glycemic control, weight loss and low hypoglycemia risk. Once-weekly semaglutide is a novel GLP-1 analog that has been associated with improved glycemic control and reduced body mass index (BMI) versus once-weekly GLP-1 receptor agonist dulaglutide in SUSTAIN 7, which is reimbursed in patients with a BMI > 35 kg/m2 in Slovakia. The aim of the present study was to evaluate the long-term cost-effectiveness of once-weekly semaglutide 0.5 mg and 1 mg versus dulaglutide 1.5 mg in Slovakia.
Methods
Clinical and cost outcomes were projected over patient lifetimes using the IQVIA CORE Diabetes Model. Baseline cohort characteristics and treatment effects were based on the sub-group of patients with a BMI > 35 kg/m2 in SUSTAIN 7. Patients were modeled to receive once-weekly semaglutide or dulaglutide for 3 years, after which treatment was intensified to basal insulin. Treatment effects associated with once-weekly semaglutide and dulaglutide were maintained for the first 3 years before HbA1c increased to 7.0% and BMI reverted to baseline. Costs were accounted from a healthcare payer perspective in Slovakia and expressed in euros (EUR). Utilities relating to quality of life were taken from published sources.
Results
Once-weekly semaglutide 0.5 mg and 1 mg were associated with improvements in quality-adjusted life expectancy of 0.04 and 0.07 quality-adjusted life years (QALYs), respectively, versus dulaglutide 1.5 mg. Lifetime medical costs were similar, with cost savings of EUR 20 and EUR 140 per patient with once-weekly semaglutide 0.5 mg and 1 mg, respectively, versus dulaglutide 1.5 mg. Both doses of once-weekly semaglutide were therefore considered dominant versus dulaglutide 1.5 mg.
Conclusion
Both doses of once-weekly semaglutide represent cost-saving treatment options versus dulaglutide 1.5 mg for obese patients with type 2 diabetes in Slovakia.
Funding
Novo Nordisk A/S.
Keywords: Cost, Cost-effectiveness, Diabetes mellitus, Dulaglutide, GLP-1 receptor agonist, GLP-1 analog, Semaglutide, Slovakia
Introduction
The economic and clinical burden of diabetes in Slovakia is ever-increasing, with 7–10% of the population diagnosed with diabetes in 2017 and direct healthcare expenditure exceeding EUR 650 million [1–3]. This is expected to rise to over EUR 730 million by 2040, with the costs of treating diabetes-related complications representing the biggest proportion of overall expenditure [1, 4]. Therefore, choosing cost-effective therapies that reduce the incidence of long-term complications, offering improvements in health outcomes for patients while managing costs for the healthcare payer, is imperative.
Treatments for type 2 diabetes typically focus on reducing glycated hemoglobin (HbA1c) levels, as glycemic control has been shown to reduce the incidence of diabetes-related complications in the long term [5–9]. More recently, reductions in additional risk factors, such as body weight and systolic blood pressure, have been shown to provide further benefits [10–13]. In Slovakia, 64% of patients with type 2 diabetes were estimated to be overweight and 27% were estimated to be obese, while the risk of mortality from cardiovascular complications is two- to three-fold higher in patients with type 2 diabetes versus people with no history of the disease [3, 14, 15]. Even modest reductions in HbA1c and body weight have been associated with lowered cardiovascular disease risk, with a 1% reduction in HbA1c linked with risk reductions of 16%, 4% and 12% for heart failure, myocardial infarction and stroke, respectively, while weight loss of between 5% and 10% has been associated with statistically significant improvements in risk factors for cardiovascular disease [16, 17]. Therapies that target multiple risk factors are therefore becoming increasingly utilized throughout the type 2 diabetes treatment algorithm [18].
Treatment with glucagon-like peptide-1 (GLP-1) receptor agonists has been associated with multifactorial benefits, such as reductions in body weight and systolic blood pressure, in addition to improved glycemic control [19–22]. Among GLP-1 receptor agonists, the short-term efficacy of once-weekly semaglutide, in terms of greater reductions in HbA1c and body weight, has been demonstrated versus a variety of comparators throughout the SUSTAIN clinical trial program and several network meta-analyses (NMAs) [23–28]. Once-weekly semaglutide has also been associated with statistically significant reductions in the risk of major adverse cardiovascular events (MACE) versus placebo in SUSTAIN 6, akin to results seen for liraglutide in LEADER [29, 30]. However, it is unclear whether these benefits are innate to GLP-1 receptor agonists as a class, as exenatide extended-release (ER) and lixisenatide did not display benefits compared with placebo in EXSCEL and ELIXA, respectively [31, 32]. Preliminary reports from REWIND indicate that dulaglutide is associated with superior reductions in the risk of MACE versus placebo, but the extent of these reductions is unclear, with a full publication currently pending [33].
Guidelines for the treatment of type 2 diabetes in Slovakia follow international consensus statements published by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), which advise GLP-1 receptor agonists as the first-line injectable therapy, particularly following metformin failure in patients with a high risk of cardiovascular disease or seeking to minimize weight gain or achieve weight loss [18]. In the latter case, once-weekly semaglutide is indicated as the preferred treatment option with the best efficacy for weight loss [18].
In Slovakia, healthcare coverage is universal through compulsory insurance, with care provided free of charge to insured individuals at the point of delivery [34]. In this system, the only currently available once-weekly GLP-1 receptor agonist is dulaglutide 1.5 mg, which is reimbursed in patients with a body mass index (BMI) > 35 kg/m2. Both doses of once-weekly semaglutide have shown short-term benefits versus dulaglutide in the SUSTAIN 7 clinical trial [26]. The present analysis aimed to examine the impact of these benefits on long-term clinical and cost outcomes and thereby evaluate the long-term cost-effectiveness of once-weekly semaglutide 0.5 mg and 1 mg versus dulaglutide 1.5 mg in patients with inadequate glycemic control on metformin with a BMI > 35 kg/m2 in Slovakia.
