Abstract
Introduction
Patient-reported outcomes (PROs) provide valuable insights about the effectiveness of overactive bladder (OAB) treatments. The aim of PERSPECTIVE (a Prospective, non-intErventional Registry Study of PatiEnts initiating a Course of drug Therapy for overactIVE bladder) was to provide real-world evidence from the USA and Canada on patient-perceived effectiveness and safety of mirabegron and antimuscarinics for treating OAB symptoms.
Methods
This prospective, non-interventional registry followed adult patients with OAB who were starting treatment with mirabegron or antimuscarinics. All treatment decisions were made at the discretion of the treating healthcare provider with no mandatory visits after enrollment. The primary objective was to identify factors associated with improved treatment effectiveness from a patient perspective mainly using the OAB Questionnaire Short-Form (OAB-q SF). The form was sent to patients via email link at baseline and months 1, 3, 6, and 12. Treatment-emergent adverse event (TEAE) data were collated from investigator reports.
Results
Overall, 1514 patients were included (female 73.5%, mean age 62.2 years). Mirabegron was initiated by 613 patients and antimuscarinics by 901 patients. A PRO response rate of approximately 60% was achieved (575 patients did not complete baseline PROs). Similar improvements in OAB-q SF symptom bother score and health-related quality of life (HRQoL) total score were observed for mirabegron and antimuscarinic initiators. Covariate-adjusted models demonstrated that worse baseline PRO score, Hispanic ethnicity, being treatment naïve, and use of complementary/supportive OAB therapies at baseline were significantly associated with greater improvements in both scores. The most frequent TEAEs were gastrointestinal disorders (dry mouth, constipation, and nausea) and nervous system disorders (headache, somnolence, and dizziness).
Conclusion
There are no differences between mirabegron and antimuscarinics in terms of patient-reported OAB symptom bother and HRQoL.
Trial Registration
ClinicalTrials.gov identifier, NCT02386072.
Funding
Astellas Pharma Global Development, Inc.
Plain Language Summary
Plain language summary available for this article.
Electronic Supplementary Material
The online version of this article (10.1007/s12325-019-00994-7) contains supplementary material, which is available to authorized users.
Keywords: Antimuscarinic, Mirabegron, Overactive bladder syndrome, Patient-reported outcomes, Registry, Urology
Plain Language Summary
What is overactive bladder (OAB) syndrome?
People with OAB feel a greater need to urinate and urinate more frequently.
In the USA and Canada, approximately 1 in 5 people have OAB.
OAB is often treated with a group of medicines called antimuscarinics or a medicine called mirabegron.
How was the study carried out?
Men and women with OAB starting treatment with mirabegron or antimuscarinics were followed for 12 months in 91 centers in the USA/Canada.
Doctors chose which medicine people took: 613 people took mirabegron, 901 people took antimuscarinics.
People were asked to complete electronic questionnaires about their OAB symptoms and quality-of-life before, during, and after 12 months of treatment.
How well did the medicines work?
People reported that both medicines improved their symptoms and quality-of-life.
The level of improvement was similar for mirabegron and antimuscarinics, and seemed to be greater for people who:
Had worse questionnaire scores when they started treatment.
Were Hispanic.
Had not received OAB medication previously.
Were using complementary OAB treatments, such as bladder training and pelvic muscle exercises.
What were the side effects?
Both medicines had side effects.
Doctors had to report the side effects for mirabegron, but not for other therapies, so the side effects of the medicines cannot be compared directly.
Approximately 3/10 people taking mirabegron had side effects; the most common were dry mouth, headache, and constipation.
What do the results of this study mean?
Mirabegron or antimuscarinics both improve OAB symptoms and quality of life to a similar extent.
Introduction
Overactive bladder (OAB) syndrome is characterized by urinary urgency, with or without urgency urinary incontinence, usually with increased daytime frequency and nocturia, in the absence of other causes [1, 2]. The burden of OAB is significant, with an estimated prevalence in the USA and Canada of approximately 20% and a total economic burden that may exceed 100 billion USD annually in the USA [3–5]. Furthermore, OAB has a significant negative impact on patients’ health-related quality of life (HRQoL) [6, 7].
Antimuscarinics and the β3-adrenoreceptor agonist mirabegron are recommended as second-line therapies for OAB symptoms after behavioral therapy [8]. Both types of agent are well-established treatments for OAB symptoms [9–11]. Although antimuscarinics are the most widely used pharmacotherapies for OAB symptoms, anticholinergic side effects (including dry mouth, constipation, and blurred vision) are problematic in some patients [12]. Mirabegron represents an alternative to antimuscarinics for the treatment of OAB symptoms, with efficacy demonstrated across numerous studies, including in patients who had an insufficient response or intolerance to antimuscarinics [9, 10]. Furthermore, previous studies have demonstrated that mirabegron is associated with fewer anticholinergic side effects than antimuscarinics [13].
Clinical trials of OAB pharmacotherapies usually focus on objective measures of symptom improvement, such as micturition frequency and volume and the number of incontinence episodes using bladder diaries and/or urodynamic studies [14, 15]. However, there is growing recognition that subjective measures of OAB treatment effectiveness via patient-reported outcomes (PROs) also provide valuable insights. Objective measures of OAB symptom improvement may not fully represent treatment effect from the patients’ perspective, which is also impacted by several factors including treatment satisfaction, the effect on HRQoL, and adverse events (AEs) [14, 15]. Furthermore, OAB management in normal clinical practice is usually based on patients reporting their symptoms and previous treatment experiences, so translation of clinical trial efficacy into real-world effectiveness from the patient’s perspective is complicated [14].
We herein report the primary findings of PERSPECTIVE (a Prospective, non-intErventional Registry Study of PatiEnts initiating a Course of drug Therapy for overactIVE bladder; NCT02386072), a large registry of OAB patients in the USA and Canada designed to provide evidence on the real-world, patient-perceived effectiveness and safety of mirabegron and antimuscarinics for the treatment of OAB symptoms [16].
