Abstract
This retrospective study estimated healthcare resource use (HRU), symptoms and toxicities (SxTox), and costs in relapsed/refractory (R/R) patients with acute myeloid leukemia (AML), stratified by hematopoietic stem cell transplantation (HSCT) status. Claims data were used to identify adult patients with AML diagnoses from 1 January 2008 to 31 March 2016 in the USA. Patients were considered R/R if they had an AML relapse ICD-9 code (205.02) or a line of therapy consistent with R/R disease. The final R/R sample (N = 707) included 476 patients with and 231 patients without HSCT. The mean total episode cost (from relapse date to death or end of study period) for all patients was $439,104 (with HSCT $524,595 and without HSCT $263,310). Inpatient visits accounted for the greatest cost component (mean $308,978) followed by intensive care unit stays (mean $221,537), non-clinician (e.g., lab tests) visits (mean $30,909), and outpatient pharmacy utilization (mean $24,640). Patients with HSCT appeared to have longer episodes of care compared with patients without HSCT (16.8 vs 11.1 months), perhaps reflecting longer survival for HSCT patients. Mean number of visits within each category and their associated costs appeared to be higher in patients with HSCT compared with patients without HSCT. Patients with HSCT appeared to experience more SxTox compared with patients without HSCT across all categories. Results of the current study suggest that there is a substantial HRU and cost burden on R/R AML patients in the USA receiving active treatments. More effective therapies with improved tolerability would meet this tremendous unmet need in the R/R AML population.
Funding: Astellas Pharma, Inc.
Keywords: Acute myeloid leukemia, Cost, Healthcare burden, Healthcare utilization, Relapsed/refractory, Symptoms and toxicities
Introduction
Acute myeloid leukemia (AML) is a clonal disorder of hematopoietic stem cells and is estimated to affect 19,520 new patients and lead to 10,670 deaths in the USA in 2018 [1]. The 5-year survival rate after diagnosis is 27.4%, and is lower in patients 65 years or older and in patients with fms-like tyrosine kinase 3 (FLT3) mutations [2]. Despite an initial response to treatment, AML may progress rapidly and disease relapse is common [3]. Relapsed/refractory (R/R) AML is associated with a poor prognosis [4].
AML is associated with high healthcare resource use [5, 6]. Of note, the costs related to hematopoietic stem cell transplantation (HSCT) have increased dramatically over time, primarily associated with hospital costs [7]. The frequency of HSCT in the USA has increased steadily since 2000, with over 3000 allogeneic HSCTs having been performed in patients with AML in 2016 [8, 9]. Therefore, new data regarding the cost of transplant episodes is of special interest because of the increase in frequency of transplant in the disease and because of known escalating costs.
The economic burden associated with R/R AML is poorly defined and not well studied, in part because AML symptoms and treatment-related adverse events can overlap. Detailed real-world evidence regarding clinical disease burden and cost estimates associated with R/R AML treatment episodes is scarce. Given the lack of evidence, the objective of this study was to assess healthcare resource use and costs along with symptoms and toxicities (SxTox) associated with R/R AML and its treatment. Traditional claims database study methods typically rely on diagnostic codes alone to identify relapsed but not refractory AML patients, potentially including more false-positive cases, and losing false-negative cases in the process. This is mainly due to coding errors, as providers may not specify patient relapse in their coding, instead using the general code of AML throughout the disease course. By contrast, the current study was not limited to diagnostic codes, but also involved extensive analysis of treatment patterns. It was conducted to yield a sample more likely to include only patients that truly had R/R disease (i.e., excluded non-R/R), and did not erroneously filter out patients who did have R/R disease and were not coded as such.
Methods
Study Design and Data Source
This retrospective analysis was conducted using IQVIA’s Real-World Data (RWD) Adjudicated Claims Database—US [formerly known as PharMetrics Plus (P+)], using data from the study period of January 2007 through June 2016. The IQVIA RWD Adjudicated Claims Database is one of the largest US health plan claims databases. The aggregated database comprises adjudicated claims from more than 150 million unique enrollees across the USA. RWD Adjudicated Claims data have a diverse representation of geography, employers, payers, providers, and therapy areas. Records in the database are considered representative of the national, commercially insured population in terms of age and gender. Standard fields include inpatient and outpatient diagnoses and procedures, and retail and mail-order prescription records and payments. All data are Health Insurance Portability and Accountability Act (HIPAA) compliant to protect patient privacy.
Patient Selection
Eligible patients were required to have a diagnosis of AML in the Adjudicated Claims Database during the sample selection window (1 January 2008 to 31 March 2016), evidenced by at least two non-ancillary outpatient claims with an AML diagnosis more than 30 days apart or at least one inpatient claim with an AML diagnosis. Additional requirements included continuous health plan enrollment of at least 6 months pre- and at least 3 months post-initial AML diagnosis date and no observed AML diagnoses at least 6 months prior to the initial AML diagnosis date (to ensure patients were newly diagnosed with AML). Patients were excluded if they had a diagnosis of acute lymphoblastic leukemia, or a diagnosis of other primary or secondary malignancies (except for chronic myeloid leukemia, myelodysplastic syndrome, and secondary AML) during the 6-month period before the initial AML diagnosis date.