Methods
Model Overview
Cost-effectiveness was estimated using the IQVIA CORE Diabetes Model, a web-based computer model that simulates the long-term health and economic outcomes of interventions for the treatment of type 1 and type 2 diabetes [35, 36]. Model outcomes include direct medical costs, life expectancy (measured in years), quality-adjusted life expectancy [measured in quality-adjusted life years (QALYs)], the cumulative incidence and time to onset of diabetes-related complications and cost-effectiveness scatterplots and acceptability curves. In cases where an intervention is associated with increased costs and greater clinical benefits, costs and effectiveness are combined to give an incremental cost-effectiveness ratio (ICER), describing the incremental cost per unit of effectiveness gained for the tested intervention versus the comparator. In scenarios where an intervention is associated with reduced costs and increased clinical benefits, it is considered dominant versus the comparator. The model has been validated versus real-life data at both the time of release in 2004 and more recently in 2014 and is frequently used and widely accepted by health technology assessment bodies globally [36, 37].
Analyses were performed over patient lifetimes, as per the guidelines for the assessment of cost-effectiveness of diabetes interventions, capturing all relevant long-term complications experienced by patients and the associated treatment costs [38]. All analyses utilized Slovakia-specific life tables for background mortality, with remaining mortality considered as a result of diabetes-related complications [39]. The risk of cardiovascular complications was predicted using the UKPDS 68 equations [40]. All base case and sensitivity analyses took a first-order Monte Carlo approach, while a second-order Monte Carlo approach was used for the probabilistic sensitivity analysis (PSA). Discounting for clinical and cost outcomes was set at 5.0% annually, in line with guidelines for health economic analyses in Slovakia [41].
Clinical Data
Baseline cohort characteristics were based on the sub-group of patients with a BMI > 35 kg/m2, extracted post-hoc from the SUSTAIN 7 clinical trial (Table 1). The number of cigarettes smoked per day and mean weekly alcohol consumption were unavailable from the trial data, and these were therefore based on Slovakia-specific data for the general population [42, 43].
Table 1.
Baseline cohort characteristics of patients with a BMI > 35 kg/m2 in SUSTAIN 7
| Characteristic | Mean (standard deviation) |
|---|---|
| Start age (years) | 53.94 (10.10) |
| Duration of diabetes (years) | 7.05 (5.34)a |
| Percentage male (%) | 48.28 |
| HbA1c (%) | 8.23 (0.92) |
| Systolic blood pressure (mmHg) | 134.83 (13.79) |
| Diastolic blood pressure (mmHg) | 82.54 (9.32) |
| Total cholesterol (mg/dl) | 180.00 (41.71) |
| HDL cholesterol (mg/dl) | 43.84 (10.51) |
| LDL cholesterol (mg/dl) | 99.96 (35.69) |
| Triglycerides (mg/dl) | 191.82 (109.02) |
| BMI (kg/m2) | 40.49 (5.26) |
| Percentage smokers (%) | 15.33 |
| Cigarettes per day | 14.70b |
| Alcohol consumption (oz/week) | 6.87c |
All data were taken from SUSTAIN 7, unless otherwise indicated
BMI body mass index, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein
aRounded to 7.00 in the analysis, as the model only accepts integer values for duration of diabetes
bBased on data published by the Statistical Office of the Slovak Republic [42]
cBased on data published by the World Health Organization [43]
Treatment effects relating to physiological parameters were also based on the sub-group of patients with a BMI > 35 kg/m2 in SUSTAIN 7 (Table 2). While pre-specified analyses assessed the statistical significance of treatment effect differences between once-weekly semaglutide 1 mg and dulaglutide 1.5 mg, post-hoc analyses were required to assess the statistical significance of treatment effect differences between once-weekly semaglutide 0.5 mg and dulaglutide 1.5 mg. These showed that once-weekly semaglutide 0.5 mg was associated with non-statistically significant reductions in HbA1c, cholesterol and BMI, while once-weekly semaglutide 1 mg was associated with statistically significant reductions in HbA1c and BMI and non-statistically significant reductions in diastolic blood pressure and cholesterol compared with dulaglutide 1.5 mg. Both statistically significant and non-statistically significant differences were applied, in line with modeling guidelines [44].
Table 2.
Treatment effects and adverse event rates included in the analysis
| Parameter | Mean (standard error) | ||
|---|---|---|---|
| Once-weekly semaglutide 0.5 mg | Once-weekly semaglutide 1 mg | Dulaglutide 1.5 mg | |
| Physiological parameters (applied in the first year of the analysis) | |||
| HbA1c (%) | − 1.52 (0.08) | − 1.72 (0.09)* | − 1.36 (0.09) |
| Systolic blood pressure (mmHg) | − 2.82 (1.13) | − 3.93 (1.18) | − 4.19 (1.16) |
| Diastolic blood pressure (mmHg) | − 0.83 (0.69) | − 2.03 (0.71) | − 1.04 (0.70) |
| Total cholesterol (mg/dl) | − 8.24 (2.43) | − 4.59 (2.52) | − 1.45 (2.48) |
| HDL cholesterol (mg/dl) | − 1.19 (0.46) | 0.35 (0.47) | 0.80 (0.46) |
| LDL cholesterol (mg/dl) | − 3.49 (2.07) | − 0.74 (2.14) | 2.17 (2.11) |
| Triglycerides (mg/dl) | − 23.71 (5.72) | − 24.66 (5.90) | − 25.54 (5.82) |
| BMI (kg/m2) | − 1.95 (0.17) | − 2.73 (0.18)* | − 1.32 (0.18) |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | − 2.71 (0.83) | − 3.15 (0.88) | − 3.39 (0.83) |
| Adverse event rates (applied in the first 3 years of the analysis) | |||
| Non-severe hypoglycemia events, per 100 patient years | 3.37 | 2.49 | 1.13 |
| Severe hypoglycemia events, per 100 patient years | 0.00 | 1.24 | 1.13 |
| Proportion of non-severe hypoglycemic events that are nocturnal | 0.00 | 0.00 | 0.00 |
| Proportion of severe hypoglycemic events that are nocturnal | 0.00 | 0.00 | 0.00 |
BMI body mass index, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein
*Statistically significant difference at 95% confidence level versus dulaglutide 1.5 mg
Treatment Duration, Intensification and Long-Term Parameter Progression
Patients were modeled to receive once-weekly semaglutide or dulaglutide for the first 3 years of the analysis, based on a 2017 review of the current treatment landscape of type 2 diabetes, which reported that the average duration of GLP-1 receptor agonist treatment was 29.4 months [45]. As treatment switching is only possible at the end of a given year in the IQVIA CORE Diabetes Model, this was rounded up to 3 years. This approach is in line with previous long-term cost-effectiveness analyses of GLP-1 receptor agonists submitted to the Scottish Medicines Agency (SMC) and the National Institute for Health and Care Excellence (NICE) and published in peer reviewed journals [46–51]. After 3 years, once-weekly semaglutide or dulaglutide treatment was discontinued, with patients intensifying to insulin glargine U100 (Abasaglar®). This assumption recognizes the progressive nature of type 2 diabetes, with intensification to basal insulin therapy often needed for patients to maintain glycemic control in the long term.