Methods
The rationale, design, and methodology of PERSPECTIVE have been previously described in detail [16]. In brief, this study was a prospective, multicenter, non-interventional registry, following adult patients in routine clinical practice diagnosed with OAB initiating a new course of treatment with either mirabegron or antimuscarinic medication between January 2015 and August 2017. Patients could be either treatment naïve or switching from a different OAB pharmacotherapy. The primary objective was to identify factors associated with improved effectiveness of pharmacologic therapy for OAB from a patient perspective measured primarily by the OAB Questionnaire Short-Form (OAB-q SF) [17].
This study was conducted in accordance with all applicable laws and regulations, and the ethical principles originating from the Declaration of Helsinki. The protocol and any associated documents were approved by the institutional review board or independent ethics committee at each site prior to enrollment (Table S1). All patients (or their legally authorized representative) provided informed consent before participation in the study.
Recruitment
Enrollment of 1500 patients with OAB, primarily patients treated by urologists and primary care physicians, was planned from healthcare centers and sites across the USA (85%) and Canada (15%). Of these 1500 patients, it was intended that 600 patients treated with mirabegron and 900 patients treated with an antimuscarinic would be enrolled (as antimuscarinics were anticipated to be prescribed more frequently). Patient access to discounted and free medications varied by healthcare provider (HCP) and location, and initiation of therapy may have been either through prescription or samples. In Canada, except for British Columbia and Quebec, a patient-support program was available that provided supplemental coverage of up to 100% of the total cost for mirabegron. The program was in place from December 2014 and continued in each province until public reimbursement was initiated in that province. No such program was available in the USA during the study.
Inclusion/Exclusion Criteria
Patients aged at least 18 years who were diagnosed with OAB (with or without urgency urinary incontinence) by the treating HCP with symptoms for at least 3 months prior to study enrollment and who were initiating a new course of treatment with mirabegron or antimuscarinic medication (including transdermal patch formulations) for OAB were included. Patients had to be willing and able to provide informed consent and complete PRO questionnaires via email link with minimal assistance. Patients were excluded if they were receiving treatment with more than one OAB medication at the time of enrollment or had a history of OAB treatment with onabotulinum toxin A, sacral neuromodulation, percutaneous tibial nerve stimulation, external beam radiation therapy, urinary stents, surgery, or intermittent catheterization prior to or at the time of enrollment; had neurologic conditions associated with OAB symptoms; were pregnant or breastfeeding; or were resident in a nursing home.
Data Collection
All decisions regarding treatment were made at the discretion of the treating HCP in accordance with their usual practices and all clinical assessments were performed at the time of a routine appointment. Patients were followed for up to 12 months with no mandatory scheduled visits after enrollment.
At baseline (i.e., time of enrollment), patient demographic and clinical OAB characteristics were collected. PROs were sent by email link to patients at baseline (days 0–7), month 1 (days 30–45), month 3 (days 91–125), month 6 (days 182–208), and month 12 (days 336–377) to be completed electronically in their preferred language (English, French, or Spanish). Responses to baseline PROs were requested within 7 days of enrollment. Patients who failed to complete PROs at a given time point (including at baseline) remained in the study and were not excluded from analysis. PROs included the OAB-q SF [17] and the OAB treatment satisfaction questionnaire (OAB-S) [18].
The OAB-q SF [17] comprises a six-item symptom bother scale and a 13-item HRQoL scale consisting of three subscales (coping, sleep, and emotional/social). Patients rate each item on a six-point Likert scale ranging from “not at all” to “a very great deal” for symptom bother and from “none of the time” to “all of the time” for HRQoL. Scores are generated according to the scoring guidelines [17], with higher symptom bother scores indicating greater symptom bother and higher HRQoL scores indicating better HRQoL. The OAB-S [18] comprises five scales and five single-item overall assessments, including the OAB-S medication tolerability scale which captures bother associated with antimuscarinic AEs (specifically constipation, dry mouth, drowsiness, headache, nausea, and blurred vision).
A treatment-emergent AE (TEAE) was defined as an AE observed after initiation of study drug. TEAE data were based on reports from investigators. Consistent with the requirement for the study sponsor (Astellas Pharma) to report on the safety of its products, all TEAEs for mirabegron and solifenacin initiators (regardless of relationship to study treatment) were monitored and reported throughout the study (mandatory reporting), whereas identification of TEAEs for initiators of any other antimuscarinic was at the discretion of the investigator (discretionary reporting). Investigators were asked to assess the relationship of TEAEs to mirabegron/solifenacin as not related, possible, or probable. In addition, if a patient receiving mirabegron/solifenacin reported bother on the OAB-S medication tolerability scale or reported being hospitalized since the date of the last questionnaire, an email was sent to site staff to alert them to assess the side effect and report the potential TEAE. To establish whether there was differential reporting of TEAEs between the mirabegron and antimuscarinic treatment groups, reported antimuscarinic TEAEs were cross-validated with their associated bother determined using the OAB-S medication tolerability scale responses.
Statistical Analyses
The analysis population consisted of all enrolled patients who initiated a new course of treatment with either mirabegron or an antimuscarinic. The antimuscarinic data were assessed in terms of the group of therapeutics as a whole. As patients were not excluded from analysis if they failed to complete the PROs at a given time point (including at baseline), the number of patients who completed PROs at each time point varied.
Baseline demographic and clinical OAB characteristics were summarized for mirabegron and antimuscarinic initiators separately.
Analysis of covariance (ANCOVA) models with treatment group, sex, age, and treatment-naïve status as covariates were used to analyze changes from baseline to last reported score for OAB-q SF symptom bother score and HRQoL total score. Changes in the HRQoL coping, sleep, and emotional/social subscales from baseline to month 12 were also examined (data from months 1, 3, and 6 were not included in this analysis).