The R/R subgroup was further defined by either an ICD-9 diagnosis code for relapsed AML (205.02; “code” patients) or the observation of a new line of therapy (LOT). A new LOT was defined as initiation of treatment following a 90-day chemotherapy-free period after the last observed chemotherapy of interest, or upon initiation of treatment with any new chemotherapy agent following completion of the first 60 days of high- or low-intensity chemotherapy treatment. In cases where both the diagnosis code and a second LOT were observed, the earlier event defined the R/R episode start date (index date). The R/R cohort was validated by a detailed examination of observed LOTs (i.e., specific chemotherapy drugs received, temporal relationship between LOTs [onset/offset timing and the criteria used to determine each], HSCT, and relapsed ICD codes) guided by clinical expert oncologists. Patients indexed to the relapsed disease code were required to have evidence of treatment (i.e., claims for chemotherapy or HSCT) within 30 days after the R/R episode start date, to validate the occurrence of relapsed disease. Episodes of care started 2 weeks prior to the R/R diagnosis code or second LOT and ended at the end of the follow-up period.
Measures
Baseline Demographic and Clinical Characteristics
Demographic characteristics were measured at the time of the initial AML diagnosis and included age, sex, geographic region (Northeast, South, Midwest, West), and payer type (commercial, self-insured, Medicare Risk, Medicaid, unknown). Clinical characteristics were measured over the 6 months before the initial AML diagnosis date. Diagnosis codes were used to identify general comorbid conditions of interest that included cardiovascular, metabolic, hepatic, pulmonary, musculoskeletal, and nervous system illnesses. Charlson Comorbidity Index (CCI) scores were calculated excluding AML diagnoses.
Episode Durations, Resource Utilization, and Costs
All-cause healthcare resource utilization (HRU) was reported during the R/R period for the following resource categories: physician office visits, emergency department (ED) visits, inpatient visits, and pharmacy utilization (outpatient). Length of stay was defined as the number of days in the hospital per hospitalization. Hospital days were defined as the number of days in the hospital over the entire R/R episode. All-cause healthcare costs were reported as total costs incurred in addition to the following cost components: physician office visits, emergency department (ED) visits, inpatient visits, and outpatient pharmacy utilization. Healthcare resource utilization and cost accrual began 2 weeks before the observation of the R/R diagnosis code indicative of relapsed disease or a second line of therapy (whichever occurred first if both were observed) and ended at the end of the follow-up period or end of health plan eligibility. Category costs were reported for the total cohort (e.g., the mean inpatient costs for the total R/R cohort irrespective of whether or not every patient incurred inpatient costs) as well as for the subset of patients that incurred costs in each category (e.g., the mean inpatient costs for patients with at least one inpatient visit). All costs were adjusted to 2016 US dollars using the Medical Care Consumer Price Index for All Urban Consumers [10].
Symptoms and Toxicities (SxTox)
SxTox events measured during episodes were identified via diagnostic and treatment codes and reported as frequencies for the following categories of SxTox: blood and lymphatic system disorders, gastrointestinal disorders, bleeding events, infections and infestations, nervous system disorders, skin and subcutaneous tissue disorders, vascular disorders, renal disorders, liver disorders, and cardiovascular disorders.
Statistical Analyses
Descriptive analyses included reporting the frequency (number of patients, N) and percentage (%) for each cohort for categorical measures. For continuous variables, both the mean (standard deviation, SD) and median were reported. Generalized linear models (GLMs) with a gamma distribution and log link function were used to assess the contribution of SxTox to total episode costs while controlling for age, gender, and CCI score. The dependent variable was total R/R episode cost and the independent variables included age, gender, CCI score, and the SxTox categories. Bleeding events and infections were excluded from the final model as they were correlated with blood and lymphatic system disorders. The results of independent variable parameter estimates were reported as exponentiated values with corresponding 95% confidence intervals to provide the relative contribution to adverse event costs. The significance threshold was set at 0.05. Except for the GLMs, statistical comparisons between cohorts were not conducted. All analyses used SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Compliance with Ethics Guidelines
As IQVIA is the PharMetrics Plus database owner, permission to use the database was not necessary. All data are de-identified and fully compliant with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. As such, no institutional review board approval was required. Informed consent was also not applicable to this study.