Benefits in HbA1c and BMI with once-weekly semaglutide and dulaglutide were maintained during the 3 years patients received these treatments. Following intensification to basal insulin, HbA1c was brought to 7.0% in all treatment arms, as the baseline cohort characteristics justified an HbA1c target of 7.0%, according to ADA and EASD guidelines for glycemic targets in patients with type 2 diabetes (which recommend individualizing HbA1c targets based on patient factors), while BMI reverted to baseline for the remainder of patient lifetimes [52]. This resulted in a balanced cost-effectiveness analysis, with differences in HbA1c and BMI maintained only while there were differences in costs. Changes in blood pressure and serum lipids were assumed to follow the natural progression algorithms built into the IQVIA CORE Diabetes Model in all arms, based on the UKPDS or Framingham data (as described by Palmer et al.) [35]. Sensitivity analyses were conducted to explore alternate treatment switching approaches and parameter progression assumptions.
Costs, Resource Use and Utilities
Costs were accounted from a Slovakian healthcare payer perspective and expressed in euros (EUR). Unit costs of diabetes medications and consumables were based on retail prices in Slovakia, with calculations reflecting the maximum reimbursement levels for needles and self-monitoring of blood glucose (SMBG) testing equipment for patients receiving basal insulin therapy, following treatment intensification.
Diabetes medication resource use was based on the sub-group of patients with a BMI > 35 kg/m2 in the SUSTAIN 7 trial, including the ubiquitous use of concomitant metformin, which was priced according to a weighted average of metformin products used in Slovakia. No SMBG use was associated with once-weekly semaglutide or dulaglutide treatment. No needles were required for administration, as they are included in the once-weekly semaglutide and dulaglutide packs.
Following intensification after 3 years, patients were assumed to receive 40 IU insulin glargine U100 (Abasaglar), based on the defined daily dose (DDD), with universal concomitant metformin use continuing in all treatment arms. Patients were assumed to use the maximum number of reimbursable needles (200) and SMBG test strips (300) and lancets (100) annually. The impact of the cost of consumables was assessed in the sensitivity analyses, which removed consumables use for all treatment arms. Resource use was used to calculate annual treatment costs (Table 3).
Table 3.
Annual costs in the base case analysis
| Item | Once-weekly semaglutide 0.5 mg | Once-weekly semaglutide 1 mg | Dulaglutide 1.5 mg | Basal insulin (intensification) |
|---|---|---|---|---|
| Annual medication cost | 1237.94 | 1237.94 | 1237.94 | 411.90 |
| Annual metformin cost | 39.81 | 39.81 | 39.81 | 39.81 |
| Annual needle cost | 0.00 | 0.00 | 0.00 | 26.30 |
| Annual SMBG testing cost | 0.00 | 0.00 | 0.00 | 77.98 |
| Total annual cost | 1277.75 | 1277.75 | 1277.75 | 556.00 |
All costs expressed in euros (EUR). The medication cost of Abasaglar was used for basal insulin
SMBG self-monitoring of blood glucose
Slovakia-specific costs of treating diabetes-related complications, in both the year of onset and annual follow-up costs, were identified from a published source [53]. Utilities relating to quality of life were taken from a 2014 review by Beaudet et al., with the exception of hypoglycemic event disutilities, which were sourced from a 2013 publication by Evans et al. (published after the literature searches by Beaudet et al. had been conducted) [54, 55].
Sensitivity Analyses
Sensitivity analyses were conducted to test the robustness of the base case results, thereby examining the uncertainty associated with long-term extrapolation of clinical and cost outcomes from short-term data. An analysis was performed with only statistically significant differences between the treatment arms applied. The impact of shortening the time horizon was examined by conducting analyses with 10- and 20-year time horizons applied, for which it should be noted that not all diabetes-related complications and associated costs were captured, as the duration of the analyses was not sufficient for all patients to have died. The influence of discounting on cost and clinical outcomes was assessed by applying discount rates of 0% and 10% in separate simulations. The impact of differences in HbA1c on overall outcomes was evaluated in an analysis with only the difference in HbA1c between the treatment arms applied in the once-weekly semaglutide arms, with all other parameters equal to the dulaglutide arm.
Alternative HbA1c and BMI progressions were explored in separate analyses, with one applying the UKPDS progression equation for HbA1c in all treatment arms, with differences between the treatment arms gradually abolished, and the other assuming BMI differences between the treatment arms were maintained for patient lifetimes. Variations in treatment effects were assessed in separate analyses through application of the upper and lower limits of the 95% confidence intervals of the estimated treatment differences in HbA1c and BMI.
Alternative treatment switching approaches were evaluated by bringing forward treatment switching to the end of year 2 and pushing treatment switching back to the end of year 5 in all arms, as well as HbA1c progression following the UKPDS equations and treatment switching occurring when HbA1c exceeded 7.5%. The impact of over- or underestimating the costs of complications was assessed by increasing and decreasing these costs by 10% in separate analyses, while the influence of the costs of consumables (needles and SMBG testing) was tested by performing simulations with these costs excluded. Separate analyses were also conducted with alternative BMI and hypoglycemia disutilities applied, which gave greater impact to weight changes and non-severe hypoglycemic events, respectively [56, 57]. An additional analysis was performed with a diminishing hypoglycemia disutility model applied [58].
PSA, which captured statistical uncertainty by sampling around parameter inputs, was conducted with a second-order Monte Carlo approach using the predefined function in the IQVIA CORE Diabetes Model. Sampling was applied to baseline characteristics, treatment effects, event risks, costs and utilities.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Base Case Analysis
Projections of long-term clinical outcomes in patients with a BMI > 35 kg/m2 with inadequate glycemic control on OADs indicated that once-weekly semaglutide 0.5 mg and 1 mg were associated with improvements in discounted life expectancy of 0.04 and 0.06 years, respectively, and discounted quality-adjusted life expectancy of 0.04 and 0.07 QALYs, respectively, versus dulaglutide 1.5 mg (Table 4). Clinical benefits resulted from a reduced incidence and delayed time to onset of diabetes-related complications in the once-weekly semaglutide arms over the 50-year time horizon of the analysis. Benefits were observed across all included micro- and macrovascular complications (Fig. 1).