Univariate generalized linear regression models were used to identify baseline variables that were statistically significant predictors of changes in OAB-q SF symptom bother and HRQoL total scores from baseline to the last reported score in each treatment group. Covariate-adjusted generalized linear regression models were subsequently created which included the significant variables identified in the univariate models together with several pre-determined variables (age, sex, baseline PRO score, and initial treatment group). Any co-linear variables (e.g., menopause status and pregnancy were co-linear with female sex) were excluded from the models.
TEAEs were summarized descriptively by treatment group at enrollment using the system organ classes and preferred terms identified through the use of the Medical Dictionary for Regulatory Activities (MedDRA; version 19.0).
Results
Patients
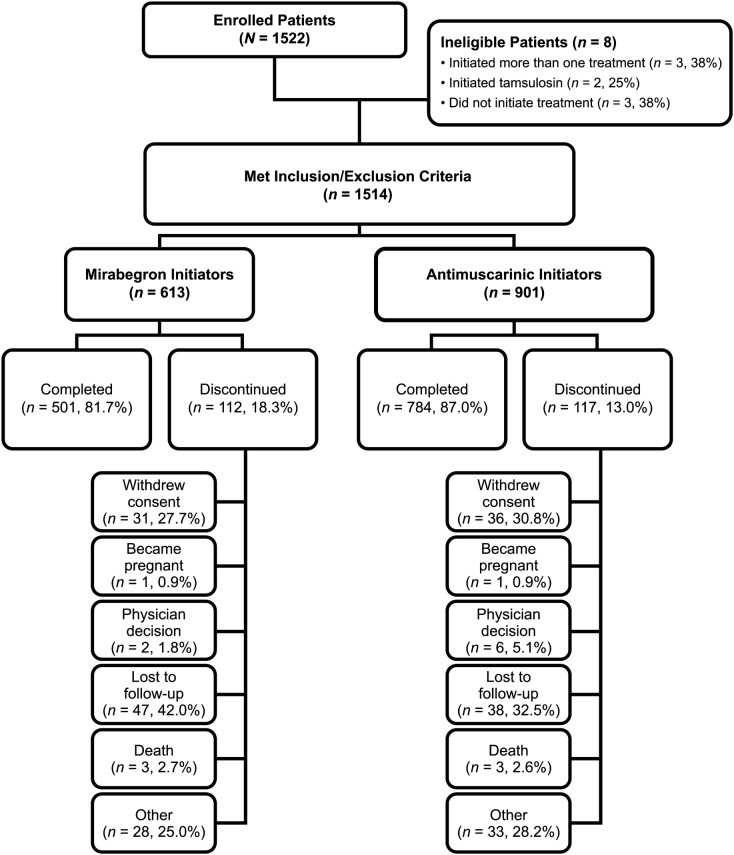
A total of 1514 eligible patients were included in PERSPECTIVE, the majority of whom were female (73.5%) and white (87.2%), with a mean age of 62.2 years (Table 1). Mirabegron was initiated by 613 patients and antimuscarinics by 901 patients (Fig. 1). The most frequently initiated antimuscarinics were oxybutynin (46.9% of antimuscarinic initiators), solifenacin (33.1%), and tolterodine (10.0%). A greater proportion of antimuscarinic initiators were treatment naïve (no OAB medication in past 12 months, 81.2%) compared with mirabegron initiators (71.0%, Table 1).
Table 1.
Baseline patient demographic and clinical overactive bladder characteristics
| Mirabegron initiators (N = 613) | Antimuscarinic initiators (N = 901) | |
|---|---|---|
| Demographic characteristics | ||
| Age at enrollment in years | ||
| Mean (SD) | 62.6 (14.6) | 62.0 (14.3) |
| Median (min, max) | 65.0 (19, 93) | 64.0 (18, 93) |
| Female sex, n (%) | 424 (69.2) | 689 (76.5) |
| BMI in kg/m2, mean (SD)a | 30.1 (7.1) | 30.4 (7.2) |
| Country, n (%) | ||
| USA | 390 (63.6) | 808 (89.7) |
| Canada | 223 (36.4) | 93 (10.3) |
| Race, n (%) | ||
| White | 544 (88.7) | 776 (86.1) |
| Black/African American | 41 (6.7) | 86 (9.5) |
| Other | 19 (3.1) | 28 (3.1) |
| Unknown/not available | 9 (1.5) | 11 (1.2) |
| Ethnicity, n (%)b | ||
| Non-Hispanic | 427 (69.7) | 425 (47.2) |
| Hispanic | 93 (15.2) | 435 (48.3) |
| French Canadian | 18 (2.9) | 11 (1.2) |
| Unknown/not available | 75 (12.2) | 30 (3.3) |
| Employed full-time, n (%) | 178 (29.0) | 214 (23.8) |
| US drug coverage, n (%)c | ||
| Private | 164 (42.1) | 222 (27.5) |
| Medicare | 169 (43.3) | 375 (46.4) |
| Medicaid | 38 (9.7) | 106 (13.1) |
| No insurance | 5 (1.3) | 67 (8.3) |
| Other | 14 (3.6) | 38 (4.7) |
| Canadian drug coverage, n (%)c | ||
| Private | 110 (49.3) | 39 (41.9) |
| Provincial (public)—open coverage | 56 (25.1) | 12 (12.9) |
| Provincial (public)—with coverage criteria | 40 (17.9) | 38 (40.9) |
| Clinical OAB characteristics | ||
| Time since OAB diagnosis in months | ||
| Mean (SD) | 49.3 (73.6) | 41.0 (67.7) |
| Median (min, max) | 20.3 (0, 610) | 15.0 (0, 579) |
| Medical specialty of HCP who made OAB diagnosis, n (%) | ||
| Primary care | 237 (38.7) | 465 (51.6) |
| Urology | 302 (49.3) | 352 (39.1) |
| Gynecology | 48 (7.8) | 55 (6.1) |
| Other | 26 (4.2) | 29 (3.2) |
| OAB with incontinence, n (%) | 436 (71.1) | 719 (79.8) |
| Stress or mixed urinary incontinence, n (%)d | 314 (72.0) | 611 (85.0) |
| Status of using pads, n (%) | ||
| In the past | 27 (4.4) | 27 (3.0) |
| Currently | 289 (47.1) | 503 (55.8) |
| Never | 264 (43.1) | 350 (38.8) |
| Unknown | 33 (5.4) | 21 (2.3) |
| Number of pads used per weeke | ||
| Mean (SD) | 15.9 (11.7) | 20.8 (12.1) |
| Median (min, max) | 14.0 (1.0, 84.0) | 21.0 (1.0, 70.0) |
| OAB treatment history, n (%) | ||
| No medication in past 12 months | 435 (71.0) | 732 (81.2) |
| ≥ 1 medication in past 12 months | 178 (29.0) | 169 (18.8) |
| Use of complementary/supportive OAB therapies, n (%) | 121 (19.7) | 131 (14.5) |
BMI body mass index, HCP healthcare provider, OAB overactive bladder, SD standard deviation
aNot available for one patient in the antimuscarinic initiator group
bAll categories are mutually exclusive
cPercentages calculated among US and Canadian patients separately
dMirabegron initiators, n = 436; antimuscarinic initiators, n = 719
eCurrent pad users only
Fig. 1.