Results
Patient Characteristics and Episodes of Care
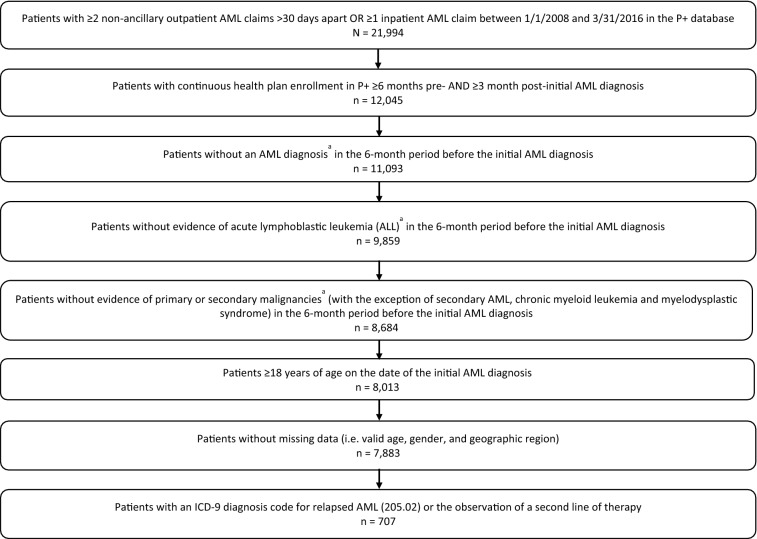
After the application of study inclusion and exclusion criteria, 7883 patients with newly diagnosed AML were initially identified. Of these, 707 (9.0%) patients who had received treatment were further identified as R/R as per the above definition. Patient attrition is described in detail in Fig. 1. Evidence of HSCT for R/R AML was observed in 476 patients. Autologous transplant was observed in 14 patients, allogeneic was observed in 242 patients, 4 patients received both autologous and allogeneic transplants, and transplant type could not be classified in 216 patients because of the lack of specificity of the observed billing code.
Fig. 1.
Study sample attrition. a≥ 2 outpatient claims > 30 days apart with the same diagnosis (at 3-digit ICD-9, 2-digit ICD-10 level) or ≥ 1 inpatient claim. AML acute myeloid leukemia
Baseline demographic and clinical characteristics are described in detail in Table 1. Mean (SD) age of the total sample was 52.0 (12.5) years and, although statistical comparisons were not performed, appeared to be similar among patients with HSCT and those without HSCT. Gender appeared to be similarly distributed among the groups. Patient distribution was weighted toward the southern USA, and the majority were commercially insured. Mean (SD) all-cause health care costs during the 6-month baseline period were $11,152 ($21,882) overall and appeared to be slightly higher among R/R patients with HSCT compared to R/R patients without HSCT ($11,259 vs $10,931). The mean (SD) CCI score (excluding cancer) was 0.9 (1.4) overall at time of the initial AML diagnosis and appeared to be slightly higher in the R/R sample without HSCT. The most frequently observed comorbid conditions were hypertension, dyslipidemia, and osteoarthritis.
Table 1.
Patient characteristics
| Characteristic | R/R total (N = 707) | R/R with HSCT (n = 476) | R/R without HSCT (n = 231) |
|---|---|---|---|
| Age, mean years (SD) | 52.0 (12.5) | 51.2 (11.6) | 53.6 (13.9) |
| Sex, n (%) male | 377 (53.3) | 258 (54.2) | 119 (51.5) |
| Geographic region, n (%) | |||
| Northeast | 175 (24.8) | 123 (25.8) | 52 (22.5) |
| Midwest | 211 (29.8) | 149 (31.3) | 62 (26.8) |
| South | 264 (37.3) | 170 (35.7) | 94 (40.7) |
| West | 57 (8.1) | 34 (7.1) | 23 (10.0) |
| Payer type, n (%) | |||
| Commercial | 440 (62.2) | 300 (63.0) | 140 (60.6) |
| Self-insured | 245 (34.7) | 167 (35.1) | 78 (33.8) |
| Medicare risk | 5 (0.7) | 1 (0.2) | 4 (1.7) |
| Medicaid | 11 (1.6) | 5 (1.1) | 6 (2.6) |
| Unknown | 6 (0.8) | 3 (0.6) | 3 (1.3) |
| Charlson Comorbidity Index (CCI), mean (SD) | 0.9 (1.4) | 0.9 (1.3) | 1.1 (1.5) |
| Baseline comorbid conditions, n (%) | |||
| Asthma | 36 (5.1) | 24 (5.0) | 12 (5.2) |
| Cardiac arrhythmia | 46 (6.5) | 27 (5.7) | 19 (8.2) |
| Chronic pain/fibromyalgia | 40 (5.7) | 23 (4.8) | 17 (7.4) |
| Depression | 39 (5.5) | 25 (5.3) | 14 (6.1) |
| Diabetes | 78 (11.0) | 47 (9.9) | 31 (13.4) |
| Dyslipidemia | 201 (28.4) | 142 (29.8) | 59 (25.5) |
| Hypertension | 218 (30.8) | 137 (28.8) | 81 (35.1) |
| Liver/gallbladder/pancreatic disease | 36 (5.1) | 22 (4.6) | 14 (6.1) |
| Myocardial infarction/CAD | 51 (7.2) | 27 (5.7) | 24 (10.4) |
| Osteoarthritis | 161 (22.8) | 110 (23.1) | 51 (22.1) |
| Sleep disorders | 47 (6.6) | 37 (7.8) | 10 (4.3) |
| Smoking or history of smoking | 61 (8.6) | 36 (7.6) | 25 (10.8) |
| Thyroid disease | 50 (7.1) | 34 (7.1) | 16 (6.9) |
CAD coronary artery disease, HSCT hematopoietic stem cell transplantation, R/R relapsed/refractory
Episodes of care started 2 weeks prior to the R/R diagnosis code or second LOT and ended at the end of the follow-up period. The follow-up period could end for any of the following reasons: loss of insurance coverage in P+ or change of insurance where the new insurance plan is not included in P+, death, or the end of study data availability. The mean (SD) duration R/R episodes of care was 15.0 (15.4) months. Patients with HSCT appeared to have longer episodes of care as compared with R/R patients without HSCT (16.8 [16.4] months vs 11.1 [12.5] months).