Table 4.
Long-term cost-effectiveness outcomes in the base case analysis
| Health outcomes | Once-weekly semaglutide 0.5 mg | Dulaglutide 1.5 mg | Difference |
|---|---|---|---|
| Discounted life expectancy (years) | 11.61 (0.15) | 11.57 (0.15) | + 0.04 |
| Discounted quality-adjusted life expectancy (QALYs) | 7.19 (0.09) | 7.16 (0.10) | + 0.04 |
| Discounted direct costs (EUR) | 18,686 (399) | 18,706 (404) | − 20 |
| ICER based on life expectancy and direct costs | Once-weekly semaglutide 0.5 mg dominant | ||
| ICER based on quality-adjusted life expectancy and direct costs | Once-weekly semaglutide 0.5 mg dominant | ||
| Health outcomes | Once-weekly semaglutide 1 mg | Dulaglutide 1.5 mg | Difference |
|---|---|---|---|
| Discounted life expectancy (years) | 11.63 (0.15) | 11.57 (0.15) | + 0.06 |
| Discounted quality-adjusted life expectancy (QALYs) | 7.23 (0.10) | 7.16 (0.10) | + 0.07 |
| Discounted direct costs (EUR) | 18,566 (402) | 18,706 (404) | − 140 |
| ICER based on life expectancy and direct costs | Once-weekly semaglutide 1 mg dominant | ||
| ICER based on quality-adjusted life expectancy and direct costs | Once-weekly semaglutide 1 mg dominant | ||
Values are means (standard deviations)
EUR euros, ICER incremental cost-effectiveness ratio, QALYs quality-adjusted life years
Fig. 1.
Mean time to onset of diabetes-related complications
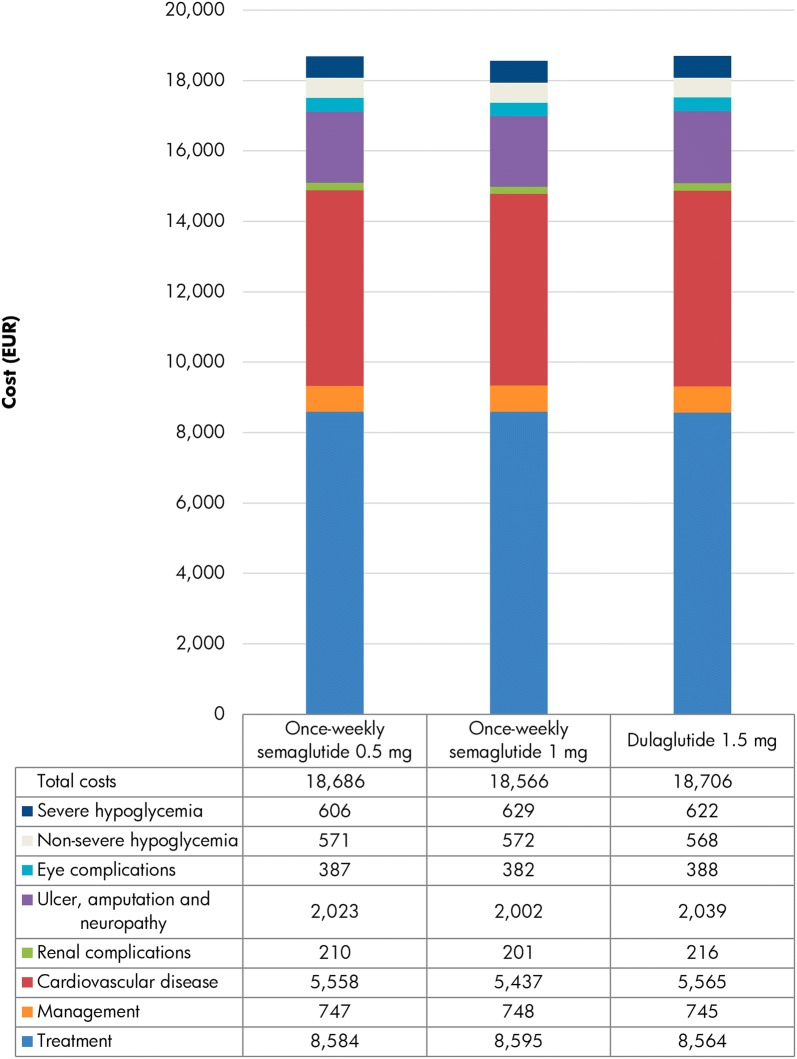
Long-term projections of direct medical costs suggested that the mean lifetime cost per patient was EUR 20 lower with once-weekly semaglutide 0.5 mg and EUR 140 lower with once-weekly semaglutide 1 mg versus dulaglutide 1.5 mg (Fig. 2). Treatment costs were EUR 20 and EUR 31 higher with once-weekly semaglutide 0.5 mg and 1 mg, respectively, versus dulaglutide 1.5 mg, as, despite equivalent unit costs, the increased survival and further treatment of patients over the long term led to increased expenditure. This was entirely offset by cost savings due to avoidance of diabetes-related complications, most notably ulcer, amputation and neuropathy complications and fewer severe hypoglycemic events with once-weekly semaglutide 0.5 mg (both yielding mean cost savings of EUR 16 per patient) and cardiovascular complications with once-weekly semaglutide 1 mg (yielding mean cost savings of EUR 128 per patient).
Fig. 2.
Direct costs over patient lifetimes. EUR, euros
With both improved life expectancy and quality-adjusted life expectancy, achieved at a reduced cost from a healthcare payer perspective, once-weekly semaglutide 0.5 mg and 1 mg were considered dominant versus dulaglutide 1.5 mg.