Patient disposition
A greater proportion of Canadian patients initiated mirabegron (70.6%) compared with US patients (32.6%). Furthermore, differences in type of drug coverage were apparent between mirabegron and antimuscarinic initiators, with fewer US patients with no coverage initiating mirabegron than antimuscarinics and a lower proportion of US antimuscarinic initiators having private insurance than mirabegron initiators (Table 1). In Canada, fewer mirabegron initiators had provincial health coverage than antimuscarinic initiators.
Most patients were recruited into the study by either primary care clinicians (48.5%) or urologists (43.7%). A greater proportion of mirabegron initiators were enrolled by urologists compared with primary care physicians, whereas the opposite was true for antimuscarinic initiators.
A total of 76.3% of patients were reported as having OAB with incontinence and, among those, 19.9% were reported to have urgency urinary incontinence (not stress or mixed urinary incontinence). A higher rate of OAB with incontinence and a higher mean number of pads used were observed for antimuscarinic initiators (79.8% had OAB with incontinence; 20–21 pads/week) compared with mirabegron initiators (71.1% had OAB with incontinence; 15–16 pads/week). The proportion of patients with OAB with incontinence was higher in the USA (79.2%) than in Canada (65.2%), as was the proportion of patients who currently used pads (55.1% vs. 41.8%, respectively).
Patient-Reported Outcomes
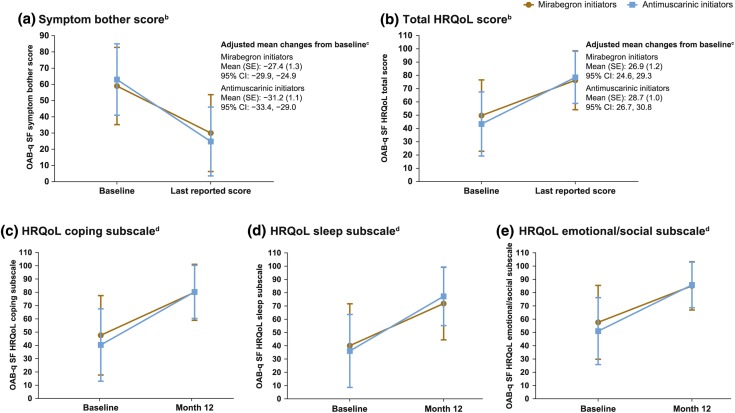
The PRO response rate was approximately 60%, with 575 patients not completing baseline PROs (277 [45.2%] mirabegron initiators and 298 [33.1%] antimuscarinic initiators). Mirabegron initiators had slightly less OAB symptom bother and better HRQoL at baseline compared with antimuscarinic initiators (Fig. 2). Mirabegron and antimuscarinic initiators had similar improvements in OAB-q SF symptom bother score and HRQoL total score from baseline to the last reported score. Similar improvements were also observed for both groups from baseline to month 12 for the coping, sleep, and emotional/social HRQoL subscales.
Fig. 2.
Mean (SD) changes in OAB-q SF questionnaire symptom bother score (a), total HRQoL score (b), and coping (c), sleep (d), and emotional/social (e) subscalesa. CI confidence interval, HRQoL health-related quality of life, OAB overactive bladder, OAB-q SF OAB Questionnaire Short-Form, SD standard deviation, SE standard error. aAll scores are transformed scores. If < 50% of the scale items were missing, the scale was retained with the mean scale score of the items present used to impute a score for the missing items. Higher symptom bother scores indicate greater symptom bother. Higher HRQoL subscale and total scores indicate better HRQoL. If ≥ 50% of the items were missing, no scale score was calculated. bBaseline: mirabegron initiators, n = 336; antimuscarinic initiators, n = 602. Last reported score: mirabegron initiators, n = 335; antimuscarinic initiators, n = 603. cGenerated from an analysis of covariance (ANCOVA) model with treatment group, sex, age, and treatment-naïve status as covariates. dChanges in the HRQoL subscales were assessed from baseline to month 12, not last reported score. Baseline: mirabegron initiators, n = 336; antimuscarinic initiators, n = 602. Month 12: mirabegron initiators, n = 310; antimuscarinic initiators, n = 548
Factors Associated with Changes in OAB-q SF Scores
Significant predictors of OAB-q SF symptom bother score improvement as examined by univariate generalized linear regression analysis were sex, ethnicity, country, medical insurance, history of prostate disease, history of urinary tract infection (UTI), number of pregnancies, type of OAB, presence of incontinence, treatment-naïve status, use of complementary/supportive OAB therapies at baseline, and use of concomitant medications at baseline. Covariate-adjusted models demonstrated that patients had statistically significantly greater improvement in OAB-q SF symptom bother score if they had a higher (worse) baseline score, were Hispanic, were treatment naïve, or used complementary/supportive OAB therapies at baseline. Whether patients initiated mirabegron or antimuscarinics was not statistically significantly associated with degree of change in OAB-q SF symptom bother score.