Resource Utilization and Costs
Most patients (97.6%) had at least one physician office visit (mean [SD] number of visits 76.8 [71.2]). The mean (SD) cost for all physician office visits during R/R episodes was $10,926 ($13,645) per patient among those patients with at least one visit. Similarly, most patients (90.1%) utilized pharmacy outpatient services at a mean (SD) cost for all prescriptions during R/R episodes of $24,640 ($46,275) per patient among those patients with at least one prescription filled. Over half of R/R patients (54.5%) had at least one ED visit (mean [SD] 3.4 [10.0] visits) that did not lead to hospital admission at a mean (SD) cost for all ED visits during R/R episodes of $4301 ($20,941) per patient among those patients with at least one visit. Hospitalization occurred in 93.9% of patients (mean [SD] number of hospitalizations 4.5 [3.9]) at a mean (SD) cost for all hospitalizations during R/R episodes of $308,978 ($306,987) per patient among those patients with at least one hospitalization (representing the highest cost category). Mean (SD) length of stay among patients with a hospitalization was 17 (32) days. Mean (SD) total episode costs were $439,104 ($405,475). Although statistical comparisons were not performed, mean (SD) total costs were nominally higher in the HSCT group compared with the non-HSCT group ($524,595 [$445,149] vs $263,310 [$222,357]). Utilization and costs across all categories appeared to be higher in patients with HSCT compared with patients without HSCT. All-cause HRU and all-cause costs are described in detail in Tables 2 and 3.
Table 2.
All-cause healthcare resource utilization during R/R episodes of care
| R/R total (N = 707) | R/R with HSCT (n = 476) | R/R without HSCT (n = 231) | |
|---|---|---|---|
| Physician office visits | |||
| Incidence of ≥ 1 visit, n (%) | 690 (97.6%) | 465 (97.7%) | 225 (97.4%) |
| Number of unique visits among all users | |||
| Mean (SD) | 76.8 (71.2) | 89.9 (77.0) | 49.7 (47.3) |
| Median | 58.0 | 71.0 | 39.0 |
| Emergency department visits | |||
| Incidence of ≥ 1 visit, n (%) | 385 (54.5%) | 259 (54.4%) | 126 (54.5%) |
| Number of unique visits among all users | |||
| Mean (SD) | 3.4 (10.0) | 3.7 (12.0) | 2.6 (2.1) |
| Median | 2.0 | 2.0 | 2.0 |
| Inpatient visits | |||
| Incidence of ≥ 1 visit, n (%) | 664 (93.9%) | 461 (96.8%) | 203 (87.9%) |
| Number of unique visits among all users | |||
| Mean (SD) | 4.5 (3.9) | 4.9 (4.3) | 3.6 (2.5) |
| Median | 3.0 | 4.0 | 3.0 |
| Length of stay (days)a | |||
| Mean (SD) | 17 (32) | 18 (38) | 14 (12) |
| Median | 13 | 14 | 12 |
| Number of hospital daysa | |||
| Mean (SD) | 75 (394) | 87 (472) | 46 (38) |
| Median | 48 | 54 | 37 |
| Pharmacy utilization (outpatient) | |||
| Incidence of ≥ 1 prescription, n (%) | 637 (90.1%) | 428 (89.9) | 209 (90.5%) |
| Number of unique prescriptions among all users | |||
| Mean (SD) | 83.1 (99.1) | 102.9 (111.1) | 42.8 (47.1) |
| Median | 50.0 | 71.0 | 25.0 |
HSCT hematopoietic stem cell transplantation, R/R relapsed/refractory
aLength of stay describes the average time in the hospital per hospitalization. Hospital days reported describes the average time in the hospital over the entire R/R episode
Table 3.