One- and Multi-Way Sensitivity Analyses
Sensitivity analyses, conducted around model inputs and assumptions, showed that the base case findings were robust to changes in these parameters, with once-weekly semaglutide 0.5 mg and 1 mg remaining dominant versus dulaglutide 1.5 mg in the majority of scenarios (Table 5). Application of only statistically significant differences between the treatment arms led to no differences between the once-weekly semaglutide 0.5 mg and dulaglutide 1.5 mg arms, as all differences in treatment effects between these arms observed in SUSTAIN 7 were not significant, while once-weekly semaglutide 1 mg was associated with comparable clinical benefits and cost savings to the base case analysis and remained dominant. Application of 10- and 20-year time horizons reduced the clinical benefits associated with once-weekly semaglutide treatment, but both doses remained dominant versus dulaglutide 1.5 mg. These outcomes demonstrate how once-weekly semaglutide is associated with long-term benefits, not all of which are captured with shorter time horizons. Altering the discount rate also reflected this, with clinical benefits for both doses of once-weekly semaglutide greatly increased with discount rates of 0% applied. Once-weekly semaglutide 1 mg remained cost saving and dominant, but once-weekly semaglutide 0.5 mg was associated with small cost increases, yielding an ICER of EUR 160 per QALY gained. Applying discount rates of 10% decreased the clinical benefits associated with once-weekly semaglutide 0.5 mg and 1 mg, but both remained cost saving and dominant versus dulaglutide 1.5 mg.
Table 5.
Sensitivity analysis results
| Analysis | Once-weekly semaglutide 0.5 mg versus dulaglutide 1.5 mg | Once-weekly semaglutide 1 mg versus dulaglutide 1.5 mg | ||||
|---|---|---|---|---|---|---|
| Difference in discounted quality-adjusted life expectancy (QALYs) | Difference in discounted direct costs (EUR) | ICER (EUR per QALY gained) | Difference in discounted quality-adjusted life expectancy (QALYs) | Difference in discounted direct costs (EUR) | ICER (EUR per QALY gained) | |
| Base case | + 0.04 | − 20 | Once-weekly semaglutide dominant | + 0.07 | − 140 | Once-weekly semaglutide dominant |
| Statistically significant differences only | 0.00 | 0 | Once-weekly semaglutide equally effective and equally costly | + 0.07 | − 99 | Once-weekly semaglutide dominant |
| 20-year time horizon | + 0.01 | − 21 | Once-weekly semaglutide dominant | + 0.04 | − 105 | Once-weekly semaglutide dominant |
| 10-year time horizon | + 0.02 | − 36 | Once-weekly semaglutide dominant | + 0.04 | − 53 | Once-weekly semaglutide dominant |
| 0% discount rates | + 0.08 | + 13 | 160 | + 0.14 | − 240 | Once-weekly semaglutide dominant |
| 5% discount rates | + 0.02 | − 26 | Once-weekly semaglutide dominant | + 0.05 | − 93 | Once-weekly semaglutide dominant |
| HbA1c difference only | + 0.02 | − 61 | Once-weekly semaglutide dominant | + 0.03 | − 130 | Once-weekly semaglutide dominant |
| BMI difference maintained for patient lifetimes | + 0.07 | − 20 | Once-weekly semaglutide dominant | + 0.15 | − 138 | Once-weekly semaglutide dominant |
| UKPDS HbA1c creep for duration of the analysis (no change upon treatment intensification) | + 0.02 | − 107 | Once-weekly semaglutide dominant | + 0.06 | − 116 | Once-weekly semaglutide dominant |
| Upper 95% CI of HbA1c estimated treatment difference | + 0.04 | − 113 | Once-weekly semaglutide dominant | + 0.09 | – 140 | Once-weekly semaglutide dominant |
| Lower 95% CI of HbA1c estimated treatment difference | + 0.02 | − 56 | Once-weekly semaglutide dominant | + 0.06 | − 82 | Once-weekly semaglutide dominant |
| Upper 95% CI of BMI estimated treatment difference | + 0.05 | − 74 | Once-weekly semaglutide dominant | + 0.08 | − 133 | Once-weekly semaglutide dominant |
| Lower 95% CI of BMI estimated treatment difference | + 0.02 | − 35 | Once-weekly semaglutide dominant | + 0.06 | − 143 | Once-weekly semaglutide dominant |
| Treatment switching at 2 years | + 0.03 | − 72 | Once-weekly semaglutide dominant | + 0.06 | − 64 | Once-weekly semaglutide dominant |
| Treatment switching at 5 years | + 0.02 | − 71 | Once-weekly semaglutide dominant | + 0.07 | − 111 | Once-weekly semaglutide dominant |
| Treatment switching at 7.5% HbA1c threshold (using UKPDS progression) | + 0.02 | − 157 | Once-weekly semaglutide dominant | + 0.10 | + 312 | 3137 |
| Cost of complications + 10% | + 0.04 | − 24 | Once-weekly semaglutide dominant | + 0.07 | − 158 | Once-weekly semaglutide dominant |
| Cost of complications − 10% | + 0.04 | − 16 | Once-weekly semaglutide dominant | + 0.07 | − 123 | Once-weekly semaglutide dominant |
| Cost of consumables excluded | + 0.04 | − 24 | Once-weekly semaglutide dominant | + 0.07 | − 146 | Once-weekly semaglutide dominant |
| Lee et al. BMI disutility applied | + 0.03 | − 20 | Once-weekly semaglutide dominant | + 0.08 | − 140 | Once-weekly semaglutide dominant |
| Diminishing hypoglycemia disutility applied | + 0.04 | − 20 | Once-weekly semaglutide dominant | + 0.07 | − 140 | Once-weekly semaglutide dominant |
| Currie et al. hypoglycemia disutility applied | + 0.04 | − 20 | Once-weekly semaglutide dominant | + 0.07 | − 140 | Once-weekly semaglutide dominant |
BMI body mass index, CI confidence interval, EUR euros, HbA1c glycated hemoglobin, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life year
Analyses conducted with only the treatment differences in HbA1c applied showed that these reductions were a substantial contributor to overall clinical outcomes, with both doses of once-weekly semaglutide remaining dominant versus dulaglutide 1.5 mg. Maintaining the BMI differences after treatment intensification to cover patient lifetimes resulted in increased incremental clinical benefits with once-weekly semaglutide, with incremental costs remaining similar to the base case analysis. Modeling HbA1c using the UKPDS progression equation following application of treatment effects in the first year of the analysis led to decreased quality-adjusted life expectancy in all treatment arms, but both doses of once-weekly semaglutide remained dominant.