Significant predictors of OAB-q SF HRQoL total score improvement from baseline as examined by univariate generalized linear regression analysis, and included in covariate-adjusted models, were sex, ethnicity, country, medical insurance, prostate disease, history of UTI, type of OAB, treatment-naïve status, use of complementary/supportive OAB therapies at baseline and use of concomitant medications at baseline. Covariate-adjusted models demonstrated that patients had statistically significantly greater improvement in OAB-q SF HRQoL total score if they had a lower (worse) baseline score, were Hispanic, were treatment naïve, or used complementary/supportive therapies at baseline. Whether patients initiated mirabegron or antimuscarinics was not statistically significantly associated with degree of change in HRQoL total score.
Safety
A total of 529 TEAEs were reported in 203 (33.1%) patients who initiated mirabegron (mandatory reporting) and 394 TEAEs in 152 (16.9%) patients who initiated antimuscarinics (mandatory reporting for solifenacin, otherwise discretionary). The most frequent TEAEs were gastrointestinal disorders (dry mouth, constipation, and nausea) and nervous system disorders (headache, somnolence, and dizziness) (Table 2). Serious TEAEs were reported in 25 (4.1%) of mirabegron initiators and 16 (1.8%) antimuscarinic initiators. The most frequent serious TEAEs were respiratory, thoracic, and mediastinal disorders in mirabegron initiators and benign, malignant, and unspecified neoplasms in antimuscarinic initiators. There were eight fatal TEAEs (none deemed to be related to study drug); three among mirabegron initiators (metastatic neoplasm, dyspnea, lung infiltration) and five among antimuscarinic initiators (anemia, cardiac arrest, myocardial infarction, toxicity to various agents, small cell lung cancer).
Table 2.
Treatment-emergent adverse events
| System organ classa Preferred termb |
Number of patients with TEAE, n (%) | |
|---|---|---|
| Mirabegron initiators (N = 613)c | Antimuscarinic initiators (N = 901)d | |
| Any TEAE | 203 (33.1) | 152 (16.9) |
| Gastrointestinal disorders | 116 (18.9) | 77 (8.5) |
| Dry mouth | 78 (12.7) | 57 (6.3) |
| Constipation | 48 (7.8) | 39 (4.3) |
| Nausea | 19 (3.1) | 11 (1.2) |
| Nervous system disorders | 74 (12.1) | 35 (3.9) |
| Headache | 54 (8.8) | 19 (2.1) |
| Somnolence | 30 (4.9) | 17 (1.9) |
| Dizziness | 5 (0.8) | 2 (0.2) |
| Any serious TEAE | 25 (4.1) | 16 (1.8) |
| Respiratory, thoracic, and mediastinal disorders | 7 (1.1) | 3 (0.3) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 4 (0.7) | 6 (0.7) |
TEAE treatment-emergent adverse event
aSystem organ class TEAEs reported in > 5% and serious TEAEs reported in > 0.5% of total population
bMost frequent preferred term TEAEs
cAll TEAEs for mirabegron initiators (regardless of relationship to study treatment) were monitored and reported to the study sponsor throughout the study (mandatory reporting)
dIdentification of TEAEs for antimuscarinic initiators was at the discretion of the investigator (discretionary reporting). The only exception was for solifenacin where mandatory reporting of TEAEs was required
An imbalance in TEAE reporting was evident from the OAB-S medication tolerability reporting (Table 3). Using the most frequent TEAE, dry mouth, as an example, the proportion of patients who had reported the symptom as a TEAE at the month 1 assessment was lower for patients who initiated antimuscarinics and reported medication-related symptom bother (2.5%, 4/161) compared with mirabegron initiators who reported symptom bother (7.0%, 6/86) (Table 3). A similar pattern was observed at month 3 (2.2% [4/184] vs. 6.4% [7/110] for antimuscarinics and mirabegron, respectively). There were too few TEAEs reported at the month 6 and 12 assessments to permit further comparisons.
Table 3.
Treatment-emergent adverse event reporting among patients who reported symptom bother on the OAB-S medication tolerability scale at month 1
| OAB-S medication tolerability scale TEAE | Patients reporting symptom bother on the OAB-S medication tolerability scale who had reported the symptom as a TEAE, n/N (%) | |
|---|---|---|
| Mirabegron initiators | Antimuscarinic initiators | |
| Dry mouth | 6/86 (7.0) | 4/161 (2.5) |
| Constipation | 6/72 (8.3) | 6/93 (6.5) |
| Headache | 9/78 (11.5) | 0/67 (0) |
| Drowsiness | 2/65 (3.1) | 3/67 (4.5) |
| Blurred vision | 5/42 (11.9) | 5/53 (9.4) |
| Nausea | 1/34 (2.9) | 1/38 (2.6) |
OAB overactive bladder, OAB-S OAB treatment satisfaction questionnaire, TEAE treatment-emergent adverse event
Discussion
PERSPECTIVE represents the first prospective, observational study conducted in North America involving patients with OAB initiating a new course of pharmacotherapy.