Direct all-cause healthcare costs during R/R episodes of care
| R/R total (N = 707) | R/R with HSCT (n = 476) | R/R without HSCT (n = 231) | |
|---|---|---|---|
| Total costs | |||
| Mean (SD) | $439,104 ($405,475) | $524,596 ($445,149) | $263,310 ($222,357) |
| Median | $340,862 | $425,318 | $197,209 |
| Physician office visit costs | |||
| Incidence of ≥ 1 visit, n (%) | 690 (97.6%) | 465 (97.7%) | 225 (97.4%) |
| Mean (SD) | $10,926 ($13,645) | $13,255 ($15,533) | $6133 ($6257) |
| Median | $7215 | $9070 | $4304 |
| Emergency department visit costs | |||
| Incidence of ≥ 1 visit, n (%) | 385 (54.5%) | 259 (54.4%) | 126 (54.5%) |
| Mean (SD) | $4301 ($20,941) | $5367 ($25,453) | $2151 ($3369) |
| Median | $861 | $852 | $913 |
| Inpatient visits | |||
| Incidence of ≥ 1 visit, n (%) | 664 (93.9%) | 461 (96.8%) | 203 (87.9%) |
| Mean (SD) | $308,978 ($306,987) | $357,812 ($337,066) | $197,528 ($180,061) |
| Median | $228,916 | $268,362 | $144,905 |
| Pharmacy utilization (outpatient) | |||
| Incidence of ≥ 1 prescription, n (%) | 637 (90.1%) | 428 (89.9) | 209 (90.5%) |
| Mean (SD) | $24,640 ($46,275) | $30,633 ($53,748) | $12,219 ($19,202) |
| Median | $11,548 | $16,469 | $4172 |
HSCT hematopoietic stem cell transplantation, R/R relapsed/refractory
When results were stratified by how the R/R AML diagnosis was defined, a pattern of lower utilization and costs was observed in patients identified as R/R via a relapsed AML diagnosis code (code patients) compared with those identified by line advancement (LOT patients). Compared with LOT patients, smaller proportions of code patients had physician office visits (99.7% vs 95.8%), emergency department visits (61.7% vs 48.3%), and pharmacy utilization (96.0% vs 85.0%). Mean numbers of unique visits and prescriptions among patients with utilization in each category also appeared to be lower in code patients (e.g., the mean [SD] number of unique emergency department visits among code patients with an emergency department visit was 2.6 [2.3] vs 4.1 [13.6] in LOT patients).
Compared with LOT patients, the mean (SD) total costs appeared to be slightly lower in code patients ($428,057 [$415,309] vs $451,981 [$393,946]) with similar patterns observed within the physician office and emergency department visit cost component categories (mean [SD] physician office visit costs $11,149 [$13,818] in LOT patients vs $10,726 [$13,505] in code patients; mean [SD] emergency department visit costs $5202 [$24,890] in LOT patients vs $3320 [$15,559] in code patients) though mean (SD) inpatient costs appeared to be slightly higher in the code patients compared with the LOT patients ($310,431 [$322,656] vs $307,251 [$287,782]).
Occurrence of SxTox Events and Their Impact on Total R/R Episode Costs
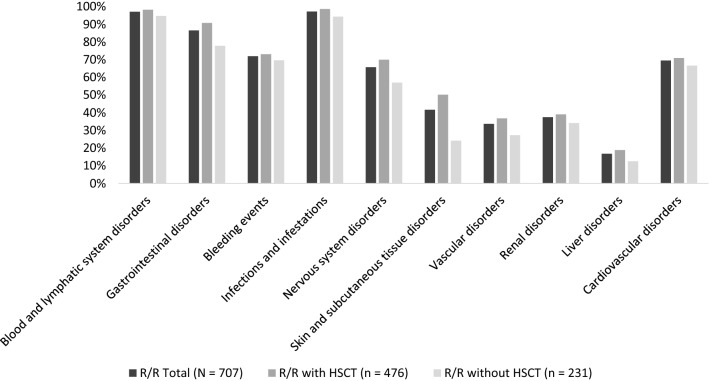
The frequency of occurrence of SxTox events is displayed in Fig. 2. Patients with R/R AML had a high occurrence and wide range of SxTox events, including infections and infestations (97.3%), blood and lymphatic system disorders (97.2%), GI disorders (86.6%), bleeding (72.0%), CV disorders (69.6%), nervous system disorders (65.8%), skin and subcutaneous tissue disorders (41.7%), renal disorders (37.5%), and vascular disorders (33.7%). Relapsed/refractory patients with HSCT appeared to experience SxTox events of interest at higher frequencies than R/R patients without HSCT. The largest discrepancies between HSCT and non-HSCT patients were seen in nervous system (70.0% vs 57.1%) and skin and subcutaneous tissue disorder categories (50.2% vs 24.2%).
Fig. 2.