Applying the upper limit of the 95% confidence interval of the estimated treatment differences in HbA1c resulted in maintained incremental benefits from the base case for once-weekly semaglutide 0.5 mg and increased incremental benefits for once-weekly semaglutide 1 mg, while use of the lower limit led to decreased clinical benefits for both doses. Application of the upper limit for differences in BMI resulted in increased incremental benefits for both once-weekly semaglutide 0.5 mg and 1 mg, while use of the lower limit had the opposite effect, with benefits reduced.
Bringing treatment switching forward to the end of year 2 resulted in reduced clinical benefits for once-weekly semaglutide 0.5 mg and 1 mg, but both remained dominant versus dulaglutide 1.5 mg. Pushing treatment switching back to the end of year 5 led to reduced incremental clinical benefits for once-weekly semaglutide 0.5 mg but increased clinical benefits for once-weekly semaglutide 1 mg, and both remained cost saving and dominant versus dulaglutide 1.5 mg. Applying the UKPDS progression equation and having treatment switching occur when HbA1c exceeded 7.5% resulted in reduced clinical benefits but increased cost savings for once-weekly semaglutide 0.5 mg and greater clinical benefits but increased incremental costs for once-weekly semaglutide 1 mg, yielding an ICER of EUR 3137 per QALY gained.
Increasing and decreasing the costs of complications by 10% resulted in only minor changes to incremental cost outcomes, and both doses of once-weekly semaglutide remained dominant. Similarly, excluding the costs of complications did not drastically alter cost outcomes, and once-weekly semaglutide 0.5 mg and 1 mg remained dominant versus dulaglutide 1.5 mg. Use of alternative disutilities for BMI and hypoglycemia resulted in only minor changes to clinical outcomes, and both doses of once-weekly semaglutide remained dominant versus dulaglutide 1.5 mg.
Probabilistic Sensitivity Analysis
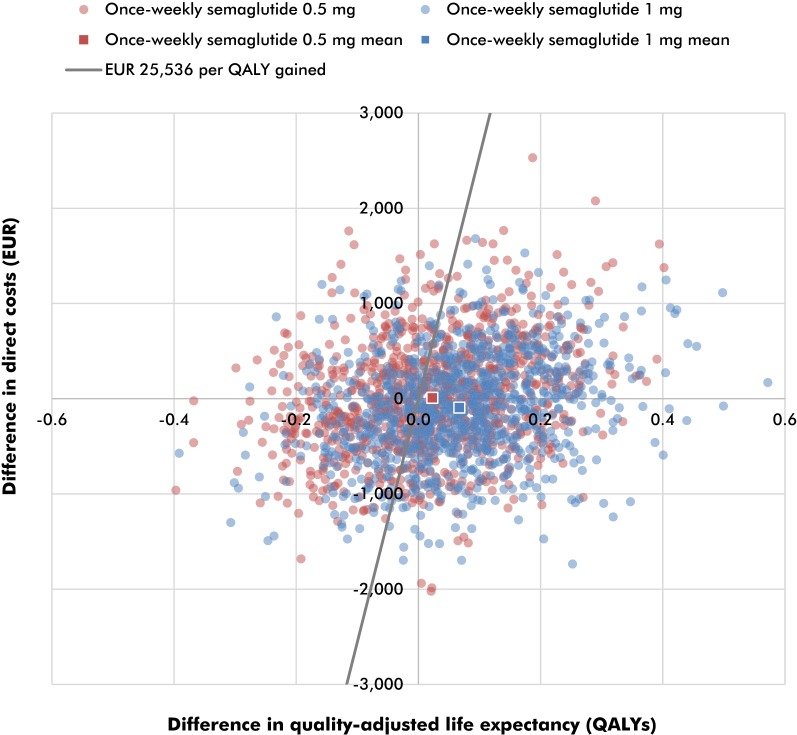
PSA, performed with sampling around cohort characteristics, treatment effects, complication costs and utilities, displayed comparable mean outcomes to the base case analysis, but yielded increased measures of variance around the mean results (Fig. 3). The mean incremental improvements in quality-adjusted life expectancy with once-weekly semaglutide 0.5 mg and 1 mg were 0.02 and 0.07 QALYs, respectively, versus dulaglutide 1.5 mg. Mean costs were estimated to be EUR 9 higher with once-weekly semaglutide 0.5 mg and EUR 93 lower with once-weekly semaglutide 1 mg versus dulaglutide 1.5 mg. Therefore, once-weekly semaglutide 0.5 mg was associated with an ICER of EUR 401 per QALY gained, while once-weekly semaglutide 1 mg was considered dominant versus dulaglutide 1.5 mg in the PSA. Based on a willingness-to-pay threshold of EUR 25,536 per QALY gained (the lowest possible threshold for an intervention in 2018, 28 times the average monthly wage in Slovakia), the modeling analysis indicated that the probabilities of once-weekly semaglutide 0.5 mg and 1 mg being cost-effective were 57.1% and 72.4%, respectively, versus dulaglutide 1.5 mg.
Fig. 3.
Probabilistic sensitivity analysis scatterplot. EUR, euros; QALY, quality-adjusted life year. Based on a willingness-to-pay threshold of EUR 25,536 per QALY gained (the lowest possible threshold for an intervention in 2018, 28 times the average monthly wage in Slovakia), the modeling analysis indicated that the probabilities of once-weekly semaglutide 0.5 mg and 1 mg being cost-effective were 57.1% and 72.4%, respectively, versus dulaglutide 1.5 mg
Discussion
The present analysis indicated that once-weekly semaglutide 0.5 mg and 1 mg are dominant treatment options versus dulaglutide 1.5 mg for the treatment of patients with type 2 diabetes with inadequate glycemic control on metformin and with a BMI > 35 kg/m2 in Slovakia. Clinical outcomes of life expectancy and quality-adjusted life expectancy were improved with once-weekly semaglutide treatment, with a delayed time to onset and reduced cumulative incidence of diabetes-related complications leading to lifetime cost savings compared with dulaglutide 1.5 mg from a healthcare payer perspective.