Both mirabegron and antimuscarinics resulted in similar, statistically significant improvements in OAB-q SF symptom bother and HRQoL total scores. This finding is consistent with the improvements in symptom bother and HRQoL that were observed in a prospective, observational study of 862 patients with OAB prescribed mirabegron during routine clinical practice in Europe (BELIEVE) [19]. Other studies have compared PROs for mirabegron and antimuscarinics in the clinical trial setting [20–23]. PRO results from a phase 3 trial demonstrated significant improvements for mirabegron over placebo in OAB-q coping, concern, and HRQoL total score, whereas no significant difference was reported for tolterodine compared with placebo [20]. A two-period crossover study (PREFER), during which patients were randomized to receive mirabegron or tolterodine in one of four sequences (mirabegron–tolterodine, tolterodine–mirabegron, mirabegron–mirabegron, or tolterodine–tolterodine), reported similar mean OAB-q SF improvements for each of the treatments. However, patients were more likely to achieve clinically relevant improvements in HRQoL during mirabegron treatment [21]. Moreover, the PREFER study demonstrated improved tolerability for mirabegron compared with tolterodine. In addition, in the phase 2 (SYMPHONY) and phase 3 (SYNERGY) trials, which involved mirabegron and solifenacin in combination, the changes in OAB-q symptom bother and HRQoL total scores were similar in the mirabegron and solifenacin monotherapy groups [22, 23]. However, in the present study, patients were grouped according to the treatment initiated at enrollment; they may have added, switched, or discontinued treatment during the study period. As such, the final PRO score during follow-up may not reflect patient experiences on the initial treatment.
Baseline PRO score, being treatment naïve, use of complementary/supportive OAB therapies at baseline, and Hispanic ethnicity were consistently associated with greater OAB-q SF symptom bother and HRQoL total score improvements. Higher (worse) OAB-q SF symptom bother score at baseline and lower (worse) HRQoL score at baseline were associated with greater score improvement, indicating that patients with more symptomatic OAB at baseline experienced greater treatment benefit. The treatment-experienced patients enrolled in this study may represent a more difficult-to-treat group, particularly if they had previously discontinued pharmacotherapy because of insufficient efficacy, which may explain the better response in treatment-naïve patients. The use of complementary therapies is consistent with American Urological Association/Society of Urodynamics, Female Pelvic Medicine, and Urogenital Reconstruction (AUA/SUFU) treatment guidelines which recommend behavioral therapies as first-line treatments [8]. As such, it is not surprising that patients who added on pharmacotherapy to behavioral therapies had greater perceived symptom and HRQoL improvements. It is notable that Hispanic ethnicity was also associated with greater OAB-q SF symptom bother and HRQoL total score improvements. This is the first study to show that ethnicity may impact OAB treatment response, which warrants further exploration.
The safety data from this study are difficult to interpret because of imbalanced reporting of TEAEs in patients initiating mirabegron/solifenacin (mandatory reporting) and any other antimuscarinic (discretionary reporting), which prevents meaningful comparisons between the treatment groups. Pharmacovigilance requirements necessitate mandatory reporting of all AEs associated with pharmacotherapies manufactured by the sponsor [24]. Hence, physicians were obliged to report the AEs for mirabegron and solifenacin (manufactured by the sponsor, Astellas Pharma), but not the AEs associated with other treatments. The imbalance was evident when examining the proportions of patients who reported bother on the OAB-S medication tolerability scale and how often it was reported as a TEAE. As reporting of bother on the OAB-S medication tolerability scale for mirabegron/solifenacin initiators resulted in an alert for site staff to assess the side effect as a potential TEAE, symptom bother was more likely to be reported as a TEAE in mirabegron initiators than in antimuscarinic initiators. This yielded results that are inconsistent with data from the large patient population (> 10,000) who received mirabegron during the clinical development studies and that are contradictory with the mechanisms of action of the two drug classes [9, 10, 25]. In particular, the results of the present study indicate that anticholinergic TEAEs were more frequent with mirabegron than antimuscarinics, which is inconsistent with previously reported data [9, 10, 13] as well as the known pharmacodynamic properties of these agents. Furthermore, the frequency of TEAEs in the present study is considerably higher than the number of adverse drug reactions reported in two real-world Japanese surveillance studies [26, 27].
Consistent with observations from another real-world study [28], fewer mirabegron initiators were treatment naïve compared with antimuscarinic initiators; the greater proportion of mirabegron initiators who were treatment experienced may suggest more frequent use of mirabegron as a second-line pharmacotherapy. Differences were also noted between mirabegron and antimuscarinic initiators with regards to the HCP specialty who made the OAB diagnosis, as well as by country and drug coverage. However, interpretation of these findings is complicated by the confounding influences of differences in patient access to discounted/free medications. These observations highlight the complex interplay of factors affecting OAB treatment choice. Of all the treatment-seeking patients in this study, 76.3% had OAB with incontinence, despite the notion that OAB without incontinence is the more prevalent form [29]. This may be due to the high proportion of women recruited, who are more likely to have incontinence than men [30–33], or the possibility that incontinence may motivate patients to seek OAB treatment, as has been suggested by other studies [34, 35].