Frequency of occurrence of SxTox
Generalized Linear Modeling
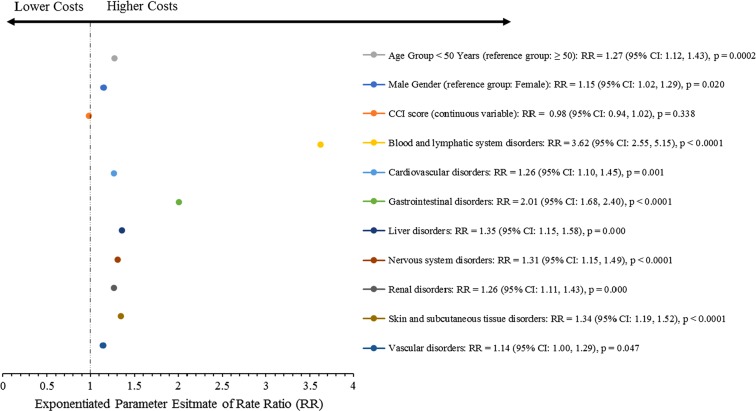
After adjustment for age, gender, and CCI score, multivariate GLM results revealed that R/R episode costs were sensitive to the occurrence of any SxTox event, as every SxTox event included in the regression model was significantly associated with higher total episode costs (p < 0.05 for all SxTox variables). Significantly increased costs (percentage increase over patients without SxTox events) were observed during R/R episodes in patients who had blood and lymphatic system disorders (262%), GI disorders (101%), liver disorders (35%), skin and subcutaneous tissue disorders (34%), nervous system disorders (31%), renal and cardiovascular disorders (both 26%), and vascular disorders (14%), compared with patients who did not have these SxTox. These results are depicted in Fig. 3.
Fig. 3.
Impact of SxTox on total R/R episode costs. Note: exponentiated parameter estimates shown represent the multiplicative effect of the variable level compared with the reference (ref.) level (e.g., the exponentiated parameter estimate of 3.62 for the blood and lymphatic system disorders variable translates to 262% higher costs in patients with blood and lymphatic system disorders compared with patients without blood and lymphatic system disorders). Patients without the specific SxTox variable reported served as the reference group for each SxTox category
Discussion
The current study provides detailed real-world HRU and cost data and an analysis of symptoms and toxicities in R/R AML patients (with and without HSCT) using a large US health insurance database. Such data suggest that patients with R/R AML incurred high HRU and economic burden (mean [SD] total episode costs $439,104 [$405,475]). Among the HRU categories examined, inpatient visits were associated with the highest costs (mean [SD] inpatient costs per episode were $308,978 [$306,987]). When cost outcomes were stratified by HSCT status, a pattern of higher costs among patients with HSCT compared with patients without HSCT emerged (mean [SD] total episode costs without HSCT $263,310 [$222,357]; with HSCT $524,596 [$445,149]). Based on data from all patients (regardless of whether or not they utilized resources in a particular category), the mean total episode cost (from relapse date to death or end of study period) for all patients was $438,483 (with HSCT $523,493 and without HSCT $263,310). Inpatient visits accounted for the greatest cost component (mean $289,749) followed by non-clinician (e.g., lab tests) visits (mean $30,384), intensive care unit stays (mean $25,068), and outpatient pharmacy utilization (mean $22,061).
Though not directly comparable, existing literature seems to be in line with the findings from the current study. Irish et al., for example, reported mean (SD) total healthcare expenditures from relapse to second remission of $142,569 ($208,307) in a small sample (N = 70) of patients with AML [11]. Compared with the Irish cohort, a higher proportion of the R/R sample in the current study had an observed hospitalization (94% vs 60%), which may explain the relatively higher overall costs in the current study. Additionally, the current study reported expenditures over a different, longer time period compared with the Irish study (i.e., through the end of the follow-up period [mean of 15.0 months] vs until second remission [mean of 1.4 months]). Lang and colleagues reported mean (SD) total costs of $51,888 ($54,825) per patient from initial diagnosis through the end of follow-up (16% of those treated eventually relapsed) [12]. However, these results were based on elderly patients (65 years or older) using Medicare data (i.e., patients were diagnosed with AML between 1991 and 1999 in the linked Surveillance, Epidemiology, and End Results [SEER]-Medicare database) and some portion of the sample may have received supportive care instead of active treatment, which may explain the observed cost differences being lower than our findings.
Approximately 70% of costs observed in the current study were driven by inpatient hospitalizations (mean [SD] inpatient costs per R/R episode $308,978 [$306,987]). These high inpatient costs appeared to be associated with in-hospital chemotherapy and HSCT-related costs. Inpatient hospitalizations have been cited by others as accounting for most of the total cost of care in patients with AML. Meyers et al., for example, estimated that hospitalizations accounted for 76% of total costs while Aly reported inpatient costs of $16,867 out of total costs of $28,148 (60%) per patient per month in patients with R/R AML [6, 13].