Treatment guidelines for type 2 diabetes in Slovakia follow international recommendations published by the ADA and EASD [18]. These position GLP-1 receptor agonists as a second-line therapy (alongside sodium-glucose cotransporter-2 [SGLT-2] inhibitors) in patients with inadequate glycemic control on metformin, particularly for patient groups with high cardiovascular disease risk or those looking to avoid weight gain. The use of data from a head-to-head trial, with a patient population with inadequate glycemic control on metformin and a BMI > 35 kg/m2, represents a key strength of the present analysis. Additionally, in Slovakia, dulaglutide is currently reimbursed only in patients with a BMI > 35 kg/m2, and so the choice of this sub-group from SUSTAIN 7 provides pertinent information for a Slovakian healthcare payer considering alternative GLP-1 receptor agonist therapies. The clinical benefits observed with once-weekly semaglutide in this patient sub-group are also seen in the full population of SUSTAIN 7, and once-weekly semaglutide remains consistently efficacious across sub-groups versus numerous comparators throughout the SUSTAIN trial program [59–61]. Long-term analyses conducted in the UK and Canada have shown that these short-term benefits in the full population of SUSTAIN 7 translate to further benefits over patient lifetimes [51, 62]. Once-weekly semaglutide is therefore likely to remain a cost-effective treatment option versus dulaglutide in Slovakia if weight restrictions are removed from the reimbursement criteria.
An aspect of treatment that could not be incorporated into the present analysis was the cardiovascular benefits offered by GLP-1 receptor agonists, as health economic models for diabetes are yet to incorporate risk equations based on cardiovascular outcome studies. Once-weekly semaglutide has been associated with statistically significant reductions in the risk of MACE versus placebo in SUSTAIN 6, while preliminary results from REWIND suggest dulaglutide is also associated with superiority, although the extent of these improvements are yet to be fully disseminated [29, 33]. GLP-1 analog liraglutide has also been associated with improved cardiovascular outcomes versus placebo in LEADER, but exenatide ER and lixisenatide did not display benefits compared with placebo in EXSCEL and ELIXA, respectively, indicating that cardiovascular benefits are not innate across the GLP-1 receptor agonist class and are instead specific to certain medications [30–32]. Further studies are needed to elucidate differences in cardiovascular outcomes between GLP-1 receptor agonist therapies. However, given the benefits in other aspects of treatment once-weekly semaglutide offers, the current evidence supports reimbursement of once-weekly semaglutide in Slovakia.
A limitation of the study, inherent to all long-term health economic analyses, was the projection of long-term outcomes from relatively short-term clinical trial data. However, in the absence of long-term data, this method represents the best available option for evaluating diabetes medications, and projecting outcomes over patient lifetimes is recommended in the guidelines for assessing the cost-effectiveness of diabetes interventions [38]. The use of a published and extensively validated model, as well as numerous sensitivity analyses that test the assumptions and data inputs used, also gives weight to the results and supports the conclusion that once-weekly semaglutide is cost-effective versus dulaglutide 1.5 mg [36, 37].
Conclusions
Once-weekly semaglutide 0.5 mg and 1 mg represent cost-saving treatment options versus dulaglutide 1.5 mg for the treatment of patients with type 2 diabetes with inadequate glycemic control on metformin and a BMI > 35 kg/m2 in Slovakia.
Acknowledgements
Funding
The present cost-effectiveness analysis, open access fee and article processing charges were supported by funding from Novo Nordisk A/S. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Monika Russel-Szymczyk is an employee of Novo Nordisk Pharma Sp. z o.o. Marek Psota is an employee of Pharm-In spol. s.r.o, which received consulting fees from Novo Nordisk s.r.o to support preparation of the analysis. Lucia Hlavinkova is an employee of Novo Nordisk Slovakia s.r.o. Samuel Malkin is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis. Barnaby Hunt is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8010869.
References
- 1.International Diabetes Federation. Diabetes Atlas—8th Edition. 2017. http://www.diabetesatlas.org/across-the-globe.html. Accessed Dec 14, 2018.
- 2.International Diabetes Federation. IDF Europe Members—Slovakia. 2018. https://www.idf.org/our-network/regions-members/europe/members/157-slovakia.html. Accessed Dec 19, 2018.
- 3.World Health Organization. Diabetes country profiles—Slovakia. 2016. https://www.who.int/diabetes/country-profiles/svk_en.pdf. Accessed Dec 20, 2018.
- 4.Jönsson B, CODE-2 Advisory Board. Revealing the cost of Type II diabetes in Europe. Diabetologia. 2002;45(7):S5–12. [DOI] [PubMed]
- 5.Ismail-Beigi F, Craven T, Banerji MA, ACCORD trial group et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel A, MacMahon S, Chalmers J, ADVANCE Collaborative Group et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMicm066227. [DOI] [PubMed] [Google Scholar]
- 7.Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38. doi: 10.1016/j.ahj.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 10.Kearney PM, Blackwell L, Collins R, Cholesterol Treatment Trialists’ Collaborators et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 13.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- 16.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell-Jones D, Vaag A, Schmitz O, Liraglutide Effect and Action in Diabetes 5 [LEAD-5] met+SU Study Group et al. (Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152–160. doi: 10.2337/diaspect.29.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 Diabetes in a Randomized Controlled Trial (AWARD-5) Diabetes Care. 2014;37(8):2149–58. doi: 10.2337/dc13-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 23.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 24.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide er in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 25.Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355–366. doi: 10.1016/S2213-8587(17)30085-2. [DOI] [PubMed] [Google Scholar]
- 26.Pratley RE, Aroda VR, Lingvay I, SUSTAIN 7 investigators et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 27.Witkowski M, Wilkinson L, Webb N, Weids A, Glah D, Vrazic H. A systematic literature review and network meta-analysis comparing once-weekly semaglutide with other GLP-1 receptor agonists in patients with type 2 diabetes previously receiving 1-2 oral anti-diabetic drugs. Diabetes Ther. 2018;9(3):1149–1167. doi: 10.1007/s13300-018-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma R, Wilkinson L, Vrazic H, et al. Comparative efficacy of once-weekly semaglutide and SGLT-2 inhibitors in type 2 diabetic patients inadequately controlled with metformin monotherapy: a systematic literature review and network meta-analysis. Curr Med Res Opin. 2018;34(9):1595–1603. doi: 10.1080/03007995.2018.1476332. [DOI] [PubMed] [Google Scholar]
- 29.Marso SP, Bain SC, Consoli A, SUSTAIN-6 Investigators et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 30.Marso SP, Daniels GH, Brown-Frandsen K, LEADER Trial Investigators et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 33.Eli Lilly. Trulicity® (dulaglutide) demonstrates superiority in reduction of cardiovascular events for broad range of people with type 2 diabetes. 2018. https://investor.lilly.com/node/39796/pdf. Accessed Nov 22, 2018.