The strengths of this study include the generation of real-world data across a large, diverse patient group, with inclusion criteria more closely representing patients receiving OAB treatment in routine clinical practice than those enrolled according to the more restrictive requirements of a randomized clinical trial (RCT). Furthermore, the use of PROs to reflect patients’ perception of treatment effect provides insights relevant to routine patient management. Some limitations should be noted. As participation was voluntary for patients and doctors, the representativeness of the enrolled study sample with the general population is unknown. However, as it was based on both primary care and specialty sites, and enrollment was for patients in whom a decision had already been made to start a medication, it is probable that the present study was more representative of the real-world situation with OAB patients than RCTs. Although the demographics of the patients appear similar to other studies involving patients with OAB and a wide age range of patients were enrolled, the need for the patients to complete the PROs by email link may have introduced selection bias into this study. In addition, the number of patients who did not complete PROs or who were lost to follow-up may have reduced the strength of the study findings and also led to potential bias in the results. Although baseline characteristics were generally similar between patients who did/did not complete the study, patients who did not complete the study were more likely to be non-Hispanic, employed full-time, and have private insurance. Baseline characteristics were also generally similar between patients who did/did not complete any PROs, although patients who did not complete any PROs were more likely to be Hispanic, employed full-time, have private insurance, have OAB with incontinence, and be using pads. Furthermore, a greater proportion of mirabegron initiators discontinued the study compared with antimuscarinic initiators (18.3% vs. 13.0%), mostly prior to completion of the first PRO. In addition, the requirement for electronic completion of PROs and the 7-day time scale for completing baseline PROs may have been challenging for patients. The high level of mirabegron prescribing in Canada, combined with a high dropout rate before completion of the first PRO, may reflect lack of patient engagement in the patient-support program that may have resulted in study discontinuation. The response rate was lower than in the non-interventional BELIEVE study, which included scheduled follow-up visits [19]. The lack of scheduled follow-up visits was intended to interfere as little as possible with routine care, but likely contributed to the loss of information from both treatment groups and incomplete patient-reported information such as OAB medication usage and compliance. As for all observational studies, unmeasured variables related to mirabegron and antimuscarinic use and outcomes have the potential to confound the study results. In addition, antimuscarinic data were assessed for the entire group of therapeutics and analyses by individual agent were not conducted. Finally, TEAEs were not systematically collected for both mirabegron and antimuscarinic initiators, resulting in imbalanced reporting that prevented meaningful comparisons between the treatment groups and provided results inconsistent with previously reported studies [9, 10, 13].
Conclusion
The results from PERSPECTIVE affirm that mirabegron and antimuscarinics offer patients multiple medication options that are associated with clinically important improvements in patient-reported OAB symptom bother and HRQoL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the PERSPECTIVE study investigators and all patients who took part in the study. The authors would also like to thank Gretchen Otermat (Planet Pharma, LLC), Feng Zhan, and Eva Oakkar (IQVIA) for their support with the conduct of this study.
Funding
This study was funded by Astellas Pharma Global Development, Inc., Northbrook, IL, USA. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing Assistance
Medical writing support was provided by Emily Howard, CMPP of Elevate Scientific Solutions and funded by Astellas Pharma Global Development.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Kevin V. Carlson is a member of the PERSPECTIVE Scientific Advisory Committee that receive compensation from Astellas. Eric S. Rovner is a member of the PERSPECTIVE Scientific Advisory Committee that receive compensation from Astellas. Kavita V. Nair is a member of the PERSPECTIVE Scientific Advisory Committee that receive compensation from Astellas. Anna S. Deal is a member of the PERSPECTIVE Scientific Advisory Committee that receive compensation from Astellas. Rita M. Kristy is an employee of Astellas Pharma Global Development, Medical Affairs. Carol R. Schermer is an employee of Astellas Pharma Global Development, Medical Affairs.
Compliance with Ethics Guidelines
This study was conducted in accordance with all applicable laws and regulations, and the ethical principles originating from the Declaration of Helsinki. The protocol and any associated documents were approved by the institutional review board or independent ethics committee at each site prior to enrollment (Table S1). All patients (or their legally authorized representative) provided informed consent before participation in the study.
Data Availability
Access to anonymized individual participant level data will not be provided for this trial as it meets one or more of the exceptions described on http://www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas”.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.8118923.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014;33(5):622–624. doi: 10.1002/nau.22609. [DOI] [PubMed] [Google Scholar]
- 3.Powell LC, Szabo SM, Walker D, Gooch K. The economic burden of overactive bladder in the United States: a systematic literature review. Neurourol Urodyn. 2018;37(4):1241–1249. doi: 10.1002/nau.23477. [DOI] [PubMed] [Google Scholar]
- 4.Corcos J, Schick E. Prevalence of overactive bladder and incontinence in Canada. Can J Urol. 2004;11(3):2278–2284. [PubMed] [Google Scholar]
- 5.Herschorn S, Gajewski J, Schulz J, Corcos J. A population-based study of urinary symptoms and incontinence: the Canadian Urinary Bladder Survey. BJU Int. 2008;101(1):52–58. doi: 10.1111/j.1464-410X.2007.07198.x. [DOI] [PubMed] [Google Scholar]
- 6.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int. 2008;101(11):1388–1395. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 7.Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health-related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology. 2012;80(1):90–96. doi: 10.1016/j.urology.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193(5):1572–1580. doi: 10.1016/j.juro.2015.01.087. [DOI] [PubMed] [Google Scholar]
- 9.Chapple CR, Siddiqui E. Mirabegron for the treatment of overactive bladder: a review of efficacy, safety and tolerability with a focus on male, elderly and antimuscarinic poor-responder populations, and patients with OAB in Asia. Expert Rev Clin Pharmacol. 2017;10(2):131–151. doi: 10.1080/17512433.2017.1275570. [DOI] [PubMed] [Google Scholar]
- 10.Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17–30. doi: 10.1002/nau.22505. [DOI] [PubMed] [Google Scholar]
- 11.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54(3):543–562. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 12.Vouri SM, Kebodeaux CD, Stranges PM, Teshome BF. Adverse events and treatment discontinuations of antimuscarinics for the treatment of overactive bladder in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2017;69:77–96. doi: 10.1016/j.archger.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelleher C, Hakimi Z, Zur R, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol. 2018;74(3):324–333. doi: 10.1016/j.eururo.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Abrams P, Artibani W, Gajewski JB, Hussain I. Assessment of treatment outcomes in patients with overactive bladder: importance of objective and subjective measures. Urology. 2006;68(2 Suppl):17–28. doi: 10.1016/j.urology.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Chapple CR, Kelleher CJ, Evans CJ, et al. A narrative review of patient-reported outcomes in overactive bladder: what is the way of the future? Eur Urol. 2016;70(5):799–805. doi: 10.1016/j.eururo.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Rovner ES, Carlson KV, Deal AS, et al. A Prospective, non-intErventional Registry Study of PatiEnts initiating a Course of drug Therapy for overactIVE bladder (PERSPECTIVE): rationale, design, and methodology. Contemp Clin Trials. 2018;70:83–87. doi: 10.1016/j.cct.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Coyne KS, Thompson CL, Lai J-S, Sexton CC. An overactive bladder symptom and health-related quality of life short-form: validation of the OAB-q SF. Neurourol Urodyn. 2015;34(3):255–263. doi: 10.1002/nau.22559. [DOI] [PubMed] [Google Scholar]
- 18.Piault E, Evans CJ, Espindle D, Kopp Z, Brubaker L, Abrams P. Development and validation of the overactive bladder satisfaction (OAB-S) questionnaire. Neurourol Urodyn. 2008;27(3):179–190. doi: 10.1002/nau.20455. [DOI] [PubMed] [Google Scholar]
- 19.Freeman R, Foley S, Rosa Arias J, et al. Mirabegron improves quality-of-life, treatment satisfaction, and persistence in patients with overactive bladder: a multi-center, non-interventional, real-world, 12-month study. Curr Med Res Opin. 2018;34(5):785–793. doi: 10.1080/03007995.2017.1419170. [DOI] [PubMed] [Google Scholar]
- 20.Khullar V, Amarenco G, Angulo JC, et al. Patient-reported outcomes with the β3-adrenoceptor agonist mirabegron in a phase III trial in patients with overactive bladder. Neurourol Urodyn. 2016;35(8):987–994. doi: 10.1002/nau.22844. [DOI] [PubMed] [Google Scholar]
- 21.Herschorn S, Staskin D, Tu LM, et al. Patient-reported outcomes in patients with overactive bladder treated with mirabegron and tolterodine in a prospective, double-blind, randomized, two-period crossover, multicenter study (PREFER) Health Qual Life Outcomes. 2018;16:69. doi: 10.1186/s12955-018-0892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrams P, Kelleher C, Staskin D, et al. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: exploratory responder analyses of efficacy and evaluation of patient-reported outcomes from a randomized, double-blind, factorial, dose-ranging, phase II study (SYMPHONY) World J Urol. 2017;35(5):827–838. doi: 10.1007/s00345-016-1908-1. [DOI] [PubMed] [Google Scholar]
- 23.Robinson D, Kelleher C, Staskin D, et al. Patient-reported outcomes from SYNERGY, a randomized, double-blind, multicenter study evaluating combinations of mirabegron and solifenacin compared with monotherapy and placebo in OAB patients. Neurourol Urodyn. 2018;37(1):394–406. doi: 10.1002/nau.23315. [DOI] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality (US). Section 12, adverse event detection, processing, and reporting. In: Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for evaluating patient outcomes: a user’s guide [internet]. 3rd ed. Rockville (MD); 2014. https://www.ncbi.nlm.nih.gov/books/NBK208615/. Accessed Apr 30, 2019.
- 25.Yamada S, Ito Y, Nishijima S, Kadekawa K, Sugaya K. Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol Ther. 2018;189:130–148. doi: 10.1016/j.pharmthera.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Kato D, Tabuchi H, Uno S. Three-year safety, efficacy and persistence data following the daily use of mirabegron for overactive bladder in the clinical setting: a Japanese post-marketing surveillance study. Low Urin Tract Symptoms. 2019;11(2):O152–O161. doi: 10.1111/luts.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nozawa Y, Kato D, Tabuchi H, Kuroishi K. Safety and effectiveness of mirabegron in patients with overactive bladder in a real-world clinical setting: a Japanese post-marketing study. Low Urin Tract Symptoms. 2018;10(2):122–130. doi: 10.1111/luts.12148. [DOI] [PubMed] [Google Scholar]
- 28.Wagg AS, Foley S, Peters J, Nazir J, Kool-Houweling L, Scrine L. Persistence and adherence with mirabegron vs antimuscarinics in overactive bladder: retrospective analysis of a UK general practice prescription database. Int J Clin Pract. 2017;71(10):e12996. doi: 10.1111/ijcp.12996. [DOI] [PubMed] [Google Scholar]
- 29.Tubaro A. Defining overactive bladder: epidemiology and burden of disease. Urology. 2004;64(6 Suppl 1):2–6. doi: 10.1016/j.urology.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 30.Buckley BS, Lapitan MCM, Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children—current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010;76(2):265–70. [DOI] [PubMed]
- 31.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y-S, Lee K-S, Jung JH, et al. Prevalence of overactive bladder, urinary incontinence, and lower urinary tract symptoms: results of Korean EPIC study. World J Urol. 2011;29(2):185–190. doi: 10.1007/s00345-009-0490-1. [DOI] [PubMed] [Google Scholar]
- 33.Markland AD, Richter HE, Fwu C-W, Eggers P, Kusek JW. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol. 2011;186(2):589–593. doi: 10.1016/j.juro.2011.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimenez-Cidre M, Costa P, Ng-Mak D, et al. Assessment of treatment-seeking behavior and healthcare utilization in an international cohort of subjects with overactive bladder. Curr Med Res Opin. 2014;30(8):1557–1564. doi: 10.1185/03007995.2014.918028. [DOI] [PubMed] [Google Scholar]
- 35.Irwin DE, Milsom I, Kopp Z, Abrams P, Epic Study Group. Symptom bother and health care-seeking behavior among individuals with overactive bladder. Eur Urol. 2008;53(5):1029–37. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to anonymized individual participant level data will not be provided for this trial as it meets one or more of the exceptions described on http://www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas”.