Although statistical comparisons were not performed on the unadjusted results, R/R patients with HSCT appeared to have utilized more resources and incurred higher costs compared with R/R patients without HSCT (mean [SD] costs $524,595 [$445,149] vs $263,310 [$222,357], respectively). These relatively higher costs may be attributed to the costs associated with the transplant procedure itself and the accompanying inpatient stays, as well as for pre-transplant preparation and post-transplant toxicities (acute and long-term). Indeed, others have reported relatively higher costs for AML patients receiving HSCT compared with other treatment regimens (e.g., chemotherapy) in AML. Zeidan et al., for example, estimated the mean cost of AML treatment in the USA by conducting a comprehensive literature review and calculating the average direct cost (2012 US dollars) per patient for the first 6 months of therapy. Results suggested that allogeneic HSCT was the most expensive treatment pathway ($352,682) followed by intensive chemotherapy ($324,502), low-intensity chemotherapy ($57,039), and best supportive care only ($14,014) [14].
Symptoms and toxicities of interest (SxTox) were more frequently observed in R/R patients with HSCT compared with R/R patients without HSCT (e.g., nervous system disorders were observed in 70.0% of R/R patients with HSCT vs 57.1% of R/R patients without HSCT). SxTox observed during R/R treatment episodes were associated with substantial clinical and economic burdens. For example, blood and lymphatic system disorders occurred in 97.2% of the sample and were associated with R/R episode costs that were 262% higher compared with patients without blood and lymphatic system disorders. Gastrointestinal (GI) disorders were observed in 86.6% of the sample and were associated with R/R episode costs that were 101% higher compared with patients without GI disorders. Taken together, the findings of higher SxTox in HSCT patients and SxTox as significant predictors of total episode costs help to explain the observation of higher costs in HSCT patients compared with non-HSCT patients.
The current study suggests possible differences in utilization and cost data as a function of the method used to define R/R disease status (i.e., lower utilization and costs in patients identified as R/R via diagnosis code vs line advancement), which may also account for some of the observed differences between results from the current study and those from the existing literature (e.g., [11, 12]). Indeed, many published claims-based R/R AML studies relied upon diagnosis code alone, potentially underestimating the utilization and cost burden by excluding data from R/R patients that were not coded as such.
This study utilized a novel methodology to define the R/R cohort where episodes of care were examined in detail, including chemotherapy regimens/procedures received and their temporal relationships to one another. The use of this novel methodology most likely yielded a sample of patients that are more representative of the broader population of commercially insured R/R AML patients treated in the USA compared with those that rely on diagnosis codes alone. As providers may not record a patient presenting with relapsed AML as such, or may record the diagnosis some time after the patient presents with relapsed disease, studies that only use diagnosis codes to identify relapsed disease may underrepresent the burden of relapsed AML and exclude those with refractory disease altogether. As the existing R/R AML literature does not include disease burden estimates that are based on the novel methodology described in this study, the results from the current study may provide a more appropriate estimate of the economic and clinical burdens of R/R AML.
Limitations
Results from retrospective studies should be interpreted with an understanding of their inherent limitations and in the context of results from other similar studies. The current study was subject to several limitations. First, the current study may not have captured all R/R patients. To minimize this limitation, patients were identified as having R/R disease by a diagnosis code indicative of relapse and/or the observation of a second line of therapy. The economic burden reported in this study may be higher than that seen in the total US population of patients with R/R AML because of the study requirement of active treatment (i.e., chemotherapy). The definition of active treatment is limited by the fact that certain drugs can be used as anticancer agents or supportive care agents. If a patient with AML was treated exclusively with a therapy categorized as a supportive care agent, the associated HRU and costs were not included in our disease burden estimates. In addition to excluding patients without active treatment (i.e., supportive care only) the requirement of at least 3 months of continuous health plan enrollment after the initial AML diagnosis may have introduced a survival bias (by excluding patients who died during the 3-month period following the first AML diagnosis); therefore, those patients who died soon after R/R diagnosis or treatment were excluded, which might have also affected the true economic burden. The R/R sample from the current study consisted of patients who received active treatment (chemotherapy or HSCT), which may have yielded higher costs than that of the overall R/R population in the USA—a population that also includes patients receiving supportive care only, which is a lower cost.
Administrative databases provide limited clinical detail with regard to discrete lab values and clinical outcomes (response rates, survival), which may be better assessed via electronic medical records or with prospective studies designed to collect such data. The current study only captures the direct healthcare resource utilization and costs of R/R AML as measured by administrative claims data. The data provide no insight into indirect costs, such as loss of productivity or unemployment, or insight into the quality of life impacts of AML on patients with the disease as well as their caregivers. Indirect costs and quality of life outcomes are not captured in claims data. Further studies with a focus on the larger burden of R/R AML beyond HRU and direct costs are needed to estimate the total impact of the disease. The results from the current study may be limited by potential coding errors by providers (e.g., failing to include codes for diagnoses that were made; including codes for procedures that were not performed) resulting in underestimation or overestimation of healthcare costs. As the study sample was anonymized to comply with HIPAA regulations, such errors cannot be identified or corrected. Finally, as the study sample was principally composed of commercially insured patients, the findings are not necessarily generalizable to the uninsured, fee-for-service Medicare, or Medicaid populations. This is a limitation that is especially relevant in AML, considering the median age of the disease—the younger, commercially insured composition of the sample may include more HSCT patients compared with older samples with less commercial coverage, perhaps contributing to an overestimate of the economic and clinical burden.
Conclusions
This study constitutes the largest and most current retrospective real-world database study of the economic and clinical burden of R/R AML episodes of care in the USA. Our findings suggest that R/R episodes are associated with high economic burden and substantial symptoms and toxicities, which increase the total cost of care. We also found that the use of HSCT led to a twofold increase in R/R episode (mean [SD] total episode costs with HSCT $524,596 [$445,149]; without HSCT $263,310 [$222,357]), and higher toxicity (e.g., 50.2% of HSCT patients had skin and subcutaneous tissue disorders vs 24.2% of the non-HSCT sample). As a cancer with a relatively low survival rate, there remains a need for effective therapies for R/R AML that are also well tolerated.
Acknowledgements
Funding
This study was sponsored by Astellas Pharma, Inc. Journal processing charges and Open Access fees were funded by Astellas Pharma, Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Role of Sponsor
Astellas employees were involved in the study design, interpretation of data, writing of the manuscript, and the decision to submit the manuscript for publication.
Medical Writing and Editorial Assistance
Xin Wang, Jeffrey Walter, and Sharon Suntag (IQVIA) provided assistance in drafting the manuscript. Jin Wu (IQVIA) provided analytic support. Editorial and logistical support was provided by Elizabeth Hermans, Ph.D. and Julie Bachman of OPEN Health Medical Communications and funded by Astellas Pharma, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
B. Pandya is an employee of Astellas. C-C. Chen is an employee of IQVIA. B. C. Medeiros is an employee of Genentech (employed at Stanford University while the study was conducted). C. B. McGuiness is an employee of IQVIA. S. Wilson is an employee of Astellas. L. E. Horvath Walsh is an employee of IQVIA Biotech (employed at Astellas while the study was conducted). R. L. Wade is an employee of IQVIA.
Compliance with Ethics Guidelines
As IQVIA is the PharMetrics Plus database owner, permission to use the database was not necessary. All data are de-identified and fully compliant with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. As such, no institutional review board approval was required. Informed consent was also not applicable to this study.
Data Availability
Access to anonymized individual participant level data will not be provided for this trial as it meets one or more of the exceptions described on http://www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas”.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8158901.
References
- 1.American Cancer Society . Cancer Facts and Figures 2018. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2.Stirewalt DL, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107(9):3724–3726. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos NR, et al. Current approaches in the treatment of relapsed and refractory acute myeloid leukemia. J Clin Med. 2015;4(4):665–695. doi: 10.3390/jcm4040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anders B, et al. Outcomes of six-dose high-dose cytarabine as a salvage regimen for patients with relapsed/refractory acute myeloid leukemia. Adv Hematol. 2017 doi: 10.1155/2017/6464972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society. Acute myeloid leukemia (AML); 2018. https://www.cancer.org/cancer/acute-myeloid-leukemia/causes-risks-prevention/risk-factors.html. Accessed 23 Oct 2018.
- 6.Meyers J, et al. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy. 2013;11(3):275–286. doi: 10.1007/s40258-013-0032-2. [DOI] [PubMed] [Google Scholar]
- 7.Stranges E (Thomson Reuters), Russo CA (Thomson Reuters), Friedman B (AHRQ). Procedures with the most rapidly increasing hospital costs, 2004–2007. HCUP statistical brief #82. Rockville, MD: Agency for Healthcare Research and Quality; 2009. http://www.hcupus.ahrq.gov/reports/statbriefs/sb82.pdf.
- 8.D’Souza A, Zhu X. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2017; 2017. http://www.cibmtr.org. Accessed 19 Sep 2018.
- 9.D'Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2018. https://www.cibmtr.org.
- 10.U.S. Bureau of Labor Statistics. Consumer price index—all urban consumers. US city average, medical care, not seasonally adjusted. http://www.bls.gov/data/home.htm. Accessed 6 June 2019.
- 11.Irish W, et al. Acute myeloid leukemia: a retrospective claims analysis of resource utilization and expenditures for newly diagnosed patients from first-line induction to remission and relapse. Curr Med Res Opin. 2017;33(3):519–527. doi: 10.1080/03007995.2016.1267615. [DOI] [PubMed] [Google Scholar]
- 12.Lang K, et al. Trends in the treatment of acute myeloid leukaemia in the elderly. Drugs Aging. 2005;22(11):943–955. doi: 10.2165/00002512-200522110-00004. [DOI] [PubMed] [Google Scholar]
- 13.Aly A, et al. Economic burden of relapsed/refractory (R/R) acute myeloid leukemia (AML) in the US. Blood. 2017;130(Suppl 1):3386. [Google Scholar]
- 14.Zeidan AM, et al. Economic burden associated with acute myeloid leukemia treatment. Expert Rev Hematol. 2016;9(1):79–89. doi: 10.1586/17474086.2016.1112735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to anonymized individual participant level data will not be provided for this trial as it meets one or more of the exceptions described on http://www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas”.