- 34.Smatana M, Pažitný P, Kandilaki D, et al. Slovakia: health system review. Health Syst Transit. 2016;18(6):1–210. [PubMed] [Google Scholar]
- 35.Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 36.Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–S40. doi: 10.1185/030079904X2006. [DOI] [PubMed] [Google Scholar]
- 37.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS core diabetes model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- 39.Statistical Office of the Slovak Republic. Life tables. 2017. http://www.infostat.sk/vdc/en/index.php?option=com_content&view=article&id=17&Itemid=18. Accessed 28 Sept 2018.
- 40.Clarke PM, Gray AM, Briggs A, UK Prospective Diabetes Study [UKDPS] Group, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59. [DOI] [PubMed]
- 41.Ministerstvo zdravotníctva Slovenskej republiky [Ministry of Health of the Slovak Republic]. Metodická pomôcka pre vykonávanie farmako-ekonomického rozboru lieku, medicínsko-ekonomického rozboru zdravotníckej pomôcky amedicínsko-ekonomického rozboru dietetickej potraviny [Methodologic guideline for conduction of health economic analysis of a drug, medical device and dietic food]. 2009. http://www.health.gov.sk/?farmako-ekonomicky-rozbor-lieku. Accessed Sept 28, 2018.
- 42.Eurostat, Statistical Office of the Slovak Republic. European Health Interview Survey (EHIS). 2009. https://ec.europa.eu/eurostat/web/microdata/european-health-interview-survey. Accessed 28 Sept 2018.
- 43.World Health Organization. European Health Information Gateway – Pure alcohol consumption, litres per capita, age 15+. 2014. https://gateway.euro.who.int/en/indicators/hfa_426-3050-pure-alcohol-consumption-litres-per-capita-age-15plus/visualizations/#id=19443. Accessed Sept 28, 2018.
- 44.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–6. Value Health. 2012;15(6):835–42. [DOI] [PubMed]
- 45.Heap G. Type 2 diabetes: Current Treatment. Detailed, Expanded Analysis (EU 5). http://www.decisionresourcesgroup.com. Accessed Nov 6, 2018.
- 46.Scottish Medicines Consortium (prepared by Novo Nordisk A/S). Insulin degludec/liraglutide 100 units/ml/3.6 mg/ml solution for injection pre-filled pen (Xultophy®), SMC No. (1088/15). 2015. https://www.scottishmedicines.org.uk/files/advice/insulin_degludec_liraglutide__Xultophy_FINAL_Sept_2015_for_website.pdf. Accessed Dec 13, 2017.
- 47.National Institute for Health and Care Excellence. Final appraisal determination: liraglutide for the treatment of type 2 diabetes mellitus. 2011. https://www.nice.org.uk/guidance/ta203/documents/diabetes-liraglutide-final-appraisal-determination3. Accessed Dec 13, 2017.
- 48.Hunt B, Vega-Hernandez G, Valentine WJ, Kragh N. Evaluation of the long-term cost-effectiveness of liraglutide versus lixisenatide for treatment of type 2 diabetes mellitus in the UK setting. Diabetes Obes Metab. 2017;19(6):842–849. doi: 10.1111/dom.12890. [DOI] [PubMed] [Google Scholar]
- 49.Mezquita-Raya P, Ramírez de Arellano A, Kragh N, et al. Liraglutide versus lixisenatide: long-term cost-effectiveness of GLP-1 receptor agonist therapy for the treatment of type 2 diabetes in Spain. Diabetes Ther. 2017;8(2):401–15. doi: 10.1007/s13300-017-0239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt B, Kragh N, McConnachie CC, Valentine WJ, Rossi MC, Montagnoli R. Long-term cost-effectiveness of two GLP-1 receptor agonists for the treatment of type 2 diabetes mellitus in the italian setting: liraglutide versus lixisenatide. Clin Ther. 2017;39(7):1347–1359. doi: 10.1016/j.clinthera.2017.05.354. [DOI] [PubMed] [Google Scholar]
- 51.Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for the treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–21. doi: 10.1111/dom.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–42. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 53.Psota M, Ondrušová M, Pšenková M, Martinka E, Ilavská A. Využívanie zdravotnej starostlivosti a nákladovosť liečby diabetu a jeho komplikácií pre potreby hodnotenia nákladovej efektívnosti zdravotníckych intervencií pomocou modelu CORE na Slovensku [The use of health care and the cost of diabetes treatment and its complications for the needs of assessing the cost effectiveness of health interventions using the CORE model in Slovakia]. 2. aktualizované vydanie [2nd updated edition]. Bratislava, Pharm-In. 2017. https://www.pharmin.sk (ISBN 978-80-89815-11-1).
- 54.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–470. doi: 10.1016/j.jval.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11(1):90. doi: 10.1186/1477-7525-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee AJ, Morgan CL, Morrissey M, Wittrup-Jensen KU, Kennedy-Martin T, Currie CJ. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with Type 1 diabetes, Type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22(11):1482–1486. doi: 10.1111/j.1464-5491.2005.01657.x. [DOI] [PubMed] [Google Scholar]
- 57.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]
- 58.Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–2650. doi: 10.1007/s11136-014-0712-x. [DOI] [PubMed] [Google Scholar]
- 59.Bain S, Araki E, Desouza C, et al. Semaglutide reduces HbA1c across baseline HbA1c subgroups across SUSTAIN 1–5 clinical trials. Diabetes. 2017;66(Suppl 1):A298–99. [Google Scholar]
- 60.Leiter L, Charpentier G, Chaykin L, et al. Semaglutide reduces body weight across baseline bmi subgroups across SUSTAIN 1–5. Can J Diabetes. 2017;41(5):S6. doi: 10.1016/j.jcjd.2017.08.020. [DOI] [Google Scholar]
- 61.Viljoen A, Bluher M, Chow FCC, et al. Semaglutide reduces body weight vs. dulaglutide across baseline BMI subgroups in SUSTAIN 7. Diabetes. 2018;67(Suppl 1).
- 62.Johansen P, Håkan-Bloch J, Liu AR, Bech PG, Persson S, Leiter LA. Cost Effectiveness of Once-Weekly Semaglutide Versus Once-Weekly Dulaglutide in the Treatment of Type 2 Diabetes in Canada. Pharmacoecon Open. 2019. 10.1007/s41669-019-0131-6 (epub ahead of print). